Controlling Charge Transport in Molecular Wires through Transannular π–π Interaction
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
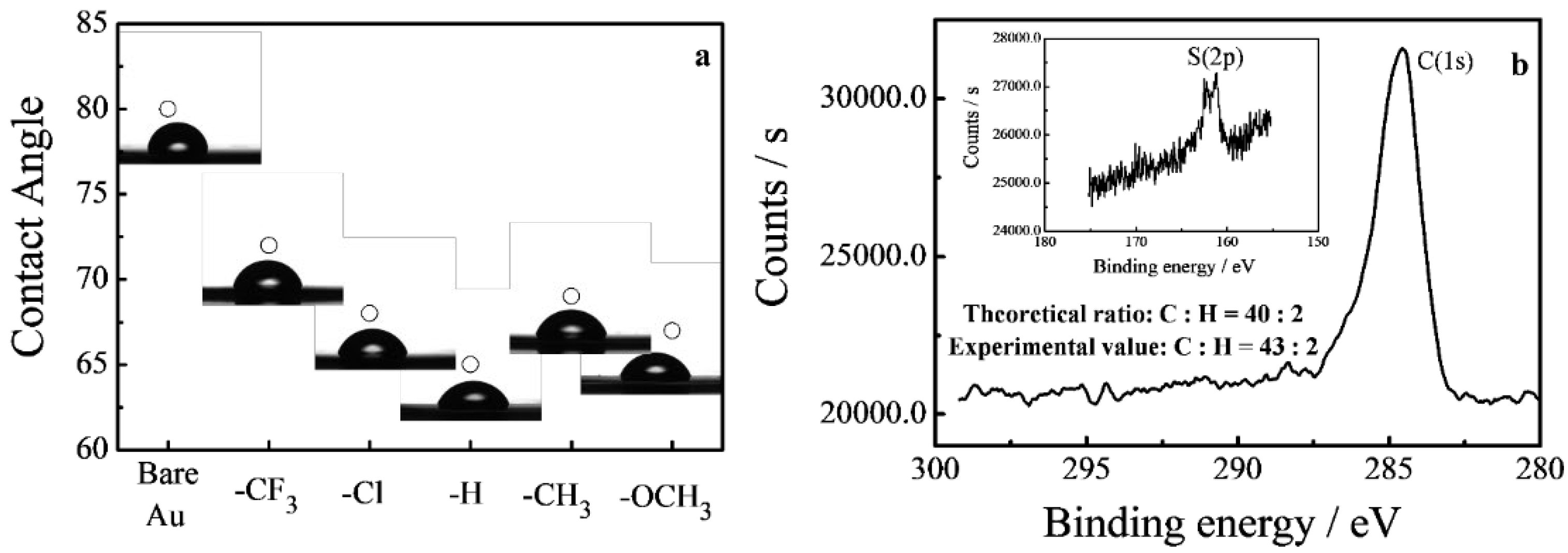
2.2. Characterizations
2.3. Fabrication of Self-Assembled Monolayers (SAMs)
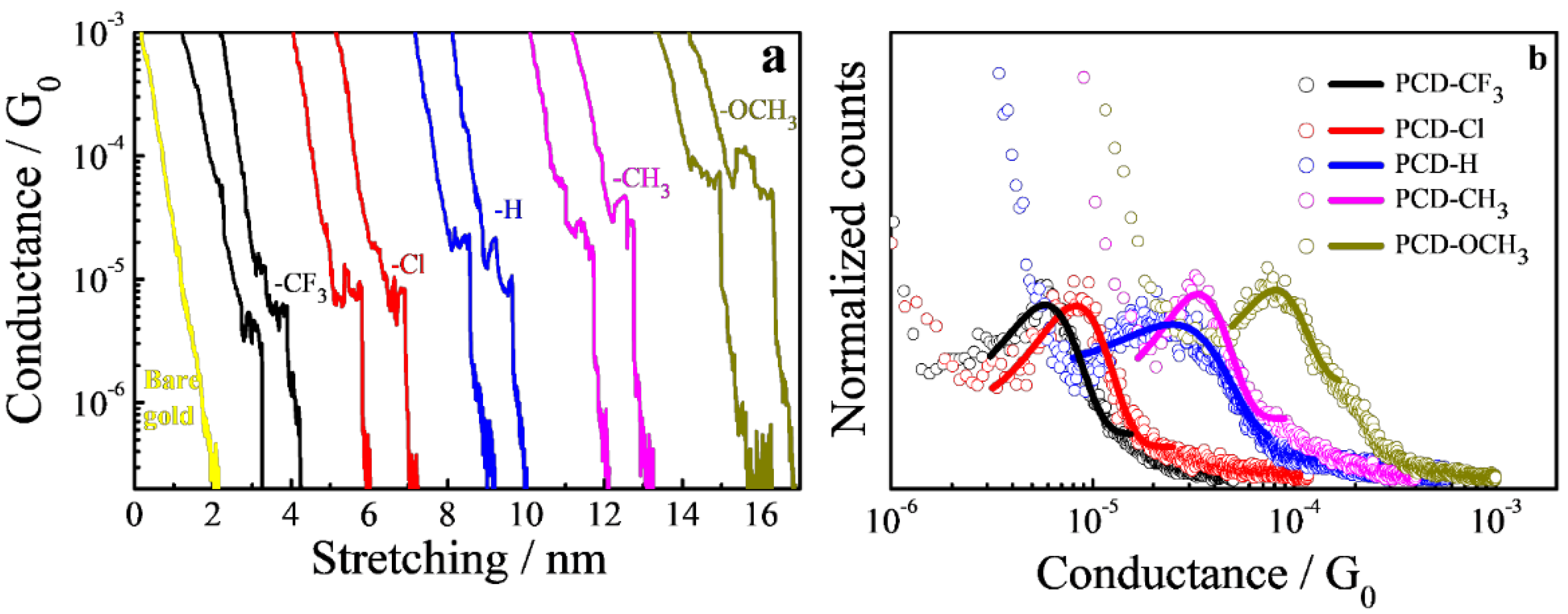
2.4. Single-Molecule Junctions by Break Junction (STM-BJ)
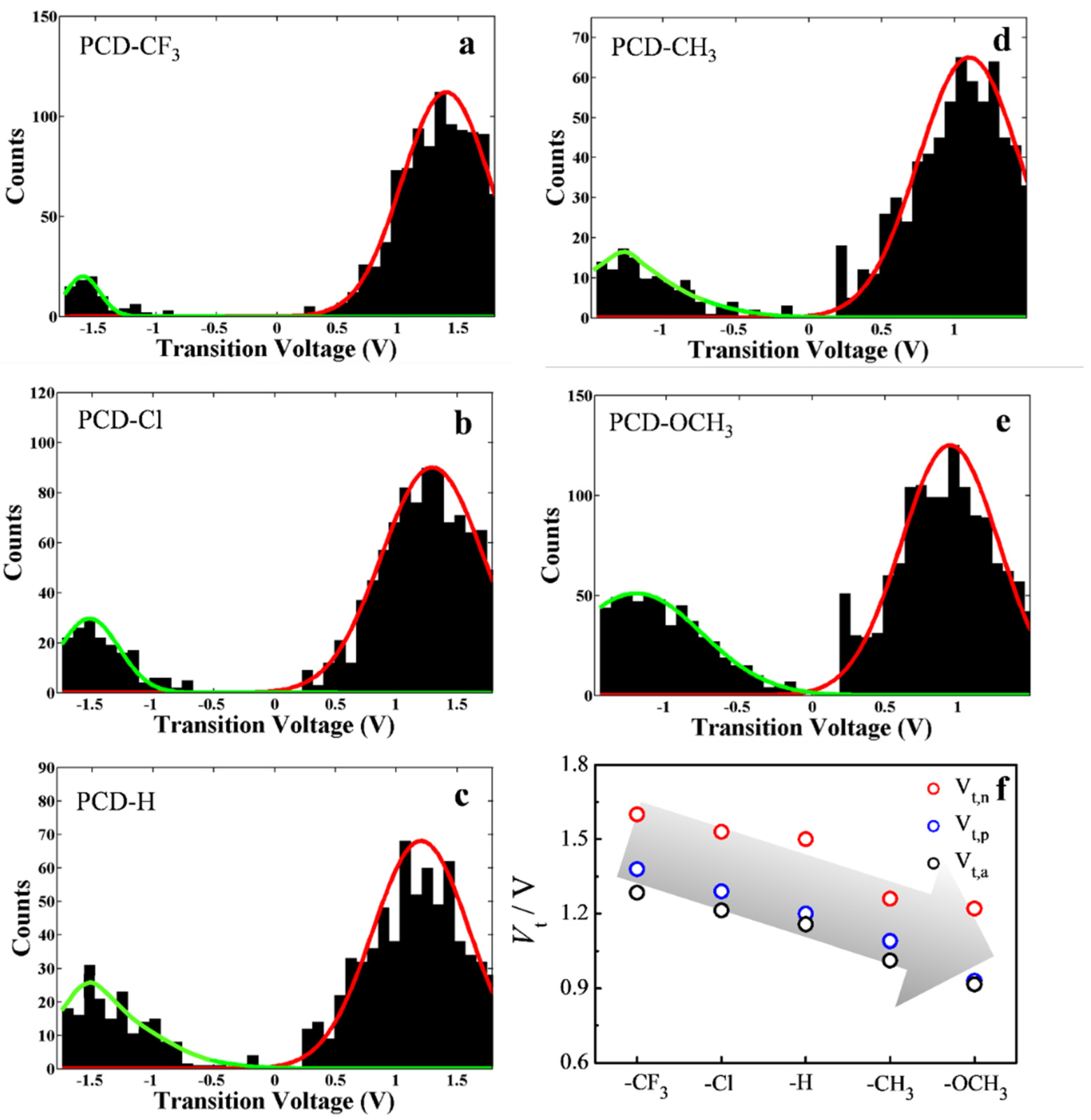
2.5. I-V Recording of Single-Molecule Junctions
3. Results
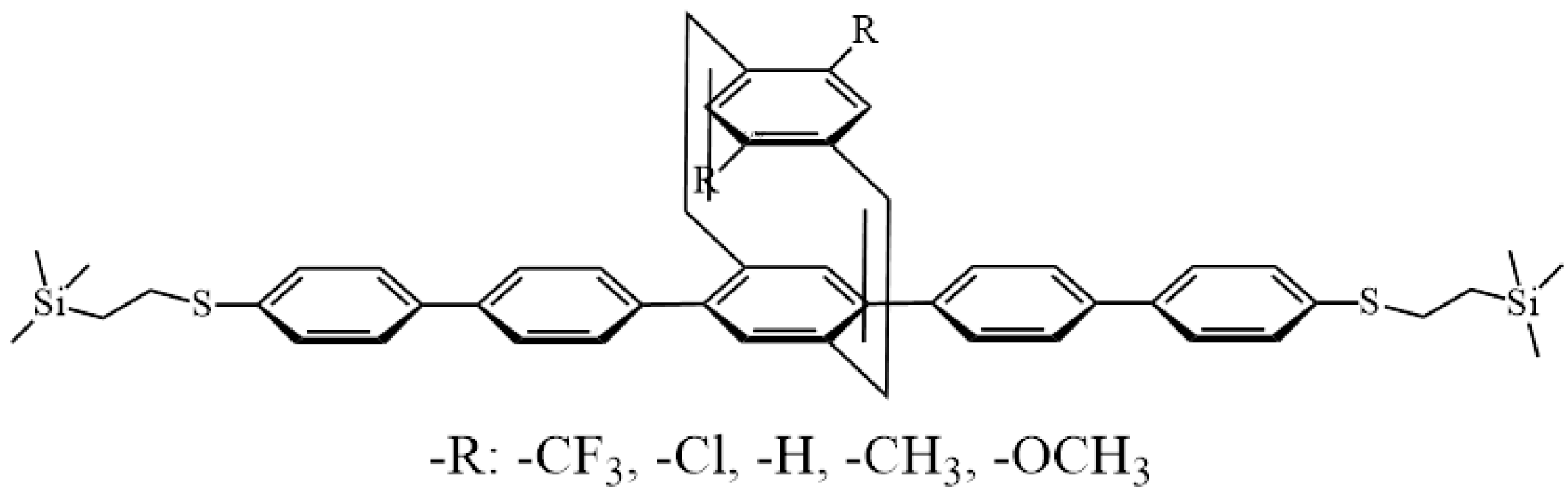
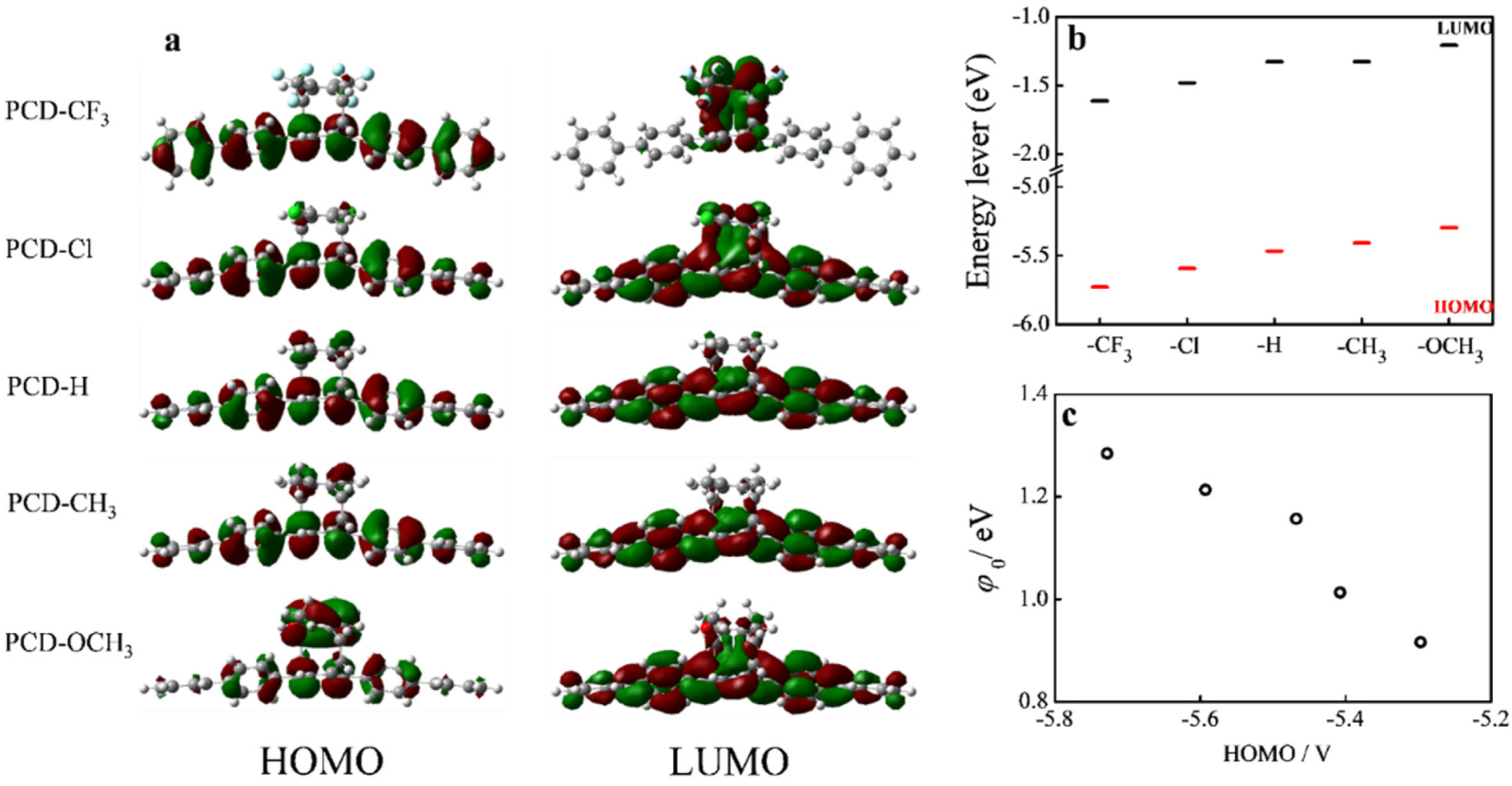
3.1. Synthesis and Characterization of PCD-Based Molecular Wires
3.2. Self-Assembled Monolayer Films on Gold
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ioffe, Z.; Shamai, T.; Ophir, A.; Noy, G.; Yutsis, I.K.K.; Cheshnovsky, O.; Selzer, Y. Detection of heating in current-carrying molecular junctions by Raman scattering. Nat. Nanotechnol. 2008, 3, 727. [Google Scholar] [CrossRef] [PubMed]
- Díez-Pérez, I.; Hihath, J.; Lee, Y.; Yu, L.; Adamska, L.; Kozhushner, M.A.; Oleynik, I.I.; Tao, N. Rectification and stability of a single molecular diode with controlled orientation. Nat. Chem. 2009, 1, 635. [Google Scholar] [CrossRef] [PubMed]
- McCold, C.E.; Domulevicz, L.; Cai, Z.; Lo, W.-Y.; Hihath, S.; March, K.; Mohammad, H.M.; Anantram, M.P.; Yu, L. Molecular Control of Charge Carrier and Seebeck Coefficient in Hybrid Two-Dimensional Nanoparticle Superlattices. J. Hihath. J. Phys. Chem. C 2020, 124, 17. [Google Scholar] [CrossRef]
- Tanaka, Y.; Kiguchi, M.; Akita, M. Inorganic and Organometallic Molecular Wires for Single-Molecule Devices. Chem. Eur. J. 2017, 23, 4741. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Morales, G.M.; You, W.; Yu, L. Synthesis of diode molecules and their sequential assembly to control electron transport. Angew. Chem. Int. Edit. 2004, 43, 4471. [Google Scholar] [CrossRef]
- Morales, G.M.; Jiang, P.; Yuan, S.W.; Lee, Y.G.; Sanchez, A.; You, W.; Yu, L. Inversion of the rectifying effect in diblock molecular diodes by protonation. J. Am. Chem. Soc. 2005, 127, 10456. [Google Scholar] [CrossRef]
- Lo, W.Y.; Zhang, N.; Cai, Z.; Li, L.; Yu, L. Beyond molecular wires: Design molecular electronic functions based on dipolar effect. Acc. Chem. Res. 2016, 49, 1852. [Google Scholar] [CrossRef]
- Duan, P.; Liu, J.; Wang, J.-Y.; Qu, K.; Cai, S.; Wang, F.; Chen, L.; Huang, X.; Li, R.; Shi, J.; et al. Enhancing single-molecule conductance of platinum (II) complexes through synergistic aromaticity-assisted structural asymmetry. Sci. China Chem. 2020, 63, 467. [Google Scholar] [CrossRef]
- Guo, S.; Zhou, G.; Tao, N. Single molecule conductance, thermopower, and transition voltage. Nano Lett. 2013, 13, 4326. [Google Scholar] [CrossRef]
- Kovalchuk, A.; Abu-Husein, T.; Fracasso, D.; Egger, D.A.; Zojer, E.; Zharnikov, M.; Terfort, A.; Chiechi, R.C. The fabrication of a supra-amphiphile for dissipative self-assembly. Chem. Sci. 2016, 7, 781. [Google Scholar] [CrossRef]
- Bâldea, I.J. Interpretation of stochastic events in single-molecule measurements of conductance and transition voltage spectroscopy. Am. Chem. Soc. 2012, 134, 7958. [Google Scholar] [CrossRef]
- Bâldea, I. Effects of stochastic fluctuations at molecule–electrode contacts in transition voltage spectroscopy. Chem. Phys. 2012, 400, 65. [Google Scholar] [CrossRef]
- Bâldea, I. Protocol for disentangling the thermally activated contribution to the tunneling-assisted charge transport. Analytical results and experimental relevance. Phys. Chem. Chem. Phys. 2005, 17, 20217. [Google Scholar] [CrossRef]
- Bléger, D.; Kreher, D.; Mathevet, F.; Attias, A.J.; Arfaoui, I.; Metgé, G.; Douillard, L.; Fiorini-Debuisschert, C.; Charra, F. Periodic Positioning of Multilayered [2.2]Paracyclophane-Based Nanopillars. Angew. Chem. Int. Edit. 2008, 47, 8412. [Google Scholar] [CrossRef]
- Guo, S.; Hihath, J.; Díez-Pérez, I.; Tao, N. Measurement and statistical analysis of single-molecule current–voltage characteristics, transition voltage spectroscopy, and tunneling barrier height. J. Am. Chem. Soc. 2011, 133, 19189. [Google Scholar] [CrossRef]
- Liu, Y.; Qiu, X.; Soni, S.; Chiechi, R.C. The chemical landscape of Chemical Physics Reviews. Chem. Phys. Rev. 2021, 2, 21303. [Google Scholar] [CrossRef]
- Feng, A.; Zhou, Y.; Al-Shebami, M.A.Y.; Chen, L.; Pan, Z.; Xu, W.; Zhao, S.; Zeng, B.; Xiao, Z.; Yang, Y.; et al. Sigma-sigma Stacked supramolecular junctions. Nat. Chem. 2022, 14, 1158. [Google Scholar] [CrossRef]
- Majumdar, P.; Tharammal, F.; Gierschner, J.; Varghese, S. Tuning Solid-State Luminescence in Conjugated Organic Materials: Control of Excitonic and Excimeric Contributions through π Stacking and Halogen Bond Driven Self-Assembly. Chem. Phys. Chem. 2020, 21, 616. [Google Scholar] [CrossRef]
- Che, Y.; Perepichka, D.F. Quantifying Planarity in the Design of Organic Electronic Materials. Angew. Chem. Int. Ed. Engl. 2021, 60, 1364. [Google Scholar] [CrossRef]
- Bennett, T.L.R.; Alshammari, M.; Au-Yong, S.; Almutlg, A.; Wang, X.; Wilkinson, L.A.; Albrecht, T.; Jarvis, S.P.; Cohen, L.F.; Ismael, A.; et al. Multi-component self-assembled molecular-electronic films: Towards new high-performance thermoelectric systems. Chem. Sci. 2022, 13, 5176. [Google Scholar] [CrossRef]
- Asakawa, M.; Ashton, P.R.; Hayes, W.; Janssen, H.M.; Meijer, E.W.; Menzer, S.; Pasini, D.; Stoddart, J.F.; White, A.J.P.; Williams, D.J. Constitutionally Asymmetric and Chiral [2]Pseudorotaxanes1. J. Am. Chem. Soc. 1998, 120, 920. [Google Scholar] [CrossRef]
- Agrawal, Y.K.; Sharma, C.R. Supramolecular assemblies and their applications. Rev. Anal. Chem. 2005, 24, 35. [Google Scholar] [CrossRef]
- Neelakandan, P.P.; Hariharan, M.; Ramaiah, D. A supramolecular ON−OFF−ON fluorescence assay for selective recognition of GTP. J. Am. Chem. Soc. 2006, 128, 11334. [Google Scholar] [CrossRef] [PubMed]
- Porz, M.; Mäker, D.; Brödner, K.; Bunz, U.H.F. Poly(para-phenylene vinylene) and Polynorbornadiene Containing Rod-Coil Block Copoylmers via Combination of Acyclic Diene Metathesis and Ring-Opening Metathesis Polymerization. Macro Rapid Commun. 2013, 34, 873. [Google Scholar] [CrossRef]
- Cram, D.J.; Steinberg, H. Macro rings. I. Preparation and spectra of the paracyclophanes. J. Am. Chem. Soc. 1951, 73, 5691. [Google Scholar] [CrossRef]
- De Meijere, A.; Kozhushkov, S.I.; Rauch, K.; Schill, H.; Verevkin, S.P.; Kümmerlin, M.; Beckhaus, H.D.; Rüchardt, C.; Yufit, D.S. Heats of formation of [2.2]paracyclophane-1-ene and [2.2]paracyclophane-1,9-diene—An experimental study. J. Am. Chem. Soc. 2003, 125, 15110. [Google Scholar] [CrossRef]
- Dodziuk, H.; Demissie, T.B.; Ruud, K.; Szymański, S.; Jaźwiński, J.; Hopf, H. Structure and NMR spectra of cyclophanes with unsaturated bridges (cyclophenes). Magn. Reson. Chem. 2012, 50, 449. [Google Scholar] [CrossRef]
- Yu, C.Y.; Horie, M.; Spring, A.M.; Tremel, K.; Turner, M.L. Homopolymers and Block Copolymers of p-Phenylenevinylene-2,5-diethylhexyloxy-p-phenylenevinylene and m-Phenylenevinylene-2,5-diethylhexyloxy-p-phenylenevinylene by Ring-Opening Metathesis Polymerization. Macromolecules 2009, 43, 222. [Google Scholar] [CrossRef]
- Menk, F.; Mondeshki, M.; Dudenko, D.; Shin, S.; Schollmeyer, D.; Ceyhun, O.; Choi, T.L.; Zentel, R. Reactivity Studies of Alkoxy-Substituted [2.2]Paracyclophane-1,9-dienes and Specific Coordination of the Monomer Repeating Unit during ROMP. Macromolecules 2015, 48, 7435. [Google Scholar] [CrossRef]
- Yu, C.Y.; Sie, C.H.; Yang, C.Y. Synthesis and through-space charge transfer of dioctyloxy diperfluorohexyl substituted [2.2]paracyclophane-1,9-diene. New J. Chem. 2014, 38, 5003. [Google Scholar] [CrossRef]
- Schneebeli, S.T.; Kamenetska, M.; Cheng, Z.; Skouta, R.; Friesner, R.A.; Venkataraman, L.; Breslow, R. Single-molecule conductance through multiple π−π-stacked benzene rings determined with direct electrode-to-benzene ring connections. J. Am. Chem. Soc. 2011, 133, 2136. [Google Scholar] [CrossRef] [PubMed]
- Lo, W.Y.; Bi, W.; Li, L.; Jung, I.H.; Yu, L. Edge-on gating effect in molecular wires. Nano Lett. 2015, 15, 958. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Lo, W.Y.; Cai, Z.; Li, L.; Yu, L. Molecular Rectification Tuned by Through-Space Gating Effect. Nano Lett. 2017, 17, 308. [Google Scholar] [CrossRef]
- Lidster, B.J.; Kumar, D.R.; Spring, A.M.; Yu, C.Y.; Helliwell, M.; Raftery, J.; Turner, M.L. Alkyl substituted [2.2]paracyclophane-1,9-dienes. Org. Biomol. Chem. 2016, 14, 6079–6087. [Google Scholar] [CrossRef] [PubMed]
- Bâldea, I. Ambipolar transition voltage spectroscopy: Analytical results and experimental agreement. Phys. Rev. B 2012, 85, 35442. [Google Scholar] [CrossRef]
- Xu, B.; Tao, N.J. Measurement of single-molecule resistance by repeated formation of molecular junctions. Science 2003, 301, 1221. [Google Scholar] [CrossRef]
- Xu, B.; Xiao, X.; Tao, N.J. Measurements of single-molecule electromechanical properties. J. Am. Chem. Soc. 2003, 125, 16164. [Google Scholar] [CrossRef]
- Wielopolski, M.; Molina-Ontoria, A.; Schubert, C.; Margraf, J.T.; Krokos, E.; Kirschner, J.; Gouloumis, A.; Clark, T.; Guldi, D.M.; Martin, N. Blending through-space and through-bond pi-pi-coupling in [2,2′]-paracyclophane-oligophenylenevinylene molecular wires. J. Am. Chem. Soc. 2013, 135, 10372. [Google Scholar] [CrossRef]
- Beebe, J.M.; Kim, B.; Gadzuk, J.W.; Frisbie, C.D.; Kushmerick, J.G. Transition from direct tunneling to field emission in metal-molecule-metal junctions. Phys. Rev. Lett. 2006, 97, 26801. [Google Scholar] [CrossRef]
- Huisman, E.H.; Guédon, C.M.; van Wees, B.J.; van der Molen, S.J. Interpretation of transition voltage spectroscopy. Nano Lett. 2009, 9, 3909. [Google Scholar] [CrossRef]
- Stern, T.E.; Gossling, B.S.; Fowler, R.H. Further studies in the emission of electrons from cold metals. Proc. R. Soc. Lon. Ser.-A 1929, 124, 699. [Google Scholar]
- Capozzi, B.; Xia, J.; Adak, O.; Dell, E.J.; Liu, Z.F.; Taylor, J.C.; Neaton, J.B.; Campos, L.M.; Venkataraman, L. Single-molecule diodes with high rectification ratios through environmental control. Nat. Nanotechnol. 2015, 10, 522. [Google Scholar] [CrossRef]
- Reddy, P.; Jang, S.; Segalman, R.A.; Majumdar, A. Thermoelectricity in molecular junctions. Science 2007, 315, 1568. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, J.; Zhu, J.; Wang, Z.; Liu, G. Controlling Charge Transport in Molecular Wires through Transannular π–π Interaction. Materials 2022, 15, 7801. https://doi.org/10.3390/ma15217801
Song J, Zhu J, Wang Z, Liu G. Controlling Charge Transport in Molecular Wires through Transannular π–π Interaction. Materials. 2022; 15(21):7801. https://doi.org/10.3390/ma15217801
Chicago/Turabian StyleSong, Jianjian, Jianglin Zhu, Zhaoyong Wang, and Gang Liu. 2022. "Controlling Charge Transport in Molecular Wires through Transannular π–π Interaction" Materials 15, no. 21: 7801. https://doi.org/10.3390/ma15217801
APA StyleSong, J., Zhu, J., Wang, Z., & Liu, G. (2022). Controlling Charge Transport in Molecular Wires through Transannular π–π Interaction. Materials, 15(21), 7801. https://doi.org/10.3390/ma15217801




