Appendix A
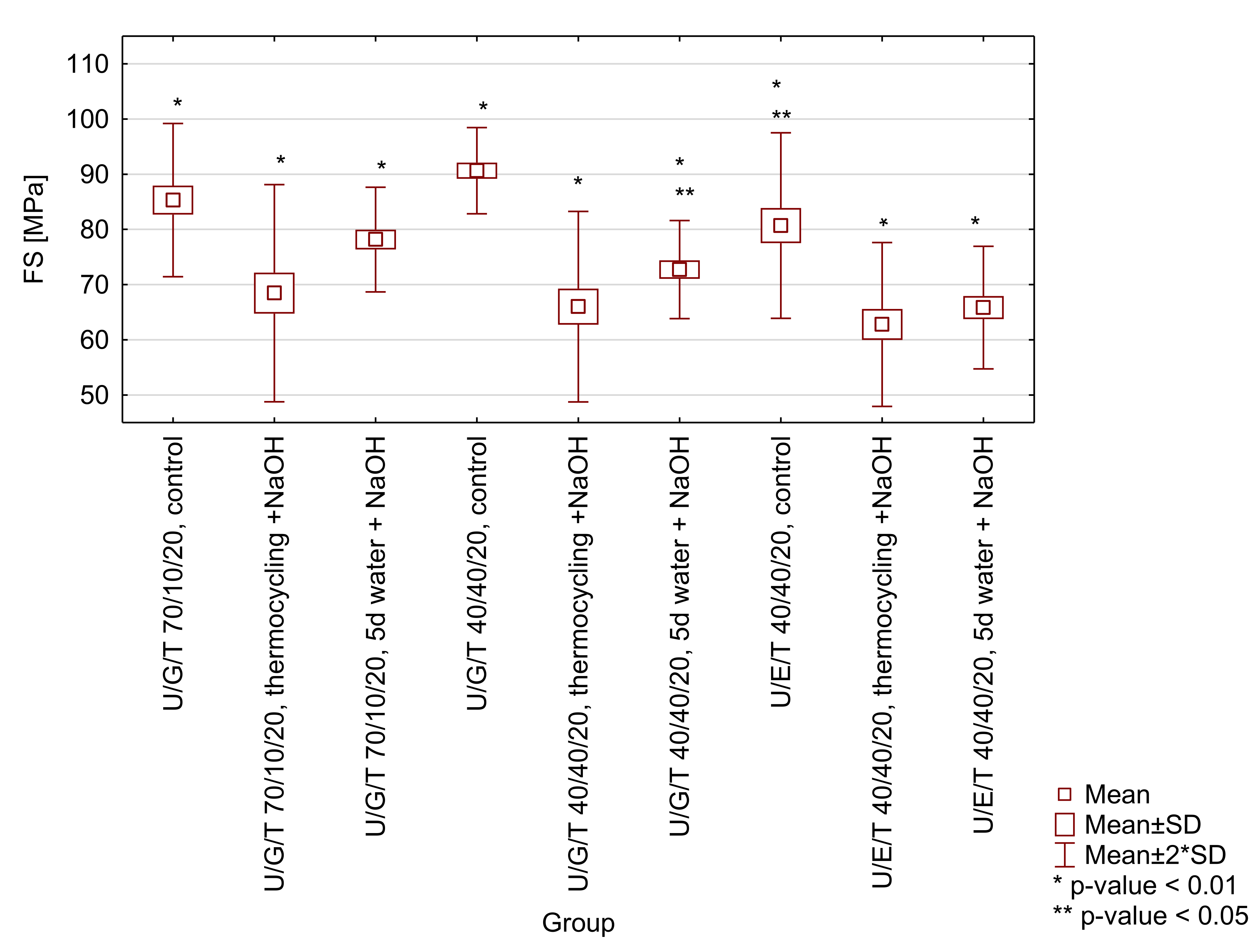
Figure A1.
Box-and-whisker plot of three-point bending flexural strength (FS) of tested matrices (UDMA/Bis-GMA/TEGDMA 70/10/20 wt.% and 40/40/20 wt.% and UDMA/Bis-EMA/TEGDMA 40/40/20 wt.%) after selected ageing protocols (control-water, 24 h, 37 °C; thermocycling + NaOH-water, 7500 thermo cycles, 5/55 °C and 0.1 M NaOH, 7 days, 60 °C; 5 d water + NaOH-water, 5 days, 55 °C and 0.1 M NaOH, 7 days). Significant differences occur in a given group with a probability of p < 0.01 (*) and/or p < 0.05 (**).
Figure A1.
Box-and-whisker plot of three-point bending flexural strength (FS) of tested matrices (UDMA/Bis-GMA/TEGDMA 70/10/20 wt.% and 40/40/20 wt.% and UDMA/Bis-EMA/TEGDMA 40/40/20 wt.%) after selected ageing protocols (control-water, 24 h, 37 °C; thermocycling + NaOH-water, 7500 thermo cycles, 5/55 °C and 0.1 M NaOH, 7 days, 60 °C; 5 d water + NaOH-water, 5 days, 55 °C and 0.1 M NaOH, 7 days). Significant differences occur in a given group with a probability of p < 0.01 (*) and/or p < 0.05 (**).
Table A1.
Probabilities for post hoc testing for three-point bending flexural strength (FS) of tested matrices. Tested group: 1-UDMA/bis-GMA/TEGDMA 70/10/20, control; 2-UDMA/bis-GMA/TEGDMA 70/10/20, thermocycling +NaOH, 3-UDMA/bis-GMA/TEGDMA 70/10/20, 5 d water + NaOH, 4-UDMA/bis-GMA/TEGDMA 40/40/20, control; 5-UDMA/bis-GMA/TEGDMA 40/40/20, thermocycling +NaOH; 6-UDMA/bis-GMA/TEGDMA 40/40/20, 5d water + NaOH; 7-UDMA/bis-EMA/TEGDMA 40/40/20, control; 8-UDMA/bis-EMA/TEGDMA 40/40/20, thermocycling +NaOH, 9-UDMA/bis-EMA/TEGDMA 40/40/20, 5 d water + NaOH.
Table A1.
Probabilities for post hoc testing for three-point bending flexural strength (FS) of tested matrices. Tested group: 1-UDMA/bis-GMA/TEGDMA 70/10/20, control; 2-UDMA/bis-GMA/TEGDMA 70/10/20, thermocycling +NaOH, 3-UDMA/bis-GMA/TEGDMA 70/10/20, 5 d water + NaOH, 4-UDMA/bis-GMA/TEGDMA 40/40/20, control; 5-UDMA/bis-GMA/TEGDMA 40/40/20, thermocycling +NaOH; 6-UDMA/bis-GMA/TEGDMA 40/40/20, 5d water + NaOH; 7-UDMA/bis-EMA/TEGDMA 40/40/20, control; 8-UDMA/bis-EMA/TEGDMA 40/40/20, thermocycling +NaOH, 9-UDMA/bis-EMA/TEGDMA 40/40/20, 5 d water + NaOH.
| | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|
| 1 | - | 0.000031 | 0.059365 | 0.156527 | 0.000003 | 0.001298 | 0.218781 | 0.000000 | 0.000003 |
| 2 | | - | 0.011409 | 0.000000 | 0.512874 | 0.254477 | 0.001710 | 0.132024 | 0.483872 |
| 3 | | | - | 0.001424 | 0.001831 | 0.147992 | 0.498258 | 0.000119 | 0.001597 |
| 4 | | | | - | 0.000000 | 0.000012 | 0.009712 | 0.000000 | 0.000000 |
| 5 | | | | | - | 0.075780 | 0.000221 | 0.387831 | 0.963301 |
| 6 | | | | | | - | 0.036092 | 0.009712 | 0.068805 |
| 7 | | | | | | | - | 0.000012 | 0.000190 |
| 8 | | | | | | | | - | 0.413359 |
| 9 | | | | | | | | | - |
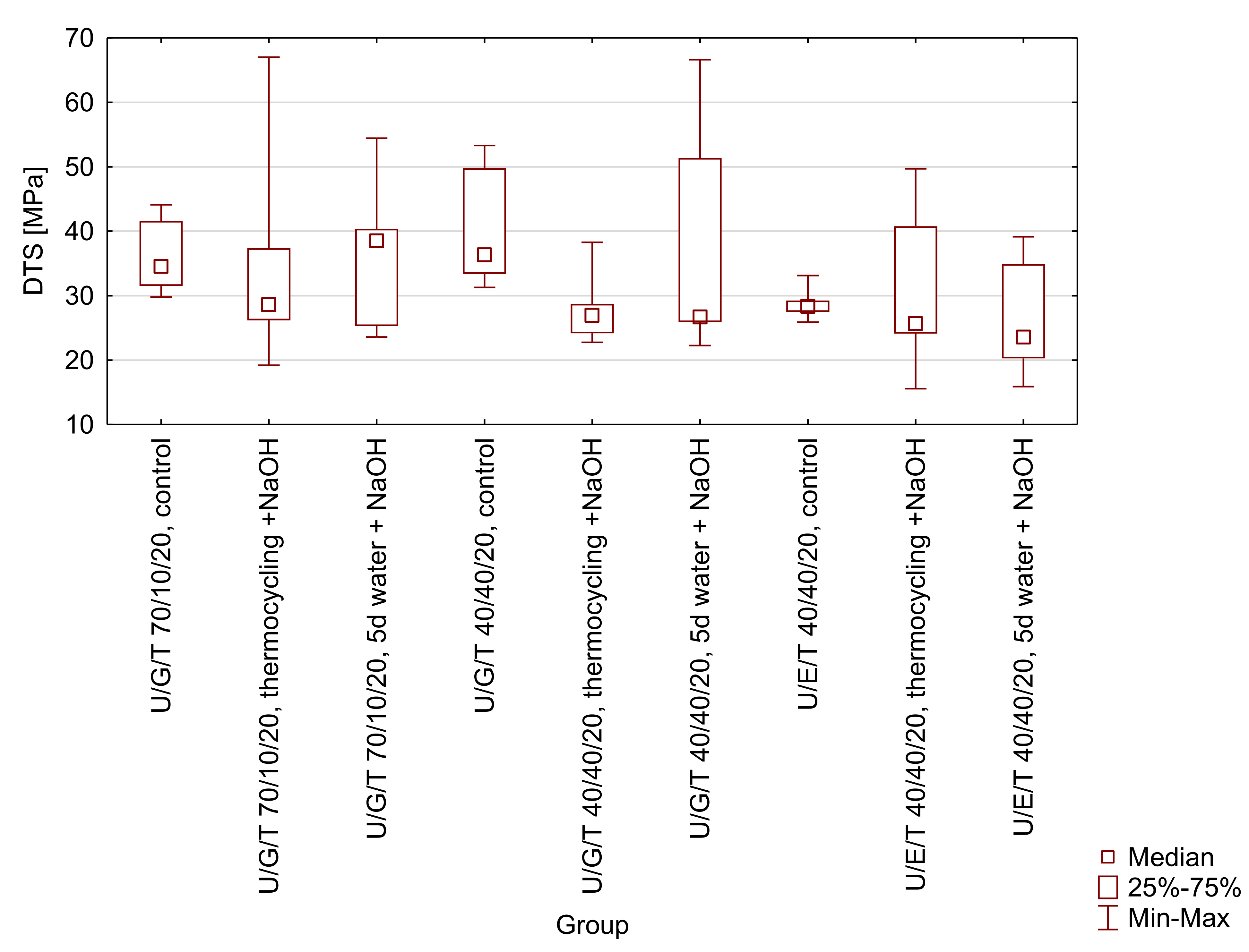
Figure A2.
Box-and-whisker plot of diametral tensile strength (DTS) of tested matrices (UDMA/Bis-GMA/TEGDMA 70/10/20 wt.% and 40/40/20 wt.% and UDMA/Bis-EMA/TEGDMA 40/40/20 wt.%) after selected ageing protocols (control-water, 24 h, 37 °C; thermocycling + NaOH-water, 7500 thermo cycles, 5/55 °C and 0.1 M NaOH, 7 days, 60 °C; 5d water + NaOH-water, 5 days, 55 °C and 0.1 M NaOH, 7 days). No significant differences were observed.
Figure A2.
Box-and-whisker plot of diametral tensile strength (DTS) of tested matrices (UDMA/Bis-GMA/TEGDMA 70/10/20 wt.% and 40/40/20 wt.% and UDMA/Bis-EMA/TEGDMA 40/40/20 wt.%) after selected ageing protocols (control-water, 24 h, 37 °C; thermocycling + NaOH-water, 7500 thermo cycles, 5/55 °C and 0.1 M NaOH, 7 days, 60 °C; 5d water + NaOH-water, 5 days, 55 °C and 0.1 M NaOH, 7 days). No significant differences were observed.
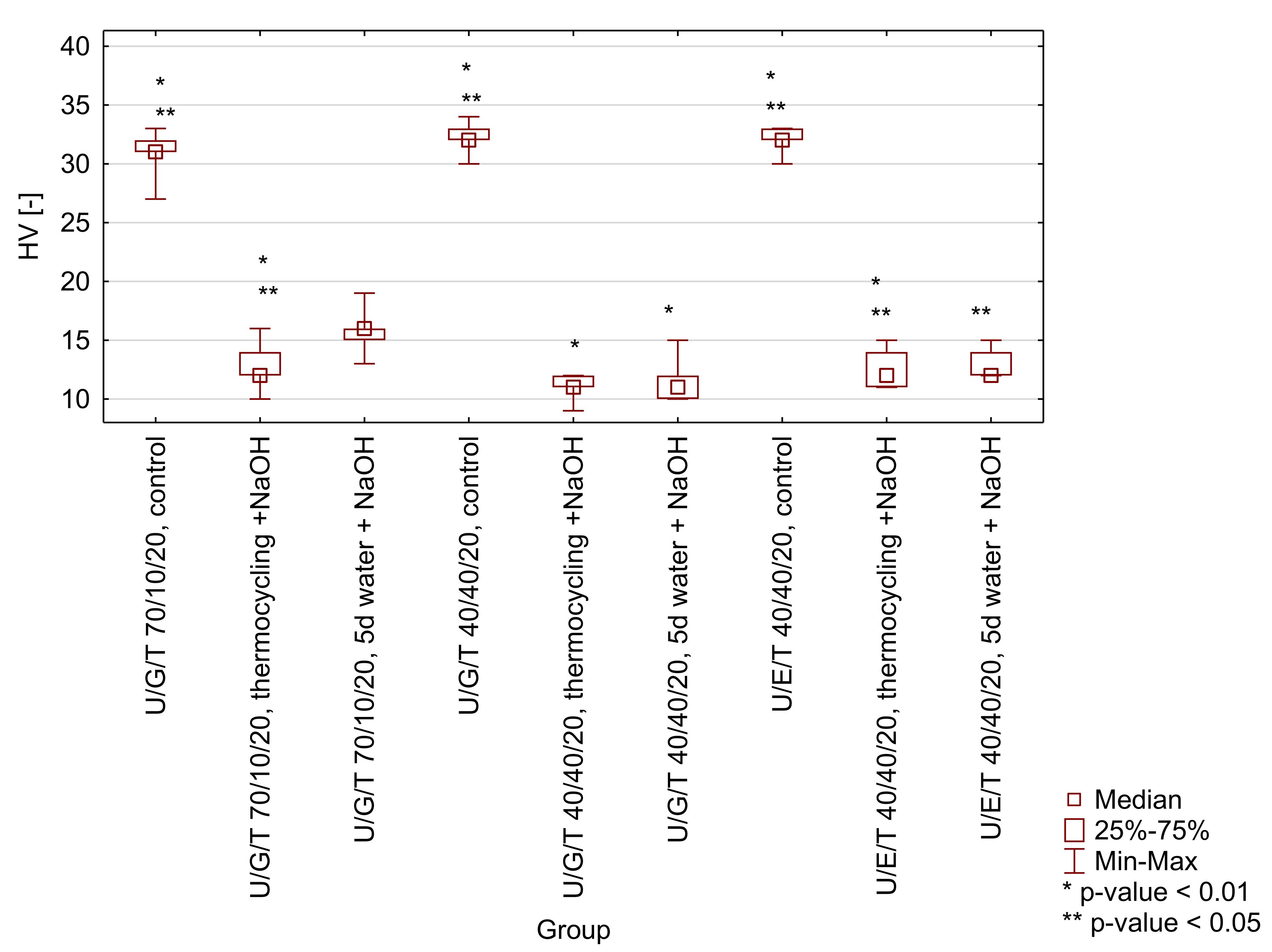
Figure A3.
Box-and-whisker plot of Vickers hardness (HV) of tested matrices (UDMA/Bis-GMA/TEGDMA 70/10/20 wt.% and 40/40/20 wt.% and UDMA/Bis-EMA/TEGDMA 40/40/20 wt.%) after selected ageing protocols (control-water, 24 h, 37 °C; thermocycling + NaOH-water, 7500 thermo cycles, 5/55 °C and 0.1 M NaOH, 7 days, 60 °C; 5 d water + NaOH-water, 5 days, 55 °C and 0.1 M NaOH, 7 days). Significant differences occur in a given group with a probability of p < 0.01 (*) and/or p < 0.05 (**).
Figure A3.
Box-and-whisker plot of Vickers hardness (HV) of tested matrices (UDMA/Bis-GMA/TEGDMA 70/10/20 wt.% and 40/40/20 wt.% and UDMA/Bis-EMA/TEGDMA 40/40/20 wt.%) after selected ageing protocols (control-water, 24 h, 37 °C; thermocycling + NaOH-water, 7500 thermo cycles, 5/55 °C and 0.1 M NaOH, 7 days, 60 °C; 5 d water + NaOH-water, 5 days, 55 °C and 0.1 M NaOH, 7 days). Significant differences occur in a given group with a probability of p < 0.01 (*) and/or p < 0.05 (**).
Table A2.
Probabilities for multiple comparison testing for Vickers hardness (HV) of tested matrices. Tested group: 1-UDMA/bis-GMA/TEGDMA 70/10/20, control; 2-UDMA/bis-GMA/TEGDMA 70/10/20, thermocycling +NaOH, 3-UDMA/bis-GMA/TEGDMA 70/10/20, 5d water + NaOH, 4-UDMA/bis-GMA/TEGDMA 40/40/20, control; 5-UDMA/bis-GMA/TEGDMA 40/40/20, thermocycling +NaOH; 6-UDMA/bis-GMA/TEGDMA 40/40/20, 5d water + NaOH; 7-UDMA/bis-EMA/TEGDMA 40/40/20, control; 8-UDMA/bis-EMA/TEGDMA 40/40/20, thermocycling +NaOH, 9-UDMA/bis-EMA/TEGDMA 40/40/20, 5d water + NaOH.
Table A2.
Probabilities for multiple comparison testing for Vickers hardness (HV) of tested matrices. Tested group: 1-UDMA/bis-GMA/TEGDMA 70/10/20, control; 2-UDMA/bis-GMA/TEGDMA 70/10/20, thermocycling +NaOH, 3-UDMA/bis-GMA/TEGDMA 70/10/20, 5d water + NaOH, 4-UDMA/bis-GMA/TEGDMA 40/40/20, control; 5-UDMA/bis-GMA/TEGDMA 40/40/20, thermocycling +NaOH; 6-UDMA/bis-GMA/TEGDMA 40/40/20, 5d water + NaOH; 7-UDMA/bis-EMA/TEGDMA 40/40/20, control; 8-UDMA/bis-EMA/TEGDMA 40/40/20, thermocycling +NaOH, 9-UDMA/bis-EMA/TEGDMA 40/40/20, 5d water + NaOH.
| | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|
| 1 | - | 0.016656 | 0.037228 | 1.000000 | 0.007118 | 0.677023 | 1.000000 | 0.000265 | 0.178237 |
| 2 | | - | 1.000000 | 0.001060 | 1.000000 | 1.000000 | 0.012059 | 1.000000 | 1.000000 |
| 3 | | | - | 0.002729 | 1.000000 | 1.000000 | 0.027433 | 1.000000 | 1.000000 |
| 4 | | | | - | 0.000393 | 0.089330 | 1.000000 | 0.000009 | 0.017621 |
| 5 | | | | | - | 1.000000 | 0.005063 | 1.000000 | 1.000000 |
| 6 | | | | | | - | 0.536834 | 1.000000 | 1.000000 |
| 7 | | | | | | | - | 0.000177 | 0.136340 |
| 8 | | | | | | | | - | 1.000000 |
| 9 | | | | | | | | | - |
Figure A4.
Box-and-whisker plot of three-point bending flexural strength (FS) of tested composites (UDMA/Bis-GMA/TEGDMA 70/10/20 wt.% and 40/40/20 wt.% and UDMA/Bis-EMA/TEGDMA 40/40/20 wt.%) after selected aging protocols (control-water, 24 h, 37 °C; thermocycling + NaOH-water, 7500 thermo cycles, 5/55 °C and 0.1 M NaOH, 7 days, 60 °C; 5d water + NaOH-water, 5 days, 55 °C and 0.1 M NaOH, 7 days). Significant differences occur in a given group with a probability of p < 0.01 (*) and/or p < 0.05 (**).
Figure A4.
Box-and-whisker plot of three-point bending flexural strength (FS) of tested composites (UDMA/Bis-GMA/TEGDMA 70/10/20 wt.% and 40/40/20 wt.% and UDMA/Bis-EMA/TEGDMA 40/40/20 wt.%) after selected aging protocols (control-water, 24 h, 37 °C; thermocycling + NaOH-water, 7500 thermo cycles, 5/55 °C and 0.1 M NaOH, 7 days, 60 °C; 5d water + NaOH-water, 5 days, 55 °C and 0.1 M NaOH, 7 days). Significant differences occur in a given group with a probability of p < 0.01 (*) and/or p < 0.05 (**).
Table A3.
Probabilities for post hoc testing for three-point bending flexural strength (FS) of tested composites. Tested group: 1-UDMA/bis-GMA/TEGDMA 70/10/20, control; 2-UDMA/bis-GMA/TEGDMA 70/10/20, thermocycling +NaOH, 3-UDMA/bis-GMA/TEGDMA 70/10/20, 5d water + NaOH, 4-UDMA/bis-GMA/TEGDMA 40/40/20, control; 5-UDMA/bis-GMA/TEGDMA 40/40/20, thermocycling +NaOH; 6-UDMA/bis-GMA/TEGDMA 40/40/20, 5d water + NaOH; 7-UDMA/bis-EMA/TEGDMA 40/40/20, control; 8-UDMA/bis-EMA/TEGDMA 40/40/20, thermocycling +NaOH, 9-UDMA/bis-EMA/TEGDMA 40/40/20, 5d water + NaOH.
Table A3.
Probabilities for post hoc testing for three-point bending flexural strength (FS) of tested composites. Tested group: 1-UDMA/bis-GMA/TEGDMA 70/10/20, control; 2-UDMA/bis-GMA/TEGDMA 70/10/20, thermocycling +NaOH, 3-UDMA/bis-GMA/TEGDMA 70/10/20, 5d water + NaOH, 4-UDMA/bis-GMA/TEGDMA 40/40/20, control; 5-UDMA/bis-GMA/TEGDMA 40/40/20, thermocycling +NaOH; 6-UDMA/bis-GMA/TEGDMA 40/40/20, 5d water + NaOH; 7-UDMA/bis-EMA/TEGDMA 40/40/20, control; 8-UDMA/bis-EMA/TEGDMA 40/40/20, thermocycling +NaOH, 9-UDMA/bis-EMA/TEGDMA 40/40/20, 5d water + NaOH.
| | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|
| 1 | - | 0.099530 | 0.261666 | 0.291457 | 0.000151 | 0.000591 | 0.386128 | 0.022297 | 0.175759 |
| 2 | | - | 0.590310 | 0.008281 | 0.019799 | 0.053406 | 0.013654 | 0.501282 | 0.762280 |
| 3 | | | - | 0.032130 | 0.004781 | 0.014857 | 0.049649 | 0.228278 | 0.813093 |
| 4 | | | | - | 0.000004 | 0.000017 | 0.848766 | 0.001205 | 0.018123 |
| 5 | | | | | - | 0.671329 | 0.000008 | 0.090340 | 0.009106 |
| 6 | | | | | | - | 0.000034 | 0.199860 | 0.026653 |
| 7 | | | | | | | - | 0.002130 | 0.028840 |
| 8 | | | | | | | | - | 0.330947 |
| 9 | | | | | | | | | - |
Figure A5.
Box-and-whisker plot of diametral tensile strength (DTS) of tested composites (UDMA/Bis-GMA/TEGDMA 70/10/20 wt.% and 40/40/20 wt.% and UDMA/Bis-EMA/TEGDMA 40/40/20 wt.%) after selected ageing protocols (control-water, 24 h, 37 °C; thermocycling + NaOH-water, 7500 thermo cycles, 5/55 °C and 0.1 M NaOH, 7 days, 60 °C; 5d water + NaOH-water, 5 days, 55 °C and 0.1 M NaOH, 7 days). Significant differences occur in a given group with a probability of p < 0.01 (*) and/or p < 0.05 (**).
Figure A5.
Box-and-whisker plot of diametral tensile strength (DTS) of tested composites (UDMA/Bis-GMA/TEGDMA 70/10/20 wt.% and 40/40/20 wt.% and UDMA/Bis-EMA/TEGDMA 40/40/20 wt.%) after selected ageing protocols (control-water, 24 h, 37 °C; thermocycling + NaOH-water, 7500 thermo cycles, 5/55 °C and 0.1 M NaOH, 7 days, 60 °C; 5d water + NaOH-water, 5 days, 55 °C and 0.1 M NaOH, 7 days). Significant differences occur in a given group with a probability of p < 0.01 (*) and/or p < 0.05 (**).
Table A4.
Probabilities for post hoc testing for diametral tensile strength (DTS) of tested composites. Tested group: 1-UDMA/bis-GMA/TEGDMA 70/10/20, control; 2-UDMA/bis-GMA/TEGDMA 70/10/20, thermocycling +NaOH, 3-UDMA/bis-GMA/TEGDMA 70/10/20, 5d water + NaOH, 4-UDMA/bis-GMA/TEGDMA 40/40/20, control; 5-UDMA/bis-GMA/TEGDMA 40/40/20, thermocycling +NaOH; 6-UDMA/bis-GMA/TEGDMA 40/40/20, 5d water + NaOH; 7-UDMA/bis-EMA/TEGDMA 40/40/20, control; 8-UDMA/bis-EMA/TEGDMA 40/40/20, thermocycling +NaOH, 9-UDMA/bis-EMA/TEGDMA 40/40/20, 5d water + NaOH.
Table A4.
Probabilities for post hoc testing for diametral tensile strength (DTS) of tested composites. Tested group: 1-UDMA/bis-GMA/TEGDMA 70/10/20, control; 2-UDMA/bis-GMA/TEGDMA 70/10/20, thermocycling +NaOH, 3-UDMA/bis-GMA/TEGDMA 70/10/20, 5d water + NaOH, 4-UDMA/bis-GMA/TEGDMA 40/40/20, control; 5-UDMA/bis-GMA/TEGDMA 40/40/20, thermocycling +NaOH; 6-UDMA/bis-GMA/TEGDMA 40/40/20, 5d water + NaOH; 7-UDMA/bis-EMA/TEGDMA 40/40/20, control; 8-UDMA/bis-EMA/TEGDMA 40/40/20, thermocycling +NaOH, 9-UDMA/bis-EMA/TEGDMA 40/40/20, 5d water + NaOH.
| | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|
| 1 | - | 0.347646 | 0.184878 | 0.277043 | 0.000159 | 0.000018 | 0.279639 | 0.013484 | 0.443427 |
| 2 | | - | 0.695213 | 0.881230 | 0.003286 | 0.000494 | 0.885909 | 0.116721 | 0.861824 |
| 3 | | | - | 0.808370 | 0.009948 | 0.001722 | 0.803782 | 0.236240 | 0.571775 |
| 4 | | | | - | 0.005070 | 0.000803 | 0.995269 | 0.154816 | 0.746411 |
| 5 | | | | | - | 0.544665 | 0.004985 | 0.150462 | 0.001949 |
| 6 | | | | | | - | 0.000788 | 0.042813 | 0.000276 |
| 7 | | | | | | | - | 0.153139 | 0.750901 |
| 8 | | | | | | | | - | 0.082238 |
| 9 | | | | | | | | | - |
Figure A6.
Box-and-whisker plot of Vickers hardness (HV) of tested composites (UDMA/Bis-GMA/TEGDMA 70/10/20 wt.% and 40/40/20 wt.% and UDMA/Bis-EMA/TEGDMA 40/40/20 wt.%) after selected ageing protocols (control-water, 24 h, 37 °C; thermocycling + NaOH-water, 7500 thermo cycles, 5/55 °C and 0.1 M NaOH, 7 days, 60 °C; 5d water + NaOH-water, 5 days, 55 °C and 0.1 M NaOH, 7 days). Statistical differences occur in a given group with a probability of p < 0.01 (*) and/or p < 0.05 (**).
Figure A6.
Box-and-whisker plot of Vickers hardness (HV) of tested composites (UDMA/Bis-GMA/TEGDMA 70/10/20 wt.% and 40/40/20 wt.% and UDMA/Bis-EMA/TEGDMA 40/40/20 wt.%) after selected ageing protocols (control-water, 24 h, 37 °C; thermocycling + NaOH-water, 7500 thermo cycles, 5/55 °C and 0.1 M NaOH, 7 days, 60 °C; 5d water + NaOH-water, 5 days, 55 °C and 0.1 M NaOH, 7 days). Statistical differences occur in a given group with a probability of p < 0.01 (*) and/or p < 0.05 (**).
Table A5.
Probabilities for multiple comparison testing for Vickers hardness (HV) of tested composites. Tested group: 1-UDMA/bis-GMA/TEGDMA 70/10/20, control; 2-UDMA/bis-GMA/TEGDMA 70/10/20, thermocycling +NaOH, 3-UDMA/bis-GMA/TEGDMA 70/10/20, 5d water + NaOH, 4-UDMA/bis-GMA/TEGDMA 40/40/20, control; 5-UDMA/bis-GMA/TEGDMA 40/40/20, thermocycling +NaOH; 6-UDMA/bis-GMA/TEGDMA 40/40/20, 5d water + NaOH; 7-UDMA/bis-EMA/TEGDMA 40/40/20, control; 8-UDMA/bis-EMA/TEGDMA 40/40/20, thermocycling +NaOH, 9-UDMA/bis-EMA/TEGDMA 40/40/20, 5d water + NaOH.
Table A5.
Probabilities for multiple comparison testing for Vickers hardness (HV) of tested composites. Tested group: 1-UDMA/bis-GMA/TEGDMA 70/10/20, control; 2-UDMA/bis-GMA/TEGDMA 70/10/20, thermocycling +NaOH, 3-UDMA/bis-GMA/TEGDMA 70/10/20, 5d water + NaOH, 4-UDMA/bis-GMA/TEGDMA 40/40/20, control; 5-UDMA/bis-GMA/TEGDMA 40/40/20, thermocycling +NaOH; 6-UDMA/bis-GMA/TEGDMA 40/40/20, 5d water + NaOH; 7-UDMA/bis-EMA/TEGDMA 40/40/20, control; 8-UDMA/bis-EMA/TEGDMA 40/40/20, thermocycling +NaOH, 9-UDMA/bis-EMA/TEGDMA 40/40/20, 5d water + NaOH.
| | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
| 1 | - | 0.037895 | 1.000000 | 1.000000 | 0.000494 | 0.000664 | 1.000000 | 0.039262 | 0.110577 |
| 2 | | - | 1.000000 | 0.005379 | 1.000000 | 1.000000 | 0.003224 | 1.000000 | 1.000000 |
| 3 | | | - | 1.000000 | 0.140756 | 0.172765 | 1.000000 | 1.000000 | 1.000000 |
| 4 | | | | - | 0.000041 | 0.000057 | 1.000000 | 0.005601 | 0.018292 |
| 5 | | | | | - | 1.000000 | 0.000022 | 1.000000 | 1.000000 |
| 6 | | | | | | - | 0.000031 | 1.000000 | 1.000000 |
| 7 | | | | | | | - | 0.003361 | 0.011382 |
| 8 | | | | | | | | - | 1.000000 |
| 9 | | | | | | | | | - |
Table A6.
Results of dispersion and polar components calculations for UDMA/bis-GMA/TEGDMA 70/10/20, UDMA/bis-GMA/TEGDMA 40/40/20 and UDMA/bis-EMA/TEGDMA 40/40/20.
Table A6.
Results of dispersion and polar components calculations for UDMA/bis-GMA/TEGDMA 70/10/20, UDMA/bis-GMA/TEGDMA 40/40/20 and UDMA/bis-EMA/TEGDMA 40/40/20.
| | Dispersion Component [mJ/m2] | SD | Polar
Component [mJ/m2] | SD |
|---|
| UDMA/bis-GMA/TEGDMA 70/10/20 | 32 | 5 | 10 | 3 |
| UDMA/bis-GMA/TEGDMA 40/40/20 | 35 | 3 | 7 | 2 |
| UDMA/bis-EMA/TEGDMA 40/40/20 | 43 | 2 | 2 | 1 |