Abstract
The structural evolution of Ho2Ce2O7 under high pressure was systematically investigated using synchrotron X-ray diffraction (up to 31.5 GPa) and Raman spectroscopy (up to 41.7 GPa). At ambient pressure, the compound adopts a common C-type cubic rare earth oxide structure (space group Ia-3). A pressure-induced phase transition was observed to commence at 23.8 GPa, characterized by a gradual structural evolution that persisted through the maximum experimental pressure of 31.5 GPa. This transition involves cation disordering accompanied by coordination environment modifications. High-pressure X-ray diffraction analysis reveals the coexistence of two distinct phases above the transition threshold: the parent cubic phase (Ia-3) and a metastable hexagonal phase (R-3c). Notably, the high-pressure phase configuration persists upon complete decompression to ambient conditions, demonstrating the irreversible nature of this pressure-induced structural transition.
1. Introduction
Rare earth-doped cerium oxide compounds have attracted significant interest due to their remarkable proton conductivity, low thermal conductivity, good mechanical properties and high thermal expansion coefficients [1,2,3,4,5,6,7,8,9]. Among these, RE2Ce2O7 (RE = rare earth elements) has gained attention as a promising candidate for applications in solid oxide fuel cells, photocatalysis and electrochemical hydrogen storage [10,11,12,13,14,15,16,17]. According to M.A. Subramanian et al. [18], the disorder of A2B2O7 solid solution where A is a trivalent lanthanide and B is a tetravalent cation is critically governed by the ionic radius ratio of A3+ and B4+ cations. The pyrochlore structure remains stable when 1.46 ≤ r(A3+)/r(B4+) ≤ 1.78, while a lower ratio of r(A3+)/r(B4+) results in the formation of a disordered fluorite structure (if it is disordered). Yamamura et al. [19] suggests that a fluorite (F-type) structure (accompanying the rare earth C-type superstructure) will form for RE2Ce2O7 when the ionic radius ratio of r(RE3+)/r(Ce4+) is lower than 1.17. For instance, Ho2Ce2O7 has garnered significant attention due to its high oxygen vacancies and proton conductivity, but its crystal structure is poorly understood. Since the tolerance factor (τ) of Ho2Ce2O7 is 0.97, which is slightly less than 1.17, it would be considered to have a disordered fluorite structure (accompanying the rare earth C-type superstructure).
As is well known, pressure is a powerful tool to modify the structure and properties of various materials. Under high pressure, the pyrochlore-type Nd2Ir2O7 oxide was reported to undergo a pressure-induced magnetic phase transition [20]. The pressure-tuned insulator-metal transition was found in Eu2Ir2O7 [21]. The photoluminescence properties of Ho2Sn2O7 were significantly enhanced as a result of the structure phase transition induced by pressure treatment [22]. The fluorite-type La2Ce2O7 oxide can occur via structural phase transition by varying temperature or pressure [23]. These extreme experimental conditions can be used to effectively change the crystal structure, including changing the interatomic distance and polyhedral distortion. At ambient pressure, RE2Ce2O7 oxides exhibit notable proton conductivity owing to their unique crystal structure with abundant oxygen vacancies [3,10,11,24,25]. We propose a pressure-induced structural engineering strategy to enhance oxygen vacancy density in the RE2Ce2O7 oxide, thereby establishing optimized proton migration channels and significantly improving the protonic conduction efficiency within this material system.
Previous studies on Ho2O3-doped CeO2 solid solutions primarily focused on the structures under different doping ratios [26]. However, the structural evolution of Ho2Ce2O7 under high pressure remains largely unexplored. In this study, we employ in situ synchrotron X-ray diffraction and Raman spectroscopy to investigate the structural and optical evolution of Ho2Ce2O7 under hydrostatic compression up to 31.5 GPa and 41.7 GPa. Our results reveal a phase transition in Ho2Ce2O7 occurring near 23.8 GPa. This transition involves cation disordering accompanied by coordination environment modifications. Furthermore, the high-pressure phase remains stable even after complete decompression, providing compelling evidence for an irreversible structural phase transition. Investigating the pressure-induced structural evolution and optical response in Ho2Ce2O7 oxide provides crucial insights for rational design of advanced functional materials with remarkable proton conductivity.
2. Materials and Methods
A high-quality Ho2Ce2O7 sample was synthesized by solid-state reaction with the raw materials Ho2O3 (99.99%) and CeO2 (99.99%) [23]. The raw materials were thoroughly mixed in a stoichiometric ratio (Ho2O3: CeO2 = 1:2) and calcined at 1673 K for 12 h. High-pressure experiments were performed using a symmetric diamond anvil cell (DAC) equipped with 400 μm diamond culet anvils. The sample was loaded into a 120 μm diameter hole that was drilled in the center of the T301 stainless steel gasket with a pre-indented thickness of 50 μm. The pressure was calibrated using the standard ruby fluorescent technique [27]. Silicone oil was employed as a pressure-transmitting medium to provide quasi-hydrostatic pressure conditions [28]. High-pressure experiments were conducted under ambient temperature.
In situ high-pressure angle-dispersive X-ray diffraction (ADXRD) measurements up to 31.5 GPa were carried out at a beam line of BL04-MSPD in the ALBA synchrotron light facility with monochromatic X-ray beam (λ = 0.4246 Å). The monochromatic X-ray beam with a wavelength of 0.4246 Å was focused to a spot size of approximately 20 × 20 µm (full width at half maximum (FWHM)). Diffraction data were collected using a Rayonix SX165 charge-coupled device (CCD) detector. For instrument calibration, high-purity LaB6 powder was employed as the standard to determine the sample-to-detector distance. DIOPTAS 0.5.7 software was used to integrate the two-dimensional diffraction patterns to one-dimensional intensity versus two theta plots. The Rietveld method was employed to analyze the structure model of diffraction patterns; the GSAS program was utilized to refine ADXRD profiles. High-pressure Raman scattering measurements were conducted up to 41.3 GPa, utilizing a micro-Raman spectrometer (Renishaw) with a 532 nm excitation laser. The Raman signals were recorded in backscattering geometry through a triple polychromator spectrometer equipped with a volume transmission grating, and the laser power was maintained at 50 mW. Sample irradiation was achieved using an Olympus objective lens (20.5 mm focal length, 0.35 numerical aperture) to focus the laser beam onto the sample surface. The Raman spectral peaks were deconvoluted using the Gaussian fitting function in OriginPro 2017 software, with their characteristic positions calibrated against standard reference spectra [29,30].
3. Results and Discussion
To investigate the structural stability of Ho2Ce2O7 under high-pressure conditions, a systematic study was conducted using synchrotron X-ray diffraction. Figure 1a shows the selected ADXRD patterns of Ho2Ce2O7 up to 31.5 GPa. At lower pressures, all diffraction peaks can be indexed to a C-type rare earth structure. As the pressure increases, all diffraction peaks shift to higher 2θ values (no the initial peaks disappear or new peaks appear). The broadening and weakening of peaks in Ho2Ce2O7 may be caused by less hydrostatic condition at pressures above 12 GPa when there is a glass transition of the pressure-transmitting medium silicone oil [28]. At 23.8 GPa, a structural phase transition was occurring with the appearance of an additional diffraction peak (~8.5°), which is the strongest diffraction peak from the high-pressure phase. Meanwhile, the new diffraction peak gradually became stronger with pressure. The pressure-induced phase transition in Ho2Ce2O7 is sluggish, similar to those observed in other pyrochlore or fluorite-structured oxides [23,31]. Remarkably, the initial crystalline phase remained predominant throughout the compression cycle, suggesting that the transformation remained incomplete at the maximum applied pressure of 31.5 GPa. A previous investigation demonstrated that the fluorite-type structure in La2Ce2O7 undergoes a partly reversible pressure-induced phase transition [23]. However, the high-pressure phase of Ho2Ce2O7 was retained after fully releasing pressure, which indicates that the structural phase transition was irreversible.
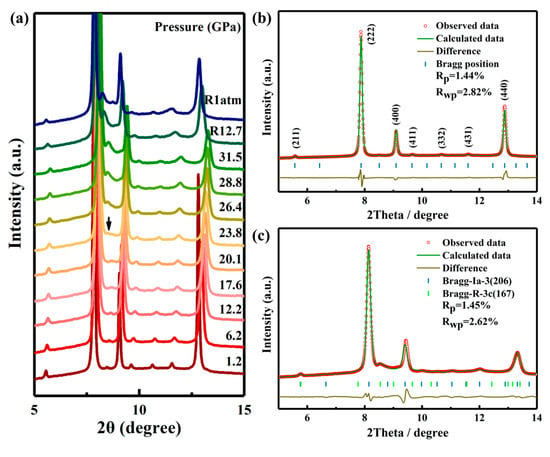
Figure 1.
(a) Representative ADXRD patterns of Ho2Ce2O7 at various pressures. (b,c) show the Rietveld refinements of ADXRD patterns collected at 1.2 and 28.8 GPa, respectively. The brown lines denote the difference between the observed (red circles) and calculated (green lines) profiles, and the blue and green verticals stand for the simulated peak positions.
In order to obtain more information about the phase transition, high-pressure structural evolution of Ho2Ce2O7 was determined by Rietveld refinement of the ADXRD pattern via GSAS. As shown in Figure 1b, the good refinement between the observed pattern and the calculated result (Rp = 1.44%, Rwp = 2.82%) can be used to illustrate that the diffraction pattern was C-type rare earth phase (Ia-3 (No. 206)) at 1.2 GPa and a lattice constant a = 10.7090 (6) Å. This is the same as the model structure of Gd2Ce2O7 [32]. Figure 2a illustrates the crystal structure of Ho2Ce2O7 oxide, where RE (Ho/Ce) cations occupy 8b and 24d Wyckoff positions with sixfold or eightfold oxygen coordination. Meanwhile, O atoms partly occupy 16c and 48e Wyckoff positions. The cation distribution is characterized by statistically equivalent local oxygen environments for all sites. All cations exhibit uniform spatial distribution and identical local oxygen coordination environments regardless of their chemical identity. Figure 2b shows the possible positions of oxygen vacancies (16c Wyckoff position of x = y = z ≈ 0.391 (2)), where partial occupancy of these sites by oxygen atoms occurs in a randomized spatial arrangement. Under high pressure, the cubic phase of pyrochlores or fluorite transformed into an orthorhombic phase with a defect cotunnite-type structure [33,34]. In2O3 with a C-type rare earth structure underwent a transformation to a hexagonal corundum-type structure under high pressure [35,36]. The crystal structure of Ho2Ce2O7 exhibited cubic symmetry for pressure values from ambient pressure to 23.8 GPa. At 23.8 GPa, the new diffraction peaks clearly corresponded to the hexagonal phase with space group (R-3c), and the lattice parameters were a = 5.7075 (8) Å and c = 16.2623 (6) Å. The phenomena are similar to the research of D. Liu et al. [35]. Above 23.8 GPa, the crystal structure of Ho2Ce2O7 indicates that that cubic phase (Ia-3) and hexagonal phase (R-3c) coexist. The high-pressure coexistent phase remained up to the highest pressure values we reached; the fitted pattern at 28.8 GPa with Rp = 1.45% and Rwp = 2.62% is shown in Figure 1c. The determined structural parameters are listed in Table 1.
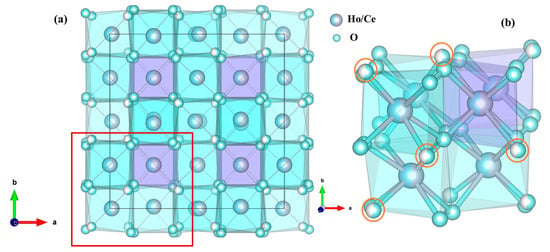
Figure 2.
(a) The corresponding crystal structure of the cubic Ho2Ce2O7. (b) A magnified view of the marked region.

Table 1.
The refined atomic coordinates of the cubic phase and the high-pressure phase of Ho2Ce2O7 at 1.2 and 28.8 GPa, respectively.
The lattice volume decreased monotonically with increasing pressure as well as a volume collapse occurred at the transition pressure of ~23.8 GPa. As presented in Figure 3a, the P-V diagrams of Ho2Ce2O7 are fitted with a third-order Birch–Murnaghan equation of state (EOS) [37,38]:
where yields the bulk modulus of B0 = 170.1 (±5.5) GPa, initial volume V0 = 1229.2 (±1.7) Å3 and pressure derivative B0′ = 14 for the cubic phase below 23.8 GPa. The large B0′ could be attributed to pressure-dependent distortions of the REO6 (RE = Ho/Ce) octahedron. The bulk modulus demonstrates slight enhancement compared to the typical fluorite-type La2Ce2O7 (144 GPa) [23]. This may be attributed to the smaller ionic radii of Ho3+ and Ce4+, which narrows the cubic structure. The bulk modulus B0 = 349.1 (±12.9) GPa (with B0′ fixed at 4) for the hexagonal phase was estimated from the patterns obtained at high pressure. As shown in Table 2, a substantial volume shrinkage was 700.5 Å3 (approximately 62%) at the phase transition pressure. The phase transition was accompanied by chemical bond cleavage between atoms and reconstruction of anion and cation sublattices. Figure 3b illustrates the pressure-dependent relative lattice parameter shrinkage (a/a0) for cubic Ho2Ce2O7. The compression ratio (a/a0) was reduced by 2.7% in the pressure range from ambient to 23.8 GPa. Notably, in the high-pressure hexagonal phase, the c/a ratio of Ho2Ce2O7 exhibited a gradual decrease from 2.849 to 2.846 as pressure increased from 23.8 to 31.5 GPa. This gradual shrinkage demonstrates that the material experiences slightly greater compressibility along the c-axis compared to the a-axis under hydrostatic compression.
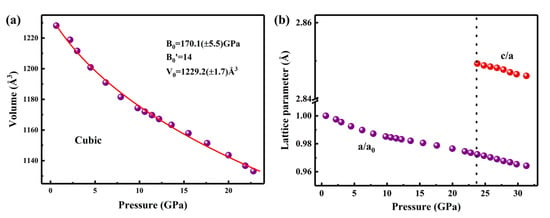
Figure 3.
(a) Evolution of cell volume at high pressure. The line refers to the fitted third-order Birch−Murnaghan equation of state for the cubic phase. (b) The pressure-dependent relative com-pression of a/a0 (violet) and c/a (red) for Ho2Ce2O7.

Table 2.
Unit cell parameters and volumes of Ho2Ce2O7 under varying pressure conditions. Above 23.8 GPa, the lattice constants of the cubic phase and the hexagonal phase are given because they both exist in significant amounts.
To investigate the local structural evolution of Ho2Ce2O7 under high pressure, Raman spectra of Ho2Ce2O7 were systematically conducted up to 41.7 GPa at ambient temperature (Figure 4a). At 1.5 GPa, six vibrational modes were observed (ν1: 200 cm−1; ν2: 272 cm−1; ν3: 387 cm−1; ν4: 489 cm−1; ν5: 569 cm−1; ν6: 618 cm−1), which correspond to the characteristic coordination environments of F-type (CeO2) and C-type (Ho2O3) phases [30]. According to A. Nakajima et al. [39], the band around 600 cm−1 split into two distinct peaks at different polarizations in similar compounds. The broad and weak ν5 band, which exhibits significant overlap and poor resolution from the ν6 peak, originates from intrinsic oxygen vacancies [30]. The high-intensity broad vibrational modes were attributed to structural disorder and the large number of oxygen vacancies in the structure. The ν4 mode was assigned to the F2g symmetric vibration mode of the Ce-O bond with Ce eightfold coordination, serving as the fingerprint of the CeO2 structure [40,41]. The C-type structure was identified through ν3 mode, which resulted from the (Ag + Fg) symmetrical stretching vibration mode of Ho-O with Ho sixfold coordination. The ν2, ν5 and ν6 modes were attributed to the interaction between the oxygen vacancies and the six next-nearest-neighbor oxygen atoms [39]. Additionally, the ν1 mode arose from the interaction between the oxygen vacancies and the four nearest-neighbor metal atoms [39].
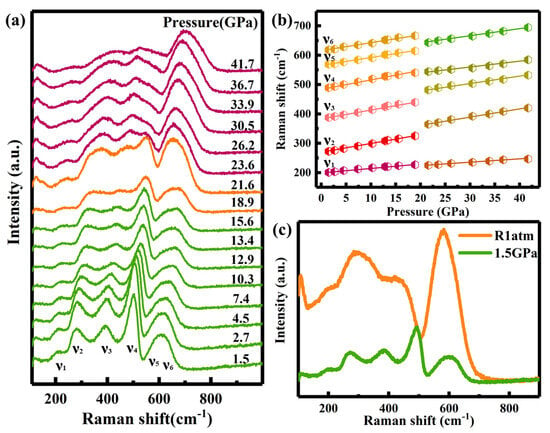
Figure 4.
(a) Raman spectra of Ho2Ce2O7 at different pressures were recorded during increasing pressure. (b) The Raman mode frequency shifts of Ho2Ce2O7 shifting as a function of pressure. (c) Raman spectra at 1.5 GPa and full decompression (R1atm) from 41.7 GPa.
As showed in Figure 4b, the observed Raman vibrational modes were monotonously shifted to higher wavenumbers with increasing pressure up to 21.6 GPa. The gradual broadening and weakening of Raman vibrational modes may be caused by the change in disorder degree and in polarizability of bonds at pressures above 10.3 GPa. Above 21.6 GPa, the Raman spectra exhibited unambiguous evidence of a change in structural symmetry. The abnormal discontinuities in the Raman vibrational modes and the weakening of the ν4 mode indicated the phase transition in Ho2Ce2O7. Above 23.6 GPa, the Raman modes of ν3 started to prevail over ν4. Meanwhile, the dominance of ν5 demonstrated that the interaction between the oxygen vacancies and the six next-nearest-neighbor oxygen atoms became increasingly active under high pressure. These results indicated that ordered cations transition to a disordered state and lead to the variation of the coordination environment. As shown in Figure 4c, the Raman modes of the high-pressure phase were retained after decompression without returning to their original states, demonstrating the irreversible phase transition. These changes in the Raman spectra were consistent with the results of X-ray diffraction data.
4. Conclusions
In this study, the crystal structure of Ho2Ce2O7 under high pressure was studied up to 31.5 GPa and 41.7 GPa by means of synchrotron X-ray diffraction and Raman spectroscopy measurements. Rietveld refinements of ADXRD showed that the ambient pressure phase (Ia-3) remained stable up to 23.8 GPa at room temperature. At 23.8 GPa, a sluggish structural phase transition occurred and remained uncompleted up to 31.5 GPa. The high-pressure phase is the coexistence of the parent cubic phase (Ia-3) and the metastable hexagonal phase (R-3c). Above 23.6 GPa, the Raman mode changes showed that ordered cations transition to a disordered state with the variation of the coordination environment. The high-pressure phase is retained after fully releasing pressure, which indicates that the structural phase transition was irreversible.
Author Contributions
Data curation, L.Y., J.Q. and X.W.; Formal analysis, X.W. and Y.L.; Funding acquisition, X.W.; Investigation, T.L.; Resources, P.Z.; Software, J.Q., Y.L., L.Y. and Q.T.; Writing—original draft, T.L.; Writing—review and editing, P.Z.; X.W. and T.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zhang, H.; Sun, J.; Duo, S.; Zhou, X.; Yuan, J.; Dong, S.; Yang, X.; Zeng, J.; Jiang, J.; Deng, L.; et al. Thermal and mechanical properties of Ta2O5 doped La2Ce2O7 thermal barrier coatings prepared by atmospheric plasma spraying. J. Eur. Ceram. Soc. 2019, 39, 2379–2388. [Google Scholar] [CrossRef]
- Yang, G.; El Loubani, M.; Chalaki, H.R.; Kim, J.; Keum, J.K.; Rouleau, C.M.; Lee, D. Tuning Ionic Conductivity in Fluorite Gd-Doped CeO2-Bixbyite RE2O3 (RE = Y and Sm) Multilayer Thin Films by Controlling Interfacial Strain. ACS Appl. Electron. Mater. 2023, 5, 4556–4563. [Google Scholar] [CrossRef] [PubMed]
- Kalland, L.-E.; Løken, A.; Bjørheim, T.S.; Haugsrud, R.; Norby, T. Structure, hydration, and proton conductivity in 50% La and Nd doped CeO2–La2Ce2O7 and Nd2Ce2O7–and their solid solutions. Solid State Ion. 2020, 354, 115401. [Google Scholar] [CrossRef]
- Gao, L.; Guo, H.; Gong, S.; Xu, H. Plasma-sprayed La2Ce2O7 thermal barrier coatings against calcium–magnesium–alumina–silicate penetration. J. Eur. Ceram. Soc. 2014, 34, 2553–2561. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, H.; Gong, S. Thermal shock resistance and mechanical properties of La2Ce2O7 thermal barrier coatings with segmented structure. Ceram. Int. 2009, 35, 2639–2644. [Google Scholar] [CrossRef]
- Zhao, A. Novel Nanofibrous Dy2Ce2O7 as an Electrocatalyst for Methanol Oxidation. Int. J. Electrochem. Sci. 2019, 14, 8121–8130. [Google Scholar] [CrossRef]
- Ping, X.; Meng, B.; Li, C.; Lin, W.; Chen, Y.; Fang, C.; Zhang, H.; Liang, W.; Zheng, Q. Thermophysical and electrical properties of rare-earth-cerate high-entropy ceramics. J. Am. Ceram. Soc. 2022, 105, 4910–4920. [Google Scholar] [CrossRef]
- Ismail, S.A.; Han, D. Phase behavior, proton concentration, and conductivity of La2Ce2O7 doped with Y, Ho, Er, Tm, or Yb. J. Am. Ceram. Soc. 2022, 105, 7548–7557. [Google Scholar] [CrossRef]
- Han, W.; Li, Z.; Liu, H. La2Ce2O7 supported ruthenium as a robust catalyst for ammonia synthesis. J. Rare Earths 2019, 37, 492–499. [Google Scholar] [CrossRef]
- Zhu, Z.; Liu, B.; Shen, J.; Lou, Y.; Ji, Y. La2Ce2O7: A promising proton ceramic conductor in hydrogen economy. J. Alloys Compd. 2016, 659, 232–239. [Google Scholar] [CrossRef]
- Zhong, Z.; Jiang, Y.; Lian, Z.; Song, X.; Peng, K. Exploring the effects of divalent alkaline earth metals (Mg, Ca, Sr, Ba) doped Nd2Ce2O7 electrolyte for proton-conducting solid oxide fuel cells. Ceram. Int. 2020, 46, 12675–12685. [Google Scholar] [CrossRef]
- Jiang, Y.; Song, X.; Zhong, Z.; Lian, Z.; Peng, K. Sintering and electrochemical performance of Nd2Ce2O7 electrolyte with Bi7WO13.5 sintering aid for proton conductor solid oxide fuel cells. J. Alloys Compd. 2020, 836, 155539. [Google Scholar] [CrossRef]
- Zinatloo-Ajabshir, S.; Salehi, Z.; Salavati-Niasari, M. Synthesis of dysprosium cerate nanostructures using Phoenix dactylifera extract as novel green fuel and investigation of their electrochemical hydrogen storage and Coulombic efficiency. J. Clean. Prod. 2019, 215, 480–487. [Google Scholar] [CrossRef]
- Zinatloo-Ajabshir, S.; Salehi, Z.; Amiri, O.; Salavati-Niasari, M. Green synthesis, characterization and investigation of the electrochemical hydrogen storage properties of Dy2Ce2O7 nanostructures with fig extract. Int. J. Hydrog. Energy 2019, 44, 20110–20120. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, J.; Xu, X.; Xi, R.; Liu, Y.; Fang, X.; Wang, X. Tailoring La2Ce2O7 catalysts for low temperature oxidative coupling of methane by optimizing the preparation methods. Catal. Today 2020, 355, 518–528. [Google Scholar] [CrossRef]
- Dolatalizadeh, M.; Behzad, M.; Khademinia, S.; Arab, A. Experimentally Designed Natural Light Induced Photocatalytic Performance of Nanostructured Eu2Ce2O7 Synthesized by a Facile Solid State Method in Removal of Environmental Pollutant Malachite Green (MG). Proc. Natl. Acad. Sci. India Sect. A Phys. Sci. 2020, 91, 9–20. [Google Scholar] [CrossRef]
- Zinatloo-Ajabshir, S.; Salavati-Niasari, M. Preparation of magnetically retrievable CoFe2O4@SiO2@Dy2Ce2O7 nanocomposites as novel photocatalyst for highly efficient degradation of organic contaminants. Compos. Part B Eng. 2019, 174, 106930. [Google Scholar] [CrossRef]
- Subramanian, M.A.; Aravamudan, G.; Rao, G.V.S. Oxide pyrochlores—A review. Prog. Solid State Chem. 1983, 15, 55–143. [Google Scholar] [CrossRef]
- Yamamura, H.; Nishino, H.; Kakinuma, K.; Nomura, K. Crystal Phase and Electrical Conductivity in the Pyrochlore Type Composition Systems, Ln2Ce2O7 (LnüLa, Nd, Sm, Eu, Gd, Yand Yb). J. Ceram. Soc. Jpn. 2003, 111, 902–906. [Google Scholar] [CrossRef]
- Sakata, M.; Kagayama, T.; Shimizu, K.; Matsuhira, K.; Takagi, S.; Wakeshima, M.; Hinatsu, Y. Suppression of metal-insulator transition at high pressure and pressure-induced magnetic ordering in pyrochlore oxide Nd2Ir2O7. Phys. Rev. B 2011, 83, 041102. [Google Scholar] [CrossRef]
- Tafti, F.F.; Ishikawa, J.J.; McCollam, A.; Nakatsuji, S.; Julian, S.R. Pressure-tuned insulator to metal transition inEu2Ir2O7. Phys. Rev. B 2012, 85, 205104. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, K.; Li, N.; Ma, S.; Wang, Y.; Kong, Q.; Baudelet, F.; Wang, X.; Yang, W. Tricolor Ho3+ Photoluminescence Enhancement from Site Symmetry Breakdown in Pyrochlore Ho2Sn2O7 after Pressure Treatment. Phys. Rev. Lett. 2020, 125, 245701. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.X.; Tracy, C.L.; Lang, M.; Ewing, R.C. Stability of fluorite-type La2Ce2O7 under extreme conditions. J. Alloys Compd. 2016, 674, 168–173. [Google Scholar] [CrossRef]
- Tu, T.; Zhang, B.; Liu, J.; Wu, K.; Peng, K. Synthesis and conductivity behaviour of Mo-doped La2Ce2O7 proton conductors. Electrochim. Acta 2018, 283, 1366–1374. [Google Scholar] [CrossRef]
- Choudhary, B.; Anwar, S. Probing the Transport Properties, Electrophoretic Deposition, and Electrochemical Performance of La2–xSmxCe2O7 Ceramics for IT-SOFCs. ACS Appl. Energy Mater. 2023, 6, 11817–11827. [Google Scholar] [CrossRef]
- Mandal, B.P.; Roy, M.; Grover, V.; Tyagi, A.K. X-ray diffraction, μ-Raman spectroscopic studies on CeO2−RE2O3 (RE=Ho, Er) systems: Observation of parasitic phases. J. Appl. Phys. 2008, 103, 033506. [Google Scholar] [CrossRef]
- Mao, H.K.; Xu, J.; Bell, P.M. Calibration of the ruby pressure gauge to 800 kbar under quasi-hydrostatic conditions. J. Geophys. Res. 1986, 91, 4673–4676. [Google Scholar] [CrossRef]
- Klotz, S.; Chervin, J.C.; Munsch, P.; Le Marchand, G. Hydrostatic limits of 11 pressure transmitting media. J. Phys. D Appl. Phys. 2009, 42, 075413. [Google Scholar] [CrossRef]
- Banerji, A.; Grover, V.; Sathe, V.; Deb, S.K.; Tyagi, A.K. system: Unraveling of microscopic features by Raman spectroscopy. Solid State Commun. 2009, 149, 1689–1692. [Google Scholar] [CrossRef]
- Coduri, M.; Scavini, M.; Pani, M.; Carnasciali, M.M.; Klein, H.; Artini, C. From nano to microcrystals: Effects of different synthetic pathways on the defect architecture in heavily Gd-doped ceria. Phys. Chem. Chem. Phys. 2017, 19, 11612–11630. [Google Scholar] [CrossRef]
- Zhang, F.X.; Lian, J.; Becker, U.; Ewing, R.C.; Hu, J.; Saxena, S.K. High-pressure structural changes in the Gd2Zr2O7 pyrochlore. Phys. Rev. B 2007, 76, 214104. [Google Scholar] [CrossRef]
- Patel, M.K.; Baldinozzi, G.; Aguiar, J.A.; Valdez, J.A.; Vogel, S.C.; Sickafus, K.E. Structural analysis of Gd2Ce2O7. MRS Proc. 2015, 1743, mrsf14-1743. [Google Scholar] [CrossRef]
- Zhang, F.; Zhao, Y.; Zhao, X.; Li, Y.; Tao, Q.; Zhu, P.; Wang, X. Pressure-induced structural transition of pyrochlore Tm2Sn2O7. J. Alloys Compd. 2023, 963, 171248. [Google Scholar] [CrossRef]
- Turner, K.M.; Rittman, D.R.; Heymach, R.A.; Tracy, C.L.; Turner, M.L.; Fuentes, A.F.; Mao, W.L.; Ewing, R.C. Pressure-induced structural modifications of rare-earth hafnate pyrochlore. J. Phys. Condens. Matter 2017, 29, 255401. [Google Scholar] [CrossRef]
- Liu, D.; Lei, W.W.; Zou, B.; Yu, S.D.; Hao, J.; Wang, K.; Liu, B.B.; Cui, Q.L.; Zou, G.T. High-pressure x-ray diffraction and Raman spectra study of indium oxide. J. Appl. Phys. 2008, 104, 083506. [Google Scholar] [CrossRef]
- Qi, J.; Liu, J.F.; He, Y.; Chen, W.; Wang, C. Compression behavior and phase transition of cubic In2O3 nanocrystals. J. Appl. Phys. 2011, 109, 063520. [Google Scholar] [CrossRef]
- Murnaghan, F.D. The Compressibility of Media under Extreme Pressures. Proc. Natl. Acad. Sci. USA 1944, 30, 244–247. [Google Scholar] [CrossRef]
- Birch, F. Finite Elastic Strain of Cubic Crystals. Phys. Rev. 1947, 71, 809–824. [Google Scholar] [CrossRef]
- Nakajima, A.; Yoshihara, A.; Ishigame, M. Defect-induced Raman spectra in doped CeO2. Phys. Rev. B 1994, 50, 13297–13307. [Google Scholar] [CrossRef]
- Weber, W.H.; Hass, K.C.; McBride, J.R. Raman study of CeO2: Second-order scattering, lattice dynamics, and particle-size effects. Phys. Rev. B 1993, 48, 178–185. [Google Scholar] [CrossRef]
- McBride, J.R.; Hass, K.C.; Poindexter, B.D.; Weber, W.H. Raman and x-ray studies of Ce1-xREXO2-y, where RE=La, Pr, Nd, Eu, Gd and Tb. J. Appl. Phys. 1994, 76, 2435–2441. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).