Novel Artificial Scaffold for Bone Marrow Regeneration: Honeycomb Tricalcium Phosphate
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Honeycomb TCP Containing BMP-2
2.2. Animals and Implantation Procedure
2.3. Histological Procedure
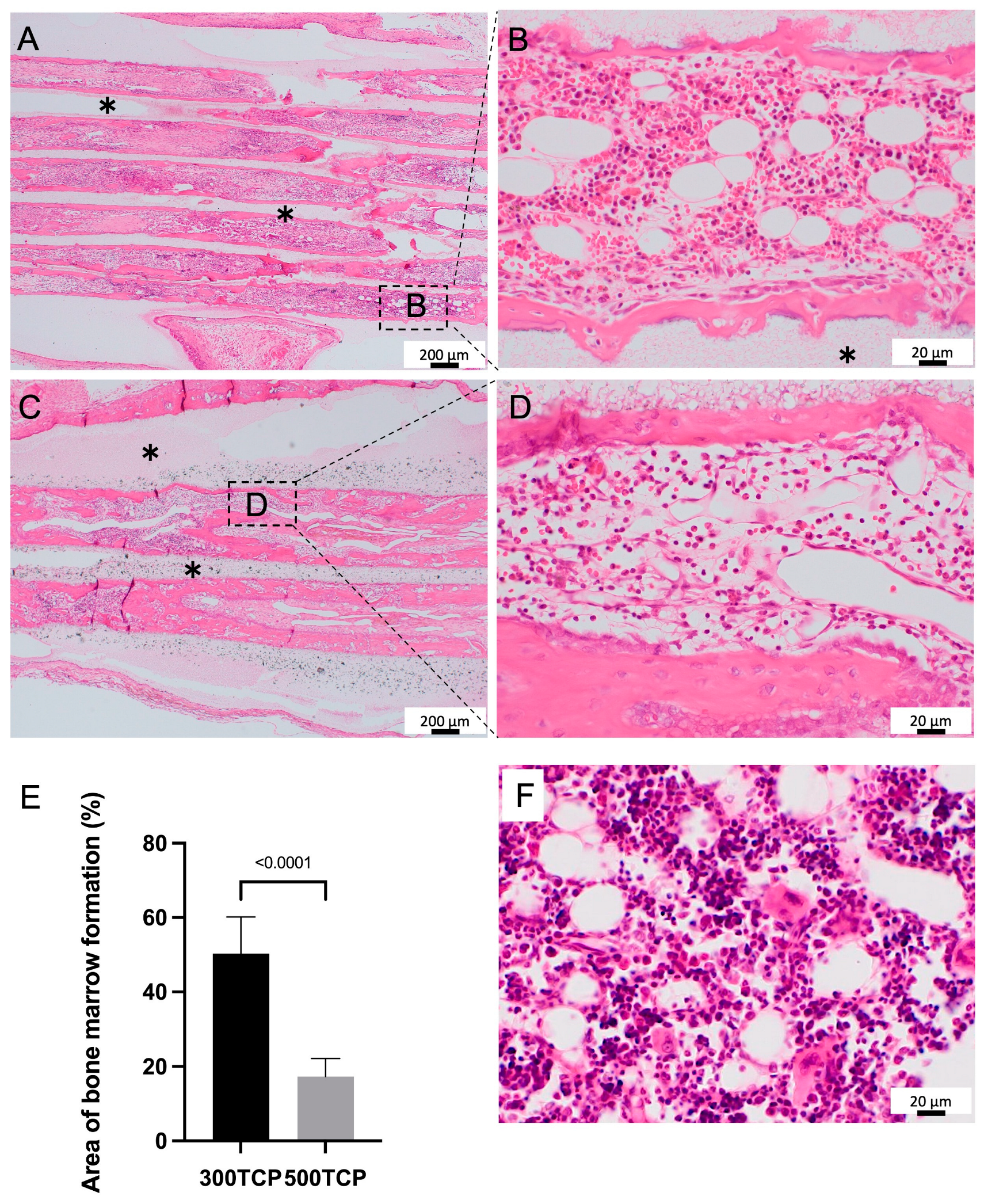
2.4. Bone Marrow Formation Evaluation by Area Measurement
2.5. Immunohistochemical (IHC) Staining
2.6. Double-Fluorescent IHC Staining
2.7. Statistical Analysis
3. Results
3.1. Comparison of Bone Marrow Induction Ability of 300TCP and 500TCP
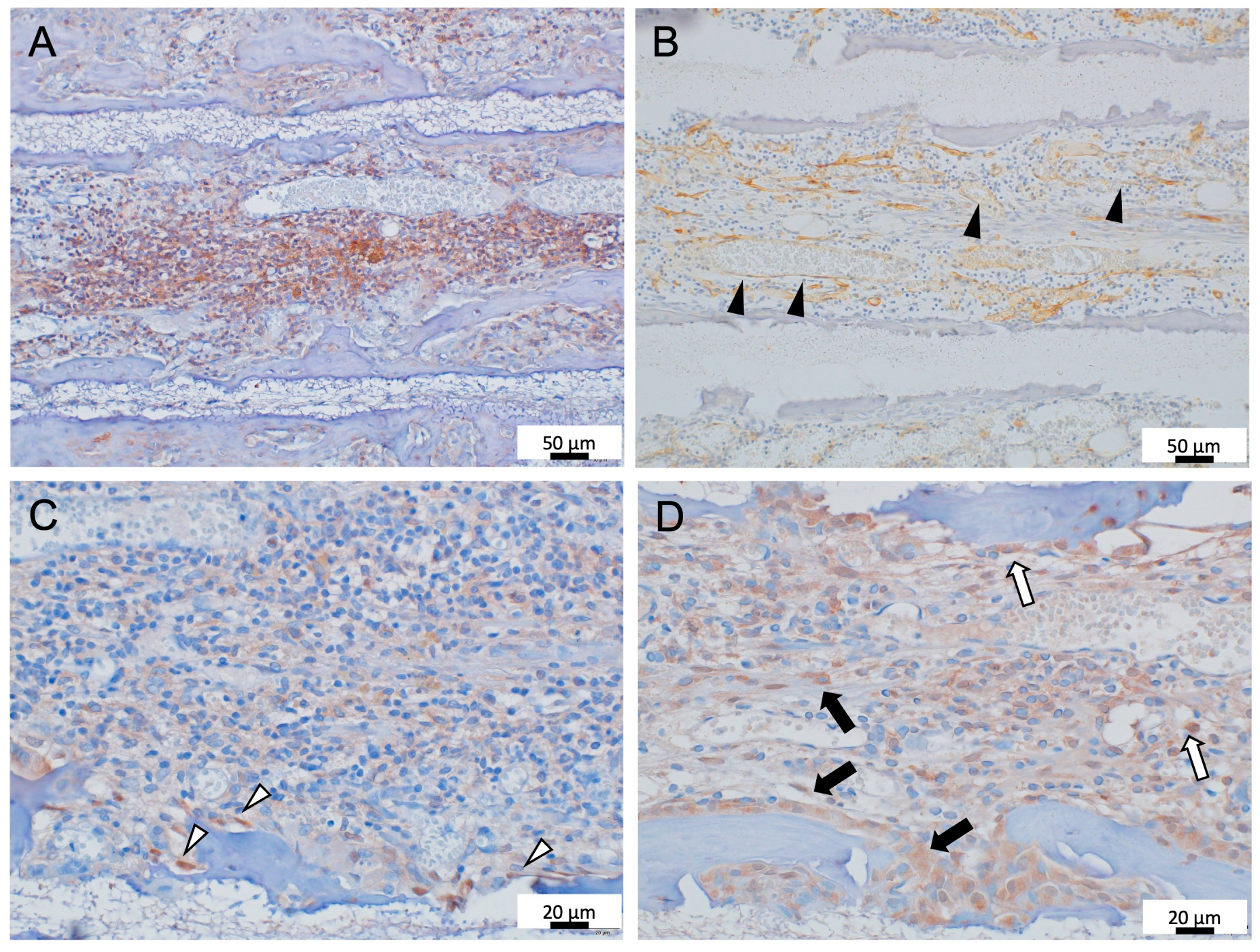
3.2. Immunohistochemistry Analysis of Formed Bone Marrow Induced by 300TCP
3.3. Evaluation of the 300TCP Function as Hematopoietic Stem Cell Niche
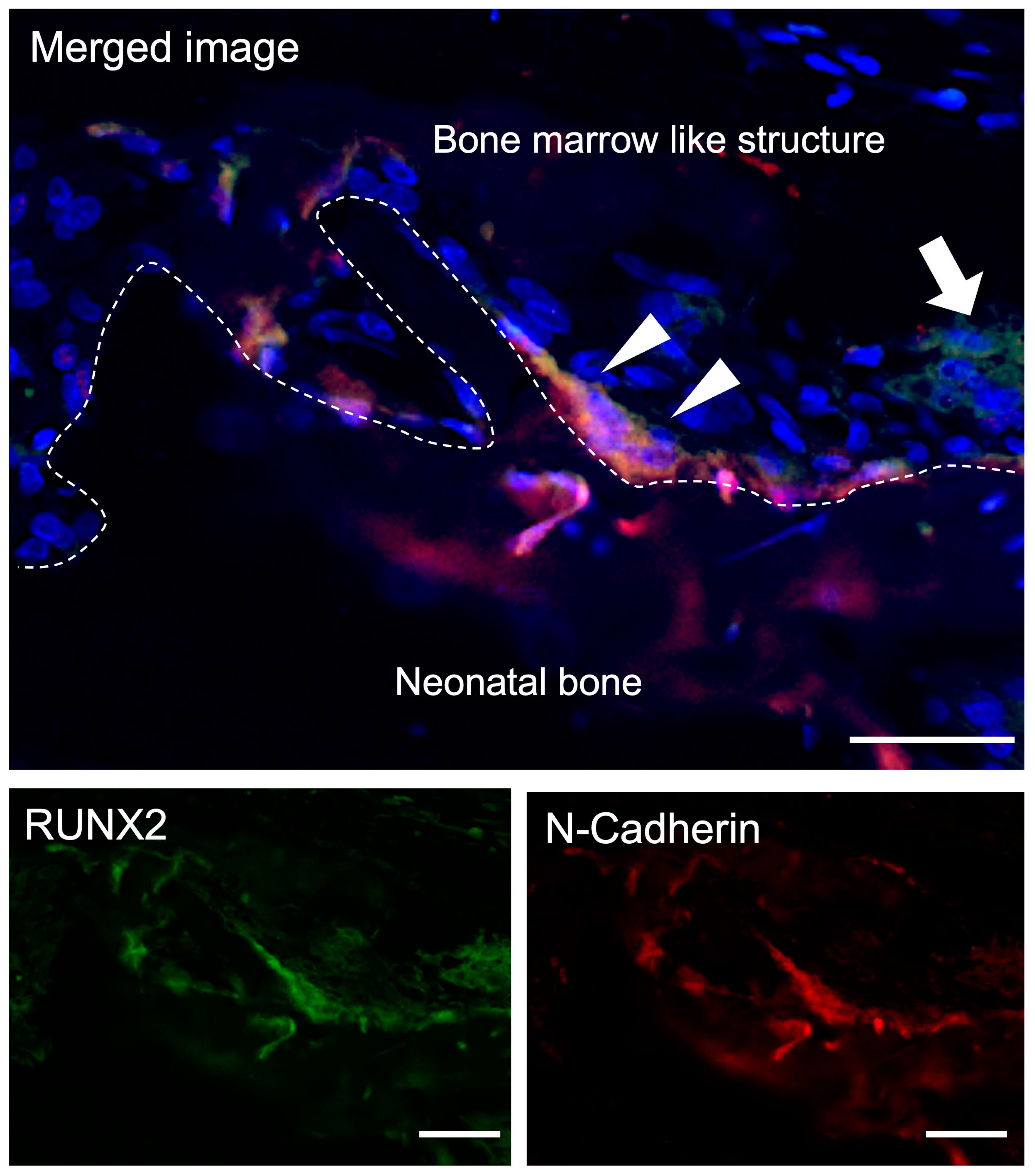
3.3.1. Double-Fluorescent IHC for Runx2-N-Cadherin
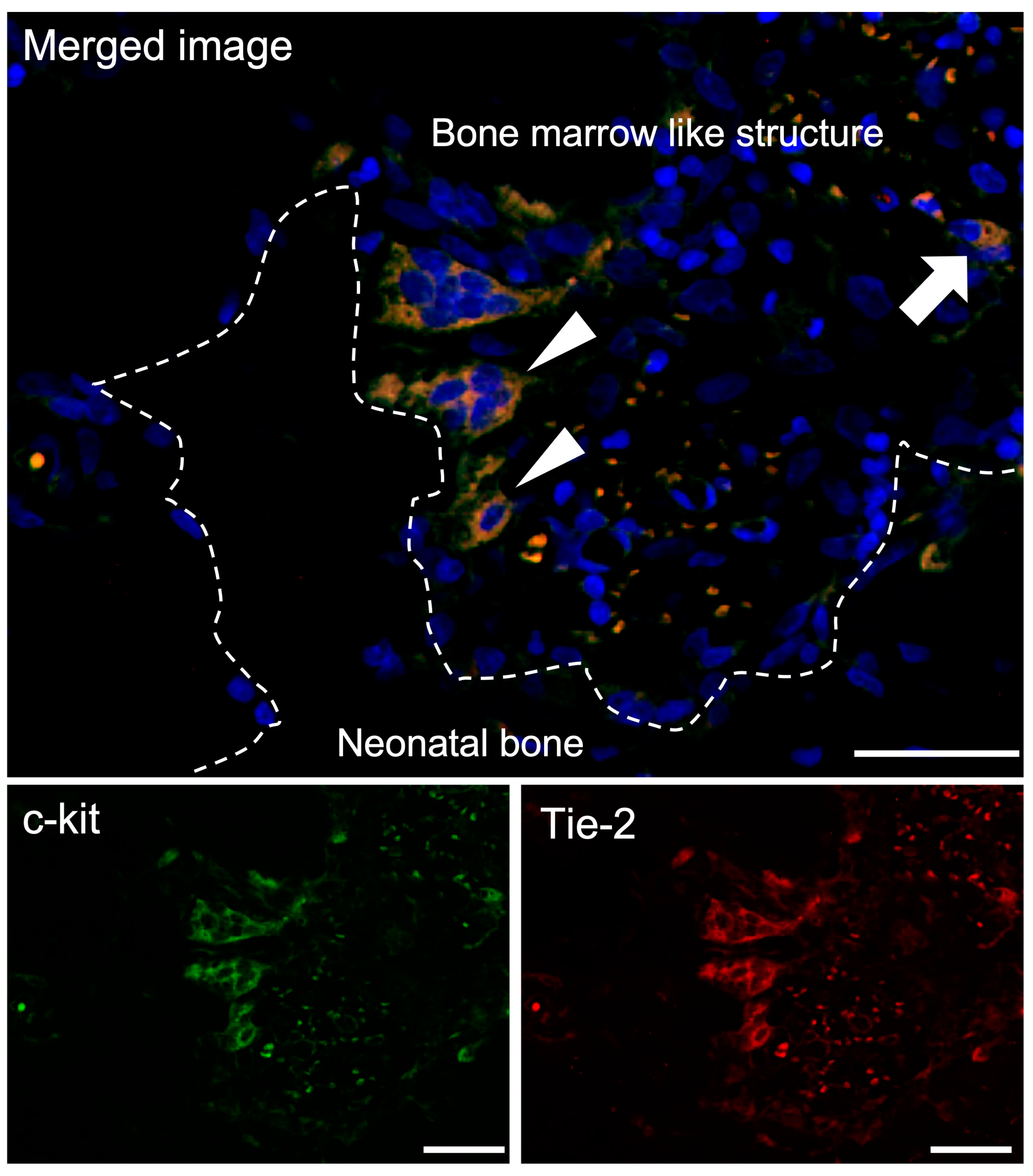
3.3.2. Double-Fluorescent IHC for c-kit-Tie-2
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Scheller, E.L.; Krebsbach, P.H.; Kohn, D.H. Tissue engineering: State of the art in oral rehabilitation. J. Oral Rehabil. 2009, 36, 368–389. [Google Scholar] [CrossRef] [PubMed]
- Torroni, A. Engineered bone grafts and bone flaps for maxillofacial defects: State of the art. J. Oral Maxillofac. Surg. 2009, 67, 1121–1127. [Google Scholar] [CrossRef] [PubMed]
- Ahmadzadeh, A.; Norozi, F.; Shahrabi, S.; Shahjahani, M.; Saki, N. Wnt/β-catenin signaling in bone marrow niche. Cell Tissue Res. 2016, 362, 321–335. [Google Scholar] [CrossRef] [PubMed]
- Maryam, M.; Alireza, S.; Bahman, Y. The roles of signaling pathways in bone repair and regeneration. J. Cell. Physiol. 2018, 233, 2937–2948. [Google Scholar]
- Lili, L.; Xianjing, L.; Wenhuan, B.; Nianqiang, J.; Yuan, M.; Yi, W.; Duan, W.; Xiaowei, X.; Ding, Z.; Hongchen, S. Carbon dots enhance extracellular matrix secretion for dentin-pulp complex regeneration through PI3K/Akt/mTOR pathway-mediated activation of autophagy. Mater. Today Bio 2022, 16, 100344. [Google Scholar]
- Yue, S.; Hao, W.; Yi, G.; Junqin, L.; Kaifeng, L.; Bin, L.; Xing, L.; Pengzhen, C.; Shuaishuai, Z.; Yixiao, W.; et al. Zinc Silicate/Nano-Hydroxyapatite/Collagen Scaffolds Promote Angiogenesis and Bone Regeneration via the p38 MAPK Pathway in Activated Monocytes. ACS Appl. Mater. Interfaces 2020, 12, 16058–16075. [Google Scholar]
- Caroline, S.; Meredith, W.; Jason, B.; Stephen, M.W. Bone tissue engineering: Current strategies and techniques-part I: Scaffolds. Tissue Eng. Part B Rev. 2012, 18, 246–257. [Google Scholar]
- Ami, R.A.; Douglas, J.A.; Cato, T.L.; Syam, P.N. Optimally porous and biomechanically compatible scaffolds for large-area bone regeneration. Tissue Eng. Part A 2012, 18, 1376–1388. [Google Scholar] [CrossRef]
- Chengde, G.; Shuping, P.; Pei, F.; Cijun, S. Bone biomaterials and interactions with stem cells. Bone Res. 2017, 5, 17059. [Google Scholar]
- Florence, B.; Clemens, A.B.; Klaas, G. Bone regeneration: Molecular and cellular interactions with calcium phosphate ceramics. J. Nanomed. 2006, 1, 317–332. [Google Scholar]
- Shuaiab, C.; Lia, P.; Liua, J.; Pengcd, S. Optimization of TCP/HAP ratio for better properties of calcium phosphate scaffold via selective laser sintering. Mater. Charact. 2013, 77, 23–31. [Google Scholar]
- Marc, B.; Bastien Le, G.S.; Nicola, D. β-tricalcium phosphate for bone substitution: Synthesis and properties. Acta Biomater. 2020, 113, 23–41. [Google Scholar]
- Marina, R.; Jolanda, R.V.; Iina, L.; Marianne, S.; Feihu, Z.; André, R.S.; Ralph, M.; Sandra, H. Scaffold Pore Geometry Guides Gene Regulation and Bone-like Tissue Formation in Dynamic Cultures. Tissue Eng. Part A 2021, 27, 1192–1204. [Google Scholar]
- Amir, A.Z. Bone tissue regeneration: The role of scaffold geometry. Biomater. Sci. 2015, 3, 231–245. [Google Scholar]
- Takabatake, K.; Yamachika, E.; Tsujigiwa, H.; Takeda, Y.; Kimura, M.; Takagi, S.; Nagatsuka, H.; Iida, S. Effect of geometry and microstructure of honeycomb TCP scaffolds on bone regeneration. J. Biomed. Mater. Res. A 2014, 102, 2952–2960. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Takabatake, K.; Tsujigiwa, H.; Watanabe, T.; Tokuyama, E.; Ito, S.; Nagatsuka, H.; Kimata, Y. Efficacy of Honeycomb TCP-induced Microenvironment on Bone Tissue Regeneration in Craniofacial Area. Int. J. Med. Sci. 2016, 13, 466–476. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, H.; Takabatake, K.; Tsujigiwa, H.; Watanabe, S.; Ito, S.; Kawai, H.; Hamada, M.; Yoshida, S.; Nakano, K.; Nagatsuka, H. Effects of the Geometrical Structure of a Honeycomb TCP on Relationship between Bone/Cartilage Formation and Angiogenesis. Int. J. Med. Sci. 2018, 15, 1582–1590. [Google Scholar] [CrossRef] [PubMed]
- Balduini, A.; Di Buduo, C.A.; Kaplan, D.L. Translational approaches to functional platelet production ex vivo. Thromb. Haemost. 2016, 115, 250–256. [Google Scholar]
- Di Buduo, C.A.; Kaplan, D.L.; Balduini, A. In vitro generation of platelets: Where do we stand? Transfus. Clin. Biol. 2017, 24, 273–276. [Google Scholar] [CrossRef]
- Malara, A.; Currao, M.; Gruppi, C.; Celesti, G.; Viarengo, G.; Buracchi, C.; Laghi, L.; Kaplan, D.L.; Balduini, A. Megakaryocytes contribute to the bone marrow-matrix environment by expressing fibronectin, type IV collagen, and laminin. Stem Cells 2014, 32, 926–937. [Google Scholar] [CrossRef]
- Gregory, S.T. Normal structure, function, and histology of the bone marrow. Toxicol. Pathol. 2006, 34, 548–565. [Google Scholar]
- Eriksen, E.F. Cellular mechanism of bone remodeling. Rev. Endocr. Metab. Disord. 2010, 11, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Kurosawa, S.; Iwama, A. Aging and leukemic evolution of hematopoietic stem cells under various stress conditions. Inflamm. Regen. 2020, 40, 29. [Google Scholar] [PubMed]
- Zhang, J.; Niu, C.; Ye, L.; Huang, H.; He, X.; Tong, W.; Ross, J.; Haug, J.; Johnson, T.; Feng, J.Q.; et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature 2003, 425, 836–841. [Google Scholar] [CrossRef] [PubMed]
- Arai, F.; Hirao, A.; Ohmura, M.; Sato, H.; Matsuoka, S.; Takubo, K.; Ito, K.; Young, G.K.; Suda, T. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell 2004, 118, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Morrison, S.J.; Scadden, D.T. The bone marrow niche for haematopoietic stem cells. Nature 2014, 505, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.D.; Wagers, A.J. Dynamic niches in the origination and differentiation of haematopoietic stem cells. Nat. Rev. Mol. Cell Biol. 2011, 12, 643–655. [Google Scholar] [CrossRef]
- Stegner, D.; van Eeuwijk, J.M.M.; Angay, O.; Gorelashvili, M.G.; Semeniak, D.; Pinnecker, J.; Schmithausen, P.; Meyer, I.; Friedrich, M.; Dütting, S.; et al. Thrombopoiesis is spatially regulated by the bone marrow vasculature. Nat. Commun. 2017, 8, 127. [Google Scholar] [CrossRef]
- Spencer, J.A.; Ferraro, F.; Roussakis, E.; Klein, A.; Wu, J.; Runnels, J.; Zaher, W.; Mortensen, L.J.; Alt, C.; Turcotte, R.; et al. Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature 2014, 508, 269–273. [Google Scholar] [CrossRef]
- Kiel, M.J.; Yilmaz, O.H.; Iwashita, T.; Terhorst, C.; Morrison, S.J. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell 2005, 121, 1109–1121. [Google Scholar] [CrossRef]
- Taichman, R.S.; Emerson, S.G. Human osteoblasts support hematopoiesis through the production of granulocyte colony-stimulating factor. J. Exp. Med. 1994, 179, 1677–1682. [Google Scholar] [CrossRef] [PubMed]
- Kiel, M.J.; Radice, G.L.; Morrison, S.J. Lack of evidence that hematopoietic stem cells depend on N-cadherin-mediated adhesion to osteoblasts for their maintenance. Cell Stem Cell 2007, 1, 204–217. [Google Scholar] [CrossRef] [PubMed]
- Noda, M.; Omatsu, Y.; Sugiyama, T.; Oishi, S.; Fujii, N.; Nagasawa, T. CXCL12-CXCR4 chemokine signaling is essential for NK-cell development in adult mice. Blood 2011, 117, 451–458. [Google Scholar] [CrossRef]
- Sugiyama, T.; Kohara, H.; Noda, M.; Nagasawa, T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity 2006, 25, 977–988. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Linheng, L. The stem cell niches in bone. J. Clin. Investig. 2006, 116, 1195–1201. [Google Scholar]
- Avecilla, S.T.; Hattori, K.; Heissig, B.; Tejada, R.; Liao, F.; Shido, K.; Jin, D.K.; Dias, S.; Zhang, F.; Hartman, T.E.; et al. Chemokine-mediated interaction of hematopoietic progenitors with the bone marrow vascular niche is required for thrombopoiesis. Nat. Med. 2004, 10, 64–71. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inada, Y.; Takabatake, K.; Tsujigiwa, H.; Nakano, K.; Shan, Q.; Piao, T.; Chang, A.; Kawai, H.; Nagatsuka, H. Novel Artificial Scaffold for Bone Marrow Regeneration: Honeycomb Tricalcium Phosphate. Materials 2023, 16, 1393. https://doi.org/10.3390/ma16041393
Inada Y, Takabatake K, Tsujigiwa H, Nakano K, Shan Q, Piao T, Chang A, Kawai H, Nagatsuka H. Novel Artificial Scaffold for Bone Marrow Regeneration: Honeycomb Tricalcium Phosphate. Materials. 2023; 16(4):1393. https://doi.org/10.3390/ma16041393
Chicago/Turabian StyleInada, Yasunori, Kiyofumi Takabatake, Hidetsugu Tsujigiwa, Keisuke Nakano, Qiusheng Shan, Tianyan Piao, Anqi Chang, Hotaka Kawai, and Hitoshi Nagatsuka. 2023. "Novel Artificial Scaffold for Bone Marrow Regeneration: Honeycomb Tricalcium Phosphate" Materials 16, no. 4: 1393. https://doi.org/10.3390/ma16041393
APA StyleInada, Y., Takabatake, K., Tsujigiwa, H., Nakano, K., Shan, Q., Piao, T., Chang, A., Kawai, H., & Nagatsuka, H. (2023). Novel Artificial Scaffold for Bone Marrow Regeneration: Honeycomb Tricalcium Phosphate. Materials, 16(4), 1393. https://doi.org/10.3390/ma16041393





