Abstract
Carbon nanomaterials (CNMs) have received tremendous interest in the area of nanotechnology due to their unique properties and flexible dimensional structure. CNMs have excellent electrical, thermal, and optical properties that make them promising materials for drug delivery, bioimaging, biosensing, and tissue engineering applications. Currently, there are many types of CNMs, such as quantum dots, nanotubes, nanosheets, and nanoribbons; and there are many others in development that promise exciting applications in the future. The surface functionalization of CNMs modifies their chemical and physical properties, which enhances their drug loading/release capacity, their ability to target drug delivery to specific sites, and their dispersibility and suitability in biological systems. Thus, CNMs have been effectively used in different biomedical systems. This review explores the unique physical, chemical, and biological properties that allow CNMs to improve on the state of the art materials currently used in different biomedical applications. The discussion also embraces the emerging biomedical applications of CNMs, including targeted drug delivery, medical implants, tissue engineering, wound healing, biosensing, bioimaging, vaccination, and photodynamic therapy.
1. Introduction
CNMs are an emerging field of nanomaterials that offer a propitious approach in drug delivery, tissue regeneration, medical implants, and other applications [1]. CNMs are defined as materials with sizes ranging from 1–100 nm. As a whole, nanotechnology has become a promising field, which has revolutionized the cure and diagnosis of diseases; this is due to the development of CNM-based materials with potential applications for disease cure and diagnosis [2]. CNMs possess unique properties that can be tuned through production methods to enhance characteristics such as optical activity, multifunctional surface morphology, surface area, drug loading efficacy, biocompatibility, and immunogenicity. This high degree of control over several key characteristics of the material offers advantages over metal-based biomaterials, such as titanium. A higher degree of freedom when engineering the material allows for more sophisticated applications, such as highly controlled drug release and drug delivery [3]. There are several types of CNMs: fullerene (or Buckyball), nano-diamond, amorphous nanocarbon, graphene nanosheets, graphene oxide (GO), single-walled carbon nanotube (SWCNTs), multi-walled carbon nanotube (MWCNTs), graphene quantum dots (GQDs), and carbon foam [4]. CNM families feature unique characteristics that can be applied to diverse biological applications [5]. They also offer potential anti-cancer and anti-inflammatory properties [6].
Recent studies have indicated that there are various reasons and factors responsible for the toxicity of CNMs. The size and shape, as well as the aspect ratio, of CNMs all affect their toxicity upon cellular uptake, interfering with cellular processes both in the cytoplasm and in the nucleus. Reports also suggest [7] that CNMs are subject to adulteration or contamination by certain substances, such as metal ions, during synthesis; these substances also contribute to the overall cytotoxicity of the final product materials. CNMs can damage lipids and DNA when they are taken up by cancer cells as they stimulate reactive oxygen species (ROS) and cause cell death [8]. Similarly, increasing ROS levels by graphene materials affects the metabolic activity of the macrophages and damages the mitochondrial membrane, which results in apoptosis [9].
The surface functionalization of CNMs has been employed to improve their biodegradability, safety, and aqueous solubility. The functionalization of CNMs improves delivery efficiency through reduced clearance and prolonged retention in the body [7]. However, some research has shown that some methods of surface functionalization, such as cationic or anionic functionalization, may lead to higher toxicity compared to non-functionalized CNMs. Evidence has shown that the functionalization of CNMs creates a more dynamic material through the “tagging” of different drugs, peptides, nucleic acid, amino acids, and proteins on the surface of CNMs, which can enhance their efficiency, and solubility while reducing their toxicity [10,11]. Various interactions, such as covalent, ionic, Van der Waal or π–π, can be used to link different functionalizing agents and/or biologically active molecules to the CNMs [11]. These interactions help to graft the functional groups over the surface of CNMs and allow for strategic placement and functionalization at specific regions of the CNMs’ shape [12]. Because these modified CNMs feature various inherent properties, they are extensively used. Therefore, we conducted a review of CNMs, emphasizing the different types of CNM, their importance in drug delivery, and their role in biomedical applications.
2. Fabrication of CNMs
Carbon is one of the most plentiful elements on the planet. It is found in nature as graphite, diamond, and coal in its basic form. Due to their unique hybridization characteristics and their susceptibility to perturbation during synthesis, nanostructured allotrope forms of carbon have been extensively studied over the last two decades, allowing the precise modification of material properties. There are various hybridization states of carbon (Figure 1). The chemical, mechanical, thermal, and electrical characteristics of various allotropic forms are closely connected to their structure and hybridization state, allowing the same material to be used for a variety of applications.
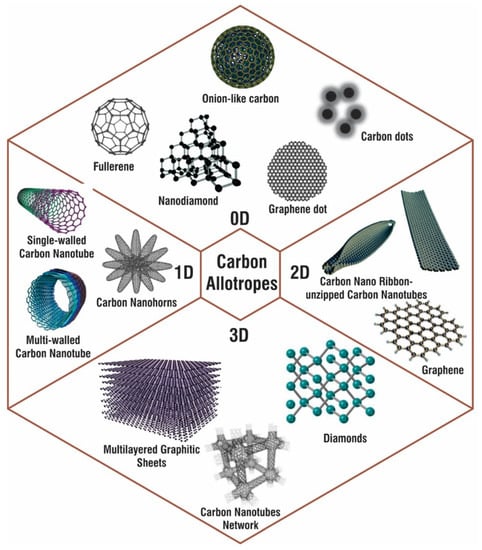
Figure 1.
Various nanoforms of carbon allotropes with examples for 0D, 1D, 2D, and 3D carbon nanostructures.
CNMs can be divided into 0D-CNMs (i.e., fullerenes, particulate diamonds, and carbon dots), 1D-CNMs (i.e., CNTs, Carbon Nanofibers (CNFs), and diamond nanorods), 2D-CNMs (i.e., graphene, graphite sheets, and diamond nanoplatelets), and 3D-CNMs. All decreased dimensionalities, including fullerenes, contain CNMs made completely of sp2-bonded graphitic carbon [12,13]. Figure 1 shows various types of carbon nanoallotropes. Table 1 shows various types of carbon nanoallotropes and their commercial application.

Table 1.
Various forms of carbons and their commercial and laboratory applications [14].
2.1. Fullerenes
Buckminsterfullerene (C60) is the third carbon allotrope, discovered in 1985 by Curl, Kroto, and Smalley. Carbon atoms make up fullerene molecules, which are hollow spheres, ellipsoids, or tubes. The bucky shapes of spherical fullerenes are also known as buckyballs. The enormous curvature of these hollow spheres’ conjugated-electron systems enables rich chemical behavior, permitting the synthesis of a wide range of derivatives, making the fullerene family a useful building block. In medicinal chemistry, it is assumed that three-dimensionality, length, hydrophobicity, and electronic configurations make them an attractive topic. The peculiar configuration of their carbon cage, along with their enormous derivatization spectrum, makes them a possible therapeutic agent. Fullerenes are insoluble in nature; thus, their biological applications have drawn growing interest. The fullerene family, especially C60, offer attractive photographic, electrochemical, and physical properties that can be used in several medical fields [27]. Fullerene derivatives have been characterized as inhibitors of human immunodeficiency virus (HIV) [28], contrast agents for magnetic resonance imaging [29], antioxidants [30], and anti-bacterial agents [31]. They are also useful as targeting vectors to mineralized bone [32] and sensitizers for photodynamic therapy [33]. The vast variety of carbon atoms utilized to make fullerenes, the diversified array of moieties that may be hooked up to the surface of fullerene, and the numerous preparation techniques have resulted in a wide range of fullerene derivatives. Among all the types of fullerenes, C60 is widely used [34]. Fullerenes’ surfaces are extensively modified to make them water-dispersible, allowing them to be utilized in medicinal and dermatological applications. Of the other fullerene derivatives, C60 may be multi-functionalized, forms NP, and serves as a drug absorbent, making it an attractive scaffold for drug administration. Fullerenes can behave as drugs in a variety of functional ways [35]. Fullerenes can produce direct bioactivity, such as antioxidant activity, when surface-functionalized [27]. Both pure and modified fullerenes can penetrate into intracellular space or accumulate at the cell membrane due to their small size (less than 1 nm), posing a threat to cell functions and integrity [36]. In a broad spectrum of applications, pristine fullerenes showed neither acute nor subacute toxicity. However, due to the techniques used for solubilization, the physicochemical characteristics of fullerenes changed, resulting in ROS-related behavior and fullerene toxicity [37]. Figure 2 shows the common approaches, such as Bingel–Hirsch reactions for cyclopropanation, polyhydroxylated for the synthesis of the hydroxyl group with fullerenes, and Prato reactions for the cycloaddition of azomethine ylides to synthesize water-soluble fullerenes. These approaches can increase the solubility of fullerenes to 100 mg/mL [35]. Fullerene derivatives are also helpful in the sustained release of drugs. Fullerene derivatives covalently attached to drugs via a linker were tested for sustained release in lung cancer and were found to be more effective than the naive drug [35].
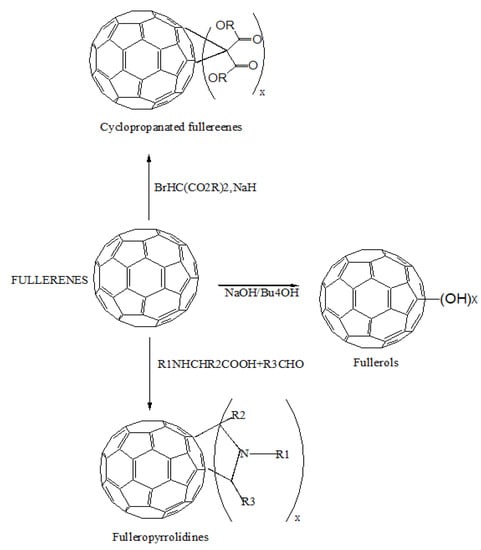
Figure 2.
The functionalization of fullerenes and their conjugation with a drug using a linker.
2.2. Nanodiamonds
Nanodiamonds (NDs) are carbon nanoparticles that are typically around 2 to 8 nm in diameter, with a truncated octahedral architecture. Generally, the surface of nanodiamonds is coated with a layer of functional groups that stabilizes the particle by reducing dangling bonds. The conversion of sp3 hybridized carbon with sp2 also results in the increasing stability of the particles. Chains and graphitic patches are formed by the carbons in sp2. Oxygen-containing groups end the majority of surface atoms. Some nanodiamonds are faceted, whereas most have a rounded shape [38]. Synthetic diamonds and nanodiamonds are relatively affordable, despite their extremely high cost. Selective uses of nanodiamonds, on the other hand, may necessitate specific physicochemical characteristics, which may necessitate additional surface modifications with various functional groups. The continued study and recognition of the methods of disaggregation, physicochemical characteristics, surface modification, biocompatibility, fluorescence, and optical dispersion of NDs has developed broad perspectives for these biomedical applications [39]. Through their various properties, such as the ability to load high amounts of drugs and the ability to penetrate cellular membranes, NDs have shown tremendous potential to emerge as a medium for transporting drugs into biological systems. The innovative properties of this biomaterial have revolutionized various drug delivery systems and supported gene delivery vehicles in the identification of the biomolecular targets of drugs [40]. Investigation of the biocompatibility, biodistribution, and biological destiny of NDs and their conjugates is required for a therapeutically relevant strategy [41]. Figure 3 displays different methods used in the functionalization of NDs. These methods help to improve the physical properties of the materials and also lead to the acquisition of various other properties, such as targeted delivery, sustained release, and pH-mediated drug delivery. NDs have been widely used for bioimaging because of their excellent optical properties and their various sensing and therapeutic components.
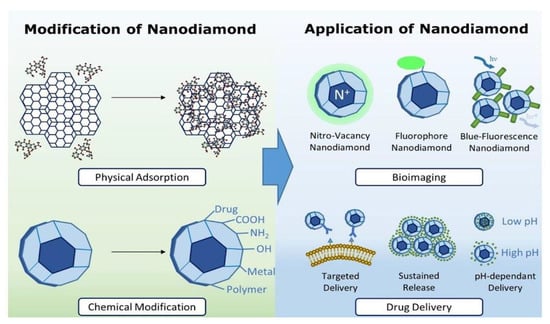
Figure 3.
Schematic representation showing the use of combinatorial nanodiamonds in pharmaceutical and biomedical applications [42]. Copyright 2016, Elsevier.
2.3. Carbon Quantum Dots
Carbon quantum dots (CQDs), which include GQDs and CQDs, are a kind of carbon nanoparticle with a size of less than 10 nanometers. CQDs have lately been the focus of research because of their fluorescent, nontoxic, and water-dispersible properties. At high temperature and pressure, CQDs can be made from graphene, cellulose, or other materials. Due to their unique properties, CQDs have received much attention. CQDs are fluorescent carbon nanostructures with two origins of the fluorescent properties, the emission of fluorescence from conjugated π-domain bandgap phases and fluorescence from surface defects. Various elements, such as nitrogen, sulphur, and phosphorus have been used in doping CQDs to improve their properties. They also help in Reactive Oxygen Species (ROS), through the hydroxyl free radical scavenging properties of doped ions [43], and photoluminescence, by increasing their fluorescence brightness and shifting their emission spectra [44,45,46]. From biological imaging to electrical and photonic devices, CQDs bring up new possibilities. Carbon QDs are notable for being constructed out of a plentiful and largely harmless element, which can aid in the ecologically responsible development of solar technology [47]. In the near-infrared (NIR) spectral area, CQDs can emit fluorescence, making them ideal for biomedical applications. CQDs have shown benefits in various sectors, such as biomedicine, solar energy conversion, photocatalysis, light-emitting diodes (LEDs), photosensors, etc. CQDs offer therapeutic uses, including bioimaging, the distribution of drugs, the delivery of DNA, and cancer treatment [48,49]. Figure 4 demonstrates the entry of peptide targeted drug-loaded graphene quantum dots into the cancer cells. These peptides bind with integrins, which are overexpressed in cancer cells. The interior of these cells can be analyzed using fluorescence; it helps in investigating the drug’s release pattern. However, while CQDs do represent an exciting possibility in the drug delivery area, there are still some drawbacks to the technology. These are that the CQDs must be modified for targeted drug delivery and therefore are only as effective as the targeting pathway, the research is quite young, and, although the evidence indicates that CQDs have a low level of toxicity, they are not completely non-toxic. The emerging field does mean that it will take some time to fully characterize and develop an understanding of the long-term effects of CQDs in an in vivo system [50].
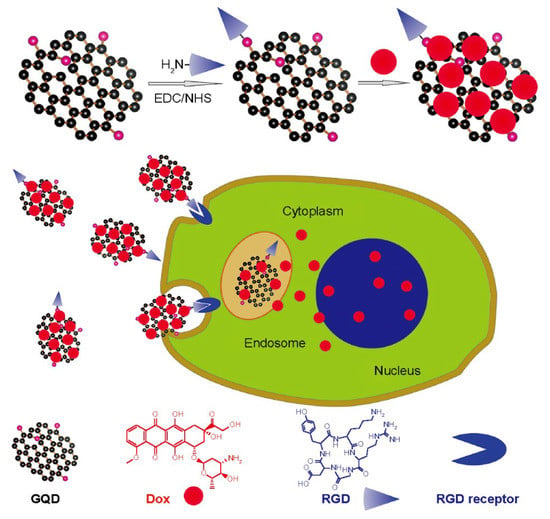
Figure 4.
Schematic presentation of cancer cells being treated by multifunctional GDQs [51]. Abbreviations: eDc/Nhs, 1-(3-(dimethylamino) propyl)-3-ethyl carbodiimide and N-hydroxysuccinimide; GQD graphene quantum dot, Dox, doxorubicin; RGD, arginine glycine-aspartic acid. Copyright Dove Press, open access to scientific and medical research.
2.4. Carbon Nanotubes
In 1993, Iijima and Ichihashi discovered nanometric range carbon, referred to as carbon nanotubes (CNTs) [52,53]. CNTs, commonly known as buckytube, are carbon-based nanoscale hollow tubes. The aspect ratios (length-to-diameter values) of these cylindrical carbon molecules are generally more than 103. These nanotubes are classified as single-walled carbon nanotubes or multiple concentric cylinders, depending on whether they are made up of one tubular nanostructure or several concentric cylinders [54]. Various methods, such as laser ablation, high-pressure carbon disproportion, chemical vapor deposition (CVD), etc., have been used for the formation of these nanocarriers [55]. The CVD method has been the most widely used method due to its high yield capacity. The carbon nanotube obtained using this method has a high degree of length and its morphological characteristics are more enhanced [56]. There are three forms of carbon nanotubes: single-, double-, and multi- walled carbon nanotubes. Single-walled carbon nanotubes (SWCNTs) and double-walled carbon nanotubes (DWCNTs) are made up of one or two (concentric) graphene cylinders, but multi-walled carbon nanotubes (MWCNTs) are made up of many concentric cylindrical shells of graphene sheets. CNTs have poor water solubility; thus, to enhance the solubility rate of CNTS, functionalization approaches are being developed. To functionalize CNTs for increasing their solubility in water, CNTs are covalently or non-covalently functionalized. Bensghaïer et al. used various dyes, such as Azure A (AA-N2+), for the surface modification of MWCNTs; the formation of hybrids was useful in different applications, such as biosensors and optically pH-responsive materials [57]. In another research study, biocomposite particles were prepared using lipase and MWNTs to enhance their solubility in solvents: lipase from Candida rugosa was covalently anchored onto acid-treated MWNTs through a self-catalytic mechanism [58]. Furthermore, a quantitative assessment of the degree of functionalization is more reliable in general. The benefits of the covalent method, however, are counterbalanced by the adverse disruption of the CNT’s conjugated backbone, which can significantly impair electrical and optical characteristics and, in many cases, limit their performance for certain applications that need such qualities. A quantitative analysis of the degree of functionalization is in general more reliably assessed. Many experiments on the use of surface-functionalized CNTs in the biomedical field have been performed during the past decade [54]. As shown in Figure 5, various medical applications are used in the biological process of carbon nanotubes, such as drug delivery, biosensing, photodynamic therapies, drug discovery, etc. [59,60,61].
Figure 5.
Schematic presentation showing carbon nanotubes alongside their precursor, graphene, and their uses in the biomedical field [63]. Copyright 2018, Elsevier.
There is a high degree of interest in the use of carbon nanomaterials in pharmaceuticals. This is due to their highly tunable characteristics and certain desirable traits that they contain inherently. CNTs have very good surface areas and, therefore, an extremely good drug loading capacity, which is naturally a very desirable trait for drug delivery. Along with their nanometric scale and the ability to functionalize them in a variety of ways, their drug delivery capabilities are a growing area of interest. Of course, there are some inherent flaws in current CNTs that hinder their use in pharmaceuticals and will need to be overcome. This is primarily that they do not show good biodegradability and, by extension, may be toxic with long-term or prolonged use [62].
2.5. Carbon Nanofibers
CNFs are members of the covalent CNM family and feature conductivity and stability similar to CNTs. The stacking of graphene sheets of various forms distinguishes CNFs from CNTs, resulting in more edge locations on the outside walls of CNFs than those of CNTs. This may make it easier for an electroactive analyte to transfer electrons [64]. CNFs are ideal candidates for next-generation on-chip connection materials, as well as potential immobilization substrates, due to their unique chemical and physical characteristics [65]. Furthermore, when compared to CNTs, CNFs are less costly; thus, they are easily used in the textile industry [66]. CNF is favored for electrical and thermal conductivity because it has a higher degree of crystallographic alignment. It not only has the same low density, high modulus, high strength, high conductivity, and thermal stability as carbon fiber manufactured using a chemical vapor deposition growth process, but it also offers benefits such as a low defect rate, a large aspect ratio, and a huge surface area [67].
CNFs are synthesized via chemical vapor deposition, phase separation electrospinning, and templating [20]. Among these, electrospinning has steadily gained popularity as a low-cost and simple method of manufacturing nanofibers [68]. CNFs can be modified for biomedical applications with many different bioactive molecules [69]. Figure 6 demonstrates a nanoelectrode array-based electrochemical technique for detecting protease activity. With a ferrocene (Fc) moiety connected at the starting end, vertically aligned carbon nanofibers were covalently bonded with legumain and cathepsin B. Fc measured the signals and identified different distinct properties from carbon electrodes.
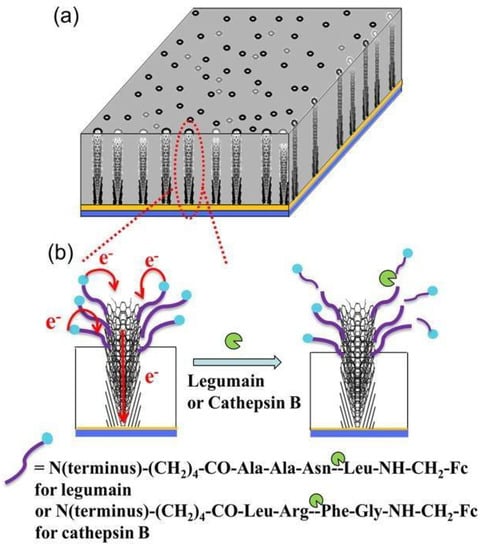
Figure 6.
Biosensors formed using carbon nanofibers showing distinct properties. (a); represent CNFs vertically aligned in SiO2 matrix. (b); shows electrons transfer and loss of electrochemical signal from ferrocene attached at the distal end of peptide. This loss of signal is due to the cleavage of peptide at a specific site [70]. Copyright 2013, American Chemical Society.
The carbon backbone and synthesis methods of CNFs allow the application of varied functionalization methods. These methods allow the material to be used for a diverse range of applications. The nanofibers can be modified and functionalized via three primary methods: firstly, through direct incorporation during the electrospinning fabrication with a variety of compounds, such as metal ions, other biomaterials, or active biomolecules and drugs; secondly, the adsorption of relevant molecules to the surface of the CNF’s fibers post-fabrication; and, finally, a combination of both techniques, in which modifications during the electrospinning fabrication are then used as covalent anchor points for surface modifications using relevant chemical pathways.
2.6. Graphene Nanosheets
Graphene is an allotrope of carbon, made up of a single sheet of atoms organized in a honeycomb lattice in two dimensions. The name is a combination of "graphite" with the suffix -ene, and it refers to the graphite allotrope of carbon, which is made up of stacked graphene layers. Graphene is a two-dimensional sheet of sp2-hybridized carbon atoms organized in a hexagonal lattice [71]. Graphene has gained traction among both the science and technical communities due to its mechanical, electrical, and thermal properties [72]. Its remarkable characteristics, on the other hand, can only be demonstrated experimentally in samples with a high degree of lattice perfection. Structural flaws are inadvertently introduced into the lattice during growth or by physicochemical treatment, which can dramatically impair the graphene’s characteristics and, as a result, the performance of graphene-based devices [73]. Melt intercalation, in situ polymerization, and solution mixing are the most common techniques for incorporating graphene into polymer matrices [74]. Recently, there has been significant progress in the creation of graphene derivatives, such as graphene oxide, porous graphene/graphene oxide, reduced graphene oxide, and GQDs. Another important kind of graphene is the zig-zag pattern found in the structure of graphene commonly called nanoribbon [75]. Various other new forms of graphene have also come into the limelight, such as aerogel graphene, gyroid graphene, etc. [76]. Figure 7 demonstrates the creation of 3D-printable graphene by combining graphene with polylactide-co-glycolide (3DG). Human mesenchymal stem cell (hMSC) adhesion, survival, multiplicity, and neurogenic differentiation have all been aided by 3D printed carbon materials, with the notable overexpression of glial and neuronal genes. Another growing area of research for graphene nanosheets and their derivatives is as a drug release mechanism. Although this application is still in its infancy, there have been initial experiments performed targeting cancerous cells and the controlled release of tegafur [77]. Other applications being investigated are the use of graphene nanosheets and their derivatives as additives in hydrogels to improve their physical characteristics, such as rigidity and conductivity, and their suitability for biological systems, i.e., cell adhesion and proliferation [16]. The current difficulty with graphene and its derivatives is the assurance that during fabrication, single-layered carbon sheets are formed and not sheets of multi-layered graphene. This is essential as the structure and symmetry of the bonds are the key characteristics of the sheet.
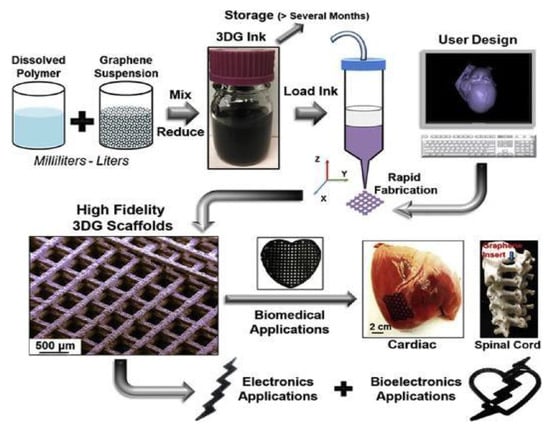
Figure 7.
Production of a 3D printable graphene composite scaffold for biomedical application [78]. Copyright 2015, American Chemical Society.
3. Drug Delivery Systems
Nanomaterials have gained enormous recognition due to their inherent capacity for drug delivery. Many research groups have studied nanomaterials (polymeric nanomaterials, CNMs, inorganic nanomaterials, and nanohybrids) for drug delivery through the loading and controlled release of medicinal products. The use of CNMs in drug carriers minimizes side-effects and reduces both the overall dosage and the dosage frequency. The ability to modulate the size, morphology, and surface functionalization of CNMs make them a useful delivery vehicle for encapsulating and transporting the drug entity to various parts of the body [79,80]. CNMs such as C60, GQDs and NDs feature distinct chemical and physical properties or biological effects compared to larger-scale counterparts (graphite), which can be beneficial for drug delivery systems. Some important advantages of CNMs are their small particle size and high surface-area-to-volume ratio, as well as their capacity to bind with drugs/biomolecules to enhance uptake across the plasma membrane. C60 and the delivery system based on it are considered very encouraging for antiviral drugs that play a pivotal function in the HIV treatment. The fullerene-based derivatives also prevent HIV protease as a result of constructing a complex, and it was shown that dendrofullerene has the highest anti-protease activity [81]. There are reports that DOX conjugated with C60 demonstrates controlled release and positive antitumor activities. Other derivatives, such as fullerenols, have high solubility in polar solvents, which results in greater carrying capacity for several drugs. Thus, they are potentially useful for delivering and improving the efficacy of anticancer drugs [82,83]. Figure 8 displays trends in drug delivery systems using different nanomaterials with their various parameters, such as morphology (spherical, cuboidal, triangular etc.), size (1 nm to 100 nm), surface area, and surface modification through the addition of functional groups (–SH, –COOH, –NH2) or the targeting of ligands (as antibodies, peptide, aptamer, RNA) as drug carriers.
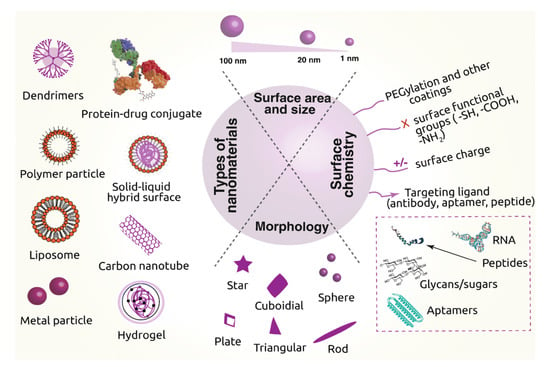
Figure 8.
Current trends in drug delivery systems employing nanomaterials as drug carriers [84]. Copyright Springer Nature, 2019. Open access.
Fine-tuning CNMs characteristics for optimal drug delivery requires the consideration of the following characteristics. Nanoparticles’ surface-area-to-volume ratio could be changed to allow more drug molecules to bind [85]. A further key design consideration is the surface functionalization of CNMs, which is routinely performed through bioconjugation or the adsorption of compounds onto the CNM’s surface. Surface-functionalized CNMs with appropriate ligands can improve drug binding, suppress the immune response, or enable the targeting/controlled release of desired molecules. By taking advantage of these features, it is possible to attain increased efficacy and reduced toxicity. More drugs are supplied to the target site, increasing efficacy and limiting the amount of the drug in the body, which reduces hazardous side effects [86]. A surface-modified and DOX-loaded CNT-based novel drug delivery system can deliver an anti-cancer drug to the tumor site with limited interaction with other systems and organs.
Until now, countless methods have been developed by many researchers to carry smaller compounds, such as chemotherapeutic anti-cancer drugs, on CNMs, either by using covalent conjugation or non-covalent adsorption. The design of CNM-based drug vehicles has also been led by theoretical modelling. Drugs are covalently conjugated with a nanocarrier and attached to functional groups on the CNM’s surface or coated with polymers on the CNM via cleavable bonds. Sahoo NG et al. [87] used folic acid (FA) to modify the surface of CNTs, which resulted in the enhanced suppression of tumor growth and minimized the side effects caused due to the DOX in the solution. The developed formulation with a modified surface of CNTs and with a high DOX loading capacity boosted anti-tumor efficacy in the in vitro study. This study offered better hope for the development of a promising therapy with lower systemic toxicity that might be utilized for tumor treatments in the future. Similarly, CNMs in conjugation with other anticancer drugs, such as docetaxel [88], tamoxifen [89], and oxaliplatin [90] have also shown encouraging results for the treatment of cancer in vitro, as well as in the in vivo study. Table 2 emphasizes different types of drug-loaded CNMs studied for the assessment of efficacy in vitro and in vivo against several diseases. Pei X et al. [91] demonstrated that anticancer drugs conjugated with PEGylated graphene oxide (GO) can be delivered to cancer cells in the cell culture. This study suggested that a novel delivery system could be used for the delivery of different aromatic, low-solubility drugs, which could support the development of better and more efficient treatments for cancer. In another in vitro study, PEGylated nano-GO loaded graphene oxide with DOX was formulated and showed it can be used as chemotherapy and photothermal therapy (PTT) for the treatment of cancer. In the safety assessment study, this delivery system did not show any cytotoxicity to the murine mammary tumor cell line EMT6 [92,93]. Figure 9 demonstrates several perspectives employing CNT-based drug delivery carrier systems. These approaches include the binding of the ligand at its particular site (such as the antibody, peptide etc) in disease, single-stranded biomolecules (such as siRNA, miRNA) or the coating of biocompatible polymers to provide intimacy in the living system (e.g., Polyethylene Glycol (PEG)), some drugs are chemically attached to the nanocarrier’s surface or PEG (e.g., chemotherapy drugs) and plasmid encapsulated by CNT that could be helpful in detection and imaging [94].

Table 2.
Some drug delivery systems based on CNMs with efficacy studies, with specific drugs and target diseases.
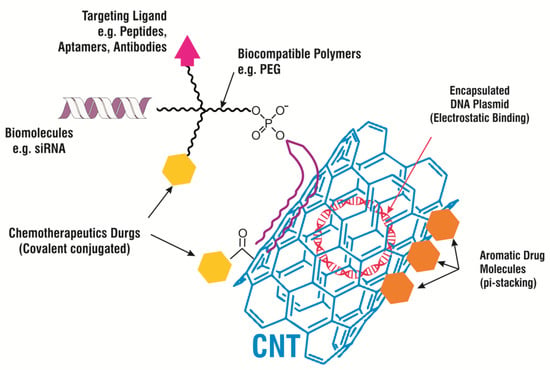
Figure 9.
A schematic diagram presenting several strategies for CNT-based drug delivery and cancer therapies.
4. Biomedical Scaffolds
Biomedical scaffolds are engineered materials that are put inside or on the surface of the body. These materials are designed specifically for interacting with the cellular environment, resulting in the formation of new tissues that are beneficial for medical purposes. Cells seeded in these scaffolds support the formation of three-dimensional structural tissues. Many scaffolds or implants deliver medication, monitor body functions, and provide support to organs and tissues. Medical scaffolds are synthetic and can be used by the human body to mimic the normal function of the missing or damaged biological structure [102]. These devices are made up of chemically inert, very strong, fatigue-resistant, cheap, and corrosion-resistant metals and alloys [103]. Metals and their alloys have been used predominantly as structural biomaterials for different applications in reconstructive surgery, mainly orthopedics, and in blood vessels [104].
CNMs in specific 1D and 2D CNMs have been used for improving the strength of orthopedic implants. CNTs and graphene nanosheets have been added to bone cement composites due to their enhanced fatigue performance, which can lead to improved longevity of the implant. The SWCNTs and MWCNTs that are used as fillers on the polymethyl methacrylate (PMMA) mixed resin matrix have been found to offer more advantageous physical characteristics, which allow growth and support the adhesion of newly formed tissue throughout the implant [105]. Krul et al., in 2007, demonstrated that nanocomposite implants made up of poly-D, L-lactide, and MWCNTs dispersed slowly in comparison to those composed of a single polymer without additives [106]. Yu Bai et al., in 2016, prepared and characterized a composite implant with reduced GO for medical applications. It was found that GO-based fluorhydroxyapatite implants restrict the adhesion and multiplication of Streptococcus mutans. The results show that the composite implant would be invaluable as dental implant material, and for other bacteria-free implants [107].
A novel biocompatible nanocrystalline diamond (NCD) coating technology was used in the generation of CNMs. NCD coating provides unique mechanical, electrical, chemical, tribological, and biocompatibility features, allowing them to be used to fabricate a new range of biomedical scaffolds and tools with higher performance as compared to the existing alternative, as well as materials that have recently been used in commercial devices and implants but whose performances are constrained [108].
The electrical and physical properties of CNMs make them a promising material for implantation where both mechanical and electrical stimulation is required. Platforms designed around these properties can benefit both tissue engineering and transition from in vitro to in vivo applications for implantable applications. Table 3 represents various types of medical implants based on CNMs. In particular, the focus has been on neuronal and peripheral nerve cell applications, as natural healing pathways in this area are limited and the damage is often permanent. Ongoing research aims to provide a means for repair or even circumvention of this damage. Work conducted by Ghosh et al. shows that electrospun nanofiber scaffolds containing MWCNTs demonstrated excellent regeneration properties for peripheral nerve cells in rats [109]. The nerve cells regenerated directionally, an important characteristic for functional nerve regeneration.

Table 3.
Different medical implants based on CNMs and their biomedical applications.
Similarly, CNMs can be used for neuronal applications and deep brain stimulation. Deep brain stimulation is a form of electrotherapeutics, in which the brain is stimulated via implanted electrodes to combat conditions such as epilepsy [110]. Conductive applications are required not only for the stimulation of the neuronal cells but also for monitoring the electrical signals of the brain and to anticipate electrical misfiring in the brain to induce the corrective stimulation pulse required. The conductivity and nanoscale of CNMs are ideal for this and development is well underway to take advantage of them for these purposes. Groups such as Alvarez et al. have been demonstrating the remarkable application of these electrodes in a variety of species, with exciting results [111].
5. Tissue Engineering
Tissue engineering is a multidimensional science that utilizes the application of biological sciences, medicine, and engineering to regenerate or mimic tissue material for organ transplantation, therapeutic and diagnostic purposes [117,118]. Biomaterials play an essential role in tissue engineering because they can stimulate certain biological processes, modify cell differentiation, and influence cell–cell interactions [119,120]. Hybrid or composite materials are used to adequately mimic the physical and biological characteristics of the original specialized cells, as a single material may not fulfill all the requirements to fabricate artificial tissues [121]. CNTs are inert and non-biodegradable, which makes them suitable for scaffold production for tissue regeneration, as well as favorable surfaces to promote the transmission of neural signals [122]. Mazzatenta et al. found that SWCNT with the hippocampal cells might be directly involved in stimulating the activity of the brain circuit, considering it is a favorable material for tissue engineering. Non-functionalized aligned MWCNTs were found to promote the growth and multiplication of pancreatic cancer cells, demonstrating a new way to analyze cancer in vitro [123]. Abarrategi et al. reported the development of CNT/chitosan meshes that supported cell recolonization and also noticed their break up in vivo through proper dispersion in the newly raised tissue [124]. CNTs resemble certain biological structures; therefore, they are used as mimicking agents. The morphological resemblance of CNTs to fibrillar/extracellular matrix protein and their capacity to promote cell adhesion makes them suitable for use as synthetic substrates for artificial bone formation.
Graphene was demonstrated to have a unique ability to adsorb nucleobases via π–π interaction and also to effectively protect nucleotides from enzymatic degradation [125]. Graphene nanosheets could be used as a suitable vector due to their easy uptake by cells. Chen et al. developed a poly (ethylenimine)-GO (PEI-GO) as a transfecting agent to deliver plasmids into HeLa cells and found that the PEI-GO enhanced the transfection efficiency by due to a proton-sponge effect [126]. In the application of tissue engineering, graphene and its derivatives could be bound with other biomaterials to improve their mechanical, physical, and electrical characteristics. The modification of the surface attributes of graphene with a coating of SiO2 enhances the proliferation and stretching of human mesenchymal stem cells [127]. Artificial fibrous structures that permit a greater transportation of nutrients are broadly employed in biomedical applications. Graphene-based fibers report higher conductivity as compared to composite fibers [78]. A biocompatible GO scaffold increased cell proliferation and HFB4 cell attachments to a fibrous composition. It also helped in enhancing mechanical properties and allowed manipulation at the nanoscale level in fibrous scaffolds for biomedical applications [128]. Table 4 shows use of CNMs in tissue engineering application.

Table 4.
Different uses of CNMs in tissue engineering applications.
Nanohydroxyapatite/graphene oxide (nHA/GO) composites, which increase cell survival, were successfully modified by polyethylene glycol, polyvinylpyrrolidone, and chitosan [129]. A nHA/GO composite modified with synthetic polymers improved the growth, viability, and proliferation of MG-63 cells for more than 14 days. The modified nHA/GO composites, which are limited when applied in traditionally-used composites of natural polymers, showed enhanced bioactivity [130].
6. Wound Healing
Wound healing is a distinct physiological process through which injured tissue is repaired within a short time. Uncoordinated wound healing is generally associated with many health problems, such as diabetes, extensive burns, and chronic wounds [137]. In wound healing, there are four coordinated and overlapping phases [138]. These phases must proceed in the correct sequence for proper healing [139]. Figure 10 indicates 4 evolutionarily conserved phases of wound healing in skin executed in a coordinated manner: hemostasis, inflammation, proliferation, and tissue remodeling. Multiple cell types (fibroblast, keratinocyte, endothelial cells etc.) and molecular events are involved in highly regulated ways by various growth factors, cytokines and chemokines [140].
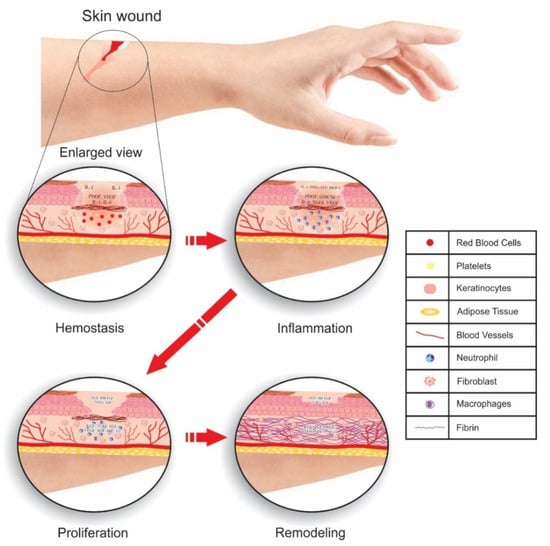
Figure 10.
Distinct phases of wound healing in skin illustrate cells and molecules in the tightly controlled process of recovering a healthy barrier.
Researchers have used graphene with ultrafine silver nanoparticles to demonstrate the antimicrobial and burn wound healing potential of the material. Tuning the formulations of the materials generated a large number of oxidative radicals that lead to the development bactericidal properties. Histopathological studies revealed that graphene-based nanomaterials can enhance the regeneration of the epidermis, thus demonstrating their promising application to burn and wound healing [141].
Silver nanoparticle (AgNPs)-guided single-stranded DNA (ssDNA) attached to graphene oxide (ssDNA-AgNPs-GO) exhibit good bactericidal activity as well as wound healing properties. This antibacterial activity has been observed through synergistic antimicrobial properties used against Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, and Bacillus subtilis at very low minimum inhibitory concentrations (MIC). Due to its improved antibacterial and wound healing properties, ssDNA-AgNPs@GO offers broad applications against bacterial infections caused at the sites of damaged tissues [142].
Fullerenes and CNTs accelerate wound healing by reversing the inflammatory and proliferative phases. Fullerenes, which are stronger antioxidants than CNTs, target and inhibit ROS and reactive nitrogen species generated at the site of damaged tissue [104]. Researchers have demonstrated that fullerene imitated accelerated wound healing in a modified scratch assay and an ex vivo human skin assay. CNMs have been used to promote cell migration, induce wound closure in human skin explants, and increase the speed of wound healing. Consequently, fullerene derivatives are promising wound healing agents that may assist the development of better treatment designs [143]. Table 5 represent different types of CNMs in wound healing application.

Table 5.
Different uses of CNMs in wound healing applications.
7. Biosensors
Biosensors are sensors whose analyte or recognition elements are of a biological nature. Thus, they can be used to track changes in biorecognition events in a diseased or abnormal condition [150]. In general, a biosensor is an element comprising a transducer producing a thermal, electrical, or optical output signal. In electrochemical biosensors, carbon materials have been used for decades [151,152]. Figure 11 shows different types of CNMs, of which two types of methods, electrochemical and optical, are most commonly used for biosensing platforms. In the electrochemical sensing platform, CNMs improved analytical performance due to the increase in electrochemically active surface area. In optical sensing platforms, CNMs are employed as fluorescence emitters and fluorescence quenchers due to changes in fluorescence intensity. CNMs offer a diverse set of uses in the clinical, agricultural, and food industries because of their simpler, portable, and less expensive disposable biosensors [153].
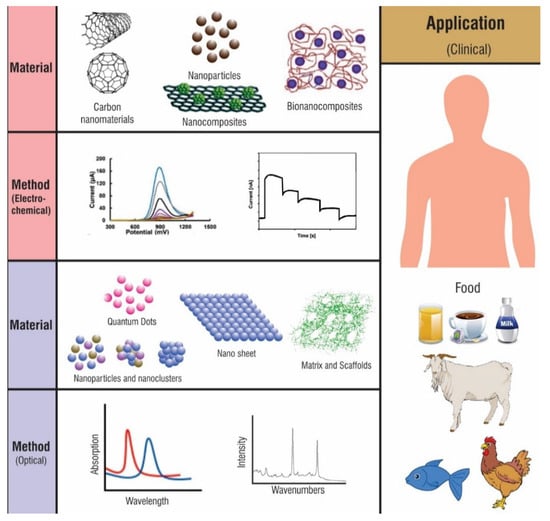
Figure 11.
Schematic representation of the possible application of CNMs implemented in biosensing applications.
A broad range of ions and compounds, such as glucose, fluoride ion, hydrogen peroxide, and other organic vapors, have been detected using functionalized fullerenes and modified electrodes carrying fullerenes [154]. To be used as a sensor, Carbon Nanodiamond (CND) needs surface functionalization to enhance its solubility and facilitate specific binding to the analytes of interest. CNDs can form emissive nitrogen-vacancy (N-V) defects where the fluorescence depends on the N-V center’s electronic spin state showing unique properties of CNDs [155,156]. In recent years, fluorescent CDs have gained a foothold as fluorescent probes for the identification of several cations, such as Cu2+, Zn2+, Al3+, Ag+, K+, Be2+, Hg2+, and Fe3+, as well as, numbers of anions, including iodides, hypochlorous acid (HClO), nitrite, oxalate, and superoxide. A number of drugs and small molecules, including tetracyclines, melamine, hydrogen peroxide, 2,4-dinitrophenol, picric acid, amoxicillin, and pentachlorophenol, have been reported by Carbon dot systems [157].
A large number of possible uses for biosensors have been exploited to develop the special physical and chemical characteristics of graphene and CNTs. Graphene and CNTs can be altered with a biological sensing component in these biosensors, such as nucleic acids, and can also be changed with suitable groups capable of biomolecular detection (e.g., proteins, such as enzymes and antibodies) or bioprocess tracking. Biosensor designs are also influenced by whether the investigation is conducted in vivo or in vitro. In many significant reviews that have reported the development of graphene and CNT biosensors, these factors have been discussed [158,159,160]. Many researchers have designed CNT-based glucose sensors that can detect glucose levels in biological samples based on glucose oxidase-impregnated polyvinyl alcohol solutions [161,162,163,164]. Researchers designed an electrochemical biosensor for glucose oxidase sensing that was helpful in studying the structure of glucose-oxidase-coated MWCNT [165]. Table 6 represent various application of CNMs in biosensing application. Nitric oxide and epinephrine can be also detected using CNT-based electrochemical biosensors [166,167]. Graphene was also used to fabricate an enzyme substrate based on an ultra-efficient probe for the sensing applications of the different biomolecules [168,169]. Researchers have also used fullerenes as materials for sensors and biosensors in the detection of deoxyribonucleic acid (DNA) [170]. The immobilized DNA was used in a fullerene-infused screen-printed electrode for the detection of 16S rDNA. The efficacy of the established procedure was examined by spotting 46S rDNA of E. coli on the fullerene-impregnated electrode with probes that were ideally aligned [171].

Table 6.
Different uses of CNMs in biosensing applications.
Carbon nanohorns (CNHs) offer an appealing alternative to CNT-based sensors [172]. Other unique properties of CNHs, such as their electrocatalytic activity against the oxidation of dihydroxybenzenes, can be used as methods of detection; for instance, the identification of food pollutants, such as bisphenol A, malachite grey, and triclosan [173,174]. CNH-modified standard glassy carbon electrodes have also been employed for the rapid identification of isomers of dihydroxybenzene catechol, hydroquinone, and resorcinol, with limits of detection of 0.1 M, 0.2 M, and 0.5 M, respectively [175]. CNH optical-based sensors typically depend on the ability of the CNH to quench an analyte’s fluorescence in the absence of intrinsic fluorescence. This technique was reported by Zhu et al. for the detection of DNA. A mix-and-detect technique for the fluorescent detection of an HIV-related oligonucleotide sequence has been demonstrated [176,177].
8. Bioimaging Applications
Bioimaging is a noninvasive imaging technique that can be used to visualize living organisms, organs, cells, or internal cell structures, based on their biological activity. It aids in the study of the function of a particular organ in different conditions in correlation with the anatomical 3D structure of specimens. These imaging techniques do not interfere with organ functions, such as respiration, movement, etc. Through the use of high-resolution imaging platforms such as Stimulated Emission Depletion (STED) microscopy, they can also facilitate observations of subcellular structures and all the tissues in multicellular organisms [184]. Bioimaging involves two steps: acquiring and processing images, and picturing the structural or functional aspects of live objects or systems. CNMs are suitable for in vivo and in vitro biological imaging because of their intrinsic optical features, which include a broad absorption spectrum in the visible and near-infrared (NIR) regions, photoluminescence in the NIR range, and significant resonance Raman scattering.
Along with their inherent physical features, particularly their optical attributes, CNMs could be used as biosensors as well as in bioimaging. GQDs, CNTs, and their derivatives possess useful optical features for bioimaging, particularly in living cells, such as visible and NIR photoluminescence, distinctive Raman bands, and photoacoustic and photothermal responses. CNMs have a high ability for fluorescence and the multimodal bioimaging of cells and tissues because of their excellent aqueous solubility, biocompatibility, and minimal cytotoxicity, and even their remarkable tolerance of photobleaching. Intracellular motor protein tagged with SWCNTs has been used to track the dynamic processes within the cytoskeleton. The kinesin-1 motor Ki5c of COS-7 cells was covalently linked to SWCNTs wrapped with DNA. The SWCNT-labelled kinesin motor protein facilitated the real-time tracking of intercellular dynamic events, such as kinesin transport along microtubules and fluctuations in the microtubule-network, using fluorescent microscopy. A complex of SWCNT—a fluorescent, aqueous soluble—and a cytocompatible polymer has been applied for bioimaging. The supramolecular assembly comprises an alkylated polymer that is coupled with neutral hydroxylated or charged sulfated dendronized perylene bisimides (PBIs) and SWCNTs as a stationary base. The backbone of the polymer imparts several new characteristics to the SWCNTs, such as increasing their solubility, making them fluorescent by adding PBIs, and improving cytocompatibility by wrapping around the SWCNT scaffold. In photophysical characterizations and biological in vitro studies, sulphated complexes show better cellular uptake, optical properties, and intracellular staining compared to their hydroxylated analogs [185,186]. Table 7 represented bioimaging application of CNMs.

Table 7.
Different uses of CNMs in bioimaging applications.
A suspension of SWCNTs and bovine serum albumin (BSA) was used for in vivo imaging of Drosophila melanogaster larvae. Protein-coated SWCNT was fed to the larvae. Fluorescent images of the digestive system clearly showed peristaltic movements. Besides, the CNDs were covalently functionalized with transferrin protein through carbodiimide and amine functional groups. The transferrin-functionalized nanodots were internalized into cancerous HeLa cells by overexpressed transferrin receptors on the cell membranes and imaged under fluorescent microscopy [187,188].
K. Yang et al. reported labeling of PEGylated GO with NIR fluorophores (e.g., Cy7) and fluorescein isothiocyanate for in vivo and in vitro imaging [189]. Furthermore, in vivo biodistribution of PEGylated GO along with the signal of 125I was evaluated by F. Yang et al. [190]. A novel protein-based GO was developed for use in ultrasonic dual-modality for imaging, and photothermal therapy in cancer [191]. Additionally, reduced GO nanocomposites tagged with quantum dots were shown to have implications for PTT, tumor imaging, and in situ monitoring of tumor treatment [192].
The photoluminescent (PL) characterization of nGOs showed that upon excitation, peak PL emission intensity is in the bluish-green region (455 nm). In one study, nGOs encapsulated in polylactic acid (PLA) were shown to be biocompatible and photoluminescent, allowing them to be employed in bioimaging applications [193]. Mesoporous silica nanoparticle (MSN) coated with fluorescent fullerene (C60-TEG-COOH) is water-soluble and biocompatible, and it is successfully used for the fabrication of nanocarriers, which have demonstrated a capacity for pH-sensitive drug release and can be put to use for fluorescent cell imaging. In an in vitro study, it was shown that the synthesized materials exhibited excellent biocompatibility. Furthermore, the DOX-loaded nanocarrier showed effective anticancer activity. This study demonstrated a straightforward way to design a dual-purpose nanomaterial, such as for pH-responsive drug delivery, as well as a bioimaging system that can be used as a therapeutic agent and for monitoring treatment responses [194].
9. Vaccination
One of the most popular strategies for preventing and reducing infectious and non-infectious diseases is vaccination. CNMs with appropriate surface functionalization can alter the way therapeutic molecules interact with target cells or tissues. In vaccinology, nanoparticles-based formulations enhance immunogenicity by improving the stability, slow-release and cell-targeted delivery of an antigen. CNMs play a critical role in vaccine delivery, since several types of CNMs have been extensively utilized [201].
CNTs offer advantages to the design of a vaccine delivery system with different approaches against viral, bacterial and protozoal disease as antigens as well as CpG adjuvants [202,203,204]. Early attempts to use CNT scaffolds as vaccine delivery vehicles include the covalent attachment of viral envelope peptides of foot-and-mouth disease to CNTs [205]. In this study, it was demonstrated that epitope structures retain their immunogenic nature when linked with CNT. In the animal model, the CNT-viral protein molecular complexes were effective in eliciting particular immune responses against the viral proteins. Pantarotto et al. showed that the peptide-CNT conjugates elicited IgG responses that were specifically able to neutralize antibodies [206]. Figure 12 represents the antigen that might be connected to the surface of the nanoparticles or could be encapsulated. The renovation of the surface of nanoparticles with targeting molecules (e.g., antibodies, fab-fragments, peptides, etc.) potentially improves particle distribution to antigen-presenting cells (APCs) in order to induce innate and adaptive immune responses.
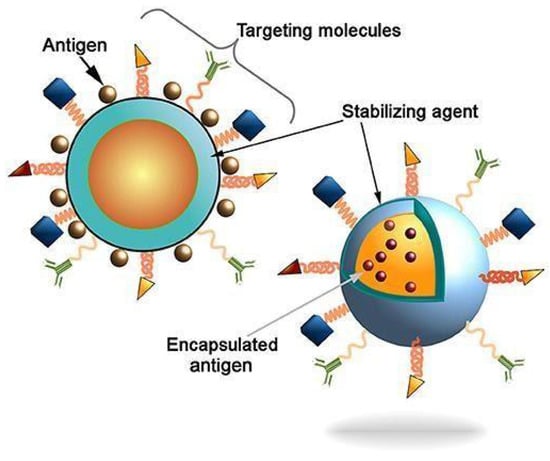
Figure 12.
Schematic depiction of antigen delivery through nanocarriers with surface modification to enhance efficacy of vaccine against specific diseases [207]. Copyright © 2021 Pati, Shevtsov and Sonawane. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY).
Meng et al. conjugated cell lysate to SWCNTs to study vaccine protection using a murine hepatoma model [208]. The SWCNT-conjugated lysate vaccine enhanced protection levels, as compared to cell lysates only, through the improved activation of cytolytic T cells. Purified protein derivative (PPD) in Freund’s adjuvant generated a predominantly Th-2 response when it was given to the mouse models while, conjugated with an SWCNT, the PPD response was shifted in the direction of a Th-1 [209]. Some researchers studied when the MWCNTs and embryonic stem cells injected into mice suppressed the growth of murine colon carcinoma and enhanced the activation CD4 and CD8 cells. In one study, Villa et al. reported that CNT-peptide constructs might enhance the immunogenicity of a less immunogenic, clinically appropriate cancer-associated peptide [210]. They utilized different methods to conjugate 19-amino acid peptides on SWCNTs, conjugated peptides with an easy uptake by dendritic cells, and macrophages in vitro, and the mice immunized with it demonstrated peptide-specific IgG responses. Some researchers have designed self-assembled fullerenol for dual DNA vaccine delivery for HIV. The conjugate showed promising activity by reducing the antigen dosage and enhanced the protection level, which helps in the activation of signaling pathways [211].
GO is widely utilized for the delivery of biomolecules. It has shown promising results in encapsulating and delivering antigens, as well as the ability to induce the immune system. The functionalization of GOs with hydrophilic groups improve their bio-solubility and biocompatibility; as delivery vehicles, the consequent hybrid GOs possess superior adjuvant properties [212].
Due to the excellent adsorbing properties of GOx, it was used in combination with protein. Consequently, it showed intracellular protein delivery, thus demonstrating its potential use in vaccination. The researcher demonstrated that adsorbed proteins on GO were selectively and effectively taken by dendritic cells and induced the presentation of antigen specifically to the cytotoxic T cells. In this way, it induced a powerful cellular antigen-specific immune response against intracellular pathogens and cancer [213]. Table 8 exhibits recently used CNMs in vaccine implementation.

Table 8.
Different CNMs’ uses in vaccination.
10. Photodynamic Therapy (PDT)
Photodynamic therapy (PDT) is an authentic and easy procedure commonly used in the treatment of cancer. PDT is composed of a combination of light, a class of drugs known as photosensitizers (PS), and molecular oxygen, in order to achieve a therapeutic effect on the target area. Broadly speaking, the therapeutic procedure involves the topical or intravenous administration of a photosensitizing agent to the patient, followed by irradiation with light of a particular wavelength that is within the absorbance range of the sensitizer [220]. Energy from the electronically excited PS is transferred to the ground state of molecular oxygen and generates excited singlet oxygen that is toxic to the cells, causing the death of cancerous cells by apoptosis or necrosis. It also causes serious damage to the cancerous microvasculature, and dramatic changes in the tumor’s surroundings [221,222]. Figure 13 represents the diverse PDT approaches of CNMs: (a) a nanocarrier loaded with PSs sensitizes in the presence of light and converts O2 to ROS, which helps in tumor destruction at that specific site; and (b) various types of CNMs are used in PDT applications as shown in Table 9 and after light eradication, PSs in cells produce ROS. The two types of photosensitized processes, Type 1 and Type 2, are based on the type of oxygen consumption.
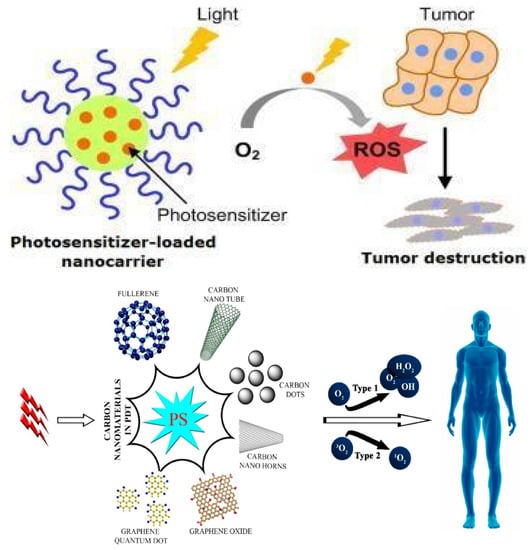
Figure 13.
Schematic illustration of the theranostic of disease photo-triggered by carbon-based CNMs, which are targeted and effective in PDT for cancer [223]. Copyright 2016, MDPI, Open Access.

Table 9.
Different CNMs used in PDT applications.
Ogbodu et al. showed the synthesis of spermidine adsorption on SWCNTS. The conjugation of spermidine, a poly-amine compound, with zinc mono-carboxy phenoxy phthalocyanine results in improving the effect of PDT. The experiment revealed that the cell viability of breast cancer cells lines decreased by 97% [224]. Shine et al. performed an experiment that showed various applications of fullerenes, including the role of PDT and magnetic targeting. The experiment also showed no toxicity of fullerenes during in vivo analysis [225]. For tumor imaging, researchers have developed carboxyl group functionalized fullerene carriers, which can penetrate into cells and can kill cancer cells [226]. Another study revealed the anti-tumor effect and MRI tumor imaging obtained by using fullerenes. The researched conjugated PEG with fullerenes along with pentetic acid, using gadolinium acetate solution. The complex showed an antitumor effect when administered through the IV route [227,228]. Table 10 shows list of patents describe CNTs used in the delivery of therapeutics, imaging and in disease diagnosis.

Table 10.
Some foremost patents describe CNTs used in the delivery of therapeutics, imaging and in disease diagnosis.
11. Conclusions
Over the past decades, carbon nanomaterials have shown great promise in the development of smart nanomaterials for biomedical applications in drug delivery, bioimaging, biosensing, and tissue engineering. Their uniqueness is due to their increased surface area, substantial mechanical strength, and atypical optical properties; these attributes may prove very beneficial for many biomedical applications. The inherent traits of CNMs, including their carbon-rich nanostructures, tiny size, ease of surface functionalization, and high purity, are key to keeping CNM-based complexes at the forefront of in vivo based studies. However, some limitations might still impede their use in certain biomedical applications in particular drug delivery. Current research focuses on improving the surface chemistry of carbon nanomaterials towards increased bioavailability and biocompatibility and reducing toxicity. Already, researchers have been able to overcome the cytotoxicity of some types of carbon nanomaterials in order to use them as nanocarriers for drugs and genes, as contrasting agents in imaging, as well as scaffolds for tissue regeneration, and various biomedical applications, with exciting results. In our opinion, more research needs to focus on the biomedical applications of CNMs. This would provide researchers in chemistry and biology with an opportunity to overcome the limitations imposed on CNMs by their toxicity and dispersibility. Surface modifications through conjugation and the grafting of more compatible materials would improve CNMs’ toxicity and solubility, which would in turn aid in the development of more biocompatible and soluble materials based on CNMs. We believe that the use of composite materials based on CNMs will expand as their limitations are overcome and they become more appropriate for use in human applications.
Author Contributions
Conceptualization, A.B.Y. and A.B.; writing—original draft preparation, C.M., M.G. and A.B.Y; writing—review and editing, A.B., S.S. and A.B.Y.; supervision, A.B.Y., M.G., S.S., F.Ó.M., M.B. and A.B.; funding acquisition, A.B., A.B.Y., F.Ó.M. and M.B. All authors have read and agreed to the published version of the manuscript.
Funding
Very special thanks to Egypt–France Joint Driver (Imhotep Program), grant number 43990SF. A. Barhoum (Head of NanoStruc Research Group) would like to thank the Irish Research Council for financing his research (2020–2022) at the School of Chemical Sciences, Dublin City University, under grant number GOIPD/2020/340. Authors would also like to thank the Department of Science and Technology, Ministry of Science and Technology, India, under Nanomission Scheme (grant number SR/NM/NS-1470/2014) for financial support.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All datasets generated for this study are included in the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kowalski, P.S.; Bhattacharya, C.; Afewerki, S.; Langer, R.S. Smart Biomaterials: Recent Advances and Future Directions. ACS Biomater. Sci. Eng. 2018, 4, 3809–3817. [Google Scholar] [CrossRef]
- Gajdosechova, Z.; Mester, Z. Recent trends in analysis of nanoparticles in biological matrices. Anal. Bioanal. Chem. 2019, 411, 4277–4292. [Google Scholar] [CrossRef]
- Mamidi, N.; Delgadillo, R.M.V.; Ortiz, A.G.; Barrera, E.V. Carbon Nano-Onions Reinforced Multilayered Thin Film System for Stimuli-Responsive Drug Release. Pharmaceutics 2020, 12, 1208. [Google Scholar] [CrossRef]
- Kumar, N.; Kumbhat, S. Carbon-Based Nanomaterials. In Essentials in Nanoscience and Nanotechnology; Wiley: Hoboken, NJ, USA, 2016; pp. 189–236. [Google Scholar]
- Das, S.; Mitra, S.; Khurana, S.M.P.; Debnath, N. Nanomaterials for biomedical applications. Front. Life Sci. 2013, 7, 90–98. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, M.; Wu, M.; Zhu, J.; Zhang, X. Multifunctional Carbon-Based Nanomaterials: Applications in Biomolecular Imaging and Therapy. ACS Omega 2018, 3, 9126–9145. [Google Scholar] [CrossRef] [PubMed]
- Maiti, D.; Tong, X.; Mou, X.; Yang, K. Carbon-Based Nanomaterials for Biomedical Applications: A Recent Study. Front. Pharmacol. 2019, 9, 1401. [Google Scholar] [CrossRef] [PubMed]
- Jacinto, T.A.; Meireles, G.S.; Dias, A.T.; Aires, R.; Porto, M.L.; Gava, A.L.; Vasquez, E.C.; Pereira, T.M.C.; Campagnaro, B.P.; Meyrelles, S.S. Increased ROS production and DNA damage in monocytes are biomarkers of aging and atherosclerosis. Biol. Res. 2018, 51, 33. [Google Scholar] [CrossRef] [PubMed]
- Sims, C.M.; Hanna, S.K.; Heller, D.A.; Horoszko, C.P.; Johnson, M.E.; Bustos, A.R.M.; Reipa, V.; Riley, K.R.; Nelson, B.C. Redox-active nanomaterials for nanomedicine applications. Nanoscale 2017, 9, 15226–15251. [Google Scholar] [CrossRef]
- Peng, Z.; Liu, X.; Zhang, W.; Zeng, Z.; Liu, Z.; Zhang, C.; Liu, Y.; Shao, B.; Liang, Q.; Tang, W.; et al. Advances in the application, toxicity and degradation of carbon nanomaterials in environment: A review. Environ. Int. 2019, 134, 105298. [Google Scholar] [CrossRef]
- Karousis, N.; Tagmatarchis, N.; Tasis, D. Current Progress on the Chemical Modification of Carbon Nanotubes. Chem. Rev. 2010, 110, 5366–5397. [Google Scholar] [CrossRef]
- Georgakilas, V.; Perman, J.; Tucek, J.; Zboril, R. Broad Family of Carbon Nanoallotropes: Classification, Chemistry, and Applications of Fullerenes, Carbon Dots, Nanotubes, Graphene, Nanodiamonds, and Combined Superstructures. Chem. Rev. 2015, 115, 4744–4822. [Google Scholar] [CrossRef]
- Patel, K.D.; Singh, R.K.; Kim, H.-W. Carbon-based nanomaterials as an emerging platform for theranostics. Mater. Horiz. 2019, 6, 434–469. [Google Scholar] [CrossRef]
- Shah, R.; Kausar, A.; Muhammad, B.; Shah, S. Progression from Graphene and Graphene Oxide to High Performance Polymer-Based Nanocomposite: A Review. Polym. Technol. Eng. 2015, 54, 173–183. [Google Scholar] [CrossRef]
- Abdel-Haleem, F.M.; Gamal, E.; Rizk, M.S.; El Nashar, R.M.; Anis, B.; Elnabawy, H.M.; Khalil, A.S.; Barhoum, A. t-Butyl calixarene/Fe2O3@MWCNTs composite-based potentiometric sensor for determination of ivabradine hydrochloride in pharmaceutical formulations. Mater. Sci. Eng. C 2020, 116, 111110. [Google Scholar] [CrossRef]
- Yan, L.; Zhou, T.; Han, L.; Zhu, M.; Cheng, Z.; Li, D.; Ren, F.; Wang, K.; Lu, X. Conductive Cellulose Bio-Nanosheets Assembled Biostable Hydrogel for Reliable Bioelectronics. Adv. Funct. Mater. 2021, 31, 2010465. [Google Scholar] [CrossRef]
- Barhoum, A.; Shalan, A.E.; El-Hout, S.I.; Ali, G.A.M.; Abdelbasir, S.M.; Abu Serea, E.S.; Ibrahim, A.H.; Pal, K. A Broad Family of Carbon Nanomaterials: Classification, Properties, Synthesis, and Emerging Applications. In Handbook of Nanofibers; Springer International Publishing: Cham, Switzerland, 2019; pp. 1–40. [Google Scholar]
- Rasouli, R.; Barhoum, A.; Bechelany, M.; Dufresne, A. Nanofibers for Biomedical and Healthcare Applications. Macromol. Biosci. 2019, 19, e1800256. [Google Scholar] [CrossRef] [PubMed]
- Abdo, G.G.; Zagho, M.M.; Al Moustafa, A.; Khalil, A.; Elzatahry, A.A. A comprehensive review summarizing the recent biomedical applications of functionalized carbon nanofibers. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 109, 1893–1908. [Google Scholar] [CrossRef] [PubMed]
- Che, G.; Lakshmi, B.B.; Martin, A.C.R.; Fisher, E.R.; Ruoff, R.S. Chemical Vapor Deposition Based Synthesis of Carbon Nanotubes and Nanofibers Using a Template Method. Chem. Mater. 1998, 10, 260–267. [Google Scholar] [CrossRef]
- Aversa, R.; Petrescu, R.V.; Apicella, A.; Petrescu, F.I. Nano-diamond hybrid materials for structural biomedical application. Am. J. Biochem. Biotechnol. 2016, 13, 34–41. [Google Scholar] [CrossRef]
- Zhang, Y.; Rhee, K.Y.; Hui, D.; Park, S.-J. A critical review of nanodiamond based nanocomposites: Synthesis, properties and applications. Compos. Part B Eng. 2018, 143, 19–27. [Google Scholar] [CrossRef]
- Amans, D.; Chenus, A.-C.; Ledoux, G.; Dujardin, C.; Reynaud, C.; Sublemontier, O.; Masenelli-Varlot, K.; Guillois, O. Nanodiamond synthesis by pulsed laser ablation in liquids. Diam. Relat. Mater. 2009, 18, 177–180. [Google Scholar] [CrossRef]
- Sawy, A.M.; Barhoumbb, A.; Gaber, S.A.A.; El-Hallouty, S.M.; Shousha, W.G.; Maarouf, A.A.; Khalilaf, A.S. Insights of doxorubicin loaded graphene quantum dots: Synthesis, DFT drug interactions, and cytotoxicity. Mater. Sci. Eng. C 2021, 122, 111921. [Google Scholar] [CrossRef] [PubMed]
- Lee, X.J.; Hiew, B.Y.Z.; Lai, K.C.; Lee, L.Y.; Gan, S.; Thangalazhy-Gopakumar, S.; Rigby, S. Review on graphene and its derivatives: Synthesis methods and potential industrial implementation. J. Taiwan Inst. Chem. Eng. 2019, 98, 163–180. [Google Scholar] [CrossRef]
- El-Beshlawy, M.M.; Abdel-Haleem, F.M.; Barhoum, A. Molecularly Imprinted Potentiometric Sensor for Nanomolar Determination of Pioglitazone Hydrochloride in Pharmaceutical Formulations. Electroanalysis 2021, 33, 1244–1254. [Google Scholar] [CrossRef]
- Bakry, R.; Vallant, R.M.; Najam-ul-Haq, M.; Rainer, M.; Szabo, Z.; Huck, C.W.; Bonn, G.K. Medicinal applications of fuller-enes. Int. J. Nanomed. 2007, 2, 639. [Google Scholar]
- Martinez, Z.S.; Castro, E.; Seong, C.-S.; Cerón, M.R.; Echegoyen, L.; Llano, M. Fullerene Derivatives Strongly Inhibit HIV-1 Replication by Affecting Virus Maturation without Impairing Protease Activity. Antimicrob. Agents Chemother. 2016, 60, 5731–5741. [Google Scholar] [CrossRef] [PubMed]
- Ghiassi, K.B.; Olmstead, M.M.; Balch, A.L. Gadolinium-containing endohedral fullerenes: Structures and function as magnetic resonance imaging (MRI) agents. Dalton Trans. 2014, 43, 7346–7358. [Google Scholar] [CrossRef]
- Roy, P.; Bag, S.; Chakraborty, D.; Dasgupta, S. Exploring the Inhibitory and Antioxidant Effects of Fullerene and Fullerenol on Ribonuclease A. ACS Omega 2018, 3, 12270–12283. [Google Scholar] [CrossRef] [PubMed]
- Lyon, D.Y.; Adams, L.K.; Falkner, J.C.; Alvarez, P.J.J. Antibacterial Activity of Fullerene Water Suspensions: Effects of Preparation Method and Particle Size. Environ. Sci. Technol. 2006, 40, 4360–4366. [Google Scholar] [CrossRef]
- Mirakyan, A.L.; Wilson, L.J. Functionalization of C60 with diphosphonate groups: A route to bone-vectored fullerenes. J. Chem. Soc. Perkin Trans. 2002, 2002, 1173–1176. [Google Scholar] [CrossRef]
- Lee, D.J.; Ahn, Y.S.; Youn, Y.S.; Lee, E.S. Poly(ethylene glycol)-crosslinked fullerenes for high efficient phototherapy. Polym. Adv. Technol. 2013, 24, 220–227. [Google Scholar] [CrossRef]
- Popov, A.A. Structures and Stability of Fullerenes, Metallofullerenes, and Their Derivatives. Handb. Comput. Chem. 2016, 1, 1–66. [Google Scholar] [CrossRef]
- Bolskar, R.D. Fullerenes for Drug Delivery. Encycl. Nanotechnol. 2016, 8, 1267–1281. [Google Scholar] [CrossRef]
- Klupp, G.; Margadonna, S.; Prassides, K. Fullerenes. Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar] [CrossRef]
- Goodarzi, S.; Da Ros, T.; Conde, J.; Sefat, F.; Mozafari, M. Fullerene: Biomedical engineers get to revisit an old friend. Mater. Today 2017, 20, 460–480. [Google Scholar] [CrossRef]
- Nafisi, S. Nanotechnology in cosmetics. In Cosmetic Science and Technology; Routledge: London, UK, 2017. [Google Scholar]
- Rao, R.N. Nanomaterials in Chromatographic Sample Preparations; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar] [CrossRef]
- Saldmann, F.; Saldmann, A.; Lemaire, M.C. Characterization and internalization of nanodiamond–trehalose conjugates into mammalian fibroblast cells of naked mole rat. Int. Nano Lett. 2020, 10, 151–157. [Google Scholar] [CrossRef]
- Mengesha, A.; Youan, B.-B. Nanodiamonds for drug delivery systems. Diam.-Based Mater. Biomed. Appl. 2013, 186–205. [Google Scholar] [CrossRef]
- Lim, D.G.; Prim, R.E.; Kim, K.H.; Kang, E.; Park, K.; Jeong, S.H. Combinatorial nanodiamond in pharmaceutical and biomedical applications. Int. J. Pharm. 2016, 514, 41–51. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, L.; Du, F.; Wu, Y.; Cai, R.; Xu, L.; Jin, H.; Zou, S.; Gong, A.; Du, F. Facile synthesis of cerium-doped carbon quantum dots as a highly efficient antioxidant for free radical scavenging. Nanotechnology 2019, 30, 325101. [Google Scholar] [CrossRef]
- Atabaev, T.S. Doped Carbon Dots for Sensing and Bioimaging Applications: A Minireview. Nanomaterials 2018, 8, 342. [Google Scholar] [CrossRef]
- Kandasamy, G. Recent Advancements in Doped/Co-Doped Carbon Quantum Dots for Multi-Potential Applications. C 2019, 5, 24. [Google Scholar] [CrossRef]
- Budimir, M.; Szunerits, S. Nanoscale Materials for the Treatment of Water Contaminated by Bacteria and Viruses; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Flores-Pacheco, A. Down-Shifting by Quantum Dots for Silicon Solar Cell Applications; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Alaghmandfard, A.; Sedighi, O.; Rezaei, N.T.; Abedini, A.A.; Khachatourian, A.M.; Toprak, M.S.; Seifalian, A. Recent advances in the modification of carbon-based quantum dots for biomedical applications. Mater. Sci. Eng. C. 2020, 27, 111756. [Google Scholar] [CrossRef]
- Su, W.; Wu, H.; Xu, H.; Zhang, Y.; Li, Y. Carbon dots: A booming material for biomedical applications. Mater. Chem. Front. 2020, 4, 821–836. [Google Scholar] [CrossRef]
- Molaei, M.J. Carbon quantum dots and their biomedical and therapeutic applications: A review. RSC Adv. 2019, 9, 6460–6481. [Google Scholar] [CrossRef]
- Liu, H.; Qiu, J.; Zhang, R.; Li, J.; Sang, Y.; Tang, W.; Gil, P.R. Fluorescent graphene quantum dots as traceable, pH-sensitive drug delivery systems. Int. J. Nanomed. 2015, 10, 6709–6724. [Google Scholar] [CrossRef]
- Iijima, S.; Ichihashi, T. Single-shell carbon nanotubes of 1-nm diameter. Nat. Cell Biol. 1993, 363, 603–605. [Google Scholar] [CrossRef]
- Bethune, D.S.; Kiang, C.H.; De Vries, M.S.; Gorman, G.; Savoy, R.; Vazquez, J.; Beyers, R. Cobalt-catalysed growth of carbon nanotubes with single-atomic-layer walls. Nat. Cell Biol. 1993, 363, 605–607. [Google Scholar] [CrossRef]
- Syrgiannis, Z.; Melchionna, M.; Prato, M. Covalent Carbon Nanotube Functionalization. Encycl. Polym. Nanomater. 2014, 1–8. [Google Scholar] [CrossRef]
- Nikolaev, P. Gas-Phase Production of Single-Walled Carbon Nanotubes from Carbon Monoxide: A Review of the HiPco Process. J. Nanosci. Nanotechnol. 2004, 4, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Schulz, M.J.; Shanov, V.N.; Yun, Y. Nanomedicine Design of Particles, Sensors, Motors, Implants, Robots, and Devices; Artech House Publishers: Norwood, MA, USA, 2009. [Google Scholar]
- Bensghaïer, A.; Truong, S.L.; Seydou, M.; Lamouri, A.; Leroy, E.; Mičušik, M.; Forro, K.; Beji, M.; Pinson, J.; Omastová, M.; et al. Efficient Covalent Modification of Multiwalled Carbon Nanotubes with Diazotized Dyes in Water at Room Temperature. Langmuir 2017, 33, 6677–6690. [Google Scholar] [CrossRef]
- Shi, Q.; Yang, D.; Su, Y.; Li, J.; Jiang, Z.; Jiang, Y.; Yuan, W. Covalent functionalization of multi-walled carbon nanotubes by lipase. J. Nanopart. Res. 2007, 9, 1205–1210. [Google Scholar] [CrossRef]
- Tran, P.; Zhang, L.; Webster, T.J. Carbon nanofibers and carbon nanotubes in regenerative medicine. Adv. Drug Deliv. Rev. 2009, 61, 1097–1114. [Google Scholar] [CrossRef]
- Harrison, B. Carbon Nanotube Applications for Tissue Engineering; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Hu, L.; Hecht, D.S.; Grüner, G. Carbon Nanotube Thin Films: Fabrication, Properties, and Applications. Chem. Rev. 2010, 110, 5790–5844. [Google Scholar] [CrossRef]
- Zare, H.; Ahmadi, S.; Ghasemi, A.; Ghanbari, M.; Rabiee, N.; Bagherzadeh, M.; Karimi, M.; Webster, T.J.; Hamblin, M.R.; Mostafavi, E. Carbon Nanotubes: Smart Drug/Gene Delivery Carriers. Int. J. Nanomed. 2021, 16, 1681–1706. [Google Scholar] [CrossRef]
- Erol, O.; Uyan, I.; Hatip, M.; Yilmaz, C.; Tekinay, A.B.; Guler, M.O. Recent advances in bioactive 1D and 2D carbon nanomaterials for biomedical applications. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 2433–2454. [Google Scholar] [CrossRef]
- Kim, Y.A.; Hayashi, T.; Endo, M.; Dresselhaus, M.S. Carbon Nanofibers; Springer: Berlin/Heidelberg, Germany, 2013; pp. 233–262. [Google Scholar] [CrossRef]
- Desmaris, V.; Saleem, A.M.; Shafiee, S.; Berg, J.; Kabir, M.S.; Johansson, A. Carbon Nanofibers (CNF) for enhanced solder-based nano-scale integration and on-chip interconnect solutions. In Proceedings of the 2014 IEEE 64th Electronic Components and Technology Conference (ECTC), Orlando, FL, USA, 27–30 May 2014; pp. 1071–1076. [Google Scholar] [CrossRef]
- Han, L.; Andrady, A.L.; Ensor, D.S. Chemical sensing using electrospun polymer/carbon nanotube composite nanofibers with printed-on electrodes. Sens. Actuators B Chem. 2013, 186, 52–55. [Google Scholar] [CrossRef]
- Ozkan, T.; Naraghi, M.; Chasiotis, I. Mechanical properties of vapor grown carbon nanofibers. Carbon 2010, 48, 239–244. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, X.; Wang, C.; Fu, S.; Liu, Y.; Shao, C. Polyacrylonitrile and Carbon Nanofibers with Controllable Nanoporous Structures by Electrospinning. Macromol. Mater. Eng. 2009, 294, 673–678. [Google Scholar] [CrossRef]
- Zhang, L.; Aboagye, A.; Kelkar, A.D.; Lai, C.; Fong, H. A review: Carbon nanofibers from electrospun polyacrylonitrile and their applications. J. Mater. Sci. 2014, 49, 463–480. [Google Scholar] [CrossRef]
- Swisher, L.Z.; Syed, L.U.; Prior, A.M.; Madiyar, F.R.; Carlson, K.R.; Nguyen, T.A.; Hua, D.H.; Li, J. Electrochemical Protease Biosensor Based on Enhanced AC Voltammetry Using Carbon Nanofiber Nanoelectrode Arrays. J. Phys. Chem. C 2013, 117, 4268–4277. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Bermea, C.; Rajukumar, L.P.; Dasgupta, A.; Lei, Y.; Hashimoto, Y.; Sepulveda-Guzman, S.; Cruz-Silva, R.; Endo, M.; Terrones, M. Two-dimensional and three-dimensional hybrid assemblies based on graphene oxide and other layered structures: A carbon science perspective. Carbon 2017, 125, 437–453. [Google Scholar] [CrossRef]
- Pyun, J. Graphene Oxide as Catalyst: Application of Carbon Materials beyond Nanotechnology. Angew. Chem. Int. Ed. 2011, 50, 46–48. [Google Scholar] [CrossRef]
- Tite, T.; Chiticaru, E.A.; Burns, J.S.; Ioniţă, M. Impact of nano-morphology, lattice defects and conductivity on the performance of graphene based electrochemical biosensors. J. Nanobiotechnol. 2019, 17, 101. [Google Scholar] [CrossRef] [PubMed]
- Milani, M.A.; González, D.; Quijada, R.; Basso, N.; Cerrada, M.L.; Azambuja, D.S.; Galland, G.B. Polypropylene/graphene nanosheet nanocomposites by in situ polymerization: Synthesis, characterization and fundamental properties. Compos. Sci. Technol. 2013, 84, 1–7. [Google Scholar] [CrossRef]
- Nurunnabi, M.; Parvez, K.; Nafiujjaman, M.; Revuri, V.; Khan, H.A.; Feng, X.; Lee, Y. Bioapplication of graphene oxide derivatives: Drug/gene delivery, imaging, polymeric modification, toxicology, therapeutics and challenges. RSC Adv. 2015, 5, 42141–42161. [Google Scholar] [CrossRef]
- Hensleigh, R.M.; Cui, H.; Oakdale, J.S.; Ye, J.C.; Campbell, P.G.; Duoss, E.B.; Spadaccini, C.M.; Zheng, X.; Worsley, M.A. Additive manufacturing of complex micro-architected graphene aerogels. Mater. Horiz. 2018, 5, 1035–1041. [Google Scholar] [CrossRef]
- Shahabi, M.; Raissi, H. Payload delivery of anticancer drug Tegafur with the assistance of graphene oxide nanosheet during biomembrane penetration: Molecular dynamics simulation survey. Appl. Surf. Sci. 2020, 517, 146186. [Google Scholar] [CrossRef]
- Jakus, A.E.; Secor, E.B.; Rutz, A.L.; Jordan, S.W.; Hersam, M.C.; Shah, R.N. Three-Dimensional Printing of High-Content Graphene Scaffolds for Electronic and Biomedical Applications. ACS Nano 2015, 9, 4636–4648. [Google Scholar] [CrossRef]
- Chen, J.; Chen, S.; Zhao, X.; Kuznetsova, L.V.; Wong, S.S.; Ojima, I. Functionalized Single-Walled Carbon Nanotubes as Rationally Designed Vehicles for Tumor-Targeted Drug Delivery. J. Am. Chem. Soc. 2008, 130, 16778–16785. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Singh, R.P.; Datir, S.R.; Jain, S. Intranuclear Drug Delivery and Effective in Vivo Cancer Therapy via Estradiol–PEG-Appended Multiwalled Carbon Nanotubes. Mol. Pharm. 2013, 10, 3404–3416. [Google Scholar] [CrossRef]
- Friedman, S.H.; DeCamp, D.L.; Sijbesma, R.P.; Srdanov, G.; Wudl, F.; Kenyon, G.L. Inhibition of the HIV-1 protease by fullerene derivatives: Model building studies and experimental verification. J. Am. Chem. Soc. 1993, 115, 6506–6509. [Google Scholar] [CrossRef]
- Bosi, S.; Da Ros, T.; Spalluto, G.; Prato, M. Fullerene Derivatives: An Attractive Tool for Biological Applications. Eur. J. Med. Chem. 2004, 35, 913–923. [Google Scholar] [CrossRef]
- Gurunathan, S.; Kang, M.-H.; Qasim, M.; Kim, J.-H. Nanoparticle-Mediated Combination Therapy: Two-in-One Approach for Cancer. Int. J. Mol. Sci. 2018, 19, 3264. [Google Scholar] [CrossRef]
- Navya, P.; Kaphle, A.; Srinivas, S.; Bhargava, S.K.; Rotello, V.M.; Daima, H.K. Current trends and challenges in cancer management and therapy using designer nanomaterials. Nano Converg. 2019, 6, 23. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured mate-rials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Y.; Sun, B.; Chen, C. Understanding the Toxicity of Carbon Nanotubes. Acc. Chem. Res. 2013, 46, 702–713. [Google Scholar] [CrossRef]
- Sahoo, N.G.; Bao, H.; Pan, Y.; Pal, M.; Kakran, M.; Cheng, H.K.F.; Li, L.; Tan, L.P. Functionalized carbon nanomaterials as nanocarriers for loading and delivery of a poorly water-soluble anticancer drug: A comparative study. Chem. Commun. 2011, 47, 5235–5237. [Google Scholar] [CrossRef]
- Thotakura, N.; Sharma, S.; Khurana, R.K.; Babu, P.V.; Chitkara, D.; Kumar, V.; Singh, B.; Raza, K. Aspartic acid tagged carbon nanotubols as a tool to deliver docetaxel to breast cancer cells: Reduced hemotoxicity with improved cytotoxicity. Toxicol. Vitr. 2019, 59, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Sharma, G.; Misra, C.; Kumar, R.; Singh, B.; Katare, O.; Raza, K. -desmethyl tamoxifen and quercetin-loaded multiwalled CNTs: A synergistic approach to overcome MDR in cancer cells. Mater. Sci. Eng. C 2018, 89, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.-C.; Lin, C.-Y.; Peng, C.-L.; Shieh, M.-J. Development of a controlled-release drug delivery system by encapsulating oxaliplatin into SPIO/MWNT nanoparticles for effective colon cancer therapy and magnetic resonance imaging. Biomater. Sci. 2016, 4, 1742–1753. [Google Scholar] [CrossRef]
- Pei, X.; Zhu, Z.; Gan, Z.; Chen, J.; Zhang, X.; Cheng, X.; Wan, Q.; Wang, J. PEGylated nano-graphene oxide as a nanocarrier for delivering mixed anticancer drugs to improve anticancer activity. Sci. Rep. 2020, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yu, D.; Dai, R.; Fu, D.; Li, W.; Guo, Z.; Cui, C.; Xu, J.; Shen, S.; Ma, K. PEGylated doxorubicin cloaked nano-graphene oxide for dual-responsive photochemical therapy. Int. J. Pharm. 2019, 557, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Chitphet, K.; Geary, S.M.; Chan, C.H.F.; Simons, A.L.; Weiner, G.J.; Salem, A.K. Combining Doxorubicin-Loaded PEGylated Poly(Lactide-co-glycolide) Nanoparticles with Checkpoint Inhibition Safely Enhances Therapeutic Efficacy in a Melanoma Model. ACS Biomater. Sci. Eng. 2019, 6, 2659–2667. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Robinson, J.; Tabakman, S.; Yang, K. Carbon Materials for Drug Delivery & Cancer Therapy; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Toudeshki, R.M.; Dadfarnia, S.; Shabani, A.M.H. Surface molecularly imprinted polymer on magnetic multi-walled carbon nanotubes for selective recognition and preconcentration of metformin in biological fluids prior to its sensitive chemiluminescence determination: Central composite design optimization. Anal. Chim. Acta 2019, 1089, 78–89. [Google Scholar] [CrossRef]
- Zakharian, T.Y.; Seryshev, A.; Sitharaman, B.; Gilbert, B.E.; Knight, V.; Wilson, L.J. A Fullerene−Paclitaxel Chemotherapeutic: Synthesis, Characterization, and Study of Biological Activity in Tissue Culture. J. Am. Chem. Soc. 2005, 127, 12508–12509. [Google Scholar] [CrossRef]
- Misra, C.; Kumar, M.; Sharma, G.; Kumar, R.; Singh, B.; Katare, O.P.; Raza, K. Glycinated fullerenes for tamoxifen intracellular delivery with improved anticancer activity and pharmacokinetics. Nanomedicine 2017, 12, 1011–1023. [Google Scholar] [CrossRef]
- Bhunia, T.; Giri, A.; Nasim, T.; Chattopadhyay, D.; Bandyopadhyay, A. A transdermal diltiazem hydrochloride delivery device using multi-walled carbon nanotube/poly(vinyl alcohol) composites. Carbon 2013, 52, 305–315. [Google Scholar] [CrossRef]
- Zhanga, X.; Meng, L.; Luab, Q.; Feic, Z.; Dyson, P. Targeted delivery and controlled release of doxorubicin to cancer cells using modified single wall carbon nanotubes. Biomaterials 2009, 30, 6041–6047. [Google Scholar] [CrossRef]
- Xu, Z.; Zhu, S.; Wang, M.-W.; Li, Y.; Shi, P.; Huang, X. Delivery of Paclitaxel Using PEGylated Graphene Oxide as a Nanocarrier. ACS Appl. Mater. Interfaces 2015, 7, 1355–1363. [Google Scholar] [CrossRef]
- Chow, E.K.; Zhang, X.-Q.; Chen, M.; Lam, R.; Robinson, E.; Huang, H.; Schaffer, D.; Osawa, E.; Goga, A.; Ho, D. Nanodiamond Therapeutic Delivery Agents Mediate Enhanced Chemoresistant Tumor Treatment. Sci. Transl. Med. 2011, 3, 73ra21. [Google Scholar] [CrossRef]
- Kalita, S.J. Nanostructured Biomaterials; Springer: Stuttgart, Germany, 2008; pp. 168–219. [Google Scholar] [CrossRef]
- Eliaz, N. Corrosion of Metallic Biomaterials: A Review. Materials 2019, 12, 407. [Google Scholar] [CrossRef]
- Prasad, K.; Bazaka, O.; Chua, M.; Rochford, M.; Fedrick, L.; Spoor, J.; Symes, R.; Tieppo, M.; Collins, C.; Cao, A.; et al. Metallic Biomaterials: Current Challenges and Opportunities. Materials 2017, 10, 884. [Google Scholar] [CrossRef] [PubMed]
- Zivic, F.; Affatato, S.; Trajanovic, M.; Schnabelrauch, M. Biomaterials in Clinical Practice: Advances in Clinical Research and Medical Devices; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Krul, L.; Volozhyn, A.; Belov, D.; Poloiko, N.; Artushkevich, A.; Zhdanok, S.; Solntsev, A.; Krauklis, A.; Zhukova, I. Nanocomposites based on poly-d,l-lactide and multiwall carbon nanotubes. Biomol. Eng. 2007, 24, 93–95. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Bai, Y.; Gao, J.; Ma, W.; Su, J.; Jia, R. Preparation and characterization of reduced graphene oxide/fluorhydroxyapatite composites for medical implants. J. Alloys Compd. 2016, 688, 657–667. [Google Scholar] [CrossRef]
- Auciello, O.; Gurman, P.; Guglielmotti, M.B.; Olmedo, D.G.; Berra, A.; Saravia, M.J. Biocompatible ultrananocrystalline diamond coatings for implantable medical devices. MRS Bull. 2014, 39, 621–629. [Google Scholar] [CrossRef]
- Ghosh, S.; Haldar, S.; Gupta, S.; Bisht, A.; Chauhan, S.; Kumar, V.; Roy, P.; Lahiri, D. Anisotropically Conductive Biodegradable Scaffold with Coaxially Aligned Carbon Nanotubes for Directional Regeneration of Peripheral Nerves. ACS Appl. Bio Mater. 2020, 3, 5796–5812. [Google Scholar] [CrossRef]
- Goyal, A.; Goetz, S.; Stanslaski, S.; Oh, Y.; Rusheen, A.E.; Klassen, B.; Miller, K.; Blaha, C.D.; Bennet, K.E.; Lee, K. The development of an implantable deep brain stimulation device with simultaneous chronic electrophysiological recording and stimulation in humans. Biosens. Bioelectron. 2021, 176, 112888. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, N.; Buschbeck, E.; Miller, S.; Le, A.D.; Gupta, V.; Ruhunage, C.; Vilinsky, I.; Ma, Y. Carbon Nanotube Fibers for Neural Recording and Stimulation. ACS Appl. Bio Mater. 2020, 3, 6478–6487. [Google Scholar] [CrossRef]
- Kumar, A.; Suresh, B.; Ramakrishna, S. Biocompatible responsive polypyrrole/GO nanocomposite coatings for biomedical applications. RSC Adv. 2015, 5, 99866–99874. [Google Scholar] [CrossRef]
- Sirivisoot, S. Skeletal myotube formation enhanced by electrospun poly-urethane carbon nanotube scaffolds. Int. J. Nanomed. 2011, 6, 2483. [Google Scholar] [CrossRef]
- Tanaka, M.; Sato, Y.; Zhang, M.; Haniu, H.; Okamoto, M.; Aoki, K.; Takizawa, T.; Yoshida, K.; Sobajima, A.; Kamanaka, T.; et al. In Vitro and In Vivo Evaluation of a Three-Dimensional Porous Multi-Walled Carbon Nanotube Scaffold for Bone Regeneration. Nanomaterials 2017, 7, 46. [Google Scholar] [CrossRef]
- Tashakori-Miyanroudi, M.; Rakhshan, K. Conductive Carbon Nanofibers Incorporated into Collagen Bio-Scaffold Assists Myocardial Injury Repair; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Xu, J.; Khor, K.; Sui, J. Preparation and Characterization of a Novel Hydroxyapatite/Carbon Nanotubes Composite and Its Interaction with Osteoblast-Like Cells; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Tamayol, A.; Akbari, M.; Annabi, N.; Paul, A.; Khademhosseini, A.; Juncker, D. Fiber-based tissue engineering: Progress, challenges, and opportunities. Biotechnol. Adv. 2013, 31, 669–687. [Google Scholar] [CrossRef] [PubMed]
- Khademhosseini, A.; Vacanti, J.P.; Langer, R. Progress in Tissue Engineering. Sci. Am. 2009, 300, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Hubbell, J.A. Biomaterials in Tissue Engineering. Nat. Biotechnol. 1995, 13, 565–576. [Google Scholar] [CrossRef]
- Lutolf, M.P.; Hubbell, J.A. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tis-sue engineering. Nat. Biotechnol. 2005, 23, 47–55. [Google Scholar] [CrossRef]
- Berger, C.; Song, Z.; Li, X.; Wu, X.; Brown, N.; Naud, C.; Mayou, D.; Hass, J.; Marchenkov, A.N.; Conrad, E.H.; et al. Electronic Confinement and Coherence in Patterned Epitaxial Graphene. Science 2006, 312, 1191–1196. [Google Scholar] [CrossRef] [PubMed]
- Lovat, V.; Pantarotto, D.; Lagostena, L.; Cacciari, B.; Grandolfo, M.; Righi, M.; Spalluto, G.; Prato, M.; Ballerini, L. Carbon Nanotube Substrates Boost Neuronal Electrical Signaling. Nano Lett. 2005, 5, 1107–1110. [Google Scholar] [CrossRef]
- Mazzatenta, A.; Giugliano, M.; Campidelli, S.; Gambazzi, L.; Businaro, L.; Markram, H.; Prato, M.; Ballerini, L. Interfacing Neurons with Carbon Nanotubes: Electrical Signal Transfer and Synaptic Stimulation in Cultured Brain Circuits. J. Neurosci. 2007, 27, 6931–6936. [Google Scholar] [CrossRef]
- Abarrategi, A.; Gutierrez, M.C.; Moreno-Vicente, C.; Hortigüela, M.J.; Ramos, V.; López-Lacomba, J.L.; Ferrer, M.L.; del Monte, F. Multiwall carbon nanotube scaffolds for tissue engineering purposes. Biomaterials 2008, 29, 94–102. [Google Scholar] [CrossRef]
- Lu, C.-H.; Zhu, C.-L.; Li, J.; Liu, J.-J.; Chen, X.; Yang, H.-H. Using graphene to protect DNA from cleavage during cellular delivery. Chem. Commun. 2010, 46, 3116–3118. [Google Scholar] [CrossRef]
- Chen, B.; Liu, M.; Zhang, L.; Huang, J.; Yao, J.; Zhang, Z. Polyethylenimine-functionalized graphene oxide as an efficient gene delivery vector. J. Mater. Chem. 2011, 21, 7736–7741. [Google Scholar] [CrossRef]
- Kalbacova, M.H.; Brož, A.; Kong, J.; Kalbac, M. Graphene substrates promote adherence of human osteoblasts and mesenchymal stromal cells. Carbon 2010, 48, 4323–4329. [Google Scholar] [CrossRef]
- Ahmed, M.K.; Mansour, S.F.; Al-Wafi, R. Nanofibrous scaffolds of ϵ-polycaprolactone containing Sr/Se-hydroxyapatite/graphene oxide for tissue engineering applications. Biomed. Mater. 2021, 16, 045030. [Google Scholar] [CrossRef] [PubMed]
- Jalili, A.; Aboutalebi, S.H.; Esrafilzadeh, D.; Konstantinov, K.; Moulton, S.; Razal, J.; Wallace, G. Organic Solvent-Based Graphene Oxide Liquid Crystals: A Facile Route toward the Next Generation of Self-Assembled Layer-by-Layer Multifunctional 3D Architectures. ACS Nano 2013, 7, 3981–3990. [Google Scholar] [CrossRef] [PubMed]
- Khalili, R.; Zarintaj, P.; Jafari, S.H.; Vahabi, H.; Saeb, M.R. Electroactive poly (p-phenylene sulfide)/r-graphene oxide/chitosan as a novel potential candidate for tissue engineering. Int. J. Biol. Macromol. 2020, 154, 18–24. [Google Scholar] [CrossRef]
- Roshanbinfar, K.; Mohammadi, Z. Carbon nanotube doped pericardial matrix derived electro-conductivebiohybrid hydrogel for cardiac tissue engineering. Biomater. Sci. 2019, 7, 3906–3917. [Google Scholar] [CrossRef] [PubMed]
- Minami, K.; Kasuya, Y.; Yamazaki, T.; Ji, Q.; Nakanishi, W.; Hill, J.; Sakai, H.; Ariga, K. Highly Ordered 1D Fullerene Crystals for Concurrent Control of Macroscopic Cellular Orientation and Differentiation toward Large-Scale Tissue Engineering. Adv. Mater. 2015, 27, 4020–4026. [Google Scholar] [CrossRef]
- Zhang, Q.; Mochalin, V.; Neitzel, I.; Knoke, I. Fluorescent PLLA-Nanodiamond Composites for Bone Tissue Engineering; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Jin, L.; Ren, K.; Xu, Q.; Hong, T.; Wu, S.; Zhang, Y.; Wang, Z. Multifunctional carbon dots for live cell staining and tissue engineering applications. Polym. Compos. 2018, 39, 73–80. [Google Scholar] [CrossRef]
- Mehrabi, A.; Baheiraei, N.; Adabi, M.; Amirkhani, Z. Development of a Novel Electroactive Cardiac Patch Based on Carbon Nanofibers and Gelatin Encouraging Vascularization. Appl. Biochem. Biotechnol. 2019, 190, 931–948. [Google Scholar] [CrossRef]
- Zeimaran, E.; Pourshahrestani, S. Engineering Stiffness in Highly Porous Biomimetic Gelatin/Tertiary Bioactive Glass Hybrid Scaffolds Using Graphene Nanosheets; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Kalashnikova, I.; Das, S.; Seal, S. Nanomaterials for wound healing: Scope and advancement. Nanomedicine 2015, 10, 2593–2612. [Google Scholar] [CrossRef]
- Gosain, A.; DiPietro, L.A. Aging and Wound Healing. World J. Surg. 2004, 28, 321–326. [Google Scholar] [CrossRef]
- Mathieu, D. Handbook on Hyperbaric Medicine; Springer: Dordrecht, The Netherlands, 2006; ISBN 978-140-204-44-89. [Google Scholar]
- Hamdan, S.; Pastar, I.; Drakulich, S.; Dikici, E.; Tomic-Canic, M.; Deo, S.; Daunert, S. Nanotechnology-Driven Therapeutic Interventions in Wound Healing: Potential Uses and Applications. ACS Central Sci. 2017, 3, 163–175. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, R.; He, T.; Xu, K.; Du, D.; Zhao, N.; Cheng, X.; Yang, J.; Shi, H.; Lin, Y. Biomedical Potential of Ultrafine Ag/AgCl Nanoparticles Coated on Graphene with Special Reference to Antimicrobial Performances and Burn Wound Healing. ACS Appl. Mater. Interfaces 2016, 8, 15067–15075. [Google Scholar] [CrossRef] [PubMed]
- Tong, C.; Zou, W.; Ning, W.; Fan, J.; Li, L.; Liu, B.; Liu, X. Synthesis of DNA-guided silver nanoparticles on a graphene oxide surface: Enhancing the antibacterial effect and the wound healing activity. RSC Adv. 2018, 8, 28238–28248. [Google Scholar] [CrossRef]
- Zhou, Z.; Joslin, S.; Dellinger, A.; Ehrich, M.; Brooks, B.; Ren, Q.; Rodeck, U.; Lenk, R.; Kepley, C.L. A Novel Class of Compounds with Cutaneous Wound Healing Properties. J. Biomed. Nanotechnol. 2010, 6, 605–611. [Google Scholar] [CrossRef]
- Santos, J.C.C.; Mansur, A.; Ciminelli, V.S.T.; Mansur, H. Nanocomposites of Poly(Vinyl Alcohol)/Functionalized-Multiwall Carbon Nanotubes Conjugated With Glucose Oxidase for Potential Application as Scaffolds in Skin Wound Healing. Int. J. Polym. Mater. 2014, 63, 185–196. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, J.; Ma, H.; Bai, H.; Liu, L.; Shu, C.; Li, H.; Wang, S.; Wang, C. Designing an Amino-Fullerene Derivative C70–(EDA)8 to Fight Superbacteria. ACS Appl. Mater. Interfaces 2019, 11, 14597–14607. [Google Scholar] [CrossRef]
- Kandra, R. Synthesis, Mechanical Properties of Fluorescent Carbon Dots Loaded Nanocomposites Chitosan Film for Wound Healing and Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Fang, J.; Wang, H.; Bao, X.; Ni, Y.; Teng, Y.; Liu, J.; Carbon, X.S. Nanodiamond as Efficient Peroxidase Mimic against Periodontal Bacterial Infection; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Cam, M.; Ertas, B.; Alenezi, H. Accelerated Diabetic Wound Healing by Topical Application of Combination Oral Antidiabetic Agents-Loaded Nanofibrous Scaffolds: An In Vitro and In Vivo Evaluation; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Li, Z.; Wang, H.; Yang, B.; Sun, Y. Three-Dimensional Graphene Foams Loaded with Bone Marrow Derived Mesenchymal Stem Cells Promote Skin Wound Healing with Reduced Scarring; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Jamal, M.; Shaikh, F.M.; Aslam, B.; Razeeb, K.M. Sensor and biosensor to detect vascular graft infection: Diagnosis and challenges. Anal. Methods 2012, 4, 1865–1875. [Google Scholar] [CrossRef]
- Bhalla, N.; Jolly, P.; Formisano, N.; Estrela, P. Introduction to biosensors. Essays Biochem. 2016, 60, 1–8. [Google Scholar] [CrossRef]
- Slaughter, G. Current Advances in Biosensor Design and Fabrication. In Encyclopedia of Analytical Chemistry; John Wiley & Sons, Ltd.: Chichester, UK, 2018; pp. 1–25. [Google Scholar]
- Dervisevic, M.; Dervisevic, E.; Şenel, M. Recent progress in nanomaterial-based electrochemical and optical sensors for hypoxanthine and xanthine. A review. Microchim. Acta 2019, 186, 749. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.-H.; Shih, J.-S. Immobilized Fullerene C60-Enzyme-Based Electrochemical Glucose Sensor. J. Chin. Chem. Soc. 2011, 58, 228–235. [Google Scholar] [CrossRef]
- Krueger, A.; Lang, D. Functionality is Key: Recent Progress in the Surface Modification of Nanodiamond. Adv. Funct. Mater. 2012, 22, 890–906. [Google Scholar] [CrossRef]
- Gruber, A.; Dräbenstedt, A.; Tietz, C.; Fleury, L.; Wrachtrup, J.; von Borczyskowski, C. Scanning Confocal Optical Microscopy and Magnetic Resonance on Single Defect Centers. Science 1997, 276, 2012–2014. [Google Scholar] [CrossRef]
- Baptista, F.; Belhout, S.; Giordani, S.; Quinn, S.J. Recent developments in carbon nanomaterial sensors. Chem. Soc. Rev. 2015, 44, 4433–4453. [Google Scholar] [CrossRef]
- Balasubramanian, K.; Burghard, M. Biosensors based on carbon nanotubes. Anal. Bioanal. Chem. 2006, 385, 452–468. [Google Scholar] [CrossRef]
- Wang, J. Carbon-Nanotube Based Electrochemical Biosensors: A Review. Electroanalysis 2005, 17, 7–14. [Google Scholar] [CrossRef]
- Vashist, S.K.; Zheng, D.; Al-Rubeaan, K.; Luong, J.H.; Sheu, F.-S. Advances in carbon nanotube based electrochemical sensors for bioanalytical applications. Biotechnol. Adv. 2011, 29, 169–188. [Google Scholar] [CrossRef]
- Gupta, S.; Murthy, C.; Prabha, C.R. Recent advances in carbon nanotube based electrochemical biosensors. Int. J. Biol. Macromol. 2018, 108, 687–703. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, C.; Peairs, M. Carbon Nanotube Based Electrochemical Sensors for Biomolecules; Elsevier: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Zhu, L.; Deng, C.; Chen, P.; You, X.; Su, H.; Yuan, Y.; Carbon, M.Z. Glucose oxidase biosensors based on carbon nanotube non-woven fabrics. Carbon 2014, 1, 795–796. [Google Scholar] [CrossRef]
- Zhu, C.; Yang, G.; Li, H.; Du, D.; Lin, Y. Electrochemical Sensors and Biosensors Based on Nanomaterials and Nanostructures. Anal. Chem. 2015, 87, 230–249. [Google Scholar] [CrossRef] [PubMed]
- González-Gaitán, C.; Ruiz-Rosas, R. Effects of the surface chemistry and structure of carbon nanotubes on the coating of glucose oxidase and electrochemical biosensors performance. RSC Adv. 2017, 7, 26867–26878. [Google Scholar] [CrossRef]
- Ulissi, Z.W.; Sen, F.; Gong, X.; Sen, S.; Iverson, N.; Boghossian, A.A.; Godoy, L.C.; Wogan, G.N.; Mukhopadhyay, D.; Strano, M.S. Spatiotemporal Intracellular Nitric Oxide Signaling Captured Using Internalized, Near-Infrared Fluorescent Carbon Nanotube Nanosensors. Nano Lett. 2014, 14, 4887–4894. [Google Scholar] [CrossRef]
- Mphuthi, N.G.; Adekunle, A.; Ebenso, E.E. Electrocatalytic oxidation of Epinephrine and Norepinephrine at metal oxide doped phthalocyanine/MWCNT composite sensor. Sci. Rep. 2016, 6, 26938. [Google Scholar] [CrossRef]
- Kumar, A.; Sun, S.-S.; Lees, A.J. Directed assembly metallocyclic supramolecular systems for molecular recognition and chemical sensing. Coord. Chem. Rev. 2008, 252, 922–939. [Google Scholar] [CrossRef]
- Tang, L.A.L.; Wang, J.; Loh, K.P. Graphene-Based SELDI Probe with Ultrahigh Extraction and Sensitivity for DNA Oligomer. J. Am. Chem. Soc. 2010, 132, 10976–10977. [Google Scholar] [CrossRef]
- Pilehvar, S.; De Wael, K. Recent Advances in Electrochemical Biosensors Based on Fullerene-C60 Nano-Structured Platforms. Biosensors 2015, 5, 712–735. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, H.; Itoh, T.; Hayashi, H.; Takagi, K. Electrochemical detection of E. coli 16S rDNA sequence using air-plasma-activated fullerene-impregnated screen printed electrodes. Bioelectrochemistry 2007, 70, 481–487. [Google Scholar] [CrossRef]
- Zhu, S.; Nanoscale, G.X. Single-walled carbon nanohorns and their applications. Nanoscale 2010, 2, 2538–2549. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Gong, L.; Dai, H.; Li, X.; Zhang, S.; Lu, S. Electrochemical bisphenol A sensor based on carbon nanohorns. Anal. Methods 2013, 5, 3328–3333. [Google Scholar] [CrossRef]
- Dai, H.; Gong, L.; Xu, G.; Zhang, S.; Lu, S.; Jiang, Y. An Electrochemical Sensing Platform Structured with Carbon Nanohorns for Detecting Some Food Borne Contaminants; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Zhu, S.; Gao, W.; Zhang, L.; Zhao, J. Simultaneous Voltammetric Determination of Dihydroxybenzene Isomers at Single-Walled Carbon Nanohorn Modified Glassy Carbon Electrode; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Zhu, S.; Han, S.; Zhang, L.; Parveen, S. A novel fluorescent aptasensor based on single-walled carbon nanohorns. Nanoscale 2011, 3, 4589–4592. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Liu, Z.; Zhang, W.; Han, S.; Hu, L. Nucleic acid detection using single-walled carbon nanohorns as a fluorescent sensing platform. Chem. Commun. 2011, 47, 6099–6101. [Google Scholar] [CrossRef] [PubMed]
- Landry, M.P.; Ando, H.; Chen, A.Y.; Cao, J.; Kottadiel, V.I.; Chio, L.; Yang, D.; Dong, J.; Lu, T.K.; Strano, M.S. Single-molecule detection of protein efflux from microorganisms using fluorescent single-walled carbon nanotube sensor arrays. Nat. Nanotechnol. 2017, 12, 368–377. [Google Scholar] [CrossRef]
- Baldo, S.; Buccheri, S.; Ballo, A. Carbon Nanotube-Based Sensing Devices for Human Ar-Ginase-1 Detection; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Huang, Y.; Miao, Y.-E.; Ji, S.; Tjiu, W.W.; Liu, T. Electrospun Carbon Nanofibers Decorated with Ag–Pt Bimetallic Nanoparticles for Selective Detection of Dopamine. ACS Appl. Mater. Interfaces 2014, 6, 12449–12456. [Google Scholar] [CrossRef]
- Cheng, D.; Yang, L.; Li, X.; Zhou, J.; Chen, Q.; Yan, S.; Li, N.; Chu, M.; Dong, Y.; Xie, Z.; et al. An Electrochemical DNA Sens-ing Platform Using Carboxyl Functionalized Graphene as the Electrode Modified Material. J. Electrochem. Soc. 2017, 164, H345–H351. [Google Scholar] [CrossRef]
- Mani, V.; Devasenathipathy, R. A novel glucose biosensor at glucose oxidase immobilized graphene and bismuth nanocomposite film modified electrode. Citeseer 2015, 10, 691–700. [Google Scholar]
- Yao, D.; He, Z.; Wen, G.; Liang, A. A facile and highly sensitive resonance Rayleigh scattering-energy transfer method for urea using a fullerene probe. RSC Adv. 2018, 8, 29008–29012. [Google Scholar] [CrossRef]
- Malik, N.; Arfin, T. Graphene Nanomaterials: Chemistry and Pharmaceutical Perspectives; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Fakhri, N.; Wessel, A.D.; Willms, C.; Pasquali, M.; Klopfenstein, D.R.; MacKintosh, F.C.; Schmidt, C.F. High-resolution mapping of intracellular fluctuations using carbon nanotubes. Science 2014, 344, 1031–1035. [Google Scholar] [CrossRef] [PubMed]
- Huth, K.; Glaeske, M.; Achazi, K.; Gordeev, G.; Kumar, S.; Arenal, R.; Sharma, S.K.; Adeli, M.; Setaro, A.; Reich, S.; et al. Fluorescent Polymer-Single-Walled Carbon Nanotube Complexes with Charged and Noncharged Dendronized Perylene Bisimides for Bioimaging Studies. Small 2018, 14, e1800796. [Google Scholar] [CrossRef] [PubMed]
- Leeuw, T.K.; Reith, R.; Simonette, R.A.; Harden, M.E.; Cherukuri, P.; Tsyboulski, D.A.; Beckingham, K.M.; Weisman, R.B. Single-Walled Carbon Nanotubes in the Intact Organism: Near-IR Imaging and Biocompatibility Studies in Drosophila. Nano Lett. 2007, 7, 2650–2654. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Ohulchanskyy, T.; Liu, R.; Koynov, K.; Wu, D.; Best, A.; Kumar, R.; Bonoiu, A.; Prasad, P.N. Photoluminescent Carbon Dots as Biocompatible Nanoprobes for Targeting Cancer Cells in Vitro. J. Phys. Chem. C 2010, 114, 12062–12068. [Google Scholar] [CrossRef]
- Yang, K.; Zhang, S.; Zhang, G.; Sun, X.; Lee, S.-T.; Liu, Z. Graphene in Mice: Ultrahigh In Vivo Tumor Uptake and Efficient Photothermal Therapy. Nano Lett. 2010, 10, 3318–3323. [Google Scholar] [CrossRef]
- Yang, F.; Han, J.; Zhuo, Y.; Yang, Z.; Chai, Y.; Yuan, R. Highly sensitive impedimetric immunosensor based on single-walled carbon nanohorns as labels and bienzyme biocatalyzed precipitation as enhancer for cancer biomarker detection. Biosens. Bioelectron. 2014, 55, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Z.; Song, L.; Zheng, J.; Hu, D.; He, M.; Zheng, M.; Gao, G.; Gong, P.; Zhang, P.; Ma, Y.; et al. Protein-assisted fabrication of nano-reduced graphene oxide for combined in vivo photoacoustic imaging and photothermal therapy. Biomaterials 2013, 34, 5236–5243. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.-H.; Chen, Y.-W.; Hung, W.-T.; Chen, I.-W.; Chen, S.-Y. Quantum-Dot-Tagged Reduced Graphene Oxide Nanocomposites for Bright Fluorescence Bioimaging and Photothermal Therapy Monitored In Situ. Adv. Mater. 2012, 24, 1748–1754. [Google Scholar] [CrossRef]
- Yogesh, G.K.; Shuaib, E.; Roopmani, P.; Gumpu, M.B.; Krishnan, U.M.; Sastikumar, D. Synthesis, characterization and bioimaging application of laser-ablated graphene-oxide nanoparticles (nGOs). Diam. Relat. Mater. 2020, 104, 107733. [Google Scholar] [CrossRef]
- Kim, B.; Kim, J.; Kim, M.; Kim, B.-D.; Jo, S.; Park, S.; Son, J.; Hwang, S.; Dugasani, S.; Chang, I.; et al. Ternary and senary rep-resentations using DNA double-crossover tiles. Nanotechnology 2016, 10, 105601. [Google Scholar] [CrossRef]
- Nagai, Y.; Nakamura, K.; Yudasaka, M.; Shiraki, T.; Fujigaya, T. Radical Polymer Grafting on the Surface of Single-Walled Carbon Nanotubes Enhances Photoluminescence in the Near-Infrared Region: Implications for Bioimaging and Biosensing. ACS Appl. Nano Mater. 2020, 3, 8840–8847. [Google Scholar] [CrossRef]
- Siddique, S.; Chow, J.C.L. Application of Nanomaterials in Biomedical Imaging and Cancer Therapy. Nanomaterials 2020, 10, 1700. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, J.; Sun, M.; Yu, J.; Chen, Y. A fluorescence–Raman dual-imaging platform based on complexes of conjugated polymers and carbon nanotubes. Nanoscale 2014, 6, 1480–1489. [Google Scholar] [CrossRef]
- Chen, B.; Li, F.; Li, S.; Weng, W.; Guo, H.; Guo, T.; Zhang, X.; Chen, Y.; Huang, T.; Hong, X.; et al. Large scale synthesis of photoluminescent carbon nanodots and their application for bioimaging. Nanoscale 2013, 5, 1967–1971. [Google Scholar] [CrossRef]
- Zhang, L.; Xiao, S.; Zheng, L. Aptamer-mediated nanocomposites of semiconductor quan-tum dots and graphene oxide as well as their applications in intracellular imaging and targeted drug. J. Mater. Chem. B 2014, 2, 8558–8565. [Google Scholar] [CrossRef]
- Tan, L.; Wu, T.; Tang, Z.; Xiao, J.; Zhuo, R. Water-soluble photoluminescent fullerene capped mesoporous silica for pH-responsive drug delivery and bioimaging. Nanotechnology 2016, 27, 315104. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Hao, T.; Ou, S.; Hu, C.; Chen, L. Applications and perspectives of nanomaterials in novel vaccine development. MedChemComm 2018, 9, 226–238. [Google Scholar] [CrossRef] [PubMed]
- Carbone, M.; Valentini, F.; Caminiti, R.; Petrinca, A.R.; Donia, D.; Divizia, M.; Palleschi, G. Are PEI-coated SWCNTs conjugated with hepatitis A virus? A chemical study with SEM, Z-potential, EDXD and RT-PCR. Biomed. Mater. 2010, 5, 35001. [Google Scholar] [CrossRef][Green Version]
- Yandar, N.; Pastorin, G.; Prato, M.; Bianco, A.; Patarroyo, M.E.; Lozano, J.M. Immunological profile of a Plasmodium vivax AMA-1 N-terminus peptide-carbon nanotube conjugate in an infected Plasmodium berghei mouse model. Vaccine 2008, 26, 5864–5873. [Google Scholar] [CrossRef]
- Zeinali, M.; Jammalan, M.; Ardestani, S.K.; Mosaveri, N. Immunological and cytotoxicological characterization of tuberculin purified protein derivative (PPD) conjugated to single-walled carbon nanotubes. Immunol. Lett. 2009, 126, 48–53. [Google Scholar] [CrossRef]
- Pantarotto, D.; Partidos, C.D.; Graff, R.; Hoebeke, J.; Briand, J.-P.; Prato, M.; Bianco, A. Synthesis, Structural Characterization, and Immunological Properties of Carbon Nanotubes Functionalized with Peptides. J. Am. Chem. Soc. 2003, 125, 6160–6164. [Google Scholar] [CrossRef]
- Pantarotto, D.; Partidos, C.D.; Hoebeke, J.; Brown, F.; Kramer, E.; Briand, J.-P.; Muller, S.; Prato, M.; Bianco, A. Immunization with Peptide-Functionalized Carbon Nanotubes Enhances Virus-Specific Neutralizing Antibody Responses. Chem. Biol. 2003, 10, 961–966. [Google Scholar] [CrossRef] [PubMed]
- Pati, R.; Shevtsov, M.; Sonawane, A. Nanoparticle Vaccines Against Infectious Diseases. Front. Immunol. 2018, 9, 2224. [Google Scholar] [CrossRef]
- Meng, J.; Duan, J.; Kong, H.; Li, L.; Wang, C.; Xie, S.; Chen, S.; Gu, N.; Xu, H.; Yang, X.-D. Carbon Nanotubes Conjugated to Tumor Lysate Protein Enhance the Efficacy of an Antitumor Immunotherapy. Small 2008, 4, 1364–1370. [Google Scholar] [CrossRef]
- Maslak, P.G.; Dao, T.; Krug, L.M.; Chanel, S.; Korontsvit, T.; Zakhaleva, V.; Zhang, R.; Wolchok, J.D.; Yuan, J.; Pinilla-Ibarz, J.; et al. Vaccination with synthetic analog peptides derived from WT1 oncoprotein induces T-cell responses in patients with complete remission from acute myeloid leukemia. Blood 2010, 116, 171–179. [Google Scholar] [CrossRef]
- Villa, C.H.; Dao, T.; Ahearn, I.; Fehrenbacher, N.; Casey, E.; Rey, D.A.; Korontsvit, T.; Zakhaleva, V.; Batt, C.A.; Philips, M.R.; et al. Single-Walled Carbon Nanotubes Deliver Peptide Antigen into Dendritic Cells and Enhance IgG Responses to Tumor-Associated Antigens. ACS Nano 2011, 5, 5300–5311. [Google Scholar] [CrossRef]
- Xu, L.; Liu, Y.; Chen, Z.; Li, W.; Liu, Y.; Wang, L.; Ma, L.; Shao, Y.; Zhao, Y.; Chen, C. Morphologically Virus-Like Fullerenol Nanoparticles Act as the Dual-Functional Nanoadjuvant for HIV-1 Vaccine. Adv. Mater. 2013, 25, 5928–5936. [Google Scholar] [CrossRef]
- Cao, W.; He, L.; Huang, X.; Jia, K.; Dai, J. Recent progress of graphene oxide as a potential vaccine carrier and adjuvant. Acta Biomater. 2020, 112, 14–28. [Google Scholar] [CrossRef]
- Li, H.; Fierens, K.; Zhang, Z.; Vanparijs, N.; Schuijs, M.J.; Van Steendam, K.; Gracia, N.F.; De Rycke, R.; De Beer, T.; De Beuckelaer, A.; et al. Spontaneous Protein Adsorption on Graphene Oxide Nanosheets Allowing Efficient Intracellular Vaccine Protein Delivery. ACS Appl. Mater. Interfaces 2016, 8, 1147–1155. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Li, Y.; Wang, Q.; Wang, G.; Zhu, B. Carbon Nanotube-Based DNA Vaccine against Koi Herpesvirus Given by Intramuscular Injection; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- De Faria, P.C.B.; Dos Santos, L.I.; Coelho, J.P.; Ribeiro, H.B.; Pimenta, M.A.; Ladeira, L.O.; Gomes, D.A.; Furtado, C.A.; Gazzinelli, R.T. Oxidized Multiwalled Carbon Nanotubes as Antigen Delivery System to Promote Superior CD8+ T Cell Response and Protection against Cancer. Nano Lett. 2014, 14, 5458–5470. [Google Scholar] [CrossRef] [PubMed]
- Hassan, H.A.; Smyth, L.; Wang, T.-W.; da Costa, P.M.C.; Ratnasothy, K.; Diebold, S.S.; Lombardi, G.; Al-Jamal, K.T. Dual stimulation of antigen presenting cells using carbon nanotube-based vaccine delivery system for cancer immunotherapy. Biomaterials 2016, 104, 310–322. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Feng, X.; Chen, Z.; Yang, X.; Shen, Z. The Adjuvant Effect of C60(OH)22 Nanoparticles Promoting Both Humoral and Cellular Immune Responses to HCV Recombinant Proteins; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Li, S.; Guo, Z.; Zeng, G.; Zhang, Y.; Xue, W.; Liu, Z. Polyethylenimine-Modified Fluorescent Carbon Dots As Vaccine Delivery System for Intranasal Immunization. ACS Biomater. Sci. Eng. 2018, 4, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cao, F.; Yan, M.; Liu, Y.; Zhu, X.; Sun, H.; Ma, G. Alum-functionalized graphene oxide nanocomplexes for effective anticancer vaccination. Acta Biomater. 2019, 83, 390–399. [Google Scholar] [CrossRef]
- Dolmans, D.E.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef]
- Hwang, H.S.; Shin, H.; Han, J.; Na, K. Combination of photodynamic therapy (PDT) and anti-tumor immunity in cancer therapy. J. Pharm. Investig. 2018, 48, 143–151. [Google Scholar] [CrossRef]
- Mroz, P.; Yaroslavsky, A.; Kharkwal, G.B.; Hamblin, M.R. Cell Death Pathways in Photodynamic Therapy of Cancer. Cancers 2011, 3, 2516–2539. [Google Scholar] [CrossRef]
- Albert, K.; Hsu, H.-Y. Carbon-Based Materials for Photo-Triggered Theranostic Applications. Molecules 2016, 21, 1585. [Google Scholar] [CrossRef] [PubMed]
- Ogbodu, R.; Limson, J.; Prinsloo, E. Photophysical Properties and Photodynamic Therapy Effect of Zinc Phthalocyanine-Spermine-Single Walled Carbon Nanotube Conjugate on MCF-7 Breast Cancer Cell; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Augustine, S.; Singh, J.; Srivastava, M.; Sharma, M.; Das, A.; Malhotra, B.D. Recent advances in carbon based nanosystems for cancer theranostics. Biomater. Sci. 2017, 5, 901–952. [Google Scholar] [CrossRef] [PubMed]
- Nurunnabi; Khatun, Z.; Reeck, G.R.; Lee, D.Y.; Lee, Y.-K. Photoluminescent Graphene Nanoparticles for Cancer Phototherapy and Imaging. ACS Appl. Mater. Interfaces 2014, 6, 12413–12421. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ohta, S.; Sonoda, A. Preparation of PEG-conjugated fullerene containing Gd3+ ions for photodynamic therapy. J. Control. Release 2007, 117, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Mroz, P.; Tegos, G.P.; Gali, H.; Wharton, T.; Sarna, T.; Hamblin, M.R. Photodynamic therapy with fullerenes. Photochem. Photobiol. Sci. 2007, 6, 1139–1149. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, W.; Cui, Y.; Chu, X.; Sun, B. Magnetofluorescent Fe3O4/Carbon Quantum Dots Coated Single-Walled Carbon Nanotubes as Dual-Modal Targeted Imaging and Chemo/Photodynamic/Photothermal; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Marangon, I.; Ménard-Moyon, C.; Silva, A. Synergic Mechanisms of Photothermal and Photodynamic Therapies Mediated by Photosensitizer/Carbon Nanotube Complexes; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Jiang, B.-P.; Hu, L.-F.; Shen, X.-C.; Ji, S.-C.; Shi, Z.; Liu, C.-J.; Zhang, L.; Liang, H. One-Step Preparation of a Water-Soluble Carbon Nanohorn/Phthalocyanine Hybrid for Dual-Modality Photothermal and Photodynamic Therapy. ACS Appl. Mater. Interfaces 2014, 6, 18008–18017. [Google Scholar] [CrossRef]
- Shi, J.; Wang, B.; Wang, L.; Lu, T.; Fu, Y. Fullerene (C60)-Based Tumor-Targeting Nanoparticles with “Off-On” State for Enhanced Treatment of Cancer; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Akbari, T.; Pourhajibagher, M.; Hosseini, F.; Chiniforush, N.; Gholibegloo, E.; Khoobi, M.; Shahabi, S.; Bahador, A. The effect of indocyanine green loaded on a novel nano-graphene oxide for high performance of photodynamic therapy against Enterococcus faecalis. Photodiagn. Photodyn. Ther. 2017, 20, 148–153. [Google Scholar] [CrossRef]
- Wang, H.; Pan, X.; Wang, X.; Wang, W.; Huang, Z.; Gu, K.; Liu, S.; Zhang, F.; Shen, H.; Yuan, Q.; et al. Degradable Carbon–Silica Nanocomposite with Immunoadjuvant Property for Dual-Modality Photothermal/Photodynamic Therapy. ACS Nano 2020, 14, 2847–2859. [Google Scholar] [CrossRef]
- Harrison, R.G.; Resasco, D.E.; Neves, L.F.F. Compositions and Methods for Cancer Treatment Using Targeted Carbon Nanotubes. U.S. Patent Application No. 9,504,745, 9 December 2013. [Google Scholar]
- Harrison, R.G.; Neves, L.F.F.; Resasco, D.E. Compositions and Methods for Cancer Treatment Using Targeted Carbon Nanotubes. U.S. Patent Application No. 8,518,870, 27 August 2013. [Google Scholar]
- Carroll, D.L.; Stewart, J.H.; Levi, N.H. Wake Forest University, Wake Forest University Health Sciences, assignee. Compositions and Methods for Treating Cancer. U.S. Patent 8,501,233, 6 August 2013. [Google Scholar]
- Wilson, L.J.; Kissell, K.R.; Hartman, K.B. Carbon Nanotube Based Imaging Agents. U.S. Patent 8,986,942, 24 March 2015. [Google Scholar]
- Hirsch, A.; Sagman, U.; Wilson, S.R. Use of Buckysome or Carbon Nanotube for Drug Delivery. U.S. Patent 7,070,810, 4 July 2006. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).