Structural Characterization of Multicomponent Crystals Formed from Diclofenac and Acridines
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Compounds 1 and 2
2.2. X-ray Measurements and Refinements
3. Results
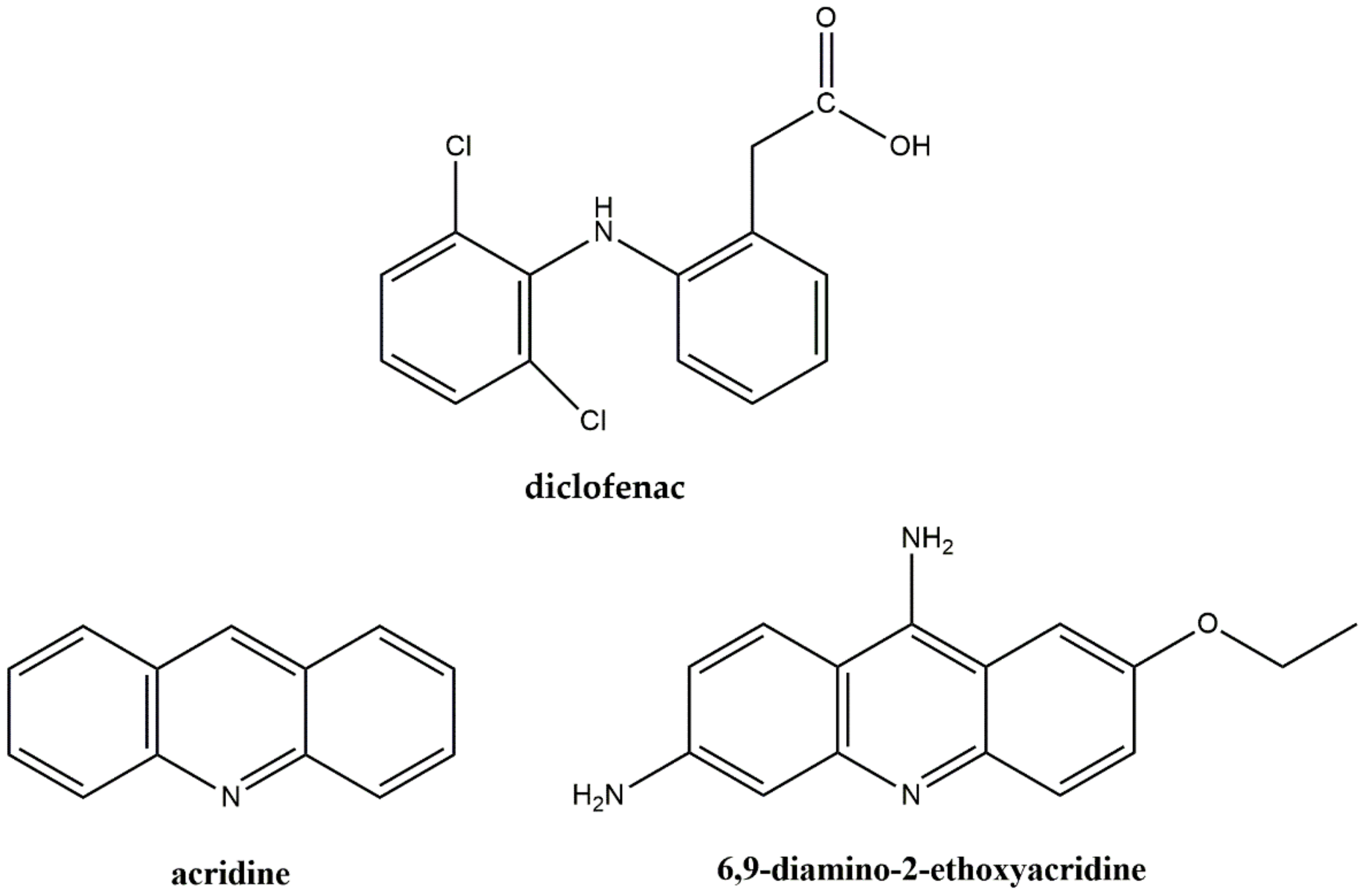
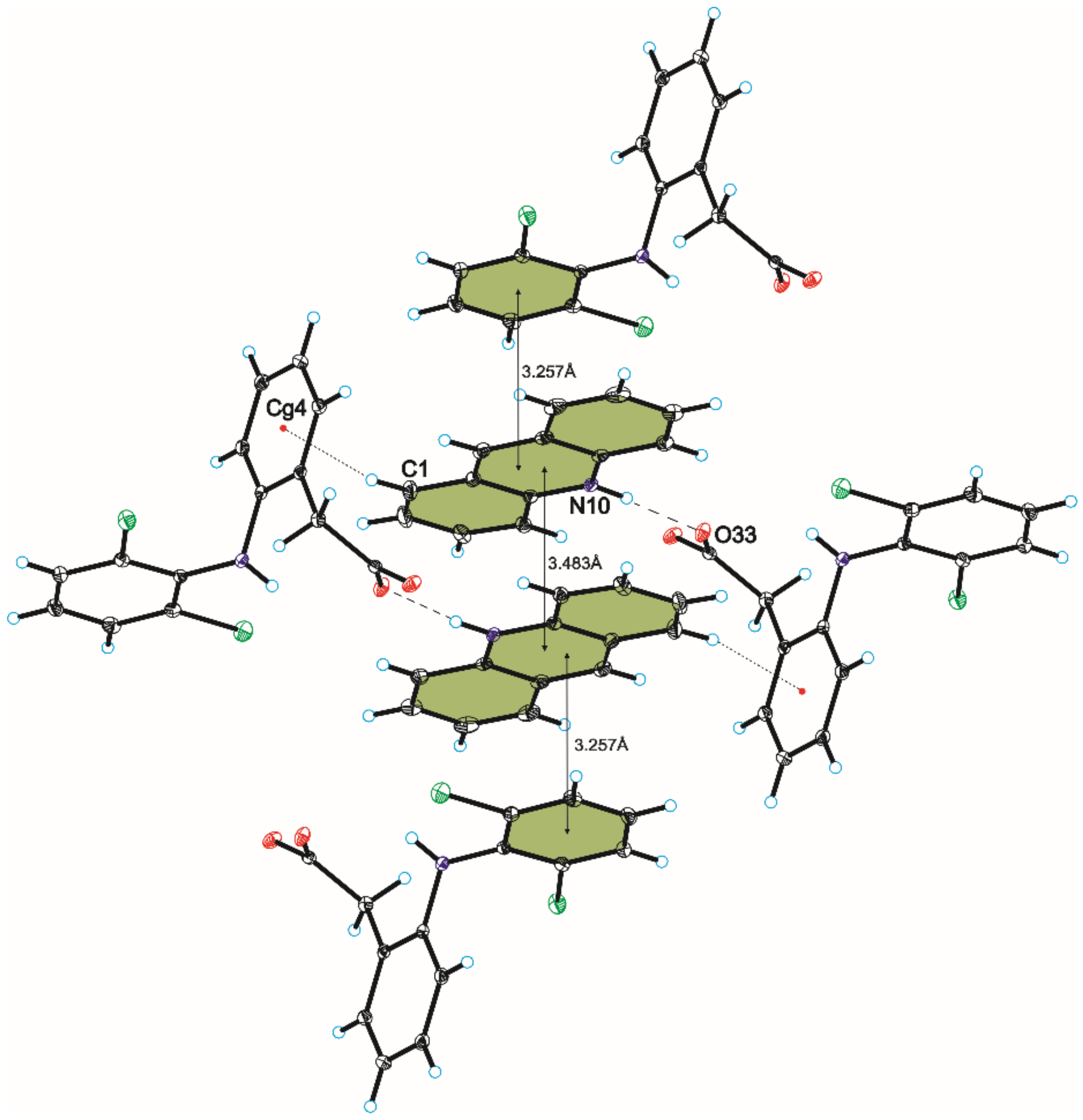
3.1. Crystal Structure of Compound 1
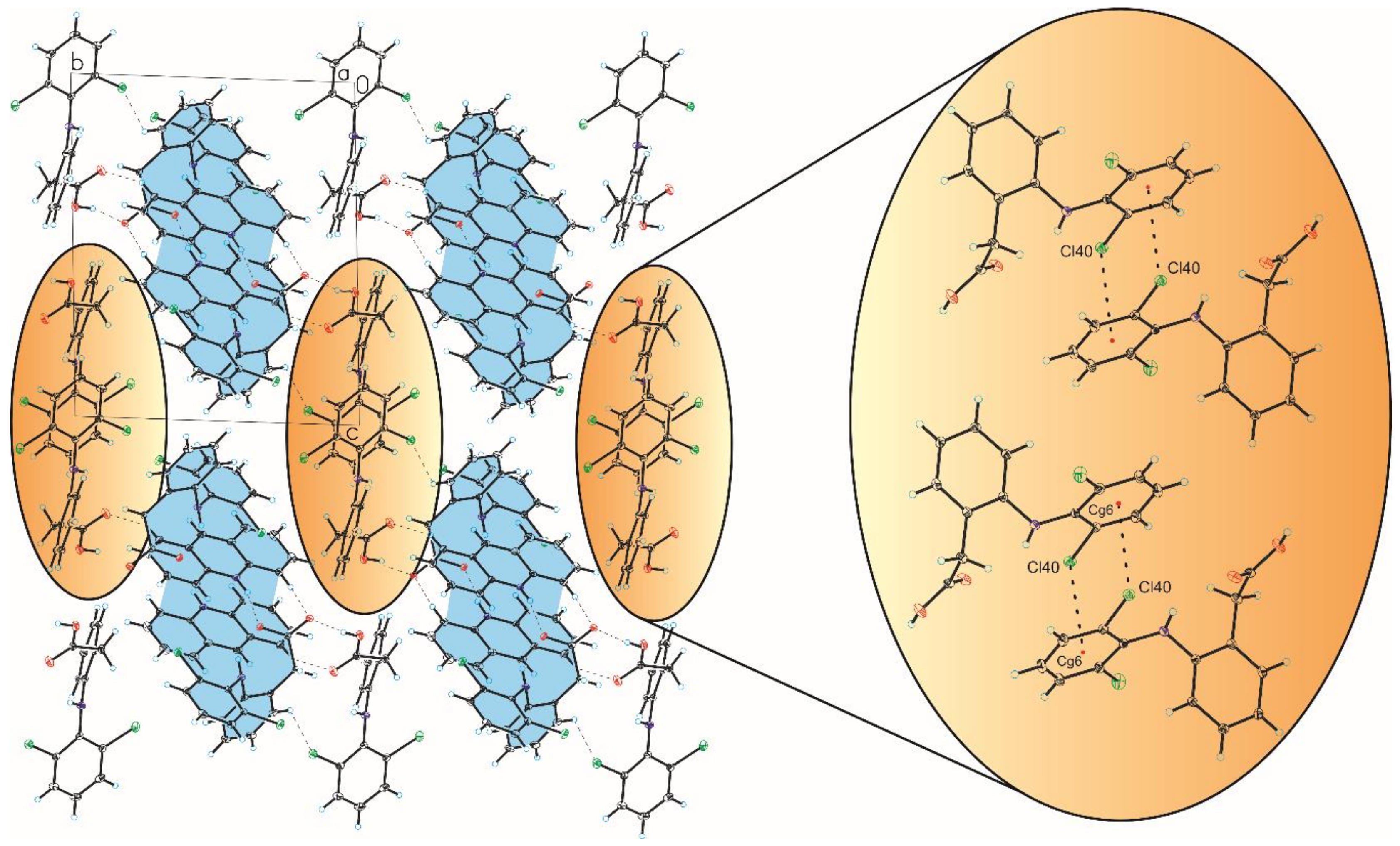
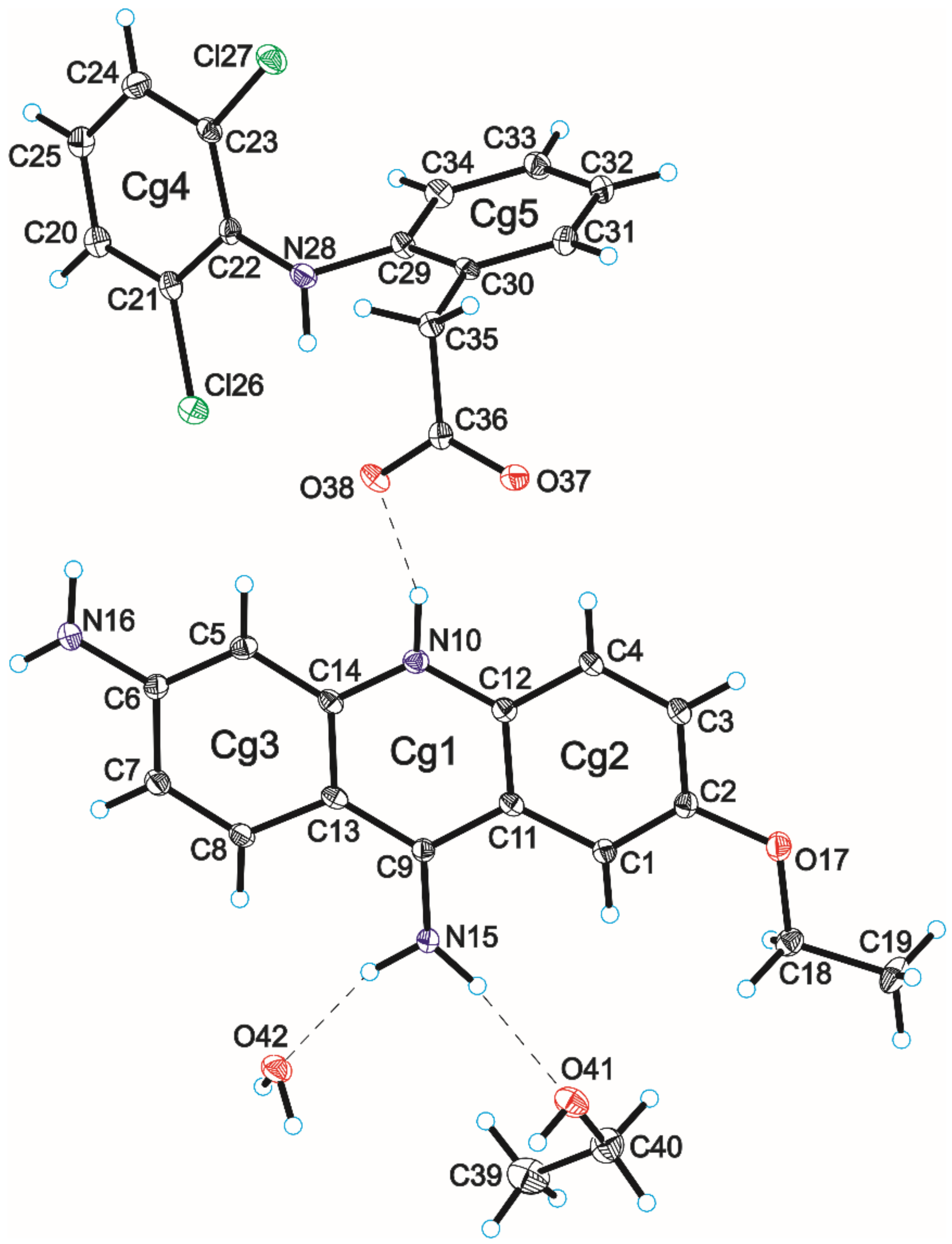
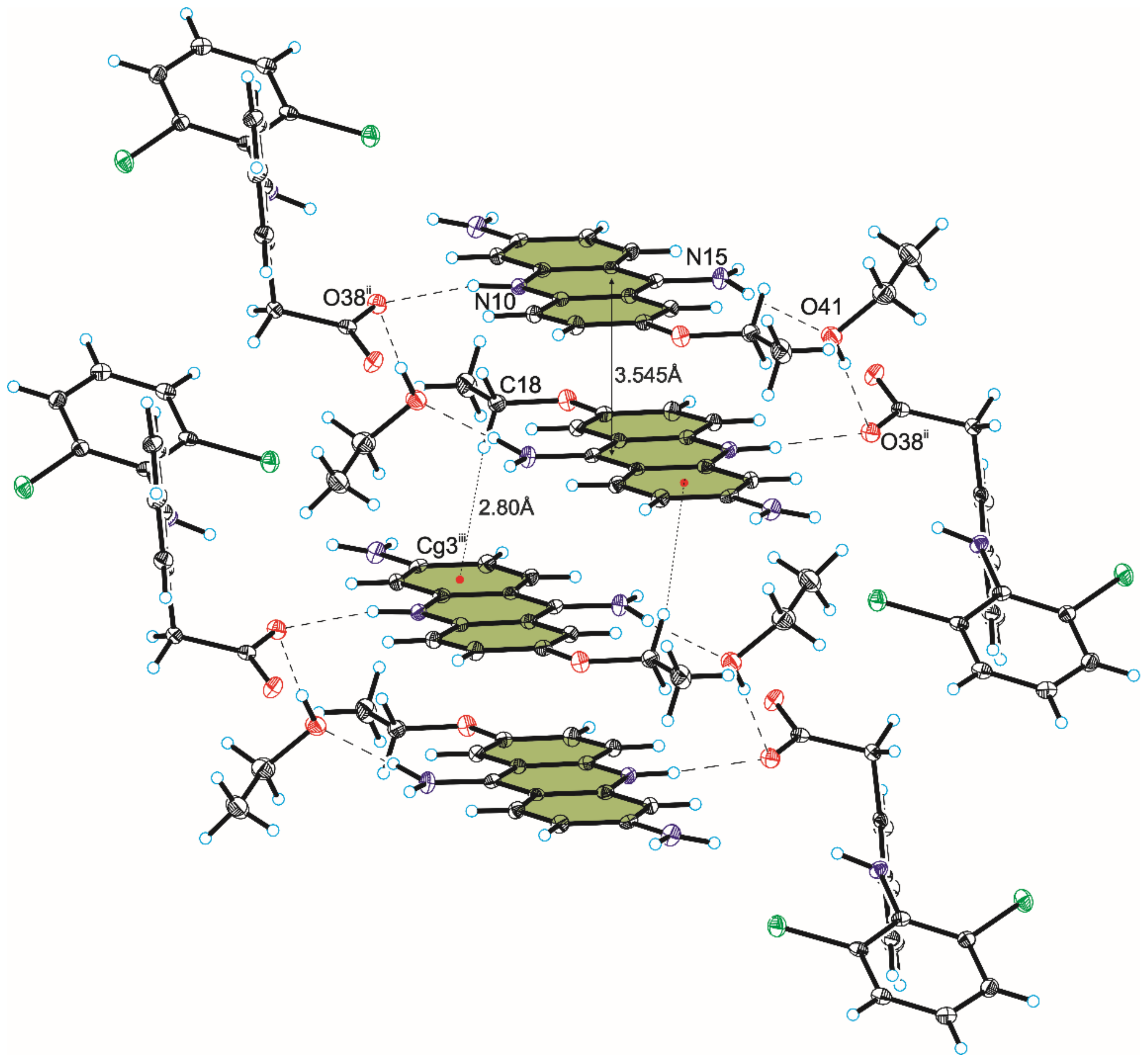
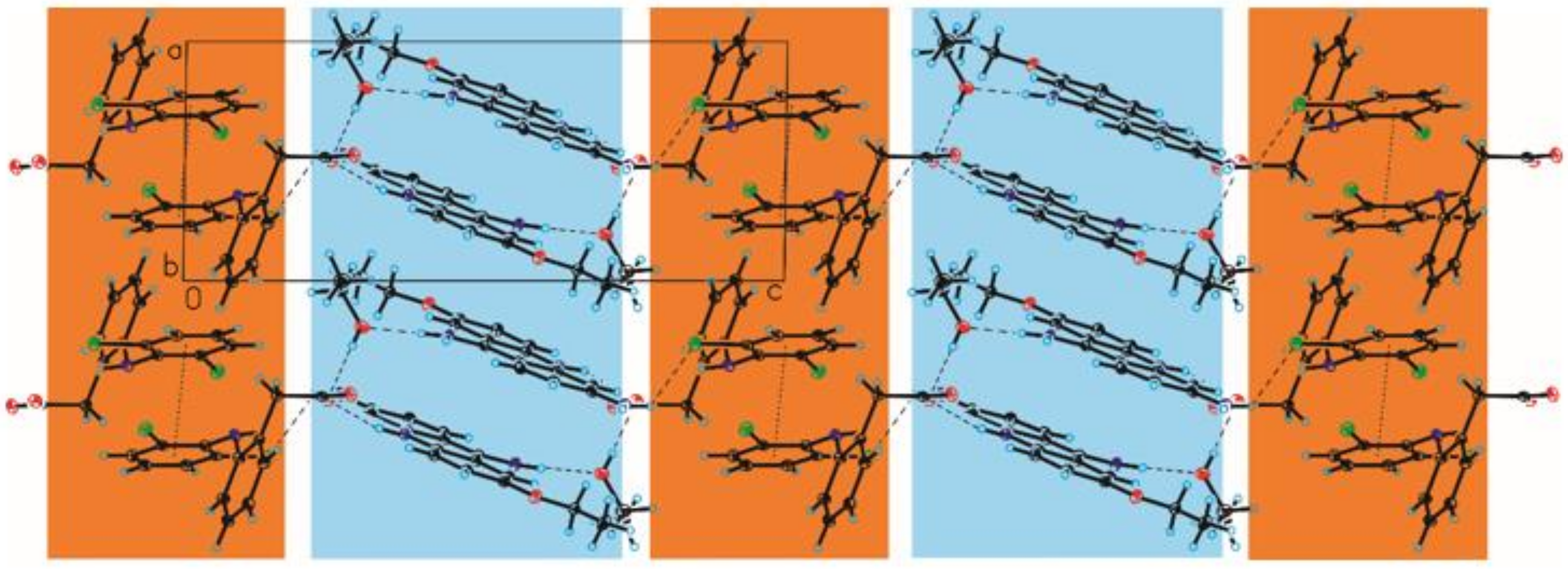
3.2. Crystal Structure of Compound 2
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jones, R. Nonsteroidal anti-inflammatory drug prescribing: Past, present, and future. Am. J. Med. 2001, 110, S4–S7. [Google Scholar] [CrossRef]
- Sostres, C.; Gargallo, C.J.; Arroyo, M.T.; Lanas, A. Adverse effects of non-steroidal anti-inflammatory drugs (NSAIDs, aspirin and coxibs) on uppergastrointestinal tract. Best Pract. Res. Clin. Gastroenterol. 2010, 24, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Heyneman, C.A.; Lawless-Liday, C.; Wall, G.C. Oral versus topical NSAIDs in rheumatic diseases: A comparison. Drugs 2000, 60, 555–574. [Google Scholar] [CrossRef] [PubMed]
- Parolini, M. Toxicity of the Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) acetylsalicylic acid, paracetamol, diclofenac, ibuprofen and naproxen towards freshwater invertebrates: A review. Sci. Total Environ. 2020, 740, 140043. [Google Scholar] [CrossRef] [PubMed]
- Khuder, S.A.; Mutgi, A.B. Breast cancer and NSAID use: A meta-analysis. Br. J. Cancer 2001, 84, 1188–1192. [Google Scholar] [CrossRef] [PubMed]
- Stewart, W.F.; Kawas, C.; Corrada, M.; Metter, E.J. Risk of Alzheimer’s disease and duration of NSAID use. Neurology 1997, 48, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Gasparini, L.; Ongini, E.; Wenk, G. Non-steroidal anti-inflammatory drugs (NSAIDs) in Alzheimer’s disease: Old and new mechanisms ofaction. J. Neurochem. 2004, 91, 521–536. [Google Scholar] [CrossRef]
- Gierse, J.K.; Hauser, S.D.; Creely, D.P.; Koboldt, C.; Rangwala, S.H.; Isakson, P.C.; Seibert, K. Expression and selective inhibition of the constitutive and inducible forms of human cyclo-oxygenase. Biochem. J. 1995, 305, 479–484. [Google Scholar] [CrossRef]
- O’Connor, J.P.; Lysz, T. Celecoxib, NSAIDs and the skeleton. Drugs Today 2008, 44, 693–709. [Google Scholar] [CrossRef]
- Gartenmann, S.; Schmidlin, P.; Maier, N.; Wiedemeier, D.B.; Attin, T. Effect of adjuvant use of nsaid in reducing probing pocket depth in the context of conventional periodontal therapy: A systematic review of randomized trials. Appl. Sci. 2020, 10, 7657. [Google Scholar] [CrossRef]
- Singh, G.; Fort, J.G.; Goldstein, J.L.; Levy, R.A.; Hanrahan, P.S.; Bello, A.E.; Andrade-Ortega, L.; Wallemark, C.; Agrawal, N.M.; Eisen, G.M.; et al. Celecoxib versus naproxen and diclofenac in osteoarthritis patients: SUCCESS-I study. Am. J. Med. 2006, 119, 255–266. [Google Scholar] [CrossRef]
- Brogden, R.N.; Heel, R.C.; Pakes, G.E.; Speight, T.M.; Avery, G.S. Diclofenac sodium: A review of its pharmacological properties and therapeutic use in rheumatic diseases and pain of varying origin. Drugs 1980, 20, 24–48. [Google Scholar] [CrossRef]
- Goa, K.L.; Chrisp, P. Ocular Diclofenac: A Review of Its Pharmacology and Clinical Use in Cataract Surgery, and Potentialin Other Inflammatory Ocular Conditions. Drugs Aging 1992, 2, 473–486. [Google Scholar] [CrossRef]
- Menassé, R.; Hedwall, P.R.; Kraetz, J.; Pericin, C.; Riesterer, L.; Sallmann, A.; Ziel, R.; Jaques, R. Pharmacological properties of diclofenac sodium and its metabolites. Scand. J. Rheum. 1978, 7, 5–16. [Google Scholar] [CrossRef]
- Small, R.E. Diclofenac sodium. Clin. Pharm. 1989, 8, 545–558. [Google Scholar]
- Goh, C.F.; Lane, M.E. Formulation of diclofenac for dermal delivery. Int. J. Pharm. 2014, 473, 607–616. [Google Scholar] [CrossRef]
- Dutta, N.K.; Dastidar, S.G.; Kumar, A.; Mazumdar, K.; Ray, R.; Chakrabarty, A.N. Antimycobacterial activity of the antiinflammatory agent diclofenac sodium, and its synergism with streptomycin. Braz. J. Microbiol. 2004, 35, 316–323. [Google Scholar] [CrossRef][Green Version]
- Mazumdar, K.; Dastidar, S.G.; Park, J.H.; Dutta, N.K. The anti-inflammatory non-antibiotic helper compound diclofenac: An antibacterial drug target. Eur. J. Clin. Microbiol. Infect. Dis. 2009, 28, 881–891. [Google Scholar] [CrossRef]
- Maitra, A.; Bates, S.; Shaik, M.; Evangelopoulos, D.; Abubakar, I.; McHugh, T.D.; Lipman, M.; Bhakta, S. Repurposing drugs for treatment of tuberculosis: A role for non-steroidal anti-inflammatory drugs. Br. Med. Bull. 2016, 118, 138. [Google Scholar] [CrossRef]
- Kroesen, V.M.; Gröschel, M.I.; Martinson, N.; Zumla, A.; Maeurer, M.; van der Werf, T.S.; Vilaplana, C. Non-steroidal anti-inflammatory drugs as host-directed therapy for tuberculosis: A systematic review. Front. Immunol. 2017, 8, 772. [Google Scholar] [CrossRef]
- Ivanyi, J.; Zumla, A. Nonsteroidal antiinflammatory drugs for adjunctive tuberculosis treatment. J. Infect. Dis. 2013, 208, 185–188. [Google Scholar] [CrossRef]
- Todd, P.A.; Sorkin, E.M. Diclofenac Sodium: A Reappraisal of Its Pharmacodynamic and Pharmacokinetic Properties, and Therapeutic Efficacy. Drugs 1988, 35, 244–285. [Google Scholar] [CrossRef] [PubMed]
- Vieno, N.; Sillanpää, M. Fate of diclofenac in municipal wastewater treatment plant—A review. Environ. Int. 2014, 69, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Acuña, V.; Ginebreda, A.; Mor, J.R.; Petrovic, M.; Sabater, S.; Sumpter, J.; Barceló, D. Balancing the health benefits and environmental risks of pharmaceuticals: Diclofenac as an example. Environ. Int. 2015, 85, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Vishnu Priyan, V.; Shahnaz, T.; Suganya, E.; Sivaprakasam, S.; Narayanasamy, S. Ecotoxicological assessment of micropollutant Diclofenac biosorption on magnetic sawdust: Phyto, Microbial and Fish toxicity studies. J. Hazard. Mater. 2021, 403, 123532. [Google Scholar] [CrossRef]
- Harshkova, D.; Majewska, M.; Pokora, W.; Baścik-Remisiewicz, A.; Tułodziecki, S.; Aksmann, A. Diclofenac and atrazine restrict the growth of a synchronous Chlamydomonas reinhardtii population via various mechanisms. Aquat. Toxicol. 2021, 230, 105698. [Google Scholar] [CrossRef]
- Lv, Y.; Liang, Z.; Li, Y.; Chen, Y.; Liu, K.; Yang, G.; Liu, Y.; Lin, C.; Ye, X.; Shi, Y.; et al. Efficient adsorption of diclofenac sodium in water by a novel functionalized cellulose aerogel. Environ. Res. 2021, 194, 11065. [Google Scholar] [CrossRef]
- Desiraju, G.R. C–H⋯O and other weak hydrogen bonds. From crystal engineering to virtual screening. Chem. Commun. 2005, 24, 2995–3001. [Google Scholar] [CrossRef]
- Steiner, T. C–H⋯O hydrogen bonding in crystals. Crystallogr. Rev. 2003, 9, 177–228. [Google Scholar] [CrossRef]
- Saha, B.K.; Nangia, A.; Jaskólski, M. Crystal engineering with hydrogen bonds and halogen bonds. CrystEngComm 2005, 7, 355–358. [Google Scholar] [CrossRef]
- Aakeröy, C.B.; Chopade, P.D.; Desper, J. Establishing a hierarchy of halogen bonding by engineering crystals without disorder. Cryst. Growth Des. 2013, 13, 4145–4150. [Google Scholar] [CrossRef]
- Mukherjee, A.; Tothadi, S.; Desiraju, G.R. Halogen bonds in crystal engineering: Like hydrogen bonds yet different. Acc. Chem. Res. 2014, 47, 2514–2524. [Google Scholar] [CrossRef]
- Alizadeh, V.; Mahmoudi, G.; Vinokurova, M.A.; Pokazeev, K.M.; Alekseeva, K.A.; Miroslaw, B.; Khandar, A.A.; Frontera, A.; Safin, D.A. Spodium bonds and metal-halogen⋯halogen-metal interactions in propagation of monomeric units to dimeric or polymeric architectures. J. Mol. Struct. 2022, 1252, 132144. [Google Scholar] [CrossRef]
- Soltani, B.; Nabipour, H.; Engle, J.T.; Ziegler, C.J. π⋯π and hydrogen bonding interactions in the crystal structure of trans-dichloro-tetrakis (1H-indazole-N2)-nickel (II). Mater. Today Proc. 2018, 5, 15845–15851. [Google Scholar] [CrossRef]
- Prohens, R.; Portell, A.; Vallcorba, O.; Font-Bardia, M.; Bauzá, A.; Frontera, A. Polymorphism in secondary squaramides: On the importance of π-interactions involving the four membered ring. CrystEngComm 2018, 20, 237–244. [Google Scholar] [CrossRef]
- Pal, P.; Das, K.; Hossain, A.; Gomila, R.M.; Frontera, A.; Mukhopadhyay, S. Synthesis and crystal structure of the simultaneous binding of Ni (ii) cation and chloride by the protonated 2, 4, 6 tris-(2-pyridyl)-1, 3, 5 triazine ligand: Theoretical investigations of anion⋯π, π⋯π and hydrogen bonding interactions. New J. Chem. 2021, 45, 11689–11696. [Google Scholar] [CrossRef]
- Basak, T.; Frontera, A.; Chattopadhyay, S. Existence of stronger CH⋯π (chelate ring) interaction compared to CH⋯ π (arene) interactions in the supramolecular assembly of dinuclear iron (III) Schiff base complexes: A theoretical insight. Inorg. Chim. Acta 2021, 516, 120081. [Google Scholar] [CrossRef]
- Frontera, A.; Gamez, P.; Mascal, M.; Mooibroek, T.J.; Reedijk, J. Putting anion–π interactions into perspective. Angew. Chem. Int. Ed. 2011, 50, 9564–9583. [Google Scholar] [CrossRef]
- Yamada, S. Cation–π interactions in organic crystals. Coord. Chem. Rev. 2020, 415, 213301. [Google Scholar] [CrossRef]
- Bhowal, R.; Biswas, S.; Thumbarathil, A.; Koner, A.L.; Chopra, D. Exploring the relationship between intermolecular interactions and solid-state photophysical properties of organic co-crystals. J. Phys. Chem. C 2018, 123, 9311–9322. [Google Scholar] [CrossRef]
- Aitipamula, S.; Banerjee, R.; Bansal, A.K.; Biradha, K.; Cheney, M.L.; Choudhury, A.R.; Zaworotko, M.J. Polymorphs, salts, and cocrystals: What’s in a name? Cryst. Growth Des. 2012, 12, 2147–2152. [Google Scholar] [CrossRef]
- Aakeröy, C.B.; Fasulo, M.E.; Desper, J. Cocrystal or salt: Does it really matter? Mol. Pharm. 2007, 4, 317–322. [Google Scholar] [CrossRef]
- Qiao, N.; Li, M.; Schlindwein, W.; Malek, N.; Davies, A.; Trappitt, G. Pharmaceutical cocrystals: An overview. Int. J. Pharm. 2011, 419, 1–11. [Google Scholar] [CrossRef]
- Desiraju, G.R. Crystal engineering: A holistic view. Angew. Chem. 2007, 46, 8342–8356. [Google Scholar] [CrossRef]
- Aakeröy, C.B.; Champness, N.R.; Janiak, C. Recent advances in crystal engineering. CrystEngComm 2010, 12, 22–43. [Google Scholar] [CrossRef]
- Barbas, R.; Bofill, L.; De Sande, D.; Font-Bardia, M.; Prohens, R. Crystal engineering of nutraceutical phytosterols: New cocrystal solid solutions. CrystEngComm 2020, 22, 4210–4214. [Google Scholar] [CrossRef]
- Zaworotko, M. Crystal engineering of the composition of API’s: Understanding polymorphs and designing pharmaceutical co-crystals. Am. Pharm. Outsourc. 2004, 5, 16–23. [Google Scholar]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Cryst. 2016, 2016. B72, 171–179. [Google Scholar] [CrossRef]
- Goswami, P.K.; Kumar, V.; Ramanan, A. Multicomponent solids of diclofenac with pyridine based coformers. J. Mol. Struct. 2020, 1210, 128066. [Google Scholar] [CrossRef]
- Zheng, Q.; Rood, S.L.; Unruh, D.K.; Hutchins, K.M. Unique supramolecular complex of diclofenac: Structural robustness, crystal-to-crystal solvent exchange, and mechanochemical synthesis. Chem. Commun. 2019, 55, 7639–7642. [Google Scholar] [CrossRef]
- Aakeröy, C.B.; Grommet, A.B.; Desper, J. Co-crystal screening of diclofenac. Pharmaceutics 2011, 3, 601–614. [Google Scholar] [CrossRef] [PubMed]
- Bodart, L.; Prinzo, M.; Derlet, A.; Tumanov, N.; Wouters, J. Taking advantage of solvate formation to modulate drug–drug ratio in clofaziminium diclofenac salts. CrystEngComm 2021, 23, 185–201. [Google Scholar] [CrossRef]
- Bhattacharya, B.; Mondal, A.; Soni, S.R.; Das, S.; Bhunia, S.; Raju, K.B.; Ghosh, A.; Reddy, C.M. Multidrug salt forms of norfloxacin with non-steroidal anti-inflammatory drugs: Solubility and membrane permeability studies. CrystEngComm 2018, 20, 6420–6429. [Google Scholar] [CrossRef]
- Surov, A.O.; Voronin, A.P.; Vener, M.V.; Churakov, A.V.; Perlovich, G.L. Specific features of supramolecular organisation and hydrogen bonding in proline cocrystals: A case study of fenamates and diclofenac. CrystEngComm 2018, 20, 6970–6981. [Google Scholar] [CrossRef]
- Goud, N.R.; Suresh, K.; Nangia, A. Solubility and stability advantage of aceclofenac salts. Cryst. Growth Des. 2013, 13, 1590–1601. [Google Scholar] [CrossRef]
- Nugrahani, I.; Kumalasari, R.A.; Auli, W.N.; Horikawa, A.; Uekusa, H. Salt cocrystal of diclofenac sodium-l-proline: Structural, pseudo polymorphism, and pharmaceutics performance study. Pharmaceutics 2020, 12, 690. [Google Scholar] [CrossRef]
- Wang, R.; Yuan, P.; Yang, D.; Zhang, B.; Zhang, L.; Lu, Y.; Du, G. Structural features and interactions of new sulfamethazine salt and cocrystal. J. Mol. Struct. 2020, 1229, 129596. [Google Scholar] [CrossRef]
- Belmont, P.; Bosson, J.; Godet, T.; Tiano, M. Acridine and acridone derivatives, anticancer properties and synthetic methods: Where are we now? Anti-Cancer Agents Med. Chem. 2007, 7, 139–169. [Google Scholar] [CrossRef]
- Stanslas, J.; Hagan, D.J.; Ellis, M.J.; Turner, C.; Carmichael, J.; Ward, W.; Hammonds, T.R.; Stevens, M.F.G. Antitumor polycyclic acridines. 7. Synthesis and biological properties of DNA affinic tetra- and pentacyclic acridines. J. Med. Chem. 2000, 43, 1563–1572. [Google Scholar] [CrossRef]
- Ferreira, R.; Aviñó, A.; Mazzini, S.; Eritja, R. Synthesis, DNA-binding and antiproliferative properties of acridine and 5-methylacridine derivatives. Molecules 2012, 17, 7067–7082. [Google Scholar] [CrossRef]
- Guendel, I.; Carpio, L.; Easley, R.; Van Duyne, R.; Coley, W.; Agbottah, E.; Dowd, C.; Kashanchi, F.; Kehn-Hall, K. 9-Aminoacridine inhibition of HIV-1 Tat dependent transcription. Virol. J. 2009, 6, 1–14. [Google Scholar] [CrossRef]
- Bongarzone, S.; Bolognesi, M.L. The concept of privileged structures in rational drug design: Focus on acridine and quinoline scaffolds in neurodegenerative and protozoan diseases. Expert Opin. Drug Discov. 2011, 6, 251–268. [Google Scholar] [CrossRef]
- Sikorski, A.; Trzybiński, D. Synthesis and structural characterization of a cocrystal salt containing acriflavine and 3,5-dinitrobenzoic acid. Tetrahedron Lett. 2014, 55, 2253–2255. [Google Scholar] [CrossRef]
- Kowalska, K.; Trzybiński, D.; Sikorski, A. Influence of the halogen substituent on the formation of halogen and hydrogen bonding in co-crystals formed from acridine and benzoic acids. CrystEngComm 2015, 17, 7199–7212. [Google Scholar] [CrossRef]
- Mirocki, A.; Sikorski, A. Influence of Halogen Substituent on the Self-Assembly and Crystal Packing of Multicomponent Crystals Formed from Ethacridine and Meta-Halobenzoic Acids. Crystals 2020, 10, 79. [Google Scholar] [CrossRef]
- Mirocki, A.; Sikorski, A. The Influence of Solvent on the Crystal Packing of Ethacridinium Phthalate Solvates. Materials 2020, 13, 5073. [Google Scholar] [CrossRef]
- Huang, T.S.; Lee, J.J.; Li, Y.S.; Cheng, S.P. Ethacridine Induces Apoptosis and Differentiation in Thyroid Cancer Cells In Vitro. Anticancer Res. 2019, 39, 4095–4100. [Google Scholar] [CrossRef]
- Koelzer, S.C.; Held, H.; Toennes, S.W.; Verhoff, M.A.; Wunder, C. Self-induced illegal abortion with Rivanol®: A medicolegal–toxicological case report. Forensic Sci. Int. 2016, 268, e18–e22. [Google Scholar] [CrossRef]
- Oie, S.; Kamiya, A. Bacterial contamination of commercially available ethacridine lactate (acrinol) product. J. Hosp. Infect. 1996, 34, 51–58. [Google Scholar] [CrossRef]
- Li, X.; Lidsky, P.V.; Xiao, Y.; Wu, C.T.; Garcia-Knight, M.; Yang, J.; Nakayama, T.; Nayak, J.V.; Jackson, P.K.; Andino, R.; et al. Ethacridine inhibits SARS-CoV-2 by inactivating viral particles. PLoS Pathog. 2021, 17, e1009898. [Google Scholar] [CrossRef]
- CrysAlis CCD and CrysAlis RED; Version 1.171.36.24; Oxford Diffraction Ltd.: Yarnton, UK, 2012.
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Spek, A.L. Structure validation in chemical crystallography. Acta Cryst. 2009, D65, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.K. ORTEP II, Report ORNL-5138; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 1976. [Google Scholar]
- Motherwell, S.; Clegg, S. PLUTO-78, Program for Drawing and Molecular Structure; University of Cambridge: Cambridge, UK, 1978. [Google Scholar]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; van de Streek, J.; Wood, P.A. Mercury CSD 2.0—New Features for the Visualization and Investigation of Crystal Structures. J. Appl. Crystallogr. 2008, 41, 466–470. [Google Scholar] [CrossRef]
- Lynch, D.E. (Coventry University, Coventry CV1 5FB, West Midlands, UK). Personal communication, (CSD Communication). 2009.

| Compound | 1 | 2 |
|---|---|---|
| Chemical formula | C41H31Cl4N3O4 | C31H34Cl2N4O5 |
| Formula weight/g·mol−1 | 771.49 | 613.52 |
| Crystal system | triclinic | triclinic |
| Space group | P-1 | P-1 |
| a/Å | 10.1582(4) | 7.6339(10) |
| b/Å | 12.3057(6) | 10.2331(17) |
| c/Å | 14.8978(7) | 19.250(3) |
| α/° | 91.050(4) | 84.860(12) |
| β/° | 99.988(3) | 89.009(11) |
| γ/° | 99.111(4) | 88.337(13) |
| V/Å3 | 1808.9(1) | 1497.0(4) |
| Z | 2 | 2 |
| T/K | 295(2) | 295(2) |
| λMo/Å | 0.71073 | 0.71073 |
| ρcalc/g·cm−3 | 1.416 | 1.361 |
| F(000) | 796 | 644 |
| µ/mm−1 | 0.375 | 0.264 |
| θ range/° | 3.31–25.00 | 3.29–25.00 |
| Completeness θ/% | 99.7 | 99.8 |
| Reflections collected | 12784 | 9800 |
| Reflections | 6353 | 5246 |
| unique | [Rint = 0.0297] | [Rint = 0.0883] |
| Data/restraints/parameters | 6353/0/485 | 5246/3/411 |
| Goodness of fit on F2 | 1.021 | 1.007 |
| Final R1 value (I > 2σ(I)) | 0.0445 | 0.0891 |
| Final wR2 value (I > 2σ(I)) | 0.0844 | 0.1855 |
| Final R1 value (all data) | 0.0703 | 0.2190 |
| Final wR2 value (all data) | 0.0945 | 0.2559 |
| CCDC number | 2128054 | 2128053 |
| Compound | D–H⋯A | d(D–H) [Å] | d(H⋯A) [Å] | d(D⋯A) [Å] | ∠D–H⋯A [°] |
|---|---|---|---|---|---|
| N23–H23⋯O33 | 0.81 (2) | 2.39 (2) | 2.977 (2) | 130 (2) | |
| N42–H42⋯O52 | 0.82 (2) | 2.36 (2) | 3.044 (3) | 142 (2) | |
| N10–H10⋯O33 | 0.87 (2) | 1.79 (2) | 2.648 (2) | 170 (2) | |
| 1 | O51–H51⋯O32 | 0.90 (4) | 1.69 (3) | 2.560 (3) | 164 (3) |
| C45–H45⋯O32 i | 0.93 | 2.71 (1) | 3.603 | 160 (1) | |
| C4–H4⋯O32 | 0.93 | 2.40 | 3.292 (3) | 160 | |
| C8–H8⋯O52 ii | 0.93 | 2.50 | 3.342 (3) | 150 | |
| Symmetry code: (i) 1 − x, −y, 1 − z; (ii) 1 − x, 1 − y, 1 − z. | |||||
| N28–H28⋯O38 | 0.91 (6) | 2.43 (6) | 3.188 (7) | 141 (6) | |
| N10–H10⋯O38 | 0.94 (6) | 2.01 (6) | 2.898 (7) | 157 (6) | |
| 2 | N15–H15B⋯O41 | 0.95 (6) | 2.00 (6) | 2.945 (8) | 173 (6) |
| N15–H15A⋯O42 | 0.95 (8) | 1.91 (8) | 2.832 (9) | 163 (7) | |
| N16–H16B⋯O37 i | 0.84 (7) | 2.19 (7) | 3.015 (1) | 171 (7) | |
| N16–H16A⋯Cl26 | 0.93 | 2.91 (9) | 3.603 | 133 (7) | |
| O41–H41⋯O38 ii | 0.82 | 2.04 | 2.864 (7) | 176 | |
| O42–H42A⋯O37 ii | 0.85 (4) | 1.95 (6) | 2.753 (6) | 158 (8) | |
| O42–H42B⋯O17 i | 0.83 (7) | 2.17 (7) | 2.941 (7) | 154 (6) | |
| C4–H4⋯O37 | 0.93 | 2.59 | 3.438 (8) | 151 | |
| C8–H8⋯O42 | 0.93 | 2.45 | 3.353 (9) | 165 | |
| Symmetry code: (i) x, 1 + y, z; (ii) 1 − x, 1 − y, 1 − z. | |||||
| Compound | C–X⋯Cg | d(X⋯Cg) [Å] | d(C⋯Cg) [Å] | ∠C–X⋯Cg [°] |
|---|---|---|---|---|
| C1–H1⋯Cg4 ii | 2.79 | 3.697 (3) | 165 | |
| C7–H7⋯Cg7 ii | 2.90 | 3.769 (3) | 156 | |
| 1 | C16–Cl21⋯Cg7 iii | 3.885 (1) | 5.138 (2) | 127.92 (8) |
| C35–Cl40⋯Cg6 iv | 3.725 (1) | 4.267 (2) | 95.89 (8) | |
| Symmetry code: (ii) 1 − x, 1 − y, 1 − z; (iii) 1 + x, 1 + y, z; (iv) 1 − x, −y, 2 − z. | ||||
| 2 | C18–H18A⋯Cg3 iii | 2.80 | 3.666 (7) | 150 |
| Symmetry code: (iii) 2 − x, 1 − y, 1 − z. | ||||
| Compound | CgI a | CgJ a | CgI⋯CgJ b [Å] | Dihedral Angle c [°] | Interplanar Distance d [Å] | Offset e [Å] |
|---|---|---|---|---|---|---|
| 1 | 1 ii | 3.737 (1) | 0.0 (1) | 3.409 (9) | 1.533 | |
| 1 | 2 ii | 3.841 (1) | 2.2 (1) | 3.404 (9) | 1.672 | |
| 1 | 2 | 3 ii | 3.796 (2) | 3.8 (1) | 3.464 (1) | 1.388 |
| 1 | 4 v | 3.662 (1) | 3.9 (1) | 3.345 (9) | 1.293 | |
| 3 | 4 v | 3.917 (1) | 2.4 (1) | 3.459 (1) | 1.883 | |
| Symmetry code: (ii) 1 − x, 1 − y, 1 − z; (v) 2 − x, 1 − y, 1 − z. | ||||||
| 1 | 1 ii | 3.835 (3) | 0.0 (3) | 3.517 (2) | 1.528 | |
| 2 | 2 | 3 ii | 3.917 (4) | 2.1 (3) | 3.530 (3) | 1.596 |
| 4 | 4 iv | 3.553 (4) | 0.0 (3) | 3.432 (3) | 0.919 | |
| Symmetry code: (ii) 1 − x, 1 − y, 1 − z; (iv) 1 − x, 1 − y, 2 − z. | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mirocki, A.; Sikorski, A. Structural Characterization of Multicomponent Crystals Formed from Diclofenac and Acridines. Materials 2022, 15, 1518. https://doi.org/10.3390/ma15041518
Mirocki A, Sikorski A. Structural Characterization of Multicomponent Crystals Formed from Diclofenac and Acridines. Materials. 2022; 15(4):1518. https://doi.org/10.3390/ma15041518
Chicago/Turabian StyleMirocki, Artur, and Artur Sikorski. 2022. "Structural Characterization of Multicomponent Crystals Formed from Diclofenac and Acridines" Materials 15, no. 4: 1518. https://doi.org/10.3390/ma15041518
APA StyleMirocki, A., & Sikorski, A. (2022). Structural Characterization of Multicomponent Crystals Formed from Diclofenac and Acridines. Materials, 15(4), 1518. https://doi.org/10.3390/ma15041518






