Preparation of Nano-Apatite Grafted Glass-Fiber-Reinforced Composites for Orthodontic Application: Mechanical and In Vitro Biofilm Analysis
Abstract
1. Introduction
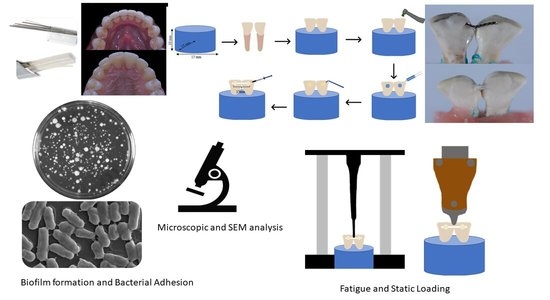
2. Materials and Methods
2.1. Synthesis of Grafted and Non-Grafted Fibers
2.2. Silanization of Fibers
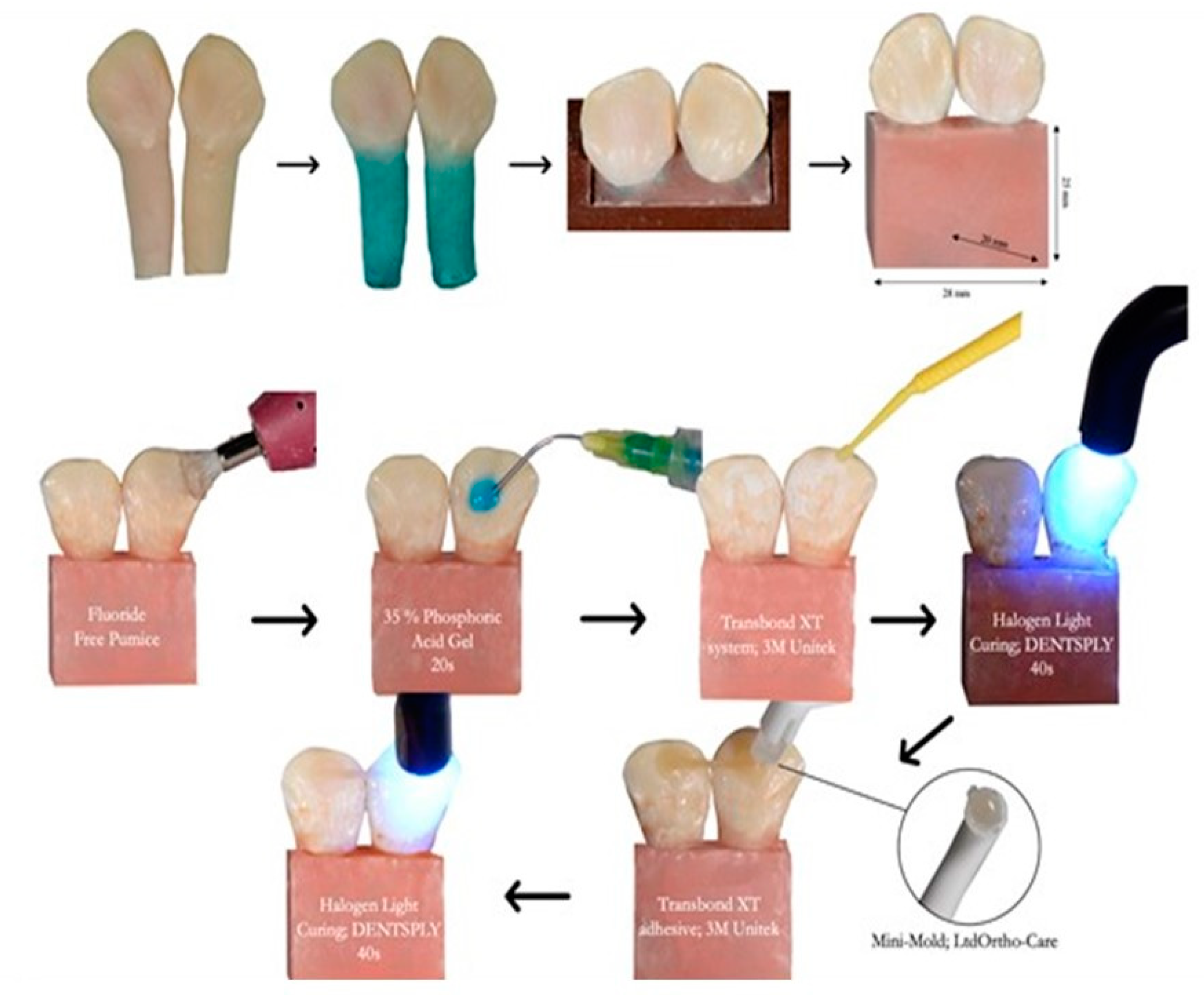
2.3. Fabrication of Experimental Fiber Posts
2.4. Preparation and Grouping of the Samples
2.5. Characterizations
2.5.1. Raman Spectroscopy
2.5.2. Cyclic Loading and Debonding Force Testing
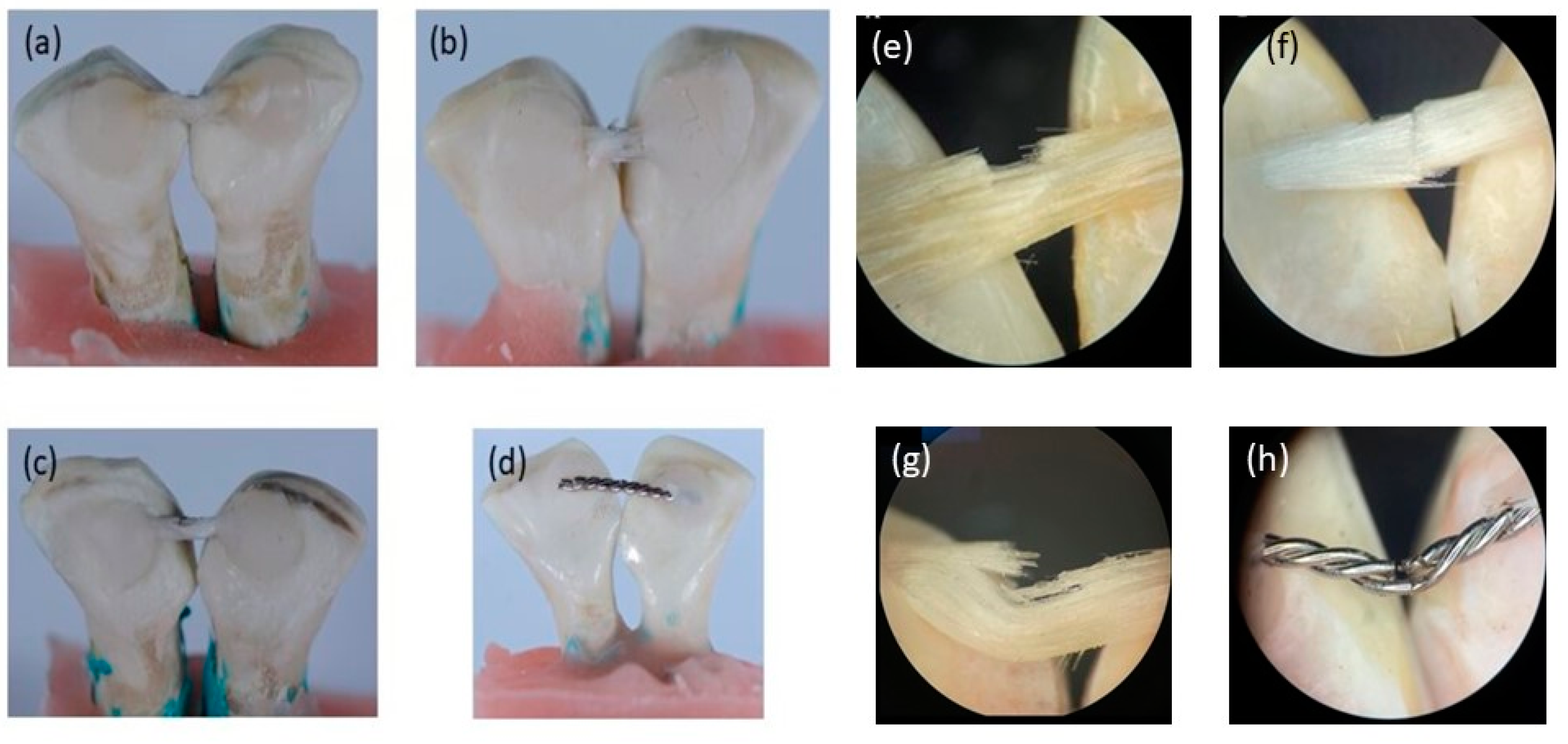
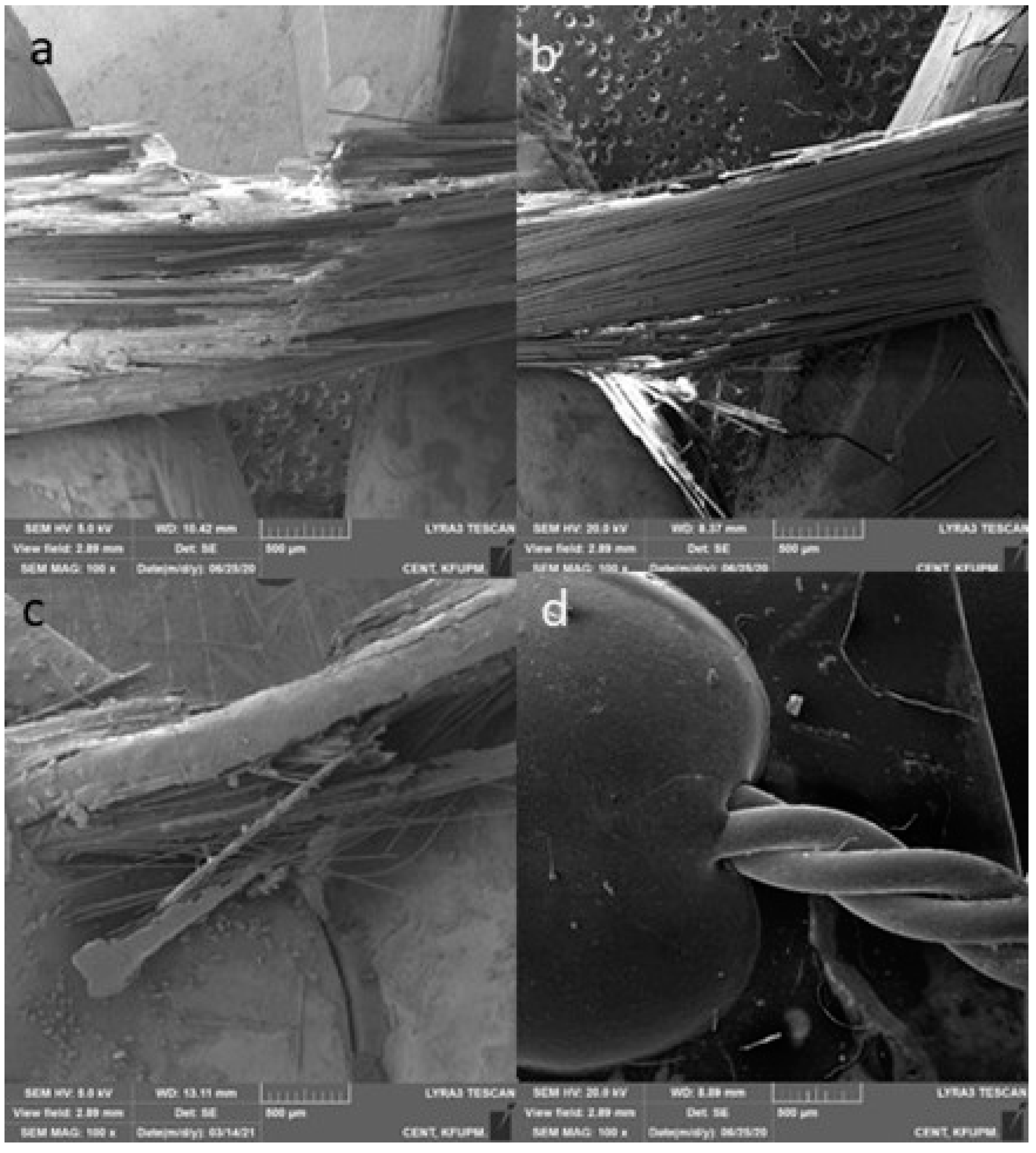
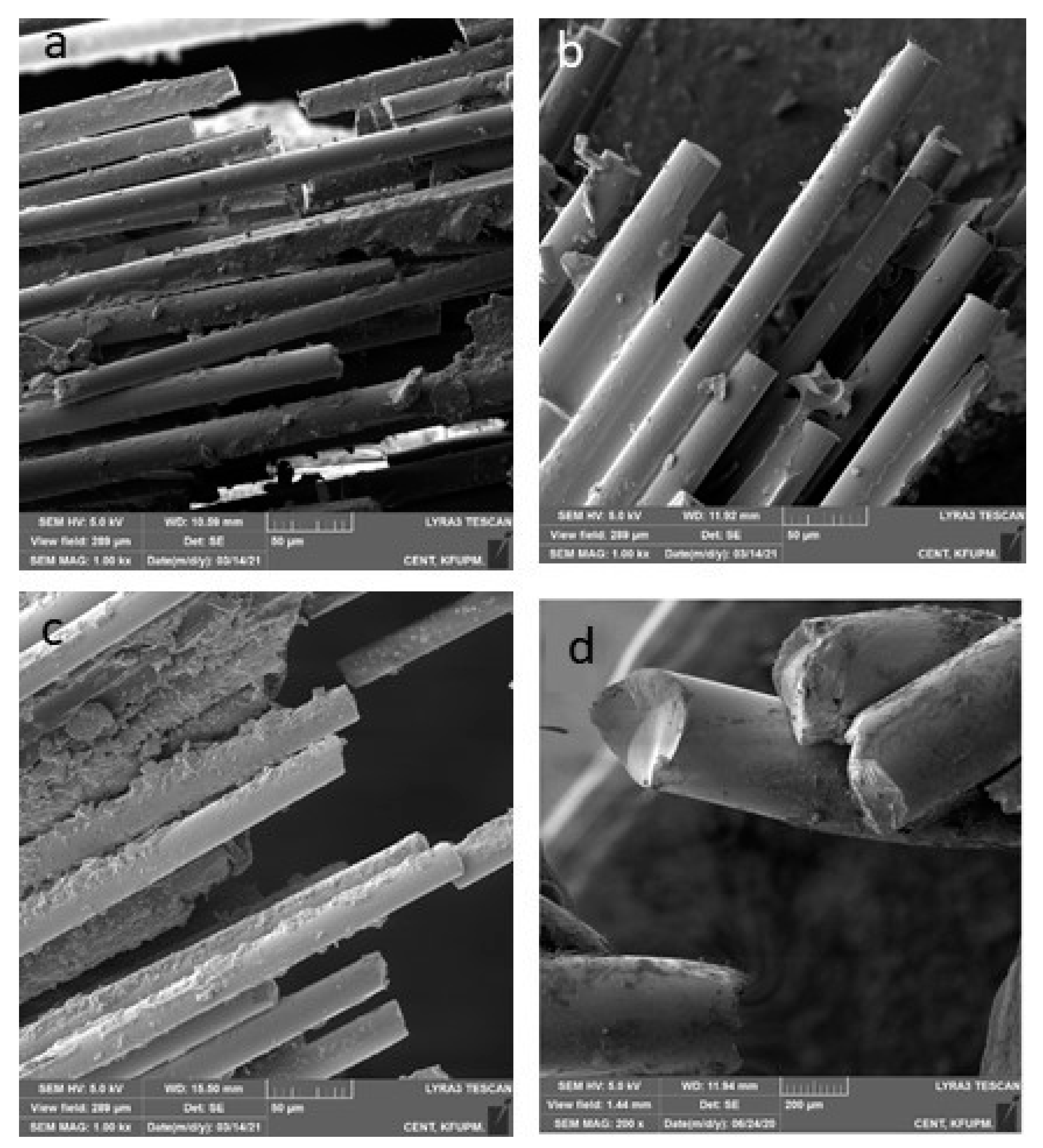
2.5.3. Fracture Mode Analysis
- Type 1: complete adhesive debonding of the retainer from the tooth surface.
- Type 2: partial adhesive detachment of the retainer from one of the teeth.
- Type 3: retainer did not debond from the tooth surface but fractured.
- Type 4: retainer did not debond from the tooth surface, but the overlying composite detached.
- Type 5: Combination of more than one type.
2.5.4. Bacterial and Fungal Growth Analysis
Preparation of Inoculum
Biofilm Assay
2.6. Statistical Analysis
3. Results
3.1. Raman Spectroscopy
3.2. Mechanical Testing
3.3. Bacterial and Fungal Growth Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Littlewood, S.J.; Kandasamy, S.; Huang, G. Retention and relapse in clinical practice. Aust. Dent. J. 2017, 62, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Johnston, C.D.; Littlewood, S.J. Retention in orthodontics. Br. Dent. J. 2015, 218, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Han, J.-Y.; Park, S.H.; Kim, J.; Hwang, K.-G.; Park, C.-J. Clinical factors affecting the longevity of fixed retainers and the influence of fixed retainers on periodontal health in periodontitis patients: A retrospective study. J. Periodontal Implant Sci. 2021, 51, 163–178. [Google Scholar] [CrossRef] [PubMed]
- Fudalej, P.S.; Renkema, A.-M. A brief history of orthodontic retention. Br. Dent. J. 2021, 230, 777–780. [Google Scholar] [CrossRef]
- Alnazeh, A.A. Orthodontic Retention Protocol—A Review; Ann Abbasi Shaheed Hospital & Khi Med Dent Coll: Sindh, Pakistan, 2020; Volume 25. [Google Scholar]
- Kartal, Y.; Kaya, B. Fixed Orthodontic Retainers: A Review. Turk. J. Orthod. 2019, 32, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Kadhum, A.S.; Alhuwaizi, A.F. The efficacy of polyether-ether-ketone wire as a retainer following orthodontic treatment. Clin. Exp. Dent. Res. 2020, 7, 302–312. [Google Scholar] [CrossRef]
- Schütz-Fransson, U.; Lindsten, R.; Bjerklin, K.; Bondemark, L. Twelve-year follow-up of mandibular incisor stability: Comparison between two bonded lingual orthodontic retainers. Angle Orthod. 2016, 87, 200–208. [Google Scholar] [CrossRef]
- Al-Moghrabi, D.; Johal, A.; O’Rourke, N.; Donos, N.; Pandis, N.; Gonzales-Marin, C.; Fleming, P.S. Effects of fixed vs. removable orthodontic retainers on stability and periodontal health: 4-year follow-up of a randomized controlled trial. Am. J. Orthod. Dentofac. Orthop. 2018, 154, 167–174.e1. [Google Scholar] [CrossRef]
- Nagani, N.I.; Ahmed, I.; Tanveer, F.; Khursheed, H.M.; Farooqui, W.A. Clinical comparison of bond failure rate between two types of mandibular canine-canine bonded orthodontic retainers—A randomized clinical trial. BMC Oral Health 2020, 20, 180. [Google Scholar] [CrossRef]
- Kotta, M.; Gorantla, S.; Muddada, V.; Lenka, R.R.; Karri, T.; Kumar, S.; Tivanani, M. Antibacterial activity and debonding force of different lingual retainers bonded with conventional composite and nanoparticle containing composite: An in vitro study. J. World Fed. Orthod. 2020, 9, 80–85. [Google Scholar] [CrossRef]
- Geserick, M.; Ball, J.; Wichelhaus, A. Bonding fiber-reinforced lingual retainers with color-reactivating flowable composite. J. Clin. Orthod. 2004, 38, 560–562. [Google Scholar] [PubMed]
- Foek, D.L.S.; Özcan, M.; Krebs, E.; Sandham, A. Adhesive properties of bonded orthodontic retainers to enamel: Stainless steel wire vs fiber-reinforced composites. J. Adhes. Dent. 2009, 11, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Renkema, A.-M.; Renkema, A.; Bronkhorst, E.; Katsaros, C. Long-term effectiveness of canine-to-canine bonded flexible spiral wire lingual retainers. Am. J. Orthod. Dentofac. Orthop. 2011, 139, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Foek, D.L.S.; Yetkiner, E.; Özcan, M. Fatigue resistance, debonding force, and failure type of fiber-reinforced composite, polyethylene ribbon-reinforced, and braided stainless steel wire lingual retainersin vitro. Korean J. Orthod. 2013, 43, 186–192. [Google Scholar] [CrossRef]
- Tacken, M.P.E.; Cosyn, J.; De Wilde, P.; Aerts, J.; Govaerts, E.; Vannet, B.V. Glass fibre reinforced versus multistranded bonded orthodontic retainers: A 2 year prospective multi-centre study. Eur. J. Orthod. 2009, 32, 117–123. [Google Scholar] [CrossRef]
- Kumbuloglu, O.; Saracoglu, A.; Özcan, M. Pilot study of unidirectional E-glass fibre-reinforced composite resin splints: Up to 4.5-year clinical follow-up. J. Dent. 2011, 39, 871–877. [Google Scholar] [CrossRef]
- Lim, B.-S.; Lee, S.-J.; Lee, J.-W.; Ahn, S.-J. Quantitative analysis of adhesion of cariogenic streptococci to orthodontic raw materials. Am. J. Orthod. Dentofac. Orthop. 2008, 133, 882–888. [Google Scholar] [CrossRef]
- Jasso-Ruiz, I.; Velazquez-Enriquez, U.; Scougall-Vilchis, R.J.; Morales-Luckie, R.A.; Sawada, T.; Yamaguchi, R. Silver nanoparticles in orthodontics, a new alternative in bacterial inhibition: In vitro study. Prog. Orthod. 2020, 21, 1–8. [Google Scholar] [CrossRef]
- Lucchese, A.; Bondemark, L. The Influence of Orthodontic Treatment on Oral Microbiology. Biol. Mech. Tooth Mov. 2021, 139–158. [Google Scholar] [CrossRef]
- Enax, J.; Epple, M. Synthetic Hydroxyapatite as a Biomimetic Oral Care Agent. Oral Health Prev. Dent. 2018, 16, 7–19. [Google Scholar]
- Najibfard, K.; Ramalingam, K.; Chedjieu, I.; Amaechi, B.T. Remineralization of early caries by a nano-hydroxyapatite dentifrice. J. Clin. Dent. 2011, 22, 139. [Google Scholar] [PubMed]
- Hannig, C.; Basche, S.; Burghardt, T.; Al-Ahmad, A.; Hannig, M. Influence of a mouthwash containing hydroxyapatite microclusters on bacterial adherence in situ. Clin. Oral Investig. 2012, 17, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Syed, M.R.; Bano, N.Z.; Ghafoor, S.; Khalid, H.; Zahid, S.; Siddiqui, U.; Hakeem, A.S.; Asif, A.; Kaleem, M.; Khan, A.S. Synthesis and characterization of bioactive glass fiber-based dental restorative composite. Ceram. Int. 2020, 46, 21623–21631. [Google Scholar] [CrossRef]
- Saleem, M.; Zahid, S.; Ghafoor, S.; Khalid, H.; Iqbal, H.; Zeeshan, R.; Ahmad, S.; Asif, A.; Khan, A.S. Physical, mechanical, and in vitro biological analysis of bioactive fibers-based dental composite. J. Appl. Polym. Sci. 2021, 138, 50336. [Google Scholar] [CrossRef]
- Siddiqui, U.; Khalid, H.; Ghafoor, S.; Javaid, A.; Asif, A.; Khan, A.S. Analyses on mechanical and physical performances of nano-apatite grafted glass fibers based dental composites. Mater. Chem. Phys. 2020, 263, 124188. [Google Scholar] [CrossRef]
- Khalid, H.; Suhaib, F.; Zahid, S.; Ahmed, S.; Jamal, A.; Kaleem, M.; Khan, A.S. Microwave-assisted synthesis and in vitro osteogenic analysis of novel bioactive glass fibers for biomedical and dental applications. Biomed. Mater. 2018, 14, 015005. [Google Scholar] [CrossRef]
- Khan, A.S.; AlMaimouni, Y.K.; Benrashed, M.A.; Alyousef, N.I.; Siddiqui, U.; Ahmad, N.; Ateeq, I.S.; Hakeem, A.S. A laboratory study to assess the physical, mechanical, and 3-D structural properties of nano-apatite grafted glass fibre-based endodontic posts. Int. Endod. J. 2021, 54, 2307–2320. [Google Scholar] [CrossRef]
- Lwanga, S.K.; Lemeshow, S.; WHO. Sample Size Determination in Health Studies: A Practical Manual; World Health Organization: Geneva, Switzerland, 1991. [Google Scholar]
- Elsorogy, M.; Hanafy, S.; Yousry, T.; Zaher, A. Comparative evaluation of failure of three different aged orthodontic bonded retainers related to vertical load: In vitro study. Egypt. Orthod. J. 2019, 56, 39–50. [Google Scholar] [CrossRef][Green Version]
- Cooke, M.E.; Sherriff, M. Debonding force and deformation of two multi-stranded lingual retainer wires bonded to incisor enamel: An in vitro study. Eur. J. Orthod. 2010, 32, 741–746. [Google Scholar] [CrossRef]
- Brosh, T.; Zary, R.; Pilo, R.; Gavish, A. Influence of periodontal ligament simulation and splints on strains developing at the cervical area of a tooth crown. Eur. J. Oral Sci. 2012, 120, 466–471. [Google Scholar] [CrossRef]
- Kocher, K.E.; Gebistorf, M.C.; Pandis, N.; Fudalej, P.S.; Katsaros, C. Survival of maxillary and mandibular bonded retainers 10 to 15 years after orthodontic treatment: A retrospective observational study. Prog. Orthod. 2019, 20, 28. [Google Scholar] [CrossRef] [PubMed]
- Jongsma, M.A.; Van Der Mei, H.C.; Atema-Smit, J.; Busscher, H.I.; Ren, Y. In vivo biofilm formation on stainless steel bonded retainers during different oral health-care regimens. Int. J. Oral Sci. 2015, 7, 42–48. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kuroiwa, A.; Nomura, Y.; Ochiai, T.; Sudo, T.; Nomoto, R.; Hayakawa, T.; Kanzaki, H.; Nakamura, Y.; Hanada, N. Antibacterial, Hydrophilic Effect and Mechanical Properties of Orthodontic Resin Coated with UV-Responsive Photocatalyst. Materials 2018, 11, 889. [Google Scholar] [CrossRef]
- Santos, C.; Luklinska, Z.B.; Clarke, R.L.; Davy, K.W.M. Hydroxyapatite as a filler for dental composite materials: Mechanical properties and in vitro bioactivity of composites. J. Mater. Sci. Mater. Electron. 2001, 12, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Aldabib, J.M.; Ishak, Z.A.M. Effect of hydroxyapatite filler concentration on mechanical properties of poly (methyl methacrylate) denture base. SN Appl. Sci. 2020, 2, 732. [Google Scholar] [CrossRef]
- Lung, C.Y.K.; Sarfraz, Z.; Habib, A.; Khan, A.S.; Matinlinna, J.P. Effect of silanization of hydroxyapatite fillers on physical and mechanical properties of a bis-GMA based resin composite. J. Mech. Behav. Biomed. Mater. 2016, 54, 283–294. [Google Scholar] [CrossRef]
- Bystrova, A.V.; Dekhtyar, Y.D.; Popov, A.I.; Coutinho, J.; Bystrov, V.S. Modified Hydroxyapatite Structure and Properties: Modeling and Synchrotron Data Analysis of Modified Hydroxyapatite Structure. Ferroelectrics 2015, 475, 135–147. [Google Scholar] [CrossRef]
- Hübner, W.; Blume, A.; Pushnjakova, R.; Dekhtyar, Y.; Hein, H.-J. The Influence of X-ray Radiation on the Mineral/Organic Matrix Interaction of Bone Tissue: An FT-IR Microscopic Investigation. Int. J. Artif. Organs 2005, 28, 66–73. [Google Scholar] [CrossRef]
- Asmussen, E.; Peutzfeldt, A. Influence of UEDMA, BisGMA and TEGDMA on selected mechanical properties of experimental resin composites. Dent. Mater. 1998, 14, 51–56. [Google Scholar] [CrossRef]
- Szczesio-Wlodarczyk, A.; Domarecka, M.; Kopacz, K.; Sokolowski, J.; Bociong, K. An Evaluation of the Properties of Urethane Dimethacrylate-Based Dental Resins. Materials 2021, 14, 2727. [Google Scholar] [CrossRef]
- Rüttermann, S.; Dluzhevskaya, I.; Großsteinbeck, C.; Raab, W.H.-M.; Janda, R. Impact of replacing Bis-GMA and TEGDMA by other commercially available monomers on the properties of resin-based composites. Dent. Mater. 2010, 26, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Pratap, B.; Gupta, R.K.; Bhardwaj, B.; Nag, M. Resin based restorative dental materials: Characteristics and future perspectives. Jpn. Dent. Sci. Rev. 2019, 55, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Poitevin, A.; De Munck, J.; Cardoso, M.V.; Mine, A.; Peumans, M.; Lambrechts, P.; Van Meerbeek, B. Dynamic versus static bond-strength testing of adhesive interfaces. Dent. Mater. 2010, 26, 1068–1076. [Google Scholar] [CrossRef] [PubMed]
- Katona, T.R.; Moore, B.K. The effects of load misalignment on tensile load testing of direct bonded orthodontic brackets—A finite element model. Am. J. Orthod. Dentofac. Orthop. 1994, 105, 543–551. [Google Scholar] [CrossRef]
- Reicheneder, C.A.; Gedrange, T.; Lange, A.; Baumert, U.; Proff, P. Shear and tensile bond strength comparison of various contemporary orthodontic adhesive systems: An in-vitro study. Am. J. Orthod. Dentofac. Orthop. 2009, 135, 422.e1–422.e6. [Google Scholar] [CrossRef]
- AlDrees, A.M.; Al-Mutairi, T.K.; Hakami, Z.W.; Al-Malki, M.M. Bonded Orthodontic Retainers: A Comparison of Initial Bond Strength of Different Wire-and-Composite Combinations. J. Orofac. Orthoped./Fortschr. Kieferorthopädie 2010, 71, 290–299. [Google Scholar] [CrossRef]
- Tezvergil, A.; Lassila, L.V.J.; Vallittu, P.K. Strength of adhesive-bonded fiber-reinforced composites to enamel and dentin substrates. J. Adhes. Dent. 2003, 5, 301–311. [Google Scholar]
- Reicheneder, C.; Hofrichter, B.; Faltermeier, A.; Proff, P.; Lippold, C.; Kirschneck, C. Shear bond strength of different retainer wires and bonding adhesives in consideration of the pretreatment process. Head Face Med. 2014, 10, 51. [Google Scholar] [CrossRef]
- Steiner, M.; Mitsias, M.E.; Ludwig, K.; Kern, M. In vitro evaluation of a mechanical testing chewing simulator. Dent. Mater. 2009, 25, 494–499. [Google Scholar] [CrossRef]
- Lamkhao, S.; Phaya, M.; Jansakun, C.; Chandet, N.; Thongkorn, K.; Rujijanagul, G.; Bangrak, P.; Randorn, C. Synthesis of Hydroxyapatite with Antibacterial Properties Using a Microwave-Assisted Combustion Method. Sci. Rep. 2019, 9, 4015. [Google Scholar] [CrossRef]
- Uskoković, V.; Iyer, M.A.; Wu, V. One ion to rule them all: The combined antibacterial, osteoinductive and anticancer properties of selenite-incorporated hydroxyapatite. J. Mater. Chem. B 2017, 5, 1430–1445. [Google Scholar] [CrossRef] [PubMed]
- Naka, S.; Wato, K.; Misaki, T.; Ito, S.; Matsuoka, D.; Nagasawa, Y.; Nomura, R.; Matsumoto-Nakano, M.; Nakano, K. Streptococcus mutans induces IgA nephropathy-like glomerulonephritis in rats with severe dental caries. Sci. Rep. 2021, 11, 5784. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.S. Staphylococci in the oral flora of healthy children and those receiving treatment for malignant disease. Microb. Ecol. Health Dis. 2000, 12, 60–64. [Google Scholar]
- Smith, A.J.; Robertson, D.; Tang, M.K.; Jackson, M.S.; MacKenzie, D.; Bagg, J. Staphylococcus aureus in the oral cavity: A three-year retrospective analysis of clinical laboratory data. Br. Dent. J. 2003, 195, 701–703. [Google Scholar] [CrossRef]
- Azmi, A.H.; Adnan, S.N.A.; Ab Malik, N. The Prevalence of Staphylococcus aureus in the Oral Cavity of Healthy Adults in Malaysia. Sains Malays. 2020, 49, 583–591. [Google Scholar] [CrossRef]
- Cepic, L.Z.; Dvorak, G.; Piehslinger, E.; Georgopoulos, A. In vitro adherence of Candida albicans to zirconia surfaces. Oral Dis. 2020, 26, 1072–1080. [Google Scholar] [CrossRef]
- McCormack, M.; Smith, A.; Akram, A.; Jackson, M.; Robertson, D.; Edwards, G. Staphylococcus aureus and the oral cavity: An overlooked source of carriage and infection? Am. J. Infect. Control 2015, 43, 35–37. [Google Scholar] [CrossRef]
- Yassen, G.H.; Platt, J.A.; Hara, A.T. Bovine teeth as substitute for human teeth in dental research: A review of literature. J. Oral Sci. 2011, 53, 273–282. [Google Scholar] [CrossRef]

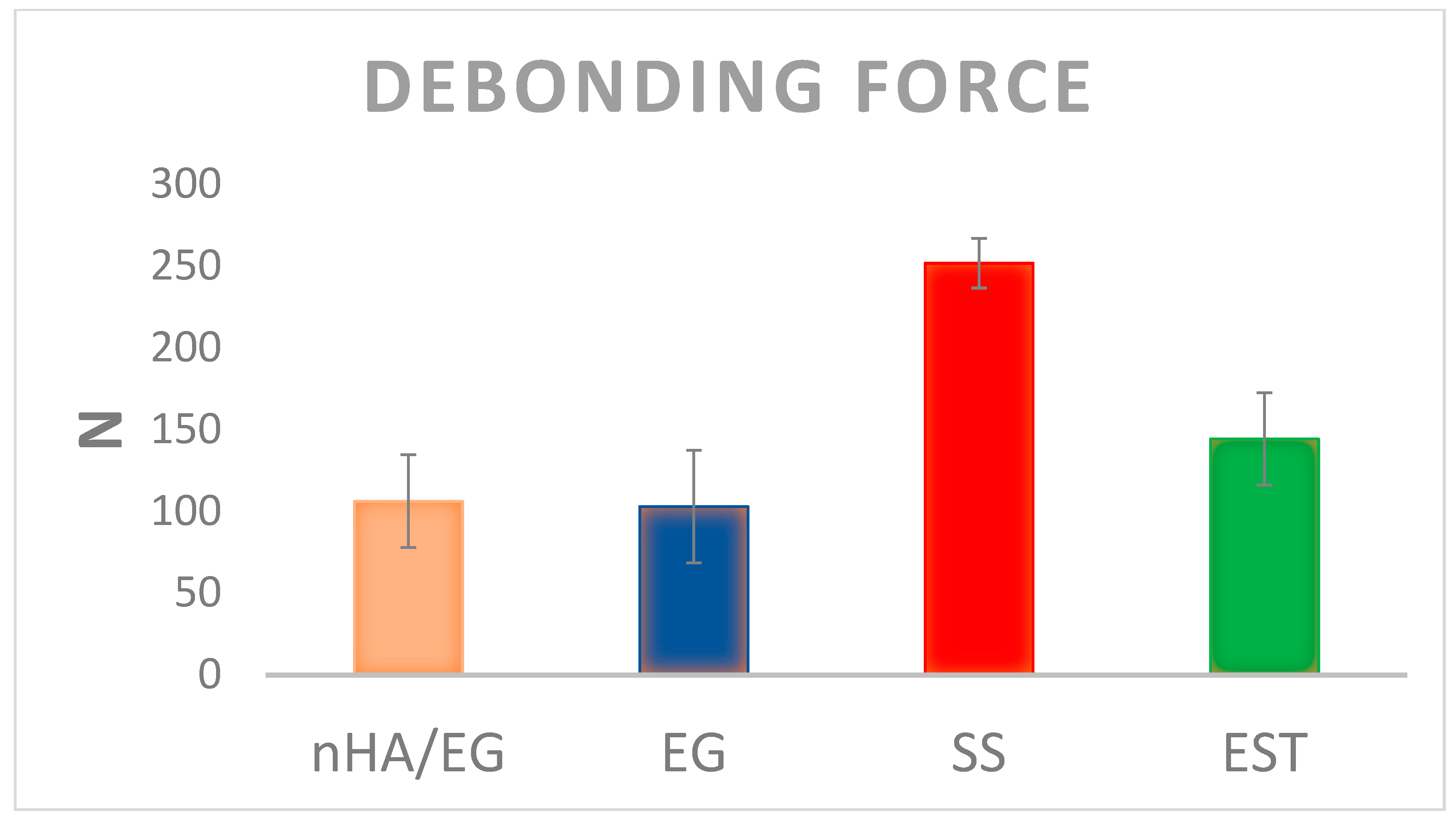


| Name | Code | Composition | Manufacturer |
|---|---|---|---|
| Nano-hydroxyapatite (nHA) grafted glass fiber | nHA/EG | Nano-hydroxyapatite, E-glass fiber, bis-GMA, UDMA | Experimental |
| Pure E-glass fiber | EG | E-glass fiber, bis-GMA, UDMA | Experimental |
| everStick Ortho | EST | E-glass, PMMA *, bis-GMA | StickTech Ltd., Turku, Finland |
| 3M lingual retainer | SS | 0.032 in. twisted bondable lingual retainer | 3M, St. Paul, MN, USA |
| Code | Type 1 | Type 2 | Type 3 | Type 4 | Combination |
|---|---|---|---|---|---|
| nHA/EG | 0% | 10% | 45% | 0% | 45% (Type 3 and 4) |
| EG | 0% | 30% | 40% | 0% | 30% (Type 3 and 4) |
| EST | 0% | 20% | 50% | 0% | 30% (Type 3 and 4) |
| SS | 50% | 50% | 0% | 0% | 0% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, A.S.; Alshaia, A.; AlDubayan, A.; Alarifi, S.; Alamri, A.; Aldossary, H.; Ahmed, S.Z.; Ateeq, I.S.; Hakeem, A.S.; Rehman, S. Preparation of Nano-Apatite Grafted Glass-Fiber-Reinforced Composites for Orthodontic Application: Mechanical and In Vitro Biofilm Analysis. Materials 2022, 15, 3504. https://doi.org/10.3390/ma15103504
Khan AS, Alshaia A, AlDubayan A, Alarifi S, Alamri A, Aldossary H, Ahmed SZ, Ateeq IS, Hakeem AS, Rehman S. Preparation of Nano-Apatite Grafted Glass-Fiber-Reinforced Composites for Orthodontic Application: Mechanical and In Vitro Biofilm Analysis. Materials. 2022; 15(10):3504. https://doi.org/10.3390/ma15103504
Chicago/Turabian StyleKhan, Abdul Samad, Alaa Alshaia, AlAnood AlDubayan, Sundus Alarifi, Abdulaziz Alamri, Hanan Aldossary, Syed Zubairuddin Ahmed, Ijlal Shahrukh Ateeq, Abbas Saeed Hakeem, and Suriya Rehman. 2022. "Preparation of Nano-Apatite Grafted Glass-Fiber-Reinforced Composites for Orthodontic Application: Mechanical and In Vitro Biofilm Analysis" Materials 15, no. 10: 3504. https://doi.org/10.3390/ma15103504
APA StyleKhan, A. S., Alshaia, A., AlDubayan, A., Alarifi, S., Alamri, A., Aldossary, H., Ahmed, S. Z., Ateeq, I. S., Hakeem, A. S., & Rehman, S. (2022). Preparation of Nano-Apatite Grafted Glass-Fiber-Reinforced Composites for Orthodontic Application: Mechanical and In Vitro Biofilm Analysis. Materials, 15(10), 3504. https://doi.org/10.3390/ma15103504