Abstract
Hybrid materials are a recent addition in the field of restorative dentistry for computer-aided design/computer-aided manufacturing (CAD/CAM) indirect restorations. The long-term clinical success of modern dental restorative materials is influenced by multiple factors. Among the characteristics affecting the longevity of a restoration, the mechanical properties and physicοchemical interactions are of utmost importance. While numerous researchers constantly evaluate mechanical properties, the biological background of resin-based CAD/CAM biomaterials is scarcely investigated and, therefore, less described in the literature. This review aims to analyze biofilm formation on the surfaces of novel, hybrid, resin-based CAD/CAM materials and evaluate the methodological protocols followed to assess microbial growth. It is demonstrated that the surface structure, the composition and the finishing and polishing procedures on the surface of a dental restorative material influence initial bacterial adhesion; however, most studies focus on in vitro protocols, and in vivo and/or in situ research of microbiomics in CAD/CAM restorative materials is lacking, obstructing an accurate understanding of the bioadhesion phenomenon in the oral cavity.
1. Introduction
Significant advances in the field of restorative dentistry have led to the transition from older metallic dental materials for direct restorations, such as the dental amalgam, to more esthetic, tooth-colored and “tooth-friendly” counterparts, namely composite resin materials. The polymerization process is the critical drawback concerning using these restorative materials for direct intraoral applications. Residual monomers and polymerization shrinkage reduce their clinical success [1]. Further disadvantages of direct resin-based restorations include inferior mechanical strengths, rapid occlusal and proximal wear, marginal discoloration, loss of integrity, a low fracture toughness and postoperative sensitivity [2]. The limitations of this direct, sensitive approach have been partially overcome by the development of nano-filled and nano-hybrid direct composite resins and by the application of indirect laboratory methods [3,4,5,6].
Furthermore, indirect restorations, either by using resin-based materials or ceramics, have proven to be a viable alternative therapeutic modality [7]. Due to the fact that ceramics have long been characterized as expensive, brittle materials that induce wear to the opposing dentition and are not repairable after fracture, indirect resin-based restorations are continuously gaining attention [8,9]. The everlasting need for more conservative, minimally invasive, and, at the same time, predictable procedures that maximize patients’ comfort has led to the incorporation of digital means in the fabrication of dental restorations. The introduction of computer-aided design/computer-aided manufacturing (CAD/CAM) appliances followed the rising demand for digital dentistry, and, subsequently, the dental market was overrun with new dental biomaterials for several types of restorations (inlays, onlays, endocrowns, etc.) [10,11,12]. The first subtractive manufacturing materials used were feldspar ceramic blocks [13]. Although they are strong, ceramics are brittle materials with a low fracture toughness and a high susceptibility to failure in the presence of flaws [9]. Therefore, using “hybrid ceramic” or resin-based CAD/CAM restorative materials has proven an ideal alternative. Their main benefit is based on adequate factory polymerization, involving high-heat and high-pressure techniques, eliminating polymerization defects and monomer release in this way. Simultaneously, incorporating a more significant amount of filler particles and altering the polymer matrix enhance their mechanical properties. The hybridity of these newly introduced CAD/CAM blocks depends on the common goal of combining the positive effects of ceramic and resin-based components [14]. Since the flexural strength of hybrid resin-based CAD/CAM blocks is higher than that of recently developed nano-filled composite resins and their elastic modulus is similar to that of dentin, a more uniform stress distribution during loading may be anticipated [15].
Through the years, researchers have constantly evaluated the mechanical properties of hybrid, ceramic, resin-based CAD/CAM blocks. The flexural strength, Vickers hardness and elastic modulus are of utmost importance for excellent clinical performance. Surface properties, such as the surface roughness and surface topography, have also been investigated, but to a lesser extent compared to mechanical properties [15,16,17,18,19,20,21,22,23,24,25]. Unfortunately, scarce evidence exists concerning bacterial attachment and subsequent biofilm formation on hybrid ceramic, resin-based CAD/CAM blocks for permanent, indirect restorations, meaning that this is a field that needs further investigation. Biofilm formation is a potential causative factor facilitating restoration failure since it promotes the appearance of secondary caries on the restoration’s margins and provokes biodegradation, thus altering the restorative material’s surface characteristics [26,27]. To the best of our knowledge, until recently, no critical reviews of the existing literature focusing on bacterial formation on CAD/CAM dental materials for indirect restorations have been published, and a comprehensive evaluation of the methodology, observations and results of current research protocols is lacking. Therefore, the aims of this review are, firstly, to introduce the resin-based CAD/CAM materials used for single indirect restorations and to present the recent data concerning biofilm formation on their surfaces, and secondly, to shed light on the methodological patterns used, as well as their limitations. Furthermore, future directions in microbiome analysis will be highlighted. A visualization of the structure of this comprehensive review is presented in Figure 1.
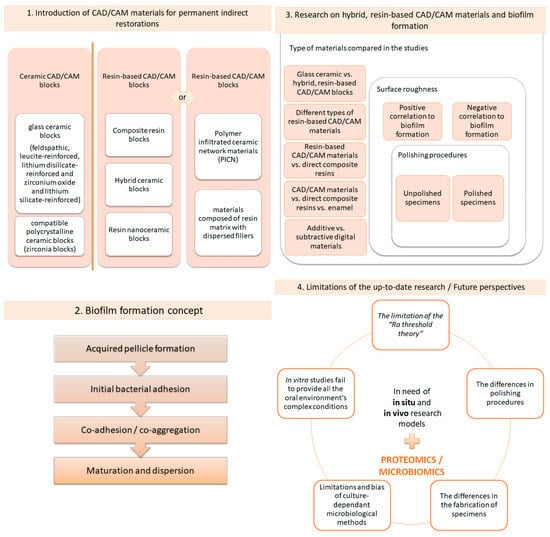
Figure 1.
Succinct description of the sections of this review.
2. “Hybrid”, Resin-Based Materials in the Digital Dentistry Era
There are a lot of different classification systems regarding CAD/CAM blocks and their application in contemporary restorative dentistry. The raw classes of CAD/CAM blocks fabricated for single, permanent indirect restorations are ceramic CAD/CAM blocks and resin-based CAD CAM blocks. According to their composition and microstructure, ceramic CAD/CAM blocks can be further divided into glass ceramics, subcategorized into feldspathic, leucite-reinforced, lithium-disilicate-reinforced and zirconium-oxide- and lithium-silicate-reinforced ceramic blocks, and compatible polycrystalline ceramics, namely zirconia CAD/CAM blocks [12]. The CAD/CAM blocks that incorporate a resin-based organic matrix can be subcategorized as follows: polymer-infiltrated ceramic network materials and materials composed of a resin matrix with dispersed fillers [28,29]. Other resin-based CAD/CAM block classes include composite resin CAD/CAM blocks, hybrid ceramic CAD/CAM blocks and resin nanoceramic CAD/CAM blocks [12,14,30]. The latter refers to polymeric networks that are reinforced with ceramic fillers (ceramics, glass ceramics, glasses, ultrafine glass particles, nanohybrid fillers, etc.). The term “hybrid” is often misinterpreted and should only be used to describe a CAD/CAM block that consists of a polymer-infiltrated ceramic network (PICN). This CAD/CAM block (VitaEnamic, VITA Zahnfabrik, Bad Säckingen, Germany) presents a double network hybrid structure composed of a porous, pre-sintered ceramic network, conditioned by a coupling agent and infiltrated with a polymer via capillary action [31,32,33]. Caution is required, since the misclassification of CAD/CAM materials in the dental literature is significant and might lead to misuse and incorrect clinical identification of CAD/CAM materials [34]. Although resin-based, hybrid ceramic, nanoceramic CAD/CAM materials exhibit inferior optical properties, their advantages compared to traditional glass ceramics are summarized as follows: they are not stiff, brittle materials; they mimic the structure of natural tooth components; they present direct composite repairability; and they are more easily and quickly fabricated [9]. Moreover, resin-based materials may be less susceptible to chipping during the milling procedure [35]. Occlusal and proximal adjustments (polishing procedures) are much more easily accomplished [14,36].
The most used resin-based CAD/CAM blocks are summarized in Table 1.

Table 1.
Commonly used hybrid, resin-based CAD/CAM materials in the dental market.
According to the manufacturer, the polymer-infiltrated ceramic network material (Vita Enamic, VITA Zahnfabrik, Bad Säckingen, Germany) consists of 86% filler by weight and 14% UDMA and TEGDMA polymer network by weight. More precisely, the inorganic fillers are primarily silicon dioxide and aluminum oxide and secondarily sodium, potassium, and calcium oxide, as well as boron trioxide and zirconia [37,38,39]. One commonly used resin nanoceramic CAD/CAM material is Lava Ultimate (3M ESPE, St. Paul, MN, USA). Nanomers of 20 nm in diameter made from silica and 4–11 nm in diameter made from zirconia, as well as zirconia and silica nanoclusters of 0.6–10 μm, comprise the approximately 80% by weight inorganic filler content, which is placed in an organic matrix of Bis-GMA, UDMA, Bis-EMA, and TEGDMA [28,40]. Shofu Block HC (Shofu Inc., Kyoto, Japan) is described as a ceramic-based restorative material, consisting of 61% silica powder, zirconium silicate and microfumed silica in a UDMA and TEGDMA organic matrix [28,41]. Cerasmart (GC Corporation, Tokyo, Japan) is now off the market and has been replaced by Cerasmart 270, which is described as a force-absorbing hybrid ceramic CAD/CAM block. Its predecessor’s composition included Bis-MEPP, UDMA, DMA, silica (20 nm) and barium glass (300 nm). Its inorganic filler load was 71% by weight [28,42]. Grandio Block (VOCO GmbH, Cuxhaven, Germany) is described as a nano-hybrid CAD/CAM block of 86% by weight nanoceramic filler particles in a UDMA and DMA organic matrix [43]. Another often used resin-based material is Brilliant Crios (Coltene Whaledent AG, Altstätten, Switzerland), described by the manufacturer as a reinforced composite block for permanent restorations. It consists of a cross-linked methacrylate resin matrix (Cross-Bis-GMA, Bis-EMA and TEGDMA) and 70.7% by weight dental glass (barium glass < 1.0 μm) and amorphous silica (<20 nm) [44]. Katana Avencia Block (Kuraray Noritake Dental, Tokyo, Japan) consists of UDMA, other methacrylate monomers and mixed fillers of colloidal silica and aluminum oxide and was launched as a hybrid, ceramic, composite resin CAD/CAM block [45]. Lastly, Tetric CAD (Ivoclar Vivadent AG, Schaan, Lichtenstein) is composed of cross-linked methacrylates, such as Bis-GMA, Bis-EMA, TEGDMA and UDMA, and 71% by weight barium glass (<1 µm) and silicon dioxide fillers [46]. As observed, resin-based CAD/CAM materials have almost the same microstructures, but in different proportions.
3. The Concept of Biofilm Formation
The oral microbiome, hosting approximately 700 different species of bacteria, represents the second largest microbiota environment, following the gut microbiome [47]. The oral cavity is a complex host with unique anatomical structures, including hard (natural teeth and restorative materials) and soft tissues (oral mucosa). The oral microbiome is the sum of the oral microbes, their genetic information, and the oral environment in which all components interact [48]. The so-called “climax community”, consisting of dietary habits, environmental conditions, host genetics and early microbial exposure, plays a pivotal role in the oral microbiota composition [49]. Biofilms are formed on every existing surface (soft and hard tissues, dental materials, etc.) in the oral cavity. The presence of biofilms is not necessarily malicious per se, since under normal circumstances, pathogenic and physiological microorganisms exhibit a phenomenon called symbiosis, which leads to the maintenance of oral health [50]. Several factors may disrupt this sensitive balance and result in dysbiosis (imbalance of the microbiome). Inadequate oral health conditions and dietary habits rich in low-molecular-weight carbohydrates, as well as inflammatory and autoimmune disorders, create the ideal environment for the establishment of pathological processes, such as the demineralization of tooth structures, tooth decay, secondary caries at the margins of restorative materials, gingivitis–periodontitis–peri-implantitis, tooth loss and/or stomatitis [51,52]. Biofilm formation (dental plaque) is a multiple-stage process [53]. When a dental biomaterial, in our case a resin-based CAD/CAM material, is adhered to a tooth structure and starts functioning in the oral cavity, it is immediately coated by saliva, and an acquired pellicle is formed [54]. After the first stage of acquired pellicle formation, the initial bacterial adhesion commences, and the formation of the dental plaque biofilm continues with the adhesion and coagulation of further microorganisms. Maturation, followed by dispersion, leads to the final dental plaque composition [55]. More precisely, the acquired pellicle is a noncellular, micellar structure that is composed of salivary glycoproteins, phosphoproteins, lipids and components of gingival crevice fluids, plus microbial products (glycosyltrasferases and glycans). The acquired pellicle modifies the surface properties of the dental biomaterial and alters the interactions between the biomaterial and the host response [56,57]. The salivary molecules activate receptors, which interact with adhesins on the surfaces of bacteria [58]. The bacterial conjunction is divided into three categories, depending on the distance between bacteria and the dental surface. If the distance is greater than 100nm, the initial bacteria are transported to the point of interest via natural salivary flow, Brownian motion (fluid dynamics) and chemotaxis (chemical signaling).
When the distance between the bacteria and the surface is 20 to 100 nm, van der Waals forces and electrostatic interactions are of utmost importance for cell attachment. Lastly, when the distance is short (<20 nm), biofilm attachment due to nonspecific and specific bonding mechanisms is observed. Signaling transactions, as well as activation of specific transmembrane receptors, are examples of specific bonding mechanisms. After the arrival of microorganisms, bacterial attachment commences and pioneer colonizers are established [59]. The initial binding is reversible due to the weak physicochemical interactions (van der Waals and electrostatic forces). The next step is the irreversible phase, where strong stereochemical interactions between microbial adhesins and receptors on the acquired pellicle occur. Adhesins expressed by secondary colonizers recognize receptors on the surfaces of pioneer colonizers, and the co-aggregation or co-adhesion phase takes place. Microbial succession, meaning the gradual replacement of initial colonizers by other bacterial species through the initial bacteria’s metabolic process, follows, and mature dental plaque is built [49,60]. Figure 2 briefly describes the dental plaque formation stages.
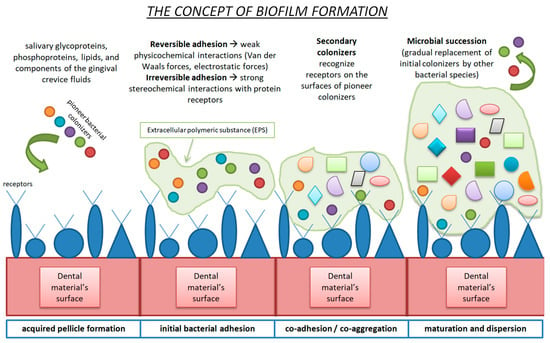
Figure 2.
Schematic representation of biofilm formation.
All in all, bacterial colonization, especially at its early stage, is contingent upon detachment shear forces and the surface energetic state of the substrate. The decisive role of surface roughness, surface free energy, surface wettability, surface topography and surface chemical composition on biofilm formation is scientifically documented, mainly by in vitro studies [61,62,63,64]. An increased surface roughness promotes greater bacterial attachment due to the greater surface contact area available for adhesion, the presence of stagnation points and the shielding of microbial cells from shear forces. Bacteria adhere easily to a surface with a high surface energy (hydrophilic), rather than to a substrate with a low surface energy [65,66]. However, since a plethora of factors has been proven to be responsible for the alterations at the interface between the substratum and biofilms, a cautious interpretation of the literature and further investigations into the correlation of surface characteristics and biofilm formation are necessary. Furthermore, it should not be forgotten that the properties of a dental material have a significant effect on the biofilm and that the biofilm may conversely affect and alter the material properties [67,68].
4. Research on Biofilm Formation on Resin-Based, Hybrid CAD/CAM Materials
Research focusing on biofilm formation on resin-based CAD/CAM materials for permanent indirect restorations predominantly originates from in vitro studies. An overall overview demonstrates a possible correlation between biofilm formation and surface characteristics (mainly surface roughness), as well as a strong association between bacterial growth, surface roughness and surface modification techniques (polishing procedures).
More precisely, after a thorough investigation of the recent literature concerning biofilm formation on resin-based CAD/CAM blocks for permanent indirect restorations, a total of eleven research articles were found [69,70,71,72,73,74,75,76,77,78,79]. These studies investigated one or more hybrid, resin-based CAD/ CAM materials with regard to biofilm attachment and growth. They evaluated either the biofilm formation as an independent variable or biofilm formation in association with surface characteristics, such as surface roughness and surface free energy. The materials investigated in each study differed. Some researchers solely examined resin-based CAD/CAM blocks [70,76,77,78]. Others compared resin-based CAD/CAM blocks to conventional composite resins [74], whereas some in vitro research incorporated direct composite resins, indirect CAD/CAM blocks and human enamel [72,73]. Moreover, other studies focused on ceramic CAD/CAM materials and hybrid resin-based CAD/CAM materials [69,71]. Lastly, a newly conducted in vitro study compared CAD/CAM-manufactured resin-based materials for indirect restorations with 3D-printed resin-based materials [79]. Other researchers investigated the potential correlation between the surface modification procedures on CAD/CAM resin-based materials and increased or decreased biofilm formation. In this kind of research, control groups were not subjected to further surface treatments, in contrast to the experimental groups, where finishing and polishing procedures with specific grinding and polishing protocols established by each researcher took place. Most in vitro studies used Streptococcus mutans (S. mutans) as the monospecies for bacterial adherence to the tested materials. Other bacterial strains used were Candida albicans (C. albicans), Streptococcus sanguis (S. sanguis), Streptococcus gordonii (S. gordonii) and Lactobacillus species. Only two in situ studies, which tried to identify the biofilm formed on smooth restorative materials, integrated hybrid resin-based CAD/CAM materials into their experimental groups [72,75].
The methods used for the evaluation of surface properties and the assessment of biofilm formation are scientifically documented by former researchers. Using a stylus profilometer or a 3D optical profilometer in contact or non-contact mode is the gold standard in the assessment of surface roughness [27,80]. Most researchers measuring surface roughness record and compare the Sa value (arithmetical mean height, expressing, as an absolute value, the difference in height of each point compared to the arithmetical mean of the surface). Scanning electron microscopy (SEM) provides qualitative information on the surface structure of a dental material [81]. Furthermore, the use of attenuated total reflectance, Fourier transform infrared spectrometry (ATR–FT–IR spectrometry) and energy-dispersive X-ray microanalysis (EDX microanalysis) enriches protocols with information concerning the molecular composition and elemental analysis of the surfaces tested (surface topography and chemical composition assessment) [82,83,84]. The sessile drop method calculates the surface free energy using contact angle measurements and customized optical goniometers [85]. For the microbiological analysis of the tested specimens, various diverse methods (direct as well as indirect) have been introduced. Still, the most commonly used method is the application of a bioreactor followed by colony-forming unit counting (CFU/mL). Scanning electron microscopy (SEM) and confocal scanning laser microscopy (CSLM) are supplementary qualitative methods for biofilm evaluation [86].
The objectives, the experimental methods and the results of these studies are analyzed on a large scale in Table 2.

Table 2.
Research focusing on bacterial adhesion on hybrid resin-based CAD/CAM materials for indirect restorations.
5. Limitations of the Current Research
Delving deeper into the aforementioned research, a cautious interpretation of their ambiguous results should be accomplished.
On the one hand, when evaluating resin-based CAD/CAM materials, a group of researchers demonstrate a definite association between biofilm formation and surface roughness or surface modification procedures [69,70,71,74,75,77,78], whereas, on the other hand, no correlation between these factors is found in research studies conducted by other groups of investigators [73,76,79]. These discrepancies are also present in previously conducted in vitro studies assessing surface roughness, different polishing techniques and their impact on biofilm formation for laboratory-fabricated indirect and direct resin-based restorative materials [27,87,88,89,90,91,92,93,94,95,96].
This divergence may rely on the following factors:
- The Ra threshold theory of 0.2 μm.
In several studies that incorporate CAD/CAM samples in their protocols, with initial Sa values of samples greater than 0.2 μm, a positive correlation between surface roughness and bacterial attachment has been found [69,70,77]. Additionally, it is further demonstrated that surface roughness has an insignificant effect on bacterial adhesion when the Sa values of the tested specimens are below this threshold [97]. In the research protocol of Ionescu et al. in 2020, where surface roughness values (Sa) were less than 0.2 μm, no strong correlation between Sa and bacterial adhesion was present [73]. Interestingly, in some research protocols with Sa values greater than the 0.2 μm threshold, no correlation between the two investigated factors has been observed [76,79], and in other research where the Sa values were lower than the established threshold, a strong correlation between surface roughness and biofilm adhesion has been demonstrated [74,78]. This fact highlights the potential influence of additional factors, such as polishing procedures, chemical composition and topography, on the outcomes of bacterial adhesion. Moreover, a systematic review by Duetra et al. in 2018 [98] concluded that the impact of roughness on bacterial adhesion is not related to a roughness threshold but rather to a range of surface roughness, which is wide and material-dependent. The majority of in vitro studies evaluating either the surface roughness as a single parameter or the relationship between surface roughness and bacterial colonization use only the Sa value, which is a single height parameter of a surface. Additional spatial, functional or hybrid (e.g., developed interfacial area ratio, Sdr) parameters, may give a greater insight into surface texture and bacterial colonization.
- 2.
- The polishing procedure may affect bacterial adhesion on resin-based CAD/CAM materials for indirect restorations.
CAD/CAM materials directly after their milling procedure present an insufficient smoothness, which may be adjusted by additional polishing protocols [99]. Although no standard protocol for polishing CAD/CAM restorations has been established [100], each company manufacturing CAD/CAM resin-based materials fabricates and promotes its finishing and polishing sets to achieve optimal surface characteristics in the final restoration. According to the literature, finishing and polishing protocols affect the surface roughness of dental materials and promote a heterogeneous impact on bacterial adhesion [98]. Comparing polished resin-based CAD/CAM blocks to unpolished control groups, statistically significant differences were found concerning the decreased amount of bacterial adhesion on polished specimens [70,71,74,78]. It is evident that different polishing techniques remove the superficial layers of the tested materials, resulting in a physically as well as chemically altered surface compared to the unpolished control group and in a subsequently reduced surface roughness [101,102]. Meanwhile, significant differences in surface roughness values were obtained while using the same polishing protocols for different resin-based CAD/CAM materials. This may be attributed to the third factor that generates variance in the results of the studies mentioned above, namely the elemental composition and the microstructure of resin-based CAD/CAM materials.
- 3.
- The chemical and topographical microstructure of hybrid, resin-based CAD/CAM materials.
More precisely, a different structural composition is present in lithium disilicate glass ceramic CAD/CAM blocks compared to polymer-infiltrated ceramic network materials, nano-ceramic filler-infiltrated polymer networks or direct resin-based materials, leading subsequently to different surface roughness and biofilm adherence values. Furthermore, biofilm formation is positively linked to the amount of the resin matrix rather than the amount of filler particles. It is scientifically proven that some released monomers stimulate bacterial growth [90]. This may explain the fact that in the research of Hassan et al. in 2022 [76], Brilliant Crios blocks exhibited more outstanding bacterial adhesion compared to Vita Enamic and Cerasmart blocks, since the former contain a greater proportion of resin matrix (29%wt). It should not be forgotten that CAD/CAM blocks are produced under a high pressure and a high temperature, improving their properties. This should be counted as an additional factor explaining the reduced biofilm formation on these materials compared to conventional composite resins [9,19].
All in all, the type of resin-based CAD/CAM material and the surface finishing and polishing techniques are significantly related to surface roughness and biofilm adherence.
- 4.
- The lack of standardization in the fabrication of specimens.
The results of the research protocols of Contreras-Guererro et al. in 2020 [74] are opposed to other similar in vitro studies evaluating biofilm formation on ceramic CAD/CAM, hybrid resin-based CAD/CAM and composite resin specimens, since they demonstrate greater surface roughness and biofilm formation values for the hybridized resin-based CAD/CAM blocks compared to conventional composite resins. Kim et al. in 2017 [69] also demonstrated that simulated intraoral adjustment and polishing procedures have a negative effect on surface roughness and on biofilm formation in hybrid resin-based materials, leucite-reinforced glass ceramics and nanoleucite-glass ceramics compared to their unpolished counterparts. Such discrepancies may be justified by the disparities in the preparation of the specimens between different research protocols. For the fabrication of conventional composite resin specimens, a universal approach has been proposed using molds with specific dimensions, glass slides, and acetate strips. On the other hand, for the fabrication of CAD/CAM samples, several approaches have been used. Some researchers generated CAD/CAM samples by the use of a diamond bur or a trepan bur under a constant water flow [73,75], whereas some others used diamond discs attached to low-speed straight handpieces [69]. In two research protocols, CAD/CAM samples were fabricated by the use of a milling unit [72,74]. Most researchers used a low-speed precision cutting machine and a diamond blade under flowing water [70,76,77,78,79]. All these different fabrication methods may result in different study outcomes.
Furthermore, in some studies, finishing and polishing were accomplished by the use of grinding and polishing devices under a constant water flow combined with silicone carbide grinding papers of different grit sizes, and the specimens were additionally polished by polishing sets of different manufacturers, whereas some others used several polishing systems on the fabricated (by the use of rotary instruments) specimens directly. These variations in the methodology of experimental protocols result in divergent outcomes in the research. All we need is the standardization of the procedures and the establishment of ideal conditions that can mimic, to the greatest extent, the intraoral environment. In vitro studies fail to provide all the oral environment’s complex conditions, and future research should focus on in situ and in vivo protocols.
- 5.
- The biofilm assessment method
Referring to intraoral conditions, another factor affecting the results of biofilm formation on resin-based CAD/CAM materials is the method of biofilm assessment. Most in vitro studies use one microbial strain (monospecies colony), mainly S. mutans, since it is a well-known predominant cariogenic species [79]. A plethora of artificial systems try to mimic the intraoral environmental conditions for biofilm development on the surface of a dental material; these systems are called bioreactors. They are used for in vitro biofilm growth and are categorized either as static or dynamic bioreactors. They can be made of artificial oral microcosms, single species or defined consortia of a few species growing together [103,104]. Most in vitro studies assessing biofilm formation on resin-based CAD/CAM surfaces use a single species, since this is a simple, controlled, inexpensive, highly reproducible technique [105]. Attempting to imitate oral conditions, most in vitro studies incorporate in their microbiological protocol the immersion of samples in mucin containing artificial saliva or whole mouth saliva, secreted from a volunteer, to form the acquired pellicle. Colony-forming unit counting (CFU/mL), combined with SEM investigations and confocal laser scanning microscopy (CLSM), is used to perform qualitative and quantitative evaluations of bacterial formation [106]. SEM and CLSM have limitations, including the high cost and complexity of their protocols, the inability of CLSM to discriminate strains, the inability of SEM to discriminate live and dead bacteria, and the fact that only a specific selected area of the substrate may be evaluated [107].
Furthermore, bacterial adhesion on the surface of a substratum is not only influenced by the surface characteristics of the materials tested but also by the selected bacterial strain, the growth medium used and the specific adhesion mechanisms of the selected monospecies. Only one in vitro study by Ionescu et al. in 2020 [74] used four models of bioreactors for microbial investigations (static, orbital shaking, continuous flow and mixed-plaque formation bioreactors) to assess biofilm formation on resin-based CAD/CAM materials, concluding that, when bioreactors with shear forces or bioreactors where multi plaque formation takes place are used, lower S. mutans formation on resin-based CAD/CAM blocks was observed compared to conventional composite resin specimens. Unfortunately, in vitro biofilm formation has only been investigated via culture-dependent, close-ended molecular methods with a great risk of bias which do not coincide with real in vivo conditions.
Until recently, only two in situ studies that evaluated biofilm adhesion and formation on different dental restorative materials used a resin-based CAD/CAM material in their experimental groups [72,75], meaning that this is a field that nowadays attracts the interest of a lot of researchers.
Lastly, it should not be forgotten that under clinical conditions, surfaces are immediately coated by saliva and the composition, the flow and the volume of saliva differ based on neural control system signaling, as well as on physical, environmental and/or pathological factors, which include circadian rhythm, age, gender, physical exercise, oral hygiene, food consumption (diet), medication and systematic diseases [108]. It is almost impossible to mimic all these above-mentioned conditions in in vitro protocols; therefore, in vivo studies incorporating parts of these factors in their study design should be conducted.
6. Conclusions
Newly introduced CAD/CAM restorative materials are gaining attention due to their more than satisfactory mechanical properties. The biological background of the tested dental materials proves to be a significant factor in dental science since bacterial adhesion is inextricably linked to secondary caries on the margins of a restoration and subsequently to the good or the poor clinical performance of a restoration. Bacterial adhesion on CAD/CAM resin-based materials is primarily investigated in in vitro studies that, unfortunately, do not represent the exact conditions of the oral environment. The current literature demonstrates a possible interaction between biofilm formation and the surface of the substratum. Surface roughness, surface free energy, surface topography and elemental and chemical composition may have a crucial impact on biofilm growth, mainly in the early stages of bacterial adherence. Further studies should be conducted in order to shed light οn the unknown phenomenon of bioadhesion.
7. Future Perspectives
When conducting an in vitro study, caution should be exercised concerning the standardization of the applied procedures. Since in vitro studies present, inter alia, culturing bias, the scientific interest of most researchers focuses on the use of culture-independent methods for the identification of the total bacterial community in the oral environment. To do so, open-ended genome sequencing technologies, such as next-generation sequencers (NGSs), as well as proteomic and metaproteomic techniques that may identify the host and the microbial proteome, are gradually being incorporated in the microbiological armamentarium. The conduction of in situ and/or in vivo studies using resin-based CAD/CAM restorative materials as experimental groups and human enamel and conventional composite resins as control groups, incorporated on oral splints worn by volunteers, may provide an insight into how surface characteristics, saliva, acquired pellicles and the oral microbiome interact. Interestingly, via 16S ribosomal RNA gene sequencing, the whole microbiome present in biofilms may be identified [109]. Furthermore, mass spectrometry (MS) devices may provide information concerning the proteomic profile of a tested material. Utilizing specific databases of bioinformatics, bacterial species adhered to a surface may be recognized using MS (metaproteomics). The “-Omics” era focuses on the principle that the whole organism works in synergy, and each bacterium is dependent on the other species present. Since biofilms are described as conglomerates, a more holistic, ecological approach to controlling dental biofilms is necessary.
Author Contributions
Conceptualization, Κ.Τ. and E.P.; methodology, K.T.; validation, K.T., C.R. and E.P.; formal analysis, K.T.; investigation, K.T.; data curation, K.T.; writing—original draft preparation, K.T.; writing—review and editing, C.R. and E.P.; visualization, K.T.; supervision, C.R. and E.P.; project administration, K.T., C.R. and E.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Watts, D.C.; Marouf, A.S.; Al-Hindi, A.M. Photo-polymerization shrinkage-stress kinetics in resin-composites: Methods development. Dent. Mater. 2003, 19, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Azeem, R.A.; Sureshbabu, N.M. Clinical performance of direct versus indirect composite restorations in posterior teeth: A systematic review. J. Conserv. Dent. 2018, 21, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.B.; Wu, D.; Holmes, B.N. An application of nanotechnology in advanced dental materials. J. Am. Dent. Assoc. 2003, 134, 1382–1390. [Google Scholar] [CrossRef] [PubMed]
- Alzraikat, H.; Burrow, M.F.; Maghaireh, G.A.; Taha, N.A. Nanofilled Resin Composite Properties and Clinical Performance: A Review. Oper. Dent. 2018, 43, 173–190. [Google Scholar] [CrossRef]
- Dejak, B.; Młotkowski, A.A. Comparison of stresses in molar teeth restored with inlays and direct restorations, including polymerization shrinkage of composite resin and tooth loading during mastication. Dent. Mater. 2015, 31, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Nandini, S. Indirect resin composites. J. Conserv. Dent. 2010, 13, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Peutzfeldt, A. Indirect Resin and Ceramic Systems. Oper. Dent. 2001, 200, 1153–1176. [Google Scholar]
- Burke, E.J.; Qualtrough, A.J. Aesthetic inlays: Composite or ceramic? Br. Dent. J. 1994, 176, 53–60. [Google Scholar] [CrossRef]
- Ruse, N.D.; Sadoun, M.J. Resin-composite blocks for dental CAD/CAM applications. J. Dent. Res. 2014, 93, 1232–1234. [Google Scholar] [CrossRef]
- van Noort, R. The future of dental devices is digital. Dent. Mater. 2012, 28, 3–12. [Google Scholar] [CrossRef]
- Fasbinder, D.J. Materials for chairside CAD/CAM restorations. Compend. Contin. Educ. Dent. 2010, 31, 702–709. [Google Scholar]
- Lambert, H.; Durand, J.C.; Jacquot, B.; Fages, M. Dental biomaterials for chairside CAD/CAM: State of the art. J. Adv. Prosthodont. 2017, 9, 486–495. [Google Scholar] [CrossRef]
- Mörmann, W.H. The evolution of the CEREC system. J. Am. Dent. Assoc. 2006, 137, 7–13. [Google Scholar] [CrossRef]
- Horvath, S.D. Key Parameters of Hybrid Materials for CAD/CAM-Based Restorative Dentistry. Compend. Contin. Educ. Dent. 2016, 37, 638–643. [Google Scholar]
- Palacios, T.; Tarancón, S.; Pastor, J.Y. On the Mechanical Properties of Hybrid Dental Materials for CAD/CAM Restorations. Polymers 2022, 14, 3252. [Google Scholar] [CrossRef] [PubMed]
- Papathanasiou, I.; Kamposiora, P.; Dimitriadis, K.; Papavasiliou, G.; Zinelis, S. In vitro evaluation of CAD/CAM composite materials. J. Dent. 2023, 136, 104623. [Google Scholar] [CrossRef] [PubMed]
- Koenig, A.; Schmidtke, J.; Schmohl, L.; Schneider-Feyrer, S.; Rosentritt, M.; Hoelzig, H.; Kloess, G.; Vejjasilpa, K.; Schulz-Siegmund, M.; Fuchs, F.; et al. Characterisation of the Filler Fraction in CAD/CAM Resin-Based Composites. Materials 2021, 14, 1986. [Google Scholar] [CrossRef] [PubMed]
- Rexhepi, I.; Santilli, M.; D’Addazio, G.; Tafuri, G.; Manciocchi, E.; Caputi, S.; Sinjari, B. Clinical Applications and Mechanical Properties of CAD-CAM Materials in Restorative and Prosthetic Dentistry: A Systematic Review. J. Funct. Biomater. 2023, 14, 431. [Google Scholar] [CrossRef] [PubMed]
- Goujat, A.; Abouelleil, H.; Colon, P.; Jeannin, C.; Pradelle, N.; Seux, D.; Grosgogeat, B. Mechanical properties and internal fit of 4 CAD-CAM block materials. J. Prosthet. Dent. 2018, 119, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Stockl, C.; Hampe, R.; Stawarczyk, B.; Haerst, M.; Roos, M. Macro- and microtopographical examination and quantification of CAD-CAM composite resin 2- and 3-body wear. J. Prosthet. Dent. 2018, 120, 537–545. [Google Scholar] [CrossRef]
- Papathanasiou, I.; Zinelis, S.; Papavasiliou, G.; Kamposiora, P. Effect of aging on color, gloss and surface roughness of CAD/CAM composite materials. J. Dent. 2023, 130, 104423. [Google Scholar] [CrossRef]
- Furtado de Mendonca, A.; Shahmoradi, M.; Gouvea, C.V.D.; De Souza, G.M.; Ellakwa, A. Microstructural and mechanical characterization of CAD/CAM materials for monolithic dental restorations. J. Prosthodont. 2019, 28, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Stawarczyk, B.; Liebermann, A.; Eichberger, M.; Güth, J.F. Evaluation of mechanical and optical behavior of current esthetic dental restorative CAD/CAM composites. J. Mech. Behav. Biomed. Mater. 2015, 55, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lauvahutanon, S.; Takahashi, H.; Shiozawa, M.; Iwasaki, N.; Asakawa, Y.; Oki, M.; Finger, W.J.; Arksornnukit, M. Mechanical properties of composite resin blocks for CAD/CAM. Dent. Mater. J. 2014, 33, 705–710. [Google Scholar] [CrossRef] [PubMed]
- Sonmez, N.; Gultekin, P.; Turp, V.; Akgungor, G.; Sen, D.; Mijiritsky, E. Evaluation of five CAD/CAM materials by microstructural characterization and mechanical tests: A comparative in vitro study. BMC Oral Health 2018, 18, 5. [Google Scholar] [CrossRef] [PubMed]
- Kramer, N.; Kunzelmann, K.H.; Garcia-Godoy, F.; Haberlein, I.; Meier, B.; Frankenberger, R. Determination of caries risk at resin composite margins. Am. J. Dent. 2007, 20, 59–64. [Google Scholar]
- Cazzaniga, G.; Ottobelli, M.; Ionescu, A.; Garcia-Godoy, F.; Brambilla, E. Surface properties of resin-based composite materials and biofilm formation: A review of the current literature. Am. J. Dent. 2015, 28, 311–320. [Google Scholar] [PubMed]
- Mainjot, A.K.; Dupont, N.M.; Oudkerk, J.C.; Dewael, T.Y.; Sadoun, M.J. From Artisanal to CAD-CAM Blocks: State of the Art of Indirect Composites. J. Dent. Res. 2016, 95, 487–495. [Google Scholar] [CrossRef]
- Della Bona, A.; Corazza, P.H.; Zhang, Y. Characterization of a polymer-infiltrated ceramic-network material. Dent. Mater. 2014, 30, 564–569. [Google Scholar] [CrossRef]
- Marchesi, G.; Camurri Piloni, A.; Nicolin, V.; Turco, G.; Di Lenarda, R. Chairside CAD/CAM Materials: Current Trends of Clinical Uses. Biology 2021, 10, 1170. [Google Scholar] [CrossRef]
- Skorulska, A.; Piszko, P.; Rybak, Z.; Szymonowicz, M.; Dobrzyński, M. Review on Polymer, Ceramic and Composite Materials for CAD/CAM Indirect Restorations in Dentistry-Application, Mechanical Characteristics and Comparison. Materials 2021, 14, 1592. [Google Scholar] [CrossRef]
- Blatz, M.B.; Conejo, J. The Current State of Chairside Digital Dentistry and Materials. Dent. Clin. N. Am. 2019, 63, 175–197. [Google Scholar] [CrossRef]
- Xie, C.; Zhang, J.F.; Li, S. Polymer Infiltrated Ceramic Hybrid Composites as Dental Materials. Oral Health Dent. Stud. 2018, 1, 2. [Google Scholar] [CrossRef]
- Rocha, M.G.; Oliveira, D.; Sinhoreti, M.A.C.; Roulet, J.F.; Zoidis, P.; Duncan, W. Assessment of CAD/CAM composites classification in abstracts using machine learning. Dent. Mater. 2023, 39, 60–61. [Google Scholar] [CrossRef]
- Tsitrou, E.A.; Northeast, S.E.; van Noort, R. Brittleness index of machinable dental materials and its relation to the marginal chipping factor. J. Dent. 2007, 35, 897–902. [Google Scholar] [CrossRef] [PubMed]
- Fasbinder, D.J.; Neiva, G.F. Surface evaluation of polishing techniques for new resilient CAD/CAM restorative materials. J. Esthet. Restor. Dent. 2016, 28, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Coldea, A.; Swain, M.V.; Thiel, N. Mechanical properties of polymer-infiltrated-ceramic-network materials. Dent. Mater. 2013, 29, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, J.F.; Migonney, V.; Ruse, N.D.; Sadoun, M. Resin composite blocks via high-pressure high-temperature polymerization. Dent. Mater. 2012, 28, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Vita Enamic®. Technical and Scientific Documentation. Available online: https://www.vita-zahnfabrik.com/en/VITA-ENAMIC-24970.html (accessed on 3 March 2024).
- Lava™ Ultimate CAD/CAM Restorative for E4D. Available online: https://multimedia.3m.com/mws/media/756863O/3m-lava-ultimate-cad-cam-restorative-for-e4d-the-edge-you-need.pdf (accessed on 2 March 2024).
- SHOFU Block & Disk HC: Instructions for Use. Available online: https://www.shofu.com/wp-content/uploads/SHOFU-Block-HC-IFU-US.pdf (accessed on 2 March 2024).
- CERASMART GC Dental Product Technical Product Profile. Available online: www.gcamerica.com (accessed on 7 July 2022).
- Grandio Blocs/Grandio Disc—Nano-Ceramic Hybrid CAD/CAM Material: Instructions for Use. Available online: https://www.voco.dental/en/portaldata/1/resources/products/instructions-for-use/e1/grandio-blocs_ifu_e1.pdf (accessed on 2 March 2024).
- Brilliant Crios: Instructions for Use. Available online: https://products.coltene.com/EN/AG/media/DOC_IFU_30003998-12-22-IFU-BRILLIANT-Crios_IND.pdf?sprache=EN (accessed on 2 March 2024).
- Katana Avencia Blocks SDS. Available online: https://katanaavencia.com/wp-content/uploads/KATANA_AVENCIA_Block_SDS_US.pdf (accessed on 2 March 2024).
- Tetric CAD Instructions for Use. Available online: https://www.ivoclar.com/en_li/eifu?brand=Tetric+CAD (accessed on 2 March 2024).
- Deo, P.N.; Deshmukh, R. Oral microbiome: Unveiling the fundamentals. J. Oral Maxillofac. Pathol. 2019, 23, 122–128. [Google Scholar] [CrossRef]
- Kilian, M.; Chapple, I.L.; Hannig, M.; Marsh, P.D.; Meuric, V.; Pedersen, A.M.; Tonetti, M.S.; Wade, W.G.; Zaura, E. The oral microbiome—An update for oral healthcare professionals. Br. Dent. J. 2016, 221, 657–666. [Google Scholar] [CrossRef]
- Samaranayake, L.; Bandara, N.; Pesee, S. Oral Biofilms: What Are They? In Oral Biofilms and Modern Dental Materials, 1st ed.; Ionescu, A.C., Hahnel, S., Eds.; Springer Nature: Cham, Switzerland, 2021; pp. 1–7. [Google Scholar] [CrossRef]
- Ptasiewicz, M.; Grywalska, E.; Mertowska, P.; Korona-Głowniak, I.; Poniewierska-Baran, A.; Niedźwiedzka-Rystwej, P.; Chałas, R. Armed to the Teeth-The Oral Mucosa Immunity System and Microbiota. Int. J. Mol. Sci. 2022, 23, 882. [Google Scholar] [CrossRef]
- Lin, N.J. Biofilm over teeth and restorations: What do we need to know? Dent. Mater. 2017, 33, 667–680. [Google Scholar] [CrossRef]
- Sterzenbach, T.; Helbig, R.; Hannig, C.; Hannig, M. Bioadhesion in the oral cavity and approaches for biofilm management by surface modifications. Clin. Oral Investig. 2020, 24, 4237–4260. [Google Scholar] [CrossRef]
- Kreth, J.; Herzberg, M.C. Molecular principles of adhesion and biofilm formation. In The Root Canal Biofilm, 1st ed.; Chávez de Paz, L.E., Sedgley, C.M., Kishen, A., Eds.; Springer Nature: Berlin, Germany, 2015; pp. 23–54. [Google Scholar] [CrossRef]
- Enax, J.; Ganss, B.; Amaechi, B.T.; Schulze Zur Wiesche, E.; Meyer, F. The composition of the dental pellicle: An updated literature review. Front. Oral Health 2023, 4, 1260442. [Google Scholar] [CrossRef]
- Kreth, J.; Merritt, J.; Pfeifer, C.S.; Khajotia, S.; Ferracane, J.L. Interaction between the Oral Microbiome and Dental Composite Biomaterials: Where We Are and Where We Should Go. J. Dent. Res. 2020, 99, 1140–1149. [Google Scholar] [CrossRef]
- Lindh, L.; Aroonsang, W.; Sotres, J.; Arnebrant, T. Salivary pellicles. Monogr. Oral Sci. 2014, 24, 30–39. [Google Scholar] [CrossRef]
- Fischer, N.G.; Aparicio, C. The salivary pellicle on dental biomaterials. Colloids Surf. B Biointerfaces 2021, 200, 111570. [Google Scholar] [CrossRef]
- Chawhuaveang, D.D.; Yu, O.Y.; Yin, I.X.; Lam, W.Y.; Mei, M.L.; Chu, C.H. Acquired salivary pellicle and oral diseases: A literature review. J. Dent. Sci. 2021, 16, 523–529. [Google Scholar] [CrossRef]
- Eliades, G.; Eliades, T.; Vavuranakis, M. General aspects of biomaterial surface alterations following exposure to biologic fluids. In Dental Materials In Vivo: Aging and Related Phenomena, 1st ed.; Eliades, G., Eliades, T., Brantley, W.A., Walts, D.C., Eds.; Quintessence Publishing Co.: Chicago, IL, USA, 2003; pp. 3–23. [Google Scholar]
- Sbordone, L.; Bortolaia, C. Oral microbial biofilms and plaque-related diseases: Microbial communities and their role in the shift from oral health to disease. Clin. Oral Investig. 2003, 7, 181–188. [Google Scholar] [CrossRef]
- Song, F.; Koo, H.; Ren, D. Effects of Material Properties on Bacterial Adhesion and Biofilm Formation. J. Dent. Res. 2015, 94, 1027–1034. [Google Scholar] [CrossRef]
- Schmalz, G.; Cieplik, F. Biofilms on Restorative Materials. Monogr. Oral Sci. 2021, 29, 155–194. [Google Scholar] [CrossRef]
- Teughels, W.; Van Assche, N.; Sliepen, I.; Quirynen, M. Effect of material characteristics and/or surface topography on biofilm development. Clin. Oral Implants Res. 2006, 17, 68–81. [Google Scholar] [CrossRef]
- Bürgers, R.; Krohn, S.; Wassmann, T. Surface Properties of Dental Materials and Biofilm Formation. In Oral Biofilms and Modern Dental Materials, 1st ed.; Ionescu, A.C., Hahnel, S., Eds.; Springer Nature: Cham, Switzerland, 2021; pp. 55–70. [Google Scholar] [CrossRef]
- Quirynen, M.; Bollen, C.M. The influence of surface roughness and surface-free energy on supra- and subgingival plaque formation in man. A review of the literature. J. Clin. Periodontol. 1995, 22, 1–14. [Google Scholar] [CrossRef]
- Zheng, S.; Bawazir, M.; Dhall, A.; Kim, H.E.; He, L.; Heo, J.; Hwang, G. Implication of Surface Properties, Bacterial Motility, and Hydrodynamic Conditions on Bacterial Surface Sensing and Their Initial Adhesion. Front. Bioeng. Biotechnol. 2021, 9, 643722. [Google Scholar] [CrossRef]
- Auschill, T.M.; Arweiler, N.B.; Brecx, M.; Reich, E.; Sculean, A.; Netuschil, L. The effect of dental restorative materials on dental biofilm. Eur. J. Oral Sci. 2002, 110, 48–53. [Google Scholar] [CrossRef]
- Padovani, G.; Fúcio, S.; Ambrosano, G.; Sinhoreti, M.; Puppin-Rontani, R. In situ surface biodegradation of restorative materials. Oper. Dent. 2014, 39, 349–360. [Google Scholar] [CrossRef]
- Kim, K.H.; Loch, C.; Waddell, J.N.; Tompkins, G.; Schwass, D. Surface Characteristics and Biofilm Development on Selected Dental Ceramic Materials. Int. J. Dent. 2017, 2017, 7627945. [Google Scholar] [CrossRef]
- Hamerschmitt, R.M.; Tomazinho, P.H.; Camporês, K.L.; Gonzaga, C.C.; da Cunha, L.F.; Correr, G.M. Surface topography and bacterial adhesion of CAD/CAM resin based materials after application of different surface finishing techniques. Braz. J. Oral Sci. 2018, 17, e18135. [Google Scholar] [CrossRef]
- Dobrzynski, M.; Pajaczkowska, M.; Nowicka, J.; Jaworski, A.; Kosior, P.; Szymonowicz, M.; Kuropka, P.; Rybak, Z.; Bogucki, Z.A.; Filipiak, J.; et al. Study of Surface Structure Changes for Selected Ceramics Used in the CAD/CAM System on the Degree of Microbial Colonization, In Vitro Tests. Biomed. Res. Int. 2019, 12, 9130806. [Google Scholar] [CrossRef]
- Conrads, G.; Wendt, L.K.; Hetrodt, F.; Deng, Z.L.; Pieper, D.; Abdelbary, M.M.H.; Barg, A.; Wagner-Döbler, I.; Apel, C. Deep sequencing of biofilm microbiomes on dental composite materials. J. Oral Microbiol. 2019, 11, 1617013. [Google Scholar] [CrossRef]
- Ionescu, A.C.; Hahnel, S.; König, A.; Brambilla, E. Resin composite blocks for dental CAD/CAM applications reduce biofilm formation in vitro. Dent. Mater. 2020, 36, 603–616. [Google Scholar] [CrossRef]
- Contreras-Guerrero, P.; Ortiz-Magdaleno, M.; Urcuyo-Alvarado, M.S.; Cepeda-Bravo, J.A.; Leyva-Del Rio, D.; Pérez-López, J.E.; Romo-Ramírez, G.F.; Sánchez-Vargas, L.O. Effect of dental restorative materials surface roughness on the in vitro biofilm formation of Streptococcus mutans biofilm. Am. J. Dent. 2020, 33, 59–63. [Google Scholar]
- Engel, A.S.; Kranz, H.T.; Schneider, M.; Tietze, J.P.; Piwowarcyk, A.; Kuzius, T.; Arnold, W.; Naumova, E.A. Biofilm formation on different dental restorative materials in the oral cavity. BMC Oral Health 2020, 20, 162. [Google Scholar] [CrossRef]
- Hassan, S.A.; Beleidy, M.; El-Din, Y.A. Biocompatibility and Surface Roughness of Different Sustainable Dental Composite Blocks: Comprehensive In Vitro Study. ACS Omega 2022, 7, 34258–34267. [Google Scholar] [CrossRef]
- Mokhtar, M.M.; Farahat, D.S.; Eldars, W.; Osman, M.F. Physico-mechanical properties and bacterial adhesion of resin composite CAD/CAM blocks: An in-vitro study. J. Clin. Exp. Dent. 2022, 14, 413–419. [Google Scholar] [CrossRef]
- Ozarslan, M.; Bilgili Can, D.; Avcioglu, N.H.; Çalışkan, S. Effect of different polishing techniques on surface properties and bacterial adhesion on resin-ceramic CAD/CAM materials. Clin. Oral Investig. 2022, 26, 5289–5299. [Google Scholar] [CrossRef]
- Ozer, N.E.; Sahin, Z.; Yikici, C.; Duyan, S.; Kilicarslan, M.A. Bacterial adhesion to composite resins produced by additive and subtractive manufacturing. Odontology 2023, 112, 460–471. [Google Scholar] [CrossRef]
- Gadelmawla, E.S.; Koura, M.M.; Maksoud, T.M.A.; Elewa, I.M.; Soliman, H.H. Roughness parameters. J. Mater. Res. Technol. 2002, 123, 133–145. [Google Scholar] [CrossRef]
- Van Meerbeek, B.; Vargas, M.; Inoue, S.; Yoshida, Y.; Perdigão, J.; Lambrechts, P.; Vanherle, G. Microscopy investigations. Techniques, results, limitations. Am. J. Dent. 2000, 13, 3–18. [Google Scholar]
- Kaczmarek, K.; Leniart, A.; Lapinska, B.; Skrzypek, S.; Lukomska-Szymanska, M. Selected Spectroscopic Techniques for Surface Analysis of Dental Materials: A Narrative Review. Materials 2021, 14, 2624. [Google Scholar] [CrossRef]
- Kaczmarek, K.; Konieczny, B.; Siarkiewicz, P.; Leniart, A.; Lukomska-Szymanska, M.; Skrzypek, S.; Lapinska, B. Surface Characterization of Current Dental Ceramics Using Scanning Electron Microscopic and Atomic Force Microscopic Techniques. Coatings 2022, 12, 1122. [Google Scholar] [CrossRef]
- Sacher, E.; França, R. Surface Analysis Techniques for Dental Materials. Dent. Biomater. 2018, 2, 1–31. [Google Scholar] [CrossRef]
- Liber-Kneć, A.; Łagan, S. Surface Testing of Dental Biomaterials-Determination of Contact Angle and Surface Free Energy. Materials 2021, 14, 2716. [Google Scholar] [CrossRef]
- Wilson, C.; Lukowicz, R.; Merchant, S.; Valquier-Flynn, H.; Caballero, J.; Sandoval, J.; Okuom, M.; Huber, C.; Brooks, T.D.; Wilson, E.; et al. Quantitative and Qualitative Assessment Methods for Biofilm Growth: A mini-review. Res. Rev. J. Eng. Technol. 2017, 6, 1–42. [Google Scholar]
- Ionescu, A.; Wutscher, E.; Brambilla, E.; Schneider-Feyrer, S.; Giessibl, F.J.; Hahnel, S. Influence of surface properties of resin-based composites on in vitro Streptococcus mutans biofilm development. Eur. J. Oral Sci. 2012, 120, 458–465. [Google Scholar] [CrossRef]
- Aykent, F.; Yondem, I.; Ozyesil, A.G.; Gunal, S.K.; Avunduk, M.C.; Ozkan, S. Effect of different finishing techniques for restorative materials on surface roughness and bacterial adhesion. J. Prosthet. Dent. 2010, 103, 221–227. [Google Scholar] [CrossRef]
- Ikeda, M.; Matin, K.; Nikaido, T.; Foxton, R.M.; Tagami, J. Effect of surface characteristics on adherence of S. mutans biofilms to indirect resin composites. Dent. Mater. J. 2007, 26, 915–923. [Google Scholar] [CrossRef]
- Ionescu, A.; Brambilla, E.; Wastl, D.S.; Giessibl, F.J.; Cazzaniga, G.; Schneider Feyrer, S.; Hahnel, S. Influence of matrix and filler fraction on biofilm formation on the surface of experimental resin-based composites. J. Mater. Sci. Mater. Med. 2015, 26, 5372. [Google Scholar] [CrossRef]
- Buergers, R.; Schneider-Brachert, W.; Hahnel, S.; Rosentritt, M.; Handel, G. Streptococcal adhesion to novel low-shrink silorane-based restorative. Dent. Mater. 2009, 25, 269–275. [Google Scholar] [CrossRef]
- Pereira, C.A.; Eskelson, E.; Cavalli, V.; Liporoni, P.C.S.; Jorge, A.O.; do Rego, M.A. Streptococcus mutans biofilm adhesion on composite resin surfaces after different finishing and polishing techniques. Oper. Dent. 2011, 36, 311–317. [Google Scholar] [CrossRef]
- Yuan, C.X.; Wang, X.; Gao, F.; Chen, X.; Liang, D.; Li, D. Effects of surface properties of polymer- based restorative materials on early adhesion of Streptococcus mutans in vitro. J. Dent. 2016, 54, 33–40. [Google Scholar] [CrossRef]
- Cazzaniga, G.; Ottobelli, M.; Ionescu, A.C.; Paolone, G.; Gherlone, E.; Ferracane, J.L.; Brambilla, E. In vitro biofilm formation on resin-based composites after different finishing and polishing procedures. J. Dent. 2017, 67, 43–52. [Google Scholar] [CrossRef]
- Bilgili, D.; Dündar, A.; Barutçugil, Ç.; Tayfun, D.; Özyurt, Ö.K. Surface properties and bacterial adhesion of bulk-fll composite resins. J. Dent. 2020, 95, 103317. [Google Scholar] [CrossRef] [PubMed]
- Hahnel, S.; Ionescu, A.C.; Cazzaniga, G.; Ottobelli, M.; Brambilla, E. Biofilm formation and release of fluoride from dental restorative materials in relation to their surface properties. J. Dent. 2017, 60, 14–24. [Google Scholar] [CrossRef]
- Bollen, C.M.; Lambrechts, P.; Quirynen, M. Comparison of surface roughness of oral hard materials to the threshold surface roughness for bacterial plaque retention: A review of the literature. Dent. Mater. 1997, 13, 258–269. [Google Scholar] [CrossRef]
- Dutra, D.; Pereira, G.; Kantorski, K.Z.; Valandro, L.F.; Zanatta, F.B. Does Finishing and Polishing of Restorative Materials Affect Bacterial Adhesion and Biofilm Formation? A Systematic Review. Oper. Dent. 2018, 43, 37–52. [Google Scholar] [CrossRef] [PubMed]
- Kara, D.; Tekçe, N.; Fidan, S.; Demirci, M.; Tuncer, S.; Balcı, S. The efects of various polishing procedures on surface topography of CAD/CAM resin restoratives. J. Prosthodont. 2021, 30, 481–489. [Google Scholar] [CrossRef] [PubMed]
- da Silva, T.M.; Salvia, A.C.R.D.; Carvalho, R.F.; Pagani, C.; Rocha, D.M.; da Silva, E.G. Polishing for glass ceramics: Which protocol? J. Prosthodont. Res. 2014, 58, 160–170. [Google Scholar] [CrossRef]
- de Oliveira, A.L.B.M.; Domingos, P.A.D.S.; Palma-Dibb, R.G.; Garcia, P.P.N.S. Chemical and morphological features of nanofilled composite resin: Influence of finishing and polishing procedures and fluoride solutions. Microsc. Res. Tech. 2012, 75, 212–219. [Google Scholar] [CrossRef]
- Kurt, A.; Cilingir, A.; Bilmenoglu, C.; Topcuoglu, N.; Kulekci, G. Effect of different polishing techniques for composite resin materials on surface properties and bacterial biofilm formation. J. Dent. 2019, 90, 103199. [Google Scholar] [CrossRef]
- Ionescu, A.C.; Brambilla, E. Bioreactors: How to Study Biofilms In Vitro. In Oral Biofilms and Modern Dental Materials, 1st ed.; Ionescu, A.C., Hahnel, S., Eds.; Springer Nature: Cham, Switzerland, 2021; pp. 37–54. [Google Scholar] [CrossRef]
- Cieplik, F.; Aparicio, C.; Kreth, J.; Schmalz, G. Development of standard protocols for biofilm-biomaterial interface testing. JADA Found. Sci. 2022, 1, 100008. [Google Scholar] [CrossRef]
- Brown, J.L.; Johnston, W.; Delaney, C.; Short, B.; Butcher, M.C.; Young, T.; Butcher, J.; Riggio, M.; Culshaw, S.; Ramage, G. Polymicrobial oral biofilm models: Simplifying the complex. J. Med. Microbiol. 2019, 68, 1573–1584. [Google Scholar] [CrossRef] [PubMed]
- Ramachandra, S.S.; Wright, P.; Han, P.; Abdal-Hay, A.; Lee, R.S.B.; Ivanovski, S. Evaluating models and assessment techniques for understanding oral biofilm complexity. MicrobiologyOpen 2023, 12, 1377. [Google Scholar] [CrossRef] [PubMed]
- Darrene, L.N.; Cecile, B. Experimental Models of Oral Biofilms Developed on Inert Substrates: A Review of the Literature. Biomed. Res. Int. 2016, 2016, 7461047. [Google Scholar] [CrossRef]
- Helmerhorst, E.J.; Dawes, C.; Oppenheim, F.G. The complexity of oral physiology and its impact on salivary diagnostics. Oral Dis. 2018, 24, 363–371. [Google Scholar] [CrossRef]
- Verma, D.; Garg, P.K.; Dubey, A.K. Insights into the human oral microbiome. Arch. Microbiol. 2018, 200, 525–540. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).