Abstract
Cow bone charcoal (CBC) was synthesized and used for the removal of metals ions (manganese, iron, nickel and copper) from aqueous solutions. Two different adsorption models were used for analyzing the data. Adsorption capacities were determined: copper ions exhibit the greatest adsorption on cow bone charcoal because of their size and pH conditions. Adsorption capacity varies as a function of pH. Adsorption isotherms from aqueous solution of heavy metals on CBC were determined. Adsorption isotherms are consistent with Langmuir´s adsorption model. Adsorbent quantity and immersion enthalpy were studied.
1. Introduction
At present, adsorption is widely accepted in environmental treatment applications throughout the world. Liquid–solid adsorption systems are based on the ability of certain solids to preferentially concentrate specific substances from solutions onto their surfaces. This principle can be used for the removal of pollutants, such as metal ions and organics, from wastewaters [1,2,3,4]. Extensive research has been carried out during the last ten years to find low-cost, high capacity adsorbents for the removal of metal ions. A wide range of adsorbents have been developed and tested, including several activated carbons [5,6,7,8]. A number of low-cost agricultural wastes; mud tire rubber and fly ash have been used for the removal of a range of metal ions. Other minerals and materials with potential for exchange sorption with cadmium, copper and zinc have been tested; among those are sodium calcium bentonite and bone char [9,10,11,12,13,14,15,16]. Several natural resources have also been studied, including tree fern, peat coal and chitosan.
Bone charcoal, a mixed adsorbent containing around 10% carbon and 90% calcium phosphate, is mainly produced by thermal treatment of bones. Structurally, calcium phosphate in bone charcoal is in the hydroxyapatite form [16]. Bone charcoal has traditionally been used in the sugar refining industry to remove color from sugar solutions. Recent studies have used bone charcoal to adsorb radioisotopes of antimony and europium ions from radioactive wastes [16,18,19,20,21,22]. Those authors suggested that chemisorption was the main operating mechanism for 152Eu3+ removal from the aqueous solution with a high degree of irreversible fixation on bone charcoal. They claimed that sorption is due to cation exchange of metal ions onto hydroxyapatite.
In the present work the adsorption of manganese, iron, nickel and copper ions from solutions, onto bone charcoal in agitated batch absorber vessels was studied. The main goal of this study is to examine the ability of bone charcoal to remove these ions from aqueous solution and therefore evaluate its potential to be used in wastewater treatment systems.
2. Experimental Section
2.1. Absorbent: Bone charcoal
Discarded cow bone residues from a cattle abatoir in Bogotá (Colombia) were tested. The bone charcoal (BC) residue is the result of a pyrolysis process according to the following conditions:
The BC residue was maintained under an inert atmosphere to avoid any oxidation. It was crushed and sieved to give uniform particle size (~ 40 mesh size) for use in the different applications. CBC was characterized by chemical analysis and the results shown in Table 1. Surface area and pore size distributions were determined from nitrogen adsorption-desorption isotherms obtained at 77 K with an automatic instrument (Quantachrome 3B). Samples were previously outgassed at 523 K for several hours. N2 adsorption data at relative pressures ranging from 10−5 to 0.99 were analyzed according to BET method for calculating apparent surface area SBET. The BET surface area of prepared cow bone charcoal was found to be 283 m2∙g–1. Pore volume was found to be 0.287 cm3·g-1.

Table 1.
CBC physical and chemical properties.
| Property | Value |
|---|---|
| Carbon content | 11% |
| Hydrogen content | 1.6% |
| Nitrogen content | 4.3% |
| Chlorine content | 2.1% |
| Ca3(PO4)2 content | 77% |
| CaCO3 content | 3.9% |
| Others (Mg, Fe, SiO2) | less than 1.0% |
| BET area | 283 m2·g−1 |
| Pore Volume, Vp | 0.287 cm3·g−1 |
| Moisture | maximum 3% |
Full-size table
2.2. Adsorbates: Metal ions
Analytical grade manganese(II) nitrate [Mn(NO3)2], iron(II) sulfate (FeSO4·7H2O), nickel(II) nitrate [Ni(NO3)2·6H2O], and copper(II) nitrate [Cu(NO3)2 ·5H2O] reagents from J.T. Baker were used in the experiments. Stock solutions of metal ions were prepared using deionized water. Metal ion solutions concentrations of were determined by atomic absorption spectrometry (AAS).
2.3. Adsorption equilibrium isotherm
Batch sorption experiments were conducted using 100 mL aliquots of pH adjusted test solutions containing 100 mg∙L–1 of each one of the ions Mn2+, Fe2+, Ni2+ and Cu2+ in monocomponent systems and placed in 250 mL amber closed bottles. A known quantity (0.01–0.15 g) of CBC was added to each bottle. Solutions were stirred at 200 rpm for periods ranging between 5 and 110 min at 298 ± 1 K. The bone charcoal was removed by filtration and the Mn2+, Fe2+, Ni2+ and Cu2+ molar concentrations were measured by atomic absorption spectroscopy (in a Perkin Elmer AAnalyst equipment) at the end of each time period. Blank solutions were also prepared and analyzed. Solution pH changes as metal ion concentration changes during the adsorption process. A previous survey was made to determine the solution pH which produces the maximum adsorption. The pH values of each metal ion solution were adjusted using either 0.01 N NaOH or 0.01 N HNO3 solutions and the volumes used were recorded to calculate the final solution volume.
2.4. Immersion enthalpy
Immersion enthalpies of CBC were determined in solutions of Mn2+, Fe2+, Ni2+ and Cu2+ with concentrations ranging from 20 to 100 mg∙L−1 for the maximum adsorption pH of 5.1. Immersion enthalpies were also determined for 100 mg∙L−1 solutions at all pH values studied. This determination was performed in a heat conduction microcalorimeter equipped with a stainless steel calorimetric cell [23]. Thirty mL of the solution to be used were pre-heated at 298 K, then placed in the cell. A sample of approximately 0.500 g CBC was weighed and placed inside the calorimetric cell in a glass ampoule. The microcalorimeter was then assembled. When the equipment reached the temperature of 298 K, potential readings were registered after a period of approximately 15 minutes, with readings every 20 seconds, the glass ampoule was broken and the generated thermal effect recorded. Electric potential readings were continue for approximately 15 minutes more and at the end of the experiment, the equipment was electrically calibrated.
3. Results and Discussion
3.1. Effect of cow bone charcoal dosage on adsorption
Figure 1 shows the removal of Mn2+, Fe2+, Ni2+ and Cu2+ from aqueous solutions of pH 5.1 as a function of added CBC. Cow bone charcoal dosage ranged from 0.01 to 0.08 g for the 100 mL of Mn2+, Fe2+, Ni2+and Cu2+ test solutions, equilibrated for 60 min. It can be seen that the maximum removal expressed as a percentage was between 75% and 98% from Mn to Cu at dosages between 0.02 g and 0.03 g of CBC. Ion removal increased quickly from 0.01 g to 0.02 g CBC dosages and reached a maximum for 0.03 g CBC. This fact may be associated with the M2+ ion availability at pH 5.1. From pKh values, it can be concluded that, at pH 5.1, Mn2+ ions have a concentration 1,500 times greater than Cu2+ ions. On the other hand, hydrated Mn2+ ions have a volume almost 30% bigger than hydrated Cu2+ ions. Mn2+ ions are more likely to be in solution rather than adsorbed. The observed constancy in percentage ion removal beyond 0.03 g/100 mL may be an indicative of a very weak interaction between adsorbent and adsorbate. This interaction appears weaker with Mn2+ ions than with Cu2+ ions. Ion solution concentration seems to attain a steady state with adsorbed species, and so, no matter the quantity of adsorbent present, there will be a residual concentration of ions in solution. This fact determines a specific relation between ion concentration and adsorbent quantity.
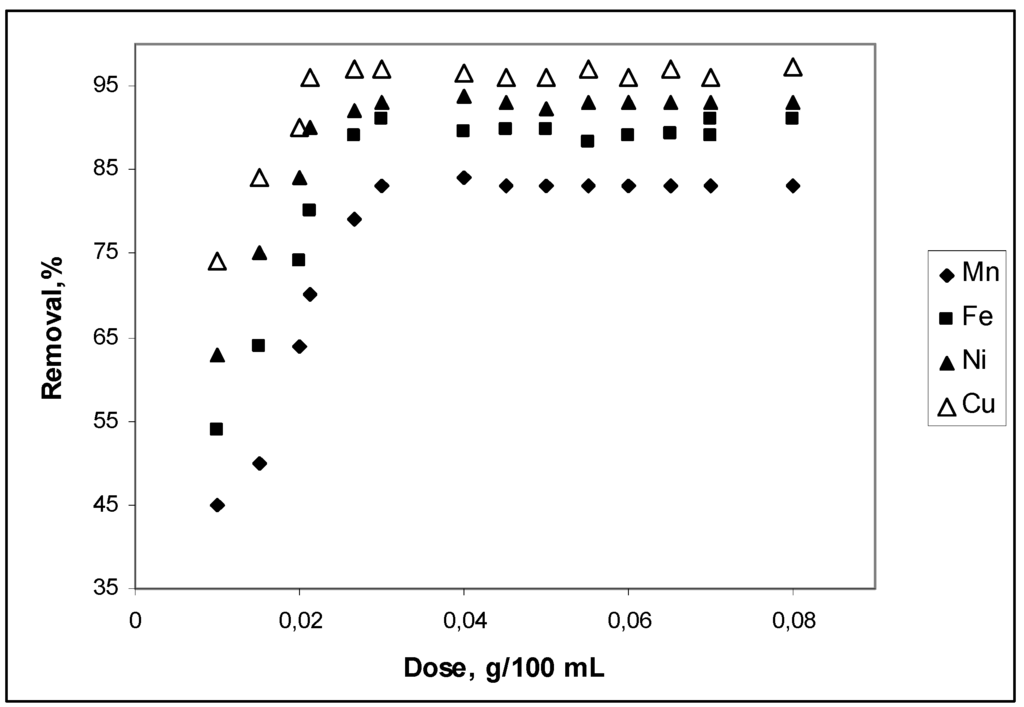
Figure 1.
CBC adsorbent dosage effect on Mn2+, Fe2+, Ni2+ and Cu2+ removal. Conditions: Co, 20 mg·L–1; time of contact, 60 min; pH 5.1 and temperature, 298 K.
Adsorption of metal ions on these types of materials is generally attributed to weak interactions between the adsorbents and adsorbates. Surface charges on substrates, as well as softness or hardness of the solutes are mostly responsible for the intensity of these interactions. Coulombic interactions can be observed for the ionic interexchange of cationic species with anionic sites in the materials and is determined by their surface areas.
3.2. Effect of contact time
Figure 2 shows contact time effect on the CBC removal of 20 mg∙L–1 Mn2+, Fe2+, Ni2+ and Cu2+. Removal increases with time and reaches a maximum after 20 min of agitation. Nevertheless, the order of affinity for the adsorbate is maintained: Mn2+ < Fe2+< Ni2+< Cu2+. This is associated with the size of the ion and the pore development in cow bone charcoal.
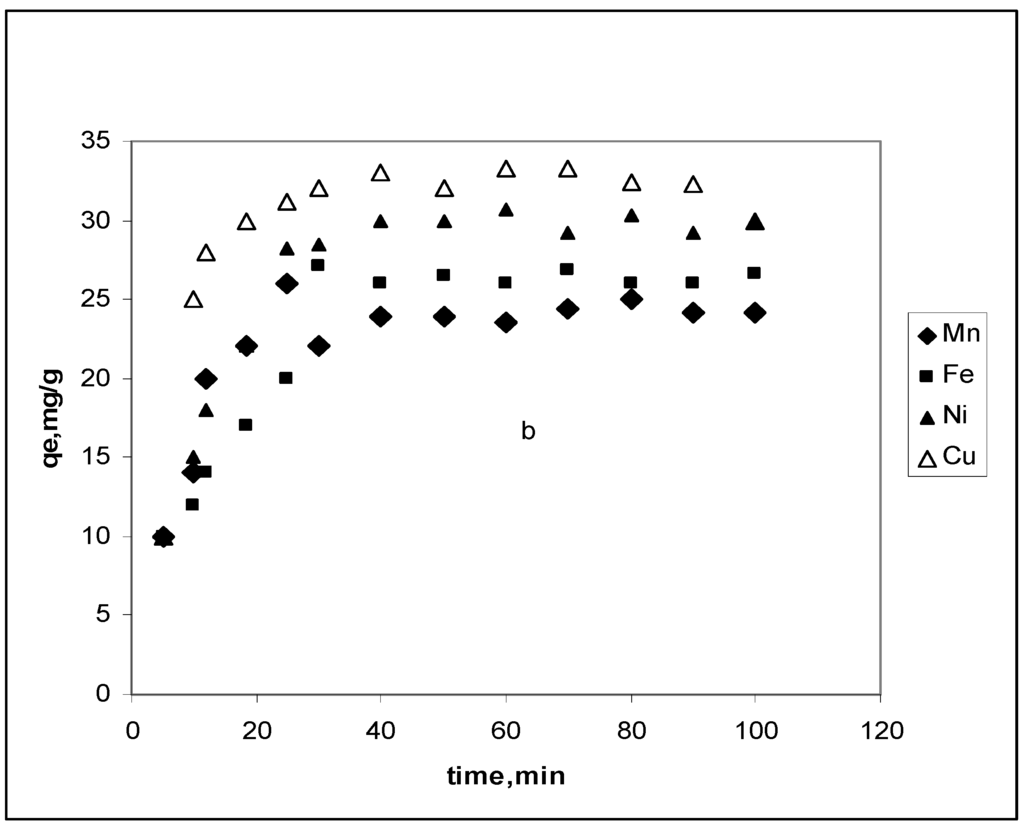
Figure 2.
CBC contact time effect on Mn2+, Fe2+, Ni2+ and Cu2+ removal. Conditions: Co, 20 mg∙L–1; CBC dose, 0.02 g; pH 5.1 and temperature, 298 K.
To analyze the sorption rates of Mn2+, Fe2+, Ni2+ and Cu2+ ions onto the CBC, two simple kinetic models were tested.
3.2.1. Pseudo-first-order model
The pseudo-first order rate expression, popularly known as the Lagergren equation, is generally described by the following equation (Lagergren, 1898) [26]:
where, qe is the amount of the metal ions adsorbed at equilibrium per unit weight of sorbent (mg∙g–1); q is the amount of metal ions adsorbed at any time (mg∙g–1). Besides, kad is the rate constant min–1. Integrating and applying boundary conditions, t = 0 and qt = 0 to t = t and q = qt, Equation 1 takes the form:
However, if the intercept does not equal the natural logarithm of the equilibrium uptake of metal ions, the reaction is not likely to be first-order, even if this plot has a high correlation coefficient with the experimental data [27]. Correlation coefficients were found to be between 0.9245 and 0.9865. The correlation coefficients are shown on Table 2, together with the Lagergren rate constants calculated from the slope of Equation 2 [26]. In order to obtain rate constants, the straight-line plots of ln (qe – qt) against t (time) were made (not shown here). This gave fairly straight lines for all four metal ions on the CBC. The intercept of this plot should give ln qe.

Table 2.
Lagergren rate equation constants and pseudo second-order rate equation constants for Mn2+, Fe2+, Ni2+ and Cu2+ adsorption on CBC.
| Lagergren rate equations constants | |||
| Metal ions | kad × min. | qe × (g·mg–1) | R2 |
| Mn2+ | 0.023 | 8.5 | 0.9443 |
| Fe2+ | 0.028 | 9.3 | 0.9765 |
| Ni2+ | 0.022 | 12.4 | 0.9865 |
| Cu2+ | 0.018 | 15.8 | 0.9245 |
| Pseudo second-order rate equation constants | |||
| Metal ions | ho × ( min·g·mg–1) | qe × (g·mg–1) | R2 |
| Mn2+ | 734.6 | 22.4 | 0.9991 |
| Fe2+ | 1546.6 | 26.7 | 0.9993 |
| Ni2+ | 1546.5 | 29.7 | 0.9999 |
| Cu2+ | 1656.6 | 33.2 | 0.9999 |
3.2.2. Pseudo-second-order model
The adsorption data was also analyzed in terms of a pseudo-second order mechanism given by [27]:
where, k2 is the rate constant (mg·g–1·min–1). Integrating the above equation and applying boundary conditions, i.e., t = 0 for q = 0 and t = t for q = qt, gives:
here, ho is the initial adsorption rate. If the second-order kinetics is applicable, the plot of t/q against t in equation 4 should give a linear relationship from which the constants qe and ho can be determined (plot not show here). Linear model gave a good fit to the experimental data. This means that the adsorption can be described by a pseudo-second order rate equation, hence qe and ho were evaluated and presented in Table 2. R2 values are approximate the same for all four metal ions on CBC with a value of 0.9999. In the limit at initial adsorption time, ho is defined as [28]:
k2 was calculated for the four metal ions and are shown in Table 2. The results obtained are similar to a previous study (Horsfall, et al., 2004) [28].

Table 3.
Hydrolysis constants and ionic volumes of metals ions.
| Heavy Metals Ions | Mn2+ | Fe2+ | Ni2+ | Cu2+ |
|---|---|---|---|---|
| pKh | 10.7 | 10.1 | 9.40 | 7.53 |
| Hydrated volume (cm3·mol–1) | 189.6 | 174.5 | 147.8 | 147.8 |
M2+ + H2O → MOH+ + H+
MOH+ + H2O → M(OH)2 + H+
M2+ + 2H2O → M(OH)2 + 2H+
It can be seen that metal ions are easily adsorbed when the hydrated ionic size decreases. Ionic sizes of the heavy metals used change in the order of Mn2+ > Fe2+ > Ni2+ > Cu2+ (Table 3). Since CBC is used is a microporous adsorbent [21], metals penetrate easily into these pores when the ionic size becomes small [22]. According to the order above, Mn2+ must be the least adsorbed and Cu2+ must be the most easily adsorbed. This is compatible with our experimental results. On the other hand, high spin, transition metals complexes exhibits stabilities according to Irving-Williams series. Studied systems agree with this behavior suggesting that metal complex with the adsorbent may play an important role in adsorption processes.
3.3. Effect of pH
Mn2+, Fe2+, Ni2+ and Cu2+ uptake as a function of hydrogen ion concentration was determined for pH values from 2 to 14. Below pH 5.1, hydrogen ions are likely to compete with manganese, iron, nickel and copper ions. At pH values above 8 manganese, iron, nickel and copper might precipitate as hidroxides. pH effects at equilibrium are presented in Figure 3. Maximum adsorption was observed about pH 5.1. In general, results indicated that the adsorption is highly pH dependant. Similar results have been reported in literature [20] S.K. Srivastava, R. Tyagi and N. Pant, Water Res. 23 (1989), pp. 1161–1165. Abstract | View Record in Scopus | Cited By in Scopus (106) [19].
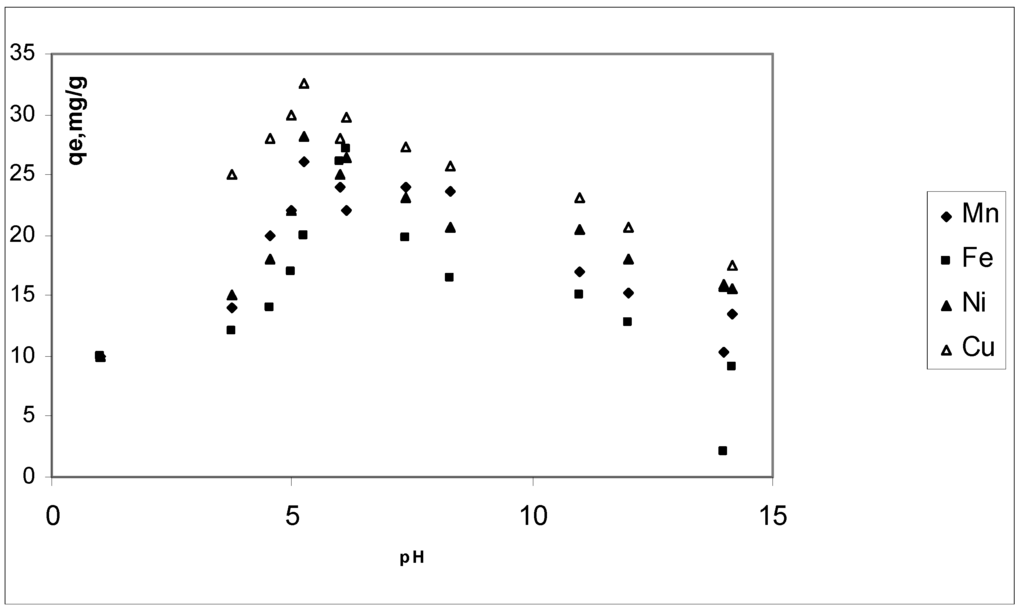
Figure 3.
pH effect on CBC adsorption of Mn2+, Fe2+, Ni2+ and Cu2+. Conditions: C0, 20 mg∙L–1; CBC dose, 0.02 g; contact time, 20 min and temperature, 298 K.
pH values affect the species of heavy metals in aqueous solutions, and heavy metal removal increases as pH value rises, reaching a maximum around 5.1. Solution pH also affects the adsorbent and the surface charge of the CBC changes. Solubility product (Ksp) calculations predict that the formation of Cu(OH)2, occurs at a pH value of 6. Precipitation occurs at pH 6, along with a qe of 26.7 mg∙g–1. On the other hand, the qe has a value of 35 mg∙g–1 when the initial pH was 5.1 (final pH of 2). This means that the removal of copper ions from the solution also contributes to the pH modification. However, at low initial pH values, below 4, the influence of adsorption is the only effect responsible for the reducing of copper ions in the solution. This suggests that the process is a suitable application for heavy metals removal because of its neutral and clean effluent.
3.4. Adsorption isotherms from aqueous solution
When the initial metal concentration rises, adsorption increases, while the binding sites are not saturated. Linear Langmuir isotherm allows the calculation of adsorption capacities and the Langmuir constants and is performed by the following equation:
Linear plots of ceq/q vs ceq (not shown), were used to calculate by means of linear regression equations, the parameters of the Langmuir isotherm. From these regression equations and the linear plots, the values of the Langmuir constants were calculated and are shown on Table 2. qmax and b were obtained from the slope and intercept of the plots. The essential characteristic of the Langmuir isotherms can be expressed in terms of a dimensionless constant separation factor or equilibrium parameter, RL, which is defined as [32]:
where b is the Langmuir constant and co is the initial concentration of the metal ions. RL value indicates the shape of the isotherm. RL values between 0 and 1 indicate favorable absorption [33]. RL equal to 0 indicate irreversible absorption, RL = 1 is linear and RL > 1 is unfavorable. From our study, RL values for Mn2+, Fe2+, Ni2+ and Cu2+ ions adsorption ranged from 0.0050 to 0.0060. This, for an initial metal ions concentration of 600 mg∙L–1, therefore, the adsorption process is favorable.
The Freundlich isotherm was chosen to estimate the adsorption intensity of the adsorbent towards the adsorbate. It is represented by the equation [34]:
where ceq is the equilibrium concentration (mg∙L–1), q is the ion amount adsorbed (mg∙g–1) and KF and n are constants incorporating all parameters affecting the adsorption process, such as adsorption capacity and intensity respectively. Linear form of Freundlich adsorption isotherm was used to evaluate the sorption data and is represented as [34]:
The linear regression equation for the Freundlich adsorption isotherm is shown on Table 4. Values of KF and n were calculated from the intercepts and slopes of the Freundlich plots respectively and are shown on this table. Adsorption is favorable for values 0.1 < 1/n < 1.0 [35].

Table 4.
Isotherm parameters of Mn2+, Fe2+,Ni2+ and Cu2 adsorption on cow bone charcoal.
| Freundlich model | Langmuir model | |||||||
|---|---|---|---|---|---|---|---|---|
| Metal | Linear KD (L/g) | KF | 1/n | R2 | qmax (mg/g) | b (L/g) | RL | R2 |
| Mn2+ | 6.76 | 14.457 | 0.315 | 0.9587 | 29.56 | 1.12 | 0.006 | 0.9987 |
| Fe2+ | 6.99 | 23.545 | 0.425 | 0.9643 | 31.43 | 1.18 | 0.005 | 0.9988 |
| Ni2+ | 7.89 | 26.876 | 0.643 | 0.9745 | 32.54 | 1.25 | 0.005 | 0.9988 |
| Cu2+ | 8.88 | 34.865 | 0.759 | 0.9876 | 35.44 | 1.34 | 0.005 | 0.9999 |
The Freundlich equation frequently gives an adequate description of adsorption data over a restricted range of concentration, even though it is not based on any theoretical background. Apart from a homogeneous surface, the Freundlich equation is also suitable for a highly heterogeneous surface and an adsorption isotherm lacking a plateau, indicating a multi-layer adsorption [36]. Values of 1/n, less than unity are an indication that significant adsorption takes place at low concentration but the increase in the amount adsorbed with concentration becomes less significant at higher concentration and vice versa [37]. The magnitude of KF and n, shows that it is possible an easy separation of heavy metal ion from aqueous solution and a high adsorption capacity. Also, as the KF value increases, the greater the adsorption intensity. Therefore, the higher KF values for Cu2+ confirms by these model that the adsorption capacity of is greater than that of the others ions. On the other hand, a relatively high R2 value indicates that this model is adjusted more confidently; this parameter is shown in the Table 4. According to the obtained values, the Langmuir model fits better the experimental data of the present study. Figure 4a and b shows adsorption isotherms related to Mn2+, Fe2+, Ni2+ and Cu2+ adsorption from aqueous solution on CBC. Continuous lines represent the non-linear regression adjustment of these isotherms fitted the Freundlich adsorption isotherm model and Langmuir isotherm model.
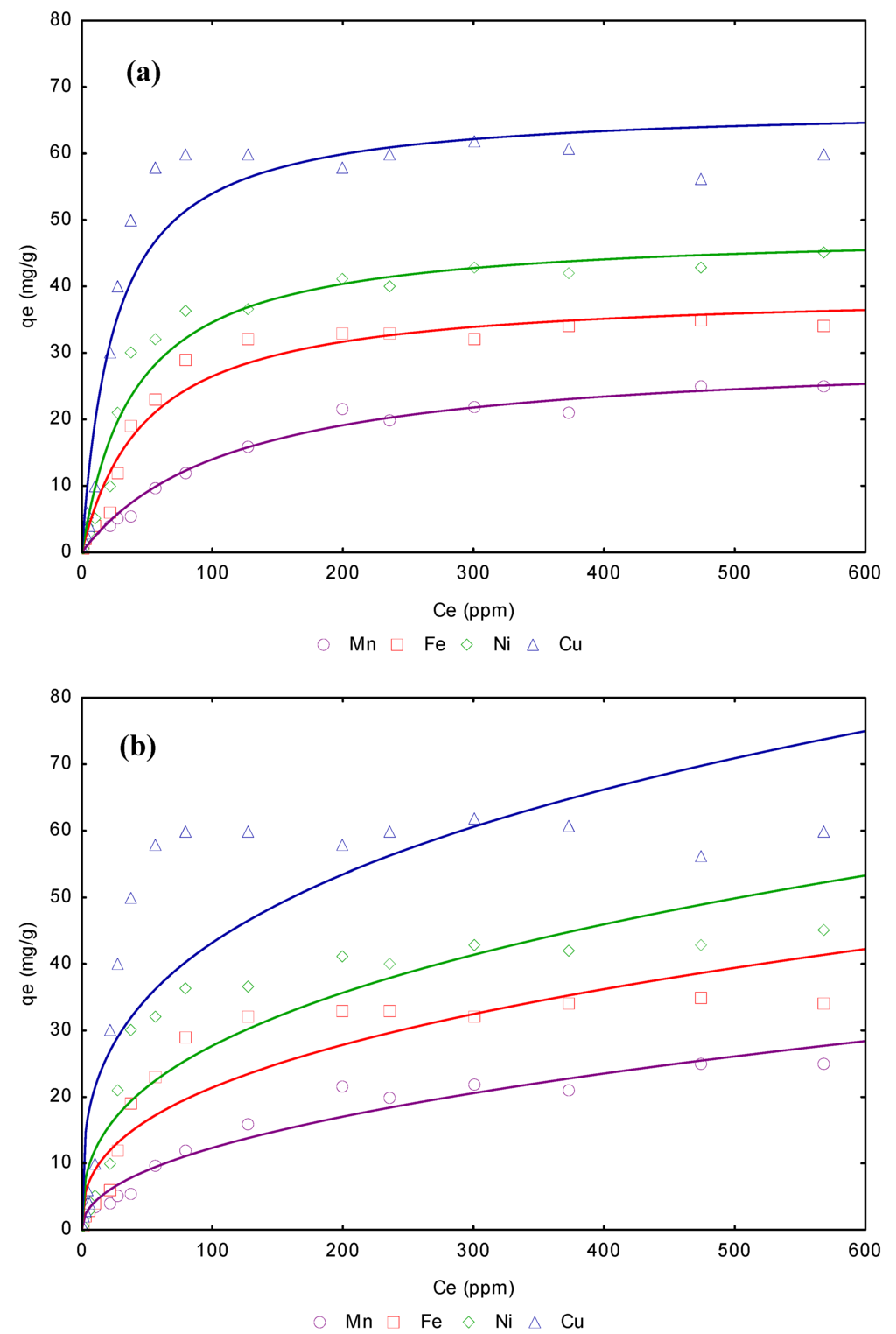
Figure 4.
(a). CBC adsorption isotherms removal of Mn2+, Fe2+,Ni2+ and Cu2+ from aqueous solution, Langmuir model. (b). CBC adsorption isotherms removal of Mn2+, Fe2+,Ni2+ and Cu2+ from aqueous solution, Freundlich model.
3.5. Immersion enthalpies
Results show that immersion enthalpies are constant at low initial concentrations. Initial concentrations above 40 mg∙L–1 exhibited a steady increase up to 90 mg∙L–1. The highest value of enthalpy was obtained for the immersion of cow bone charcoal in the copper ions solutions, while the lower value of immersion enthalpy was obtained for the immersion of cow bone charcoal in the solutions of manganese. Enthalpy values were between –60 J∙g–1 (Cu2+–CBC) and –45 J∙g–1 (Mn2+–CBC), as shown in Figure 5. This behavior agrees with textural characteristics of cow bone charcoal and the sizes of the ions under study. It should be noted that the behavior of immersion enthalpies in the solid prepared in this work, is very similar to that of an isotherm.
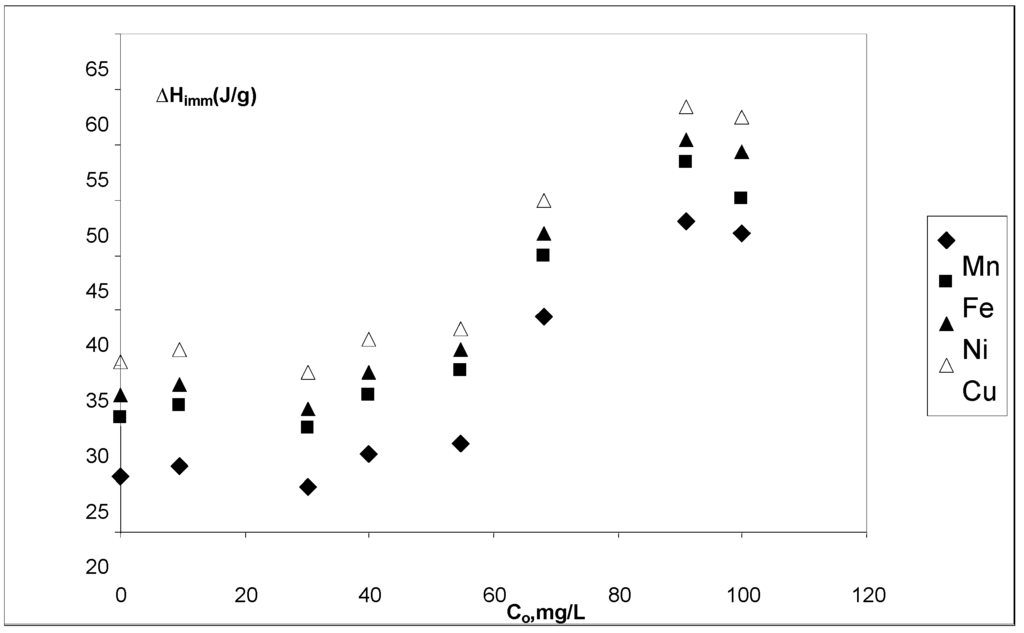
Figure 5.
Immersion enthalpies for Mn2+, Fe2+, Ni2+ and Cu2+ aqueous solutions ions concentration at pH 5.1. T = 298 K.
3.6. Removal of Mn2+, Fe2+, Ni2+ and Cu2+ from wastewater
As an approximation of the results of the present work for application to a real problem, we proposed use an industrial wastewater sample. For that purpose we chose waste from a textile industry for which the content of studied metals was determined. The sample was carefully treated with the aim of performing an analysis of each one of the ions of interest and we evaluated the adsorption capacity as the sole comparison parameter. They are analyzed one by one in order to avoid multicomponent system generation which could produce bias in the obtained results.
Wastewater samples collected in our research laboratory from a textile industry were found to contain more of 500 mg∙L–1 of Mn2+, Fe2+, Ni2+ and Cu2+, among other organic and inorganic components. Six samples were treated with nitric acid, followed by pH adjustment and sorption with CBC under optimized conditions described before. Metal ions were analyzed one at a time by atomic absorption spectrometry, using a complexing agent to avoid interference of ions different from that analyzed. Assay for manganese, iron, nickel and copper in the final effluents indicates 75.0% maximum removal of the ions originally present in the samples. The minimum removal was 53% for Mn(II). Mean standard deviation was 1.0%. These results show that CBC is an suitable material for use in the removal of these ions. However these findings should be analyzed carefully because of, in spite of procedures employed in order to avoid interferences in the assays, the sample complexity does not allow us to assure confidence in the results.
3.7. Mechanism of adsorption
Although cow bone charcoal displays relative low surface area (283 m2∙g–1), it shows high copper removal capacity (34.9 mg∙g–1). Cow bone charcoal analysis indicates that it consists of calcium phosphate as a major component (Table 1). It has been demonstrated that calcium phosphate acts not only as a source of adsorption centers but also enables ion-exchange process [24,25]:
≡PO− + H+ → ≡POH
≡CaOH2+ → ≡CaOH + H+
In the presence of Cu2+, the following reactions may occur:
≡POH + Cu2+ → ≡POCu+ + H+
≡PO− + Cu2+ → ≡POCu+
≡CaOH + Cu2+ → ≡CaOCu+ + H+
This mechanism is similar to that previously suggested for the sorption of Zn2+ and Ca2+ on calcium phosphate [24].
4. Conclusions
From the experiments, it can be concluded that the CBC has the ability to retain Mn2+, Fe2+, Ni2+ and Cu2 metals ions from aqueous solutions at the studied concentrations. Removal of heavy metals (manganese, iron, nickel and copper) from aqueous solution was possible using a activated carbon obtained from cow bone (CBC). It was seen that adsorption took place for the four metals within 25 minutes for the concentration levels studied. Under our experimental conditions and for the studied heavy metals pH plays an important role in the adsorption process, particularly on the adsorption capacity. The pH selected for an optimal rate of adsorption for all ions investigated is 5.1. It is shown that CBC has a relatively high adsorption capacity for these heavy metals; the quantities adsorbed per gram of CBC at equilibrium (qe) are 29.56 mg∙g–1 for Mn2+, 31.43 mg∙g–1 for Fe2+, 32.54 mg∙g–1 for Ni2+ and 35.44 mg∙g–1 for Cu2+. This adsorption is described by an isotherm of type I and is fully matched by the Langmuir isotherm. The kinetics of the manganese, iron, nickel and copper adsorption on the CBC was found to follow a pseudo-second-order rate equation. This method has an additional advantage, as it could be applied in developing countries due to the low cost.
Acknowledgements
Authors wish to thank Master Agreement established between Universidad de los Andes and Universidad Nacional de Colombia, and the Understanding Memorandum between Departments of Chemistry of both Universities. Special thanks to Fondo Especial de la Facultad de Ciencias and Proyecto Semilla of Universidad de los Andes for the partial financial support of this research.
References and Notes
- Netzer, A.; Hughes, D.E. Adsorption of copper, lead and cobalt by activated carbon. Water Res. 1984, 18, 927–933. [Google Scholar] [CrossRef]
- Ngah, W.S.W.; Endud, C.S.; Mayanar, R. Removal of copper(II) ions from aqueous solution onto chitosan and cross-linked chitosan beads. React. Funct. Polym. 2002, 50, 181–190. [Google Scholar] [CrossRef]
- Wang, K.; Xing, B. Adsorption and desorption of cadmium by goethite pretreated with phosphate. Chemosphere 2002, 48, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Keith, K.H.C.; Gordon, M. Sorption of cadmium, copper, and zinc ions onto bone char using Crank diffusion model. Chemosphere 2005, 60, 1141–1150. [Google Scholar] [CrossRef] [PubMed]
- Cheung, K.C.; Venkitachalam, T.H. Improving phosphate removal of sand infiltration system using alkaline fly ash. Chemosphere 2000, 41, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.; Parwate, A.V.; Bhole, A.G. Removal of Cr6+ and Ni2+ from aqueous solution using bagasse and fly ash. Waste Manag. 2002, 22, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, E. Phosphate removal from water by fly ash using crossflow micro-filtration. Sep. Purif. Technol. 2004, 35, 241–252. [Google Scholar] [CrossRef]
- Gray, C.A.; Schwab, A.P. Phosphorus-fixing ability of high pH, high calcium coal-combustion waste materials. Water Air Soil Pollut. 1993, 69, 309–320. [Google Scholar] [CrossRef]
- Keith, K.H.; McKay, G. Study of arsenic(V) adsorption on bone char from aqueous solution. J Hazard Mater. 2008, 160, 845–854. [Google Scholar]
- Purevsuren, B.; Avid, B.; Narangerel, J.; Gerelmaa, T.; Davaajav, Y. Investigation on the pyrolysis products from animal bone. J. Mater. Sci. 2004, 9, 737–740. [Google Scholar] [CrossRef]
- Wilson, J.A.; Pulford, I.D.; Thomas, S. Sorption of Cu and Zn by bone charcoal. Environ. Geochem. Health. 2003, 21, 51–56. [Google Scholar] [CrossRef]
- Jiang, J.Q. Removing arsenic from groundwater for the developing world—a review. Water Sci. Technol. 2001, 44, 89–98. [Google Scholar] [PubMed]
- Wu, F.C.; Tseng, R.L.; Juang, R.S. Comparisons of porous and adsorption properties of carbons activated by steam and KOH. Colloid Interf. Sci. 2005, 283, 49–56. [Google Scholar] [CrossRef]
- Nasiruddin, M.; Farooq, M.; Wahab, M. Characterization of chemically modified corncobs and its application in the removal of metal ions from aqueous solution. Hazard. Mater. 2007, 141, 237–244. [Google Scholar] [CrossRef]
- Liu, S.X.; Chen, X.; Chen, X.Y.; Liu, Z.F.; Wang, H.L. Activated carbon with excellent chromium(VI) adsorption performance prepared by acid–base surface modification. Hazard. Mater. 2007, 141, 315–319. [Google Scholar] [CrossRef]
- Pattanayak, J.; Mondal, K.; Mathew, S.; Lalvani, S.B. A parametric evaluation of the removal of As(V) and As(III) by carbon-based adsorbents. Carbon 2000, 38, 589–596. [Google Scholar] [CrossRef]
- Li, Q.; Zhai, J.; Zhang, W.; Wang, M.; Zhou, J. Kinetic studies of adsorption of Pb(II), Cr(III) and Cu(II) from aqueous solution by sawdust and modified peanut husk. Hazard. Mater. 2007, 141, 163–167. [Google Scholar] [CrossRef]
- Garg, U.K.; Kaur, M.P.; Garg, V.K.; Sud, D. Removal of hexavalent chromium from aqueous solution by agricultural waste biomass. J. Hazard. Mater. 2007, 40, 60–68. [Google Scholar] [CrossRef]
- Kavitha, D.; Namasivayam, D. Recycling coir pith, an agricultural solid waste, for the removal of portion orange from wastewater. Dyes Pigments 2007, 4, 237–248. [Google Scholar] [CrossRef]
- Ofomaja, A.E.; Ho, Y.S. Effect of pH on cadmium biosorption by coconut copra meal. Hazard. Mater. 2007, 139, 356–362. [Google Scholar] [CrossRef]
- Chojnacka, K.; Górecka, H.; Górecki, H. The influence of living habits and family relationships on element concentrations in human hair. Sci. Total Environ. 2006, 366, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-B.; Zhu, Y.-G.; Ma, Y.-B.; McKay, G. Effect of bone char application on Pb bioavailability in a Pb-contaminated soil. Environ. Pollut. 2006, 39, 433–439. [Google Scholar] [CrossRef]
- Giraldo, L.; Cubillos, G.I.; Moreno, J.C. Evaluación de las pérdidas térmicas en calorimetría isoperibólica. Importancia de los alrededores en la obtención de constantes instrumentales. Rev. Colomb. Quim. 2005, 34, 147–154. [Google Scholar]
- Findon, A.; Mckay, O.; Blair, H.S. Transport studies for the sorption of copper ions by chitosan. J. Environ.Sci. Heal. A 1993, 28, 173–185. [Google Scholar]
- Weber, W.J.; Digiano, F.A. Process dynamics in Environmental systems. In Environmental Science and Technology Service; Wiley and Sons: New York, NY, USA, 1996; pp. 89–94. [Google Scholar]
- Lagergren, S. About the theory of so-called adsorption of soluble substances. K. Sven. Vetenskapsakademiens Handlingar 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; McKay, G. Sorption of dye from aqueous solution by peat. Chem. Eng. J. 1998, 70, 115–124. [Google Scholar] [CrossRef]
- Horsfall, M.; Spiff, A.I. Studies on the effect of pH on the sorption of Pb2+ and Cd2+ ions from aqueous solutions by caladium bicolor (wild cocoyam) biomass. Electron. J. Biotech. 2004, 7, 1–7. [Google Scholar]
- Pearson, R.G. Chemical Hardness; Wiley-VCH: Weinheim, Germany, 1997. [Google Scholar]
- Chong, K.H.; Volesky, B. Description of two-metal biosorption equilibria by Langmuir-type models. Biotechnol. Bioeng. 1995, 47, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Ahalya, N.; Kanamadi, R.D.; Ramachandra, T.V. Biosorption of chromium (VI) from aqueous solutions by the husk of Bengal gram (Cicer arientinum). Electronic. J. Biotechnol. 2005, 8, 258–264. [Google Scholar] [CrossRef]
- Mckay, G.; Blair, H.S.; Gardener, J.R. Adsorption of dyes on chitin I. Equilibrium Studies. J. Appl. Polym. Sci. 1982, 27, 3043–3057. [Google Scholar] [CrossRef]
- Freundlich, H. Ueber die Adsorption in Loesungen. Z. Phys. Chem. 1907, 57, 385–470. [Google Scholar]
- Kardivalu, K.; Namasivayan, C. Agricutural by-product as metal adsorbent: Sorption of lead(II) from aqueous solution onto coirpith carbon. Environ. Technol. 2000, 21, 1091–1097. [Google Scholar] [CrossRef]
- Juang, R.S.; Wu, F.C.; Tseng, R.L. Adsorption removal of copper(II) using chitosan from simulated rinse solutions containing chelating agent. Water Res. 1999, 33, 2403–2409. [Google Scholar] [CrossRef]
- Hsisheng, C.-T.H. influence of surface characteristics on liquid-phase adsorption of phenol by activated carbons prepared from bituminous coal. Ind. Eng. Chem. Res. 1998, 39, 3618–3624. [Google Scholar]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).