Abstract
Electroless noble metal deposition on the conducting substrate is widely used to obtain the desired film or coating on the substrate of interest. Wire-gauge-based Pt/Pd/Pt-Pd (individually, sequentially, and simultaneously deposited) catalysts have been developed using formaldehyde and sodium formate as reducing agents. Various surface pretreatment methods like SnCl2 + PdCl2 seeding, oxalic acid etching, and HCl activation (etching) have been employed to obtain the desired noble metal coating. Minimum time duration was observed for simultaneously deposited catalysts using formaldehyde as a reducing agent. Prepared catalysts were characterized for noble metal deposition, coating kinetics, surface morphology, and binding energy. The catalyst was found to be active for H2 and O2 recombination reactions for hydrogen mitigation applications in nuclear reactors.
1. Introduction
In modern research, electroless metal plating techniques are widely used in several fields of science and technology, including metallization, galvanoplastic, microcircuits, and optoelectronics. Additionally, they are effectively employed to create catalysts for fuel cells as well as in several catalytic processes, including catalytic steam methane reforming (SMR), methanol oxidation in direct methanol fuel cells, etc. [1,2,3,4,5,6,7,8,9,10]. These include thermal deposition [11,12], sonication [13,14], UV irradiation [15,16], microwave [17,18], sputter coating [19,20], chemical vapor deposition [21,22], electrochemical plating [23,24] and electroless plating [25,26], etc. Although thermal deposition, sputter coating, and chemical vapor deposition provide excellent control over film composition, thickness, and structure, they suffer from high cost (expensive high vacuum equipment is required) and low yield. Electrochemical plating is only suitable for conducting substrates. Electroless plating provides uniform dense coatings on both conducting and non-conducting surfaces [27]. It is also a relatively inexpensive process.
Noble metal electroless deposition is an electrochemical process that is heterogeneous, autocatalytic, and redox in nature. The interaction between the reducing agents from the electroplating bath and the noble metal precursors on the surface of the metal substrate takes place at the interface between solid and liquid. The procedure can be carried out at different temperatures and pH levels, with or without the addition of stabilizing or complexing agents, depending on the type of coating required and its uses. The coating process was carried out at room temperature with or without the use of a complexing agent to meet the demand for crystalline or amorphous noble metal coating.
Deposits/thin films obtained from electroless deposition have many applications in different fields. The primary application is the protection of the underlying layers against corrosion [28,29]. Other applications include wear resistance for surfaces, ornamental coatings to improve the aesthetic appeal of objects and practical applications such as providing catalytic surfaces for electrodes used in chemical processes and low-resistance electrical connections. Metals with high melting points, such as platinum and rhodium, are primarily deposited using the electroless deposition method. One of the vast applications of these electroless deposited catalysts is in the field of hydrogen mitigation. In nuclear reactors, the catalytic mitigation of hydrogen is one of the most promising methods to keep hydrogen concentration below the combustion limit. For these purposes, wire-gauge-supported Pt/Pd catalysts prepared using the electroless deposition method found extensive use for the recombination of H2 and O2 in a nuclear reactor [30,31,32,33,34]. In this article, we report the development of an electroless deposition method for the preparation of Pt-based, Pd-based, and Pt-Pd (sequential and simultaneous) catalysts by employing sodium formate and formaldehyde as the reducing agents. It has been noted previously that the presence of complexing agents causes a bright metallic covering that is ineffective as a catalyst [35]. Depending on the type of coating required and its uses, the coating procedure can be carried out at different temperatures and pH levels, with or without the addition of stabilizing or complexing agents [36]. In this article, we report room-temperature deposition of metallic Pt-Pd nanoparticles over a wire gauge support without the use of a complexing agent, without alteration in pH, and without using any inert gas bubbling (Argon) or agitation to meet our demand for an amorphous noble metal coating with improved surface heterogeneity. Prepared catalysts were characterized for % noble metal deposition and coating kinetics using UV-Vis spectrophotometry. Further, an investigation for the study of metal–metal or metal–support interaction was carried out by XPS. Finally, the catalysts were evaluated for H2 and O2 recombination reactions.
2. Experimental
2.1. Electroless Deposition
In the present study, Hydrochloric acid (Thomas Baker, Mumbai, India, 36%) and Oxalic acid (Chemico Fine Chemicals, Delhi, India, 99.8%) were used as etching agents for the substrate. Tin Chloride (Loba Chemie, Mumbai, India, 98%) was used as a seeding agent. Sodium formate (S D Fine Chemical, Mumbai, India) and formaldehyde (Thomas Baker, Mumbai, India, 37–41%) were employed as reducing agents. Chloroplatinic acid (Hindustan Platinum Private Limited, Mumbai, India, 39.8% Pt) and palladium chloride (Hindustan Platinum Private Limited, Mumbai, India, 59.96% Pd) were employed as noble metal precursors and an SS-316 wire gauge with wire dimensions of 0.1 mm and mesh size of 80 as a substrate for deposition. The concentrations of precursor solutions used in the present study were 3.6 mg/mL or 12 mg/mL of Pd and 9.5 mg/mL of Pt. The coating procedure relies on several processes which occur at the metal support surface, including the oxidation of formaldehyde at the anode surface and the reduction of noble metal at the cathode surface [37].
A study of electroless deposition and catalytic activity evaluation was carried out over 4 cm × 6 cm and 6 cm × 8 cm dimensions of an SS wire gauge. First, 4 cm × 6 cm (or 6 cm × 8 cm) washed wire gauge pieces were subjected to HCl etching and then dipped in an electroless deposition bath. Different activation processes like electrolytic oxalic acid etching, SnCl2 + PdCl2 seeding, etc., were also employed. In general, the electroless deposition bath consisted of noble metal precursors like palladium chloride or chloroplatinic acid along with reducing agents like formaldehyde or sodium formate. The time required for the coating to complete was found to depend on the concentration of the noble metal precursor and/or reducing agent. Depending on the composition and other experimental conditions, shiny to amorphous coatings were observed. Various compositions with single or mixed noble metals were prepared. A typical electroless deposition method is shown in Figure 1a,b.
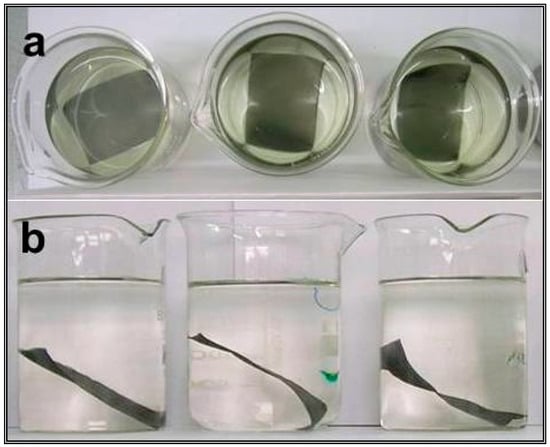
Figure 1.
Photographs for electroless reduction (a) before deposition and (b) after deposition of noble metal on 6 cm × 8 cm wire gauge support.
2.2. Catalytic Activity
Using a thermal conductivity detector-based H2 monitor, the prepared catalysts were assessed for catalytic H2-O2 recombination reaction under static air reaction conditions in a 40 L closed reactor at room temperature (RT). The time required for completion of half of the reaction (t1/2) and maximum temperature rise on the catalyst surface (Tmax) values for the H2-O2 reaction at a 4.5% H2 concentration in air were assessed. Details of catalytic activity setup were given in an earlier article reported by Sanap et al. [30].
3. Results and Discussion
3.1. Catalyst Preparation
Effective noble metal deposition on a catalyst substrate is widely influenced by the reducing agent and pre-treatment method used for activation of the substrate. The following section elaborates on the effect of the catalyst preparation route adapted and its effect on the noble metal deposition and catalytic activity.
3.1.1. Palladium on SS Wire-Gauge-Based Catalysts
The wire gauge obtained from the industry had a smooth texture and additionally, it was composed of dirt, oil, grease, etc. To obtain a good quality of coating, it was necessary to remove all contaminants from the surface. Different methods have been reported in the literature to make wire gauge surfaces clean and rough, e.g., HCl etching, [38], SnCl2 + PdCl2 seeding [39], electrolytic oxalic acid activation, etc. [40].
Work on the preparation of a Pd-supported catalyst using HCl etching was initiated from the reported process with Na-formate as a reducing agent [41]. For this purpose, initially cleaned and dried wire gauge pieces were pre-treated with 0.1 N HCl for 15 h. Afterward, they were taken out, washed with plenty of distilled water, and dried in an oven at 105 °C for 1 h. Then, the wire gauge coupons (4 cm × 6 cm) were kept in an electroless plating bath (125 mL beaker) containing a 100 mL diluted solution of precursor and reducing agent. The procedure adopted and the catalytic activity of the prepared catalysts are given below in Table 1. Thus, e.g., bath A1 consisted of 2.5 mL of PdCl2 and 1 mL of Na-formate which were then further diluted to 100 mL. After 15 h of deposition, no weight gain or catalytic activity were observed for the catalysts prepared by the direct deposition route using HCl etching treatment. Therefore, another additional surface activation step using SnCl2 + PdCl2 activation was introduced after HCl etching for the more effective deposition of noble metal. This process of activation involved dipping a wire gauge for 5 min each in SnCl2 (1 gm/L), distilled water, PdCl2 (0.1 gm/L) solution, and 0.01 M HCl. This procedure was repeated 4–10 times with intermittent rinsing with distilled water. This was followed by electroless deposition using Na-formate as a reducing agent as per the procedure given for HCl-etched catalysts. The bath was found to turn turbid after 2–3 h and the catalysts prepared were also found to be inactive for the H2-O2 recombination reaction. Catalysts prepared by this route did not show any weight gain and were also found to be catalytically inactive. Finally, another option for electrolytic oxalic acid etching (wire gauge: cathode + ve, potential: 5 V, current: 0.1 A, time: 40 s) was introduced before electroless reduction. In this case, limited noble metal coating was obtained but the prepared catalysts did not exhibit the required catalytic activity.

Table 1.
Details of palladium-based catalysts over 4 cm × 6 cm SS wire gauge coupons using sodium formate as a reducing agent.
In the case of sodium formate using SnCl2 + PdCl2 or oxalic acid activation, additional efforts were made for pH adjustment, but the bath was still found to turn turbid with time. Also, no activity was observed for the prepared catalysts.
Based on the above observations (i.e., failure of resultant noble metal deposition on the substrate), a different reducing agent, i.e., formaldehyde, was employed for further study. This was based on an earlier reported similar procedure for platinum coating [42]. Different activation steps like HCl etching, SnCl2 + PdCl2 seeding, and electrolytic etching using oxalic acid were employed as pre-treatment steps. Details are provided in Table 2.

Table 2.
Details of palladium-based catalysts over 4 cm × 6 cm SS wire gauge preparation using formaldehyde as a reducing agent.
From the data given in Table 2, it is evident that catalysts prepared using formaldehyde as a reducing agent showed the deposition of noble metal on the substrate, but catalytic activity was observed only for the catalysts that were produced after electrolytic etching in oxalic solution. Therefore, the process based on electrolytic etching followed by noble metal reduction using formaldehyde was short-listed for further studies on larger (6 cm × 8 cm) catalysts. During this process, wire gauge coupons of 6 cm × 8 cm were rotated (1 RPM) with the help of a motor in a coating bath (500 mL beaker) containing a 400 mL diluted solution of precursor and reducing agent. The details of the samples prepared are provided in Table 3. During the coating of palladium, the color of the bath was found to change from yellowish or yellowish orange to nearly colorless as coating took place on the substrate. The coating observed was uniform, black, smooth, fine, and had good adherence. All the catalysts prepared had a weight gain of ~2 wt % and exhibited good catalytic activity for the H2-O2 reaction, with t1/2 ranging from 3–3.5 min and Tmax of 200–300 °C.

Table 3.
Details of palladium-based catalysts over 6 cm × 8 cm SS wire gauge coupons.
3.1.2. Platinum on SS Wire-Gauge-Based Catalysts
Details of the Pt-based catalysts prepared and their catalytic activity data are listed in Table 4. During this process, wire gauge coupons of 6 cm × 8 cm were rotated with the help of a motor in a coating bath (500 mL beaker) containing a 400 mL diluted solution of precursor and reducing agent. During the coating of platinum, the bath color changed from yellowish or pale yellowish to colorless. The coating observed was uniform, smooth, and blackish-white without any dark patches on the wire gauge and had good adherence. Weight gain for the noble metal coating was found to vary from 1.5 to 2.0 wt %. Catalytic activities in terms of t1/2 and Tmax were found to be about 5.0 min and 245–275 °C, respectively. The inorganic acid-etched catalysts D1 and D3 (HCl-etched) were found to be superior to the organic acid-etched catalysts D2 and D4 (oxalic acid-etched). Coating took place in a short time and t1/2 was also comparable. This may be due to the strength of the acid and pH of both solutions. The coating was also smooth, fine, uniform, and had a black amorphous type with good adherence. Thus, for platinum-based catalysts, it was observed that activation of the SS wire gauge with 0.1 N HCl is sufficient for the electroless deposition procedure.

Table 4.
Details of platinum-based catalysts over 6 cm × 8 cm SS wire gauge support.
3.1.3. Mixed Noble Metal Deposition on SS Wire-Gauge-Based Catalysts
Further efforts were directed towards the development of a procedure for the deposition of mixed noble metals (Pt and Pd) on an SS wire gauge. For this purpose, three approaches were adopted: firstly, a sequential deposition of palladium over platinum. The second approach was platinum over palladium, but in this case, we observed that the process was too tedious, i.e., for the initial deposition of Pd, electrolytic oxalic acid etching was necessary, as observed in Table 2. Additionally, it is very time-consuming if we consider mass scale preparation of catalysts. Finally, simultaneous deposition of both platinum and palladium was carried out in a single electroless bath. Table 5 shows the details of sequentially (E) and simultaneously (F) prepared catalysts using formaldehyde as a reducing agent over 6 cm × 8 cm SS wire gauge support.

Table 5.
Details of sequential and simultaneous coating of platinum and palladium.
For the sequential noble metal deposition, it was observed that the coating process was very slow, taking almost ~20 h (10 + 10) for ~2% weight gain (E1, E2, and E3 samples). Catalysts produced were found to be active for H2 + O2 reaction with t1/2 = ~2.5–2.8 min and Tmax = ~295 °C.
To minimize the time of coating, we tried simultaneous deposition using HCl etching and oxalic acid etching treatment for SS support (F1, F2, and F3). In this case, coating time was observed to be only 9 h, and catalytic activity was observed only for those samples (F2 and F3) that were prepared via the HCl activation route.
The results obtained from the above study show that better noble metal deposition was observed when using formaldehyde as a reducing agent rather than sodium formate. For the individual platinum and simultaneous noble metal (Pt-Pd) deposition on the SS wire gauge, activation (etching) by (0.1 N) HCl was more appropriate than using oxalic acid and SnCl2 activation. Therefore, we recommend the use of formaldehyde as a reducing agent and hydrochloric acid for surface activation for further detailed study.
3.2. Coating Kinetics
The deposition of precursors was carried out with the electroless deposition method using formaldehyde as a reducing agent. Both precursor solutions (chloroplatinic acid and palladium chloride) have particular absorbance spectra; therefore, it is possible to interpret their rate of deposition using UV-VIS spectrophotometry. Figure 2 shows the absorbance spectra of the electroless deposition bath of simultaneously prepared Pt-Pd (F3) catalyst with the time of deposition. The absorbance spectrum corresponds to the hexachloroplatinic acid and palladium chloride precursors. It can be observed from the spectrum that the initial concentration of the deposition bath concerning chloroplatinic acid and palladium chloride fell with increasing deposition time. Figure 3 shows the decay plot of absorbance with time for the simultaneously prepared Pt-Pd catalyst and Pt-based catalysts in an electroless deposition bath. From Figure 3, it is apparent that the rate of deposition is quicker for a bi-metallic (Pt-Pd) precursor bath than for a single-metal (Pt) precursor bath. Here it is also evident that for both the catalysts, the initial rate of coating is slow for up to 1 h, and after 1 h it becomes quicker but then becomes slower again after 3 h. This may be due to the following reasons: initially, the even distribution of smaller nuclei is taking place on the substrate. These smaller nuclei act as catalytic agents for further processes and hence the method becomes autocatalytic. After 3 h, the quantity of reactants starts diminishing in the electroless deposition bath and the rate of coating again further falls.
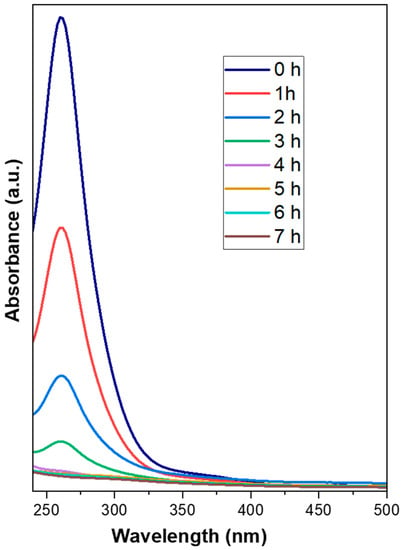
Figure 2.
Plot of absorbance of F3 catalyst.
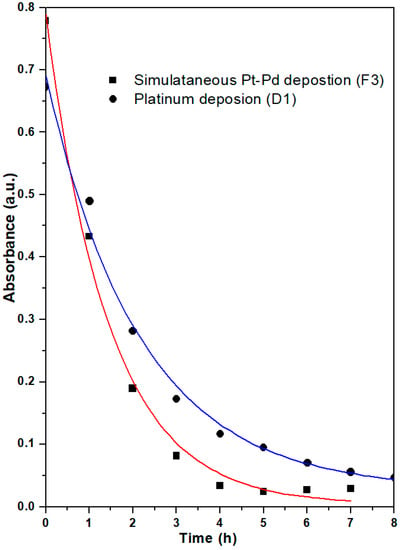
Figure 3.
Plot of absorbance against deposition time for F3 and D1 catalysts at λmax = 260.
Figure 4 shows that at the start of deposition, the noble metal deposition bath has a dark yellow color which becomes lighter as further coating takes place on the wire gauge.
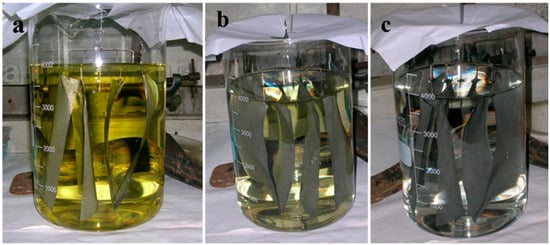
Figure 4.
Photographs for electroless reduction for F3 catalyst (a) at the start of deposition, (b) at 2 h of deposition, and (c) at 6 h of deposition of noble metal on 12 cm × 16 cm wire gauge support.
3.3. Surface Morphology
Scanning electron microscopy was employed to understand the noble metal deposition using different reducing agents and surface activation methods. Figure 5a,b show the palladium-deposited sample using sodium formate as the reducing agent. Figure 5a shows the nonuniform coating of the noble metal on the surface of the wire gauge. Figure 5b depicts the precipitation of the noble metal precursor on the surface of the wire gauge. This precipitated noble metal resulted in the loss of catalytic activity for this catalyst. Figure 5c,d pertains to the uniform coating of Pt noble metal (sample D1) on the surface of a wire gauge using formaldehyde as a reducing agent. Here, deposited noble metals are observed with perfect round and spherical geometry. Figure 5e shows the accumulation of palladium particles on the initially deposited platinum which has initiated, resulting in the formation of spike geometry on the earlier deposited Pt metal (Sample E2). Figure 5f pertains to the Pt-Pd simultaneously deposited sample (F3) using formaldehyde as a reducing agent. Here, particles are observed with well-defined cuboidal extension to the spherical particles.
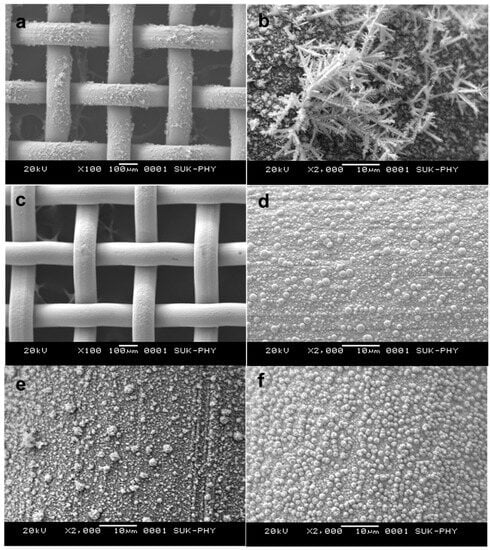
Figure 5.
Scanning electron micrograph of (a,b) Pd-deposited sample (A9) using Na formate as reducing agent, (c,d) Pt-deposited sample (D1), (e) Pt-Pd sequentially deposited sample (E2), and (f) Pt-Pd simultaneously deposited sample (F3) using formaldehyde as a reducing agent.
3.4. X-ray Photoelectron Spectroscopy
X-ray photoelectron spectroscopy (XPS) analysis was performed for Pt-based (D1), Pt-Pd sequentially prepared (E2), and simultaneously prepared Pt-Pd (F3) samples. All three samples, D1, E2, and F3, were analyzed for Pt, whereas E2 and F3 were analyzed for Pd alone. The peaks corresponding to Pt for D1 and F3 samples were deconvoluted into two peaks, i.e., 4f5/2 and 4f7/2 with the respective binding energy of ~74 and 71 eV (Figure 6c,e). The observed binding energy for Pt (4f7/2) in D1 and F3 samples is 71.4 and 71.2 eV. These values are in good agreement with the values of Pt0 reported in the literature [43,44]. Hence, our study shows that the noble metals were in the metallic state. No characteristic peak (Figure 6d) was observed for Pt in the E2 sample; this could be because of the external coating of Pd on an existing layer of Pt.
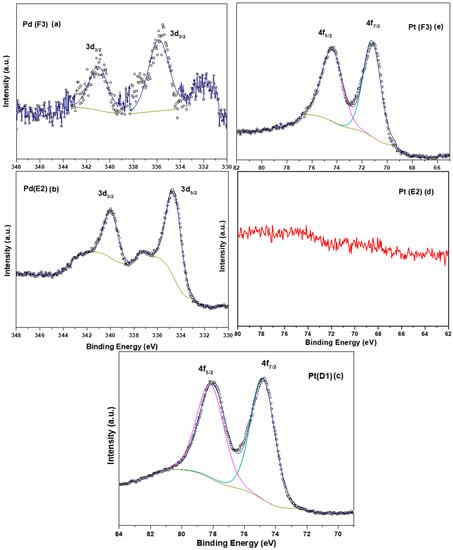
Figure 6.
(a) XPS spectra of Pd 3d region for F3 sample, (b) XPS spectra of Pd 3d region for E2 sample, (c) XPS spectra of Pt 4f region for D1 sample, (d) XPS spectra of Pt 4f region E2 samples, and (e) XPS spectra of Pt 4f region for F3 samples.
For the analysis of Pd concerning the F3 and E2 samples (Figure 6a,b), the spectrum was deconvoluted into two peaks, 3d5/2 and 3d3/2, with respective binding energy of ~335 eV and ~340 eV. The 3d5/2 binding energy contribution of Pd (F3 sample) was 335.8 eV, whereas for the E2 sample it was 334.8 eV. Both values resemble the presence of Pd in a metallic state (Pd0) [45], although a slight shift (+1 eV) is observed in binding energy for Pd in the case of the F3 sample [46]. The shift in binding energy can be explained based on the procedure of coating. The coating in F3 is carried out simultaneously so there is a possibility of homogenous solid solution formation and earlier deposition of platinum, whereas the coating of the E2 sample is carried out sequentially and therefore there is only a deposition of Pd on the existing Pt, resulting in no resultant shift in binding energy value. Therefore, in the F3 sample, electrons can transfer more easily from Pt to Pd or vice versa. The inference drawn from the XPS data supports the earlier investigation of the initial coating of platinum later followed by palladium in the case of the F3 sample.
3.5. Catalytic Activity
In a 40 L closed-vessel reactor, the prepared catalysts’ catalytic activity for the reaction of hydrogen and oxygen was assessed. A fixed-volume injector was used to inject a known amount of hydrogen into a reactor, and the hydrogen concentration and surface temperature of the catalyst were used to track the reaction’s progress. Figure 7a shows the variation in temperature on the catalyst surface and Figure 7b shows the variation in catalytic activity concerning the noble metal deposition method adapted. The palladium-based sample (A9) prepared with the pretreatment of oxalic acid and Na-formate as a reducing agent showed no catalytic activity because of the precipitation of the noble metal precursor on the surface of the wire gauge. This result goes hand in hand with the result obtained from the surface morphology study. The platinum-based sample (D1) prepared by HCl pretreatment and formaldehyde as a reducing agent showed t1/2 = 5 min and the catalyst temperature was observed to be around 250 °C. Round and spherical particles of Pt were observed to be catalytically active toward H2 and O2 reaction. Bimetallic Pt-Pd-based (E2 and F3) samples prepared by sequential deposition and simultaneous deposition showed t1/2 in the range of 2.5 min and catalyst surface temperature was observed to be around 290 °C to 313 °C. For these catalysts, a high-temperature rise was observed concerning a single Pt-based catalyst because of the synergic effects of both metals. This high temperature obtained is highly advantageous to keep catalyst poisons (e.g., H2O, reaction product) away from the catalyst surface. From the surface morphology study, it was revealed that Pt-based catalysts (D1) were perfectly round in shape, whereas catalyst F (simultaneously prepared bimetallic catalyst) showed surface heterogeneity. The reduced catalytic activity for the catalyst was because of heterogeneity in the surface morphology as compared to the smooth surface of a Pt-based catalyst. Higher surface heterogeneity resulted in the availability of more catalytic active sites on the catalyst surface, which resulted in lower catalytic activity.
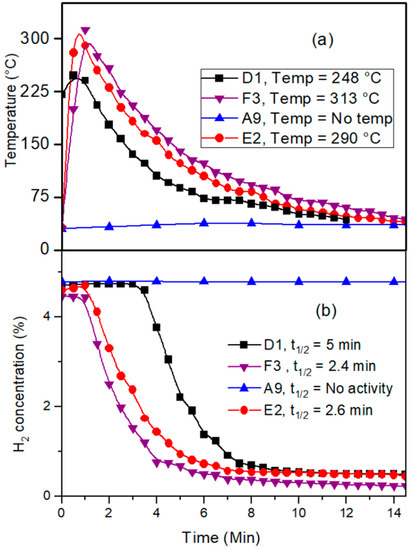
Figure 7.
(a) Change in catalyst temperature with time, (b) Variation in catalytic activity concerning H2 concentration for D1, E2, A9, and F3 catalysts.
4. Conclusions
By using a simple electroless deposition method, an effective noble metal deposition method has been developed. Formaldehyde is a superior reducing agent compared to Na-formate. To obtain the desired catalytically active noble metal coating, HCl activation (etching) was found to be more efficient than SnCl2 + PdCl2 and oxalic acid activation. Simultaneous mixed noble metal (platinum–palladium) catalysts were effectively prepared using formaldehyde as a reducing agent. The rate of the coating was found to be slower at earlier and later stages of deposition in the case of both single Pt-based and mixed noble metal (F3) catalysts. The rate of coating was found to be faster for the simultaneously prepared catalyst (F3) as compared to that of individual noble metal (D1) catalysts. All catalysts, D1, E2, and F3, were observed to be of a metallic nature from the XPS study. Catalysts with surface heterogeneity were found to be more active for H2 and O2 reactions as compared to the catalysts with smooth and spherical surfaces. For further mass-scale synthesis of catalysts to be used in the passive autocatalytic recombiner, simultaneous deposition of Pt and Pd with formaldehyde as a reducing agent and hydrochloric acid as an etching agent has been suggested, considering lower deposition time and better coating characteristics.
Author Contributions
Conceptualization, K.K.S. and S.V.; methodology, K.K.S. and S.V.; software, S.V.; validation, S.B.W.; formal analysis, A.N.S.; investigation, K.K.S. and S.V.; resources, S.B.P.; data curation, D.T.; writing—original draft preparation, K.K.S.; writing—review and editing, D.T.; visualization, S.B.W.; supervision, S.V.; project administration, D.T.; funding acquisition, S.S.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This research was supported by the National Research Foundation of Korea (NRF)–RS-2023-00248067.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Duflek, E.F.; Baudrand, D.W.; Donaldson, J.G. Electronic Applications of Electroless Nickel. In Electroless Plating: Fundamentals and Applications; Mallory, G.O., Hajdu, J.B., Eds.; American Electroplaters and Surface Finishers Society: Orlando, FL, USA, 1990. [Google Scholar]
- Okinaka, Y.; Osaka, T. Advances in Electrochemical Science and Engineering; Gerischer, H., Tobias, C.W., Eds.; VCH Publishers: Weinheim, Germany, 1994; Volume 3. [Google Scholar]
- Vaškelis, A. Coatings Technology Handbook, 2nd ed.; Satas, D., Tracton, A.A., Eds.; Marcel Dekker: New York, NY, USA, 2001; p. 213. [Google Scholar]
- O’Sullivan, E.J. Fundamental and Practical Aspects of the Electroless Deposition Reaction. In Advances in Electrochemical Science and Engineering; Alkire, R.C., Kolb, D.M., Eds.; Wiley-VCH: Weinheim, Germany, 2002; Volume 7, pp. 225–273. [Google Scholar]
- Djokić, S.S.; Cavallotti, P.L. Modern Aspects of Electrochemistry, Electroless Deposition: Theory and Applications; Djokić, S.S., Ed.; Springer: New York, NY, USA,, 2010; Volume 48, pp. 251–289. [Google Scholar]
- Mustain, W.E.; Kim, H.; Narayanan, V.; Osborn, T.; Kohl, P.A. Electroless deposition and characterization of PtxRu1−x catalysts on Pt/C nanoparticles for methanol oxidation. J. Fuel Cell Sci. Technol. 2010, 7, 041013. [Google Scholar] [CrossRef]
- Wongkaew, L.A.; Zhang, Y.; Tengco, J.M.M.; Blom, D.A.; Sivasubramanian, P.; Fanson, P.T.; Regalbuto, J.R.; Monnier, J.R. Characterization and evaluation of Pt-Pd electrocatalysts prepared by electroless deposition. Appl. Catal. B Environ. 2016, 188, 367–375. [Google Scholar] [CrossRef]
- Tate, G.; Kenvin, A.; Diao, W.; Monnier, J.R. Preparation of Pt-containing bimetallic and trimetallic catalysts using continuous electroless deposition methods. Catal. Today 2019, 334, 113–121. [Google Scholar] [CrossRef]
- Egelske, B.T.; Keels, J.M.; Monnier, J.R.; Regalbuto, J.R. An analysis of electroless deposition derived Ni-Pt catalysts for the dry reforming of methane. J. Catal. 2020, 381, 374–384. [Google Scholar] [CrossRef]
- Stoian, M.; Maurer, T.; Lamri SFechete, I. Techniques of Preparation of Thin Films: Catalytic Combustion. Catalyst 2021, 11, 1530. [Google Scholar] [CrossRef]
- Mehta, A.; Vasudev, H.; Singh, S.; Prakash, C.; Saxena, K.K.; Linul, E.; Buddhi, D.; Xu, J. Processing and Advancements in the Development of Thermal Barrier Coatings: A Review. Coat 2022, 12, 1318. [Google Scholar] [CrossRef]
- Widyastuti, E.; Hsu, J.L.; Lee, Y.C. Insight on Photocatalytic and Photoinduced Antimicro-bial Properties of ZnO Thin Films Deposited by HiPIMS through Thermal Oxidation. Nanomaterials 2022, 12, 463. [Google Scholar] [CrossRef]
- Niesz, K.; Morse, D.E. Sonication-accelerated catalytic synthesis of oxide nanoparticles. Nanotoday 2010, 5, 99–105. [Google Scholar] [CrossRef]
- Asadi, A.; Pourfattah, F.; Szilágyi, I.M.; Afrand, M.; Żyła, G.; Ahn, H.S.; Wongwises, S.; Nguyen, H.M.; Arabkoohsar, A.; Mahian, O. Effect of sonication characteristics on stability, thermophysical properties, and heat transfer of nanofluids: A comprehensive review. Ultrason. Sonochemistry 2019, 58, 104701. [Google Scholar] [CrossRef]
- Bretos, I.; Jiménez, R.; Ricote, J.; Calzada, M.L. Low-temperature crystallization of solution-derived metal oxide thin films assisted by chemical processes. Chem. Soc. Rev. 2018, 47, 291–308. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Saito, Y.K.; Takahashi, K.; Konagai, M. Preparation of boron-doped ZnO thin films by photo-atomic layer deposition. Sol. Energy Mater. Sol. 2001, 65, 125–132. [Google Scholar] [CrossRef]
- Shore, G.; Yoo, W.J.; Li, C.J.; Organ, M.G. Propargyl Amine Synthesis Catalysed by Gold and Copper Thin Films by Using Microwave-Assisted Continuous-Flow Organic Synthesis (MACOS). Eur. J. Org. Chem. 2010, 16, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Liu, Y.; Ren, Y.; Wei, Q.; Sun, Y. Microwave synthesis of single-phase nanoparticles made of multi-principal element alloys. Nano Res. 2022, 15, 4886–4892. [Google Scholar] [CrossRef]
- Gryaznov, V.H.; Serebryannikova, O.S.; Serov, Y.M.; Ermilova, M.M.; Karavanov, A.N.; Mischenko, A.P.; Orekhova, N.V. Preparation and catalysis over palladium composite membranes. Appl. Catal. A 1993, 96, 15–23. [Google Scholar] [CrossRef]
- Mazur, M.; Obstarczyk, A.; Posadowski, W.; Domaradzki, J.; Kiełczawa, S.; Wiatrowski, A.; Wojcieszak, D.; Kalisz, M.; Grobelny, M.; Szmidt, J. Investigation of the Microstructure, Optical, Electrical and Nanomechanical Properties of ZnOx Thin Films Deposited by Magnetron Sputtering. Materials 2022, 15, 6551. [Google Scholar] [CrossRef]
- Banciu, C.A.; Nastase, F.; Istrate, A.I.; Veca, L.M. 3D Graphene Foam by Chemical Vapor Deposition: Synthesis, Properties, and Energy-Related Applications. Molecules 2022, 27, 3634. [Google Scholar] [CrossRef]
- Huang, B.R.; Hung, S.C.; Ho, Y.S.; Chen, Y.S.; Yang, W.D. The Efficiency Study of Graphene Synthesis on Copper Substrate via Chemical Vapor Deposition Method with Methanol Precursor. Nanomaterials 2023, 13, 1136. [Google Scholar] [CrossRef]
- Endo, N.; Furukawa, Y.; Goshome, K.; Yaegashi, S.; Mashiko, K.; Tetsuhiko, M. Characterization of mechanical strength and hydrogen permeability of a PdCu alloy film prepared by one-step electroplating for hydrogen separation and membrane reactors. Int. J. Hydrogen Energy 2019, 44, 8290–8297. [Google Scholar] [CrossRef]
- Li, H.; Han, X.; Zhao, W.; Azhar, A.; Jeong, S.; Jeong, D.; Na, J.; Wang, S.; Yu, J.; Yamauchi, Y. Electrochemical preparation of nano/micron structure transition metal-based catalysts for the oxygen evolution reaction. Mater. Horiz. 2022, 9, 1788–1824. [Google Scholar] [CrossRef]
- Lazarus, N.; Tyler, J.B.; Cardenas, J.A.; Hanrahan, B.; Tsang, H.; Bedair, S.S. Direct electroless plating of conductive thermoplastics for selective metallization of 3D printed parts. Addit. Manuf. 2022, 55, 102793. [Google Scholar] [CrossRef]
- Shoaib, M.; Ul-Haq, N.; ul Haq, A. The Influence of pH on the Morphology and Electrochemical Behavior of Pd–Pt thin Films Prepared by Electro-less Deposition. Prot. Met. Phys. Chem. Surf. 2020, 56, 785–792. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, D.; Zhang, X.; Tian, A.; Hui, X.; Yang, J. Fabrication of Cu/Nafion-Based Ionic Polymer Metal Composites by Electroless Plating Method. Integr. Ferroelectr. 2019, 209, 48–57. [Google Scholar] [CrossRef]
- Fayomi, O.S.I.; Akande, I.G.; Sode, A.A. Corrosion Prevention of Metals via Electroless Nickel Coating: A review. J. Phys. 2019, 1378, 022063. [Google Scholar] [CrossRef]
- Fang, R.; Liu, R.; Xie, Z.-H.; Wu, L.; Ouyang, Y.; Li, M. Corrosion-resistant and superhydrophobic nickel phosphorus/nickel/PFDTMS triple-layer coating on magnesium alloy. Surf. Coat. Technol. 2022, 432, 128054. [Google Scholar] [CrossRef]
- Sanap, K.K.; Varma, S.; Waghmode, S.B.; Dalavi, D.; Patil, P.S.; Bharadwaj, S.R. Variation in noble metal morphology and its impact on the functioning of hydrogen mitigation catalyst. Int. J. Hydrogen Energy 2011, 36, 10455–10467. [Google Scholar] [CrossRef]
- Sanap, K.K.; Varma, S.; Waghmode, S.B.; Sharma, P.; Manoj, N.; Vatsa, R.K.; Bharadwaj, S.R. Bimetallic Wiregauze Supported Pt-Ru Nanocatalysts for Hydrogen Mitigation. J. Nanosci. Nanotechnol. 2015, 15, 3522–3529. [Google Scholar] [CrossRef]
- Sanap, K.K.; Varma, S.; Waghmode, S.B.; Bharadwaj, S.R. Wire gauze and cordierite sup-ported noble metal catalysts for passive autocatalytic recombiner. Nucl. Eng. Des. 2015, 294, 226–232. [Google Scholar] [CrossRef]
- Shukla, V.; Tyagi, D.; Gera, B.; Varma, S.; Ganju, S.; Maheshwari, N.K. Development and vali-dation of CFD model for catalytic recombiner against experimental results. Chem. Eng. J. 2021, 407, 127216. [Google Scholar] [CrossRef]
- Shukla, V.; Ganju, S.; Varma, S.; Sengupta, S.; Maheshwari, N.K. Affect of recombiner location on its performance inclosed containment under dry and steam conditions. Int. J. Hydrogen Energy 2019, 44, 25957–25973. [Google Scholar] [CrossRef]
- Pai, M.R.; Varma, S.; Belapurkar, A.D.; Gupta, N.M. Development of catalysts for the mitigation of hydrogen in water cooled nuclear power reactors. Part 2: Poisoning characteristics. Power Plant Chem. 2001, 3, 518–521. [Google Scholar]
- Loreta, T.-T.; Yezdi, D.; Eugenijus, N.; Ina, S.; Aldona, J.; Arnas, N.; Liudas, T.; Vytenis, B.; Laurynas, M. Electroless Platinum Deposition Using Co3+/Co2+ Redox Couple as a Reducing Agent. Materials 2021, 14, 1893. [Google Scholar]
- Barker, B.D. Electroless deposition of metal. Surf. Technol. 1981, 12, 77–88. [Google Scholar] [CrossRef]
- Fan, Q.T.; Wang, B.; Liu, Q.; Bo, Y.; Qian, L. Preparation and Application of Superhydrophobic Copper Mesh by Chemical Etching and In-situ Growth. Front. Chem. 2021, 9, 737550. [Google Scholar]
- Shu, J.; Grandjean BP, A.; Ghali, E.; Kaliaguine, S. Simultaneous deposition of Pd and Ag on porous stainless steel by electroless plating. J. Membr. Sci. 1993, 77, 181–195. [Google Scholar] [CrossRef]
- Reichert, E.; Wintringer, R.; Volmer, D.A.; Hempelmann, R. Elec-tro-catalytic oxidative cleavage of lignin in a protic ionic liquid. Phys. Chem. Chem. Phys. 2012, 15, 5214–5221. [Google Scholar] [CrossRef]
- Neikov, O.D.; Naboychenko, S.; Dowson, G. Handbook of Non-Ferrous Metal Powders: Technologies and Applications; Elsevier: Amsterdam, The Netherlands, 2009; p. 430. ISBN 978-1-85617-422-0. [Google Scholar]
- Sriring, N.; Tantavichet, N.; Pruksathorn, K. Preparation of Pt/C catalysts by electroless deposition for proton exchange membrane fuel cells. Korean J. Chem. Eng. 2010, 27, 439–445. [Google Scholar] [CrossRef]
- X-ray Photoelectron Spectroscopy (XPS). Available online: http://www.xpsfitting.com/2012/01/platinum.html (accessed on 25 July 2023).
- Shukla, A.K.; Neergat, M.; Bera, P.; Jayaram, V.; Hegde, M.S. An XPS study on binary and ternary alloys of transition metals with platinized carbon and its bearing upon oxygen electroreduction in direct methanol fuel cells. J. Electroanal. Chem. 2001, 504, 111–119. [Google Scholar] [CrossRef]
- Moussa, S.; Abdelsayed, V.; Samy El-Shall, M. Laser synthesis of Pt, Pd, CoO, and Pd–CoO nanoparticle catalysts supported on graphene. Chem. Phys. Lett. 2011, 510, 179–184. [Google Scholar] [CrossRef]
- Navarro, R.M.; Pawelec, B.; Trejo, J.M.; Mariscal, R.; Fierro, J.L.G. Hydrogenation of Aromatics on Sulfur-Resistant PtPd Bimetallic Catalysts. J. Catal. 2000, 189, 184–194. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).