Tuning Stoichiometry and Structure of Pd-WO3−x Thin Films for Hydrogen Gas Sensing by High-Power Impulse Magnetron Sputtering
Abstract
1. Introduction
2. Experimental Section
2.1. Material Synthesis
2.2. Film Characterization
3. Results and Discussion
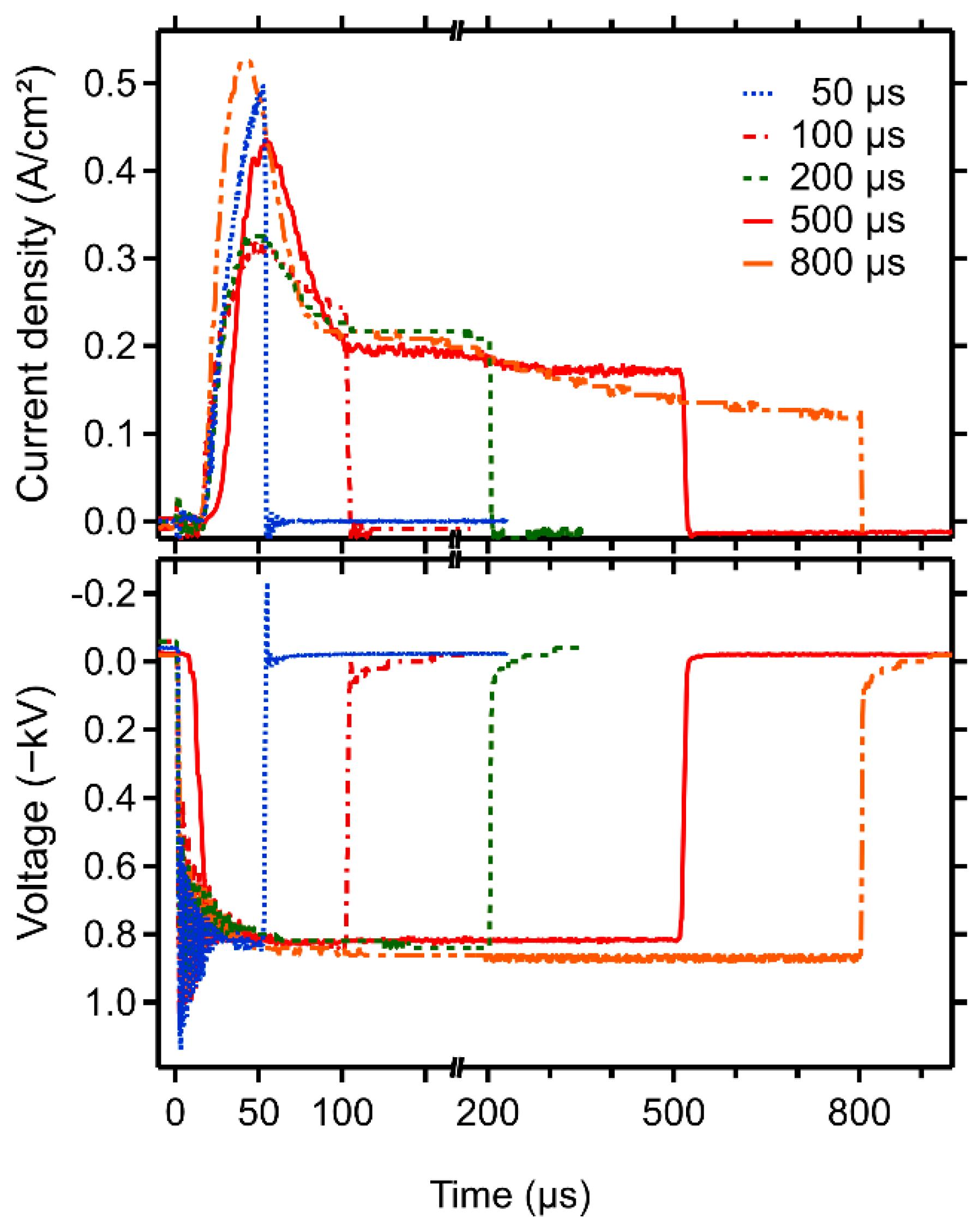
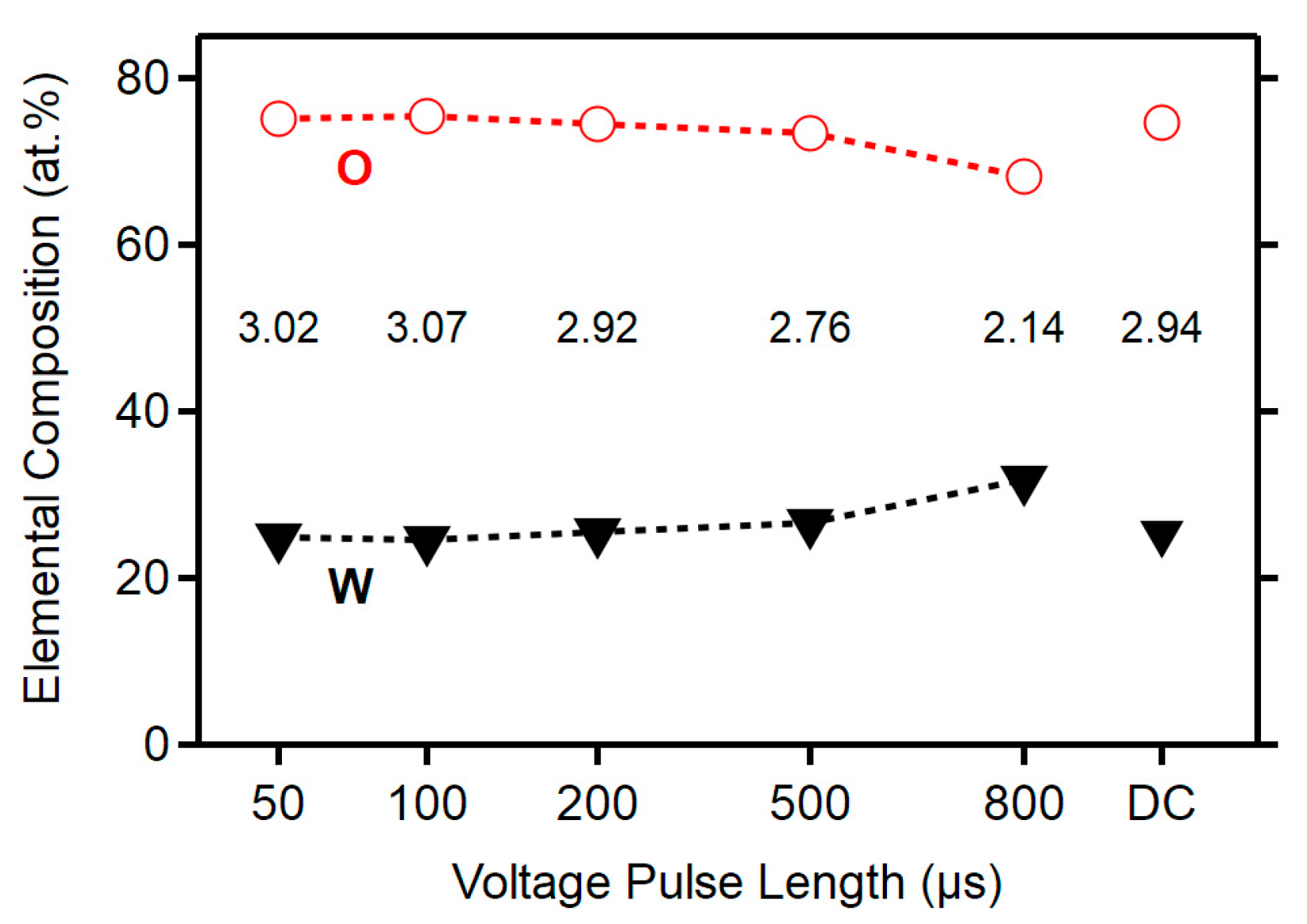
3.1. Deposition Parameters and Composition
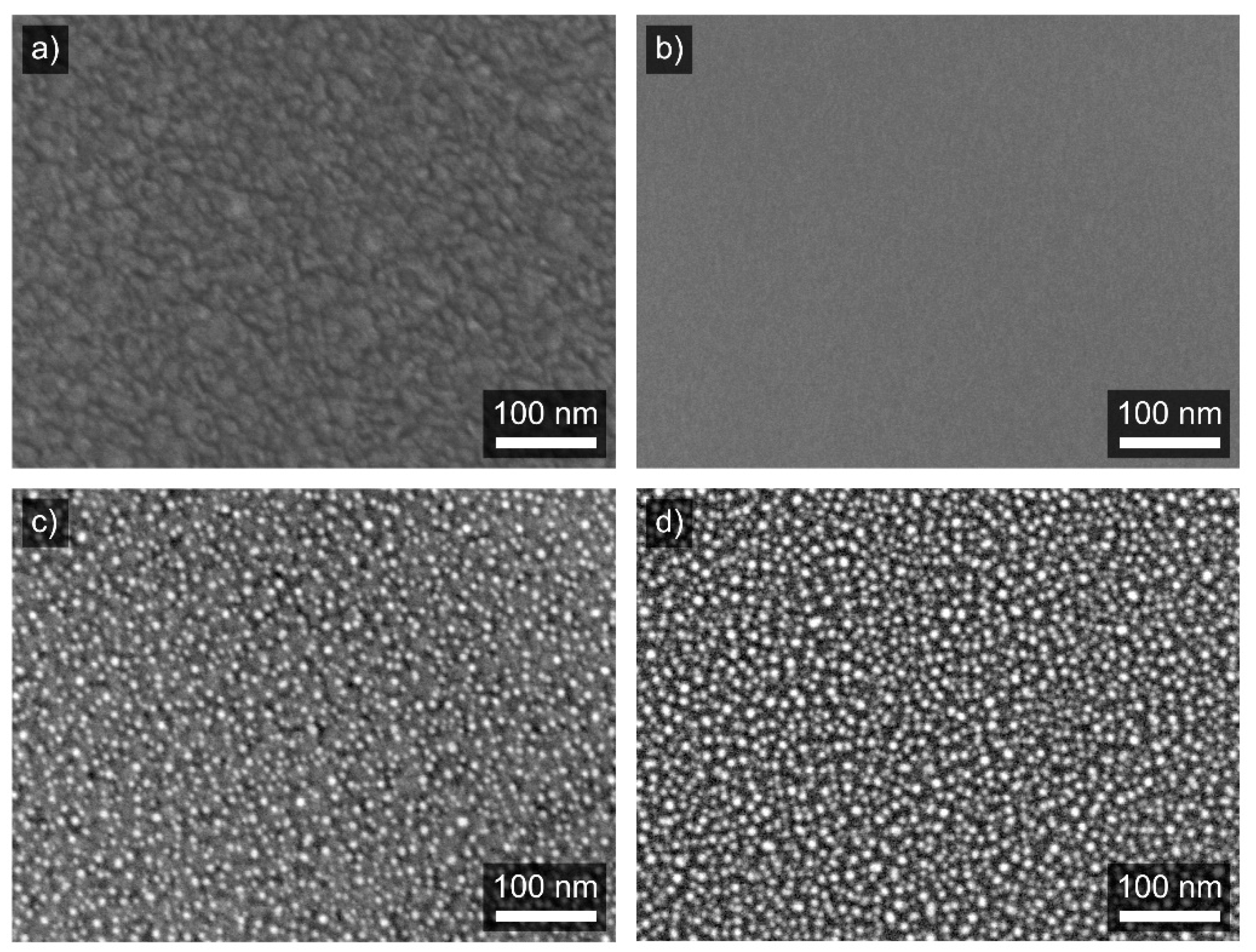
3.2. Structure
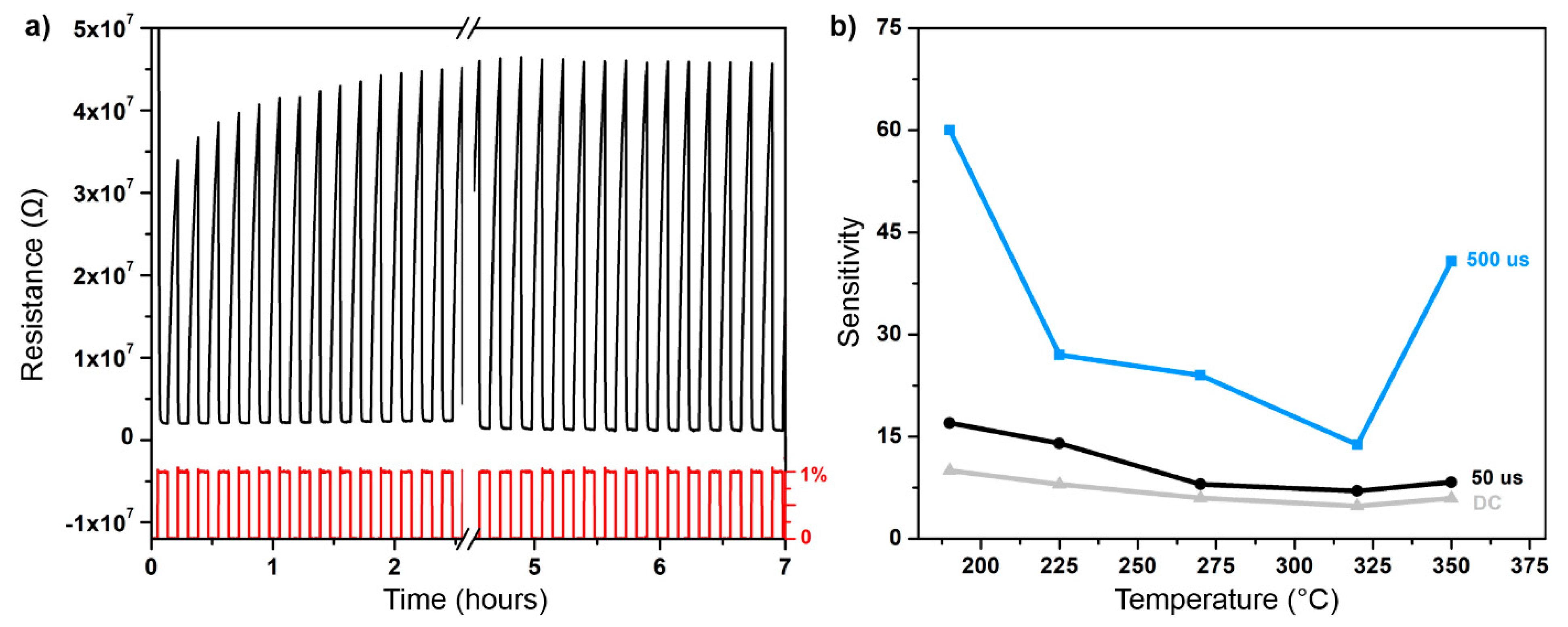
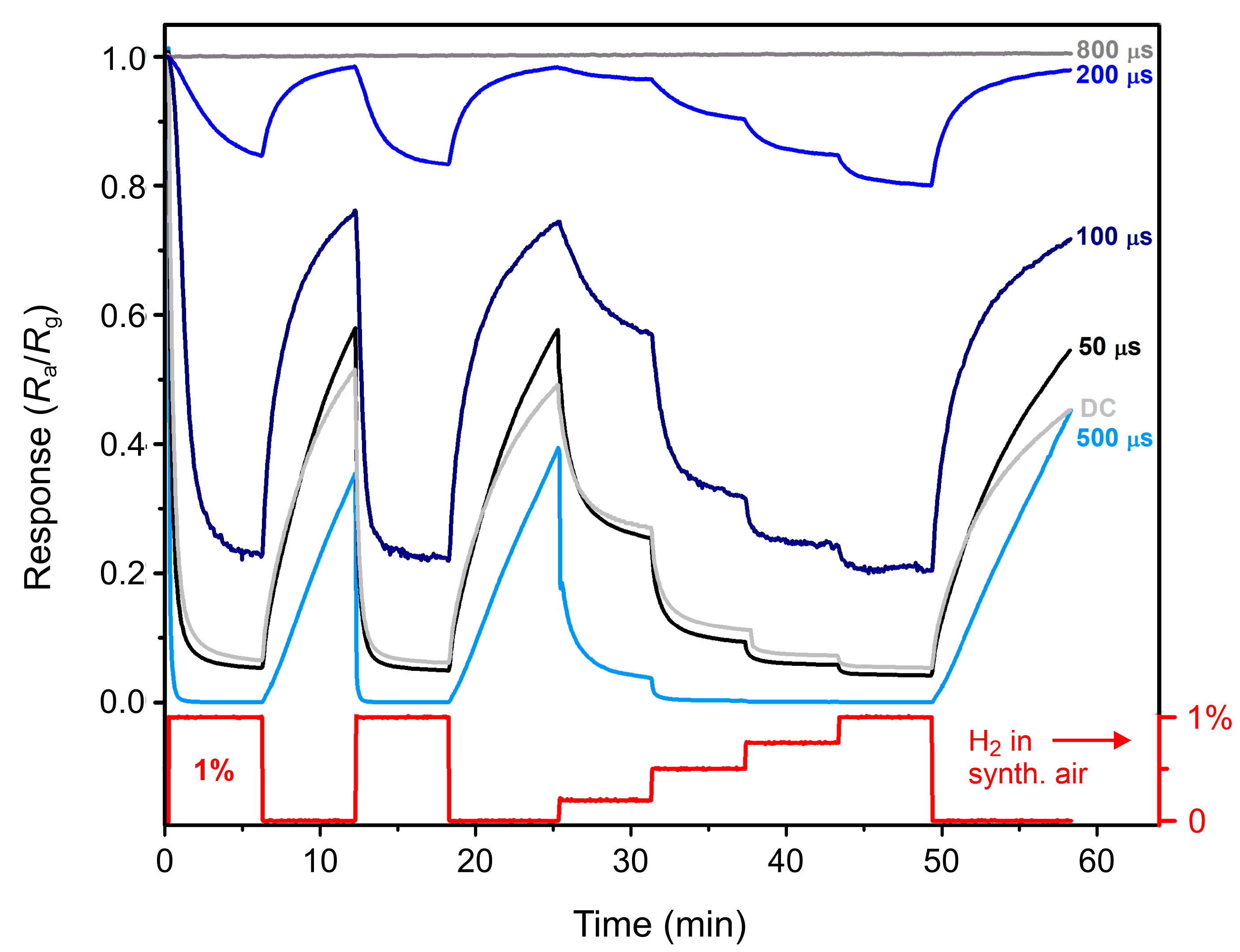
3.3. Sensory Response and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Moseley, P.T. Progress in the development of semiconducting metal oxide gas sensors: A review. Meas. Sci. Technol. 2017, 28, 082001. [Google Scholar] [CrossRef]
- Phanichphant, S. Semiconductor Metal Oxides as Hydrogen Gas Sensors. Procedia Eng. 2014, 87, 795–802. [Google Scholar] [CrossRef]
- Boudiba, A.; Roussel, P.; Zhang, C.; Olivier, M.-G.; Snyders, R.; Debliquy, M. Sensing mechanism of hydrogen sensors based on Pd loaded tungsten oxide (Pd-WO 3). Sens. Actuators B Chem. 2013, 187, 84–93. [Google Scholar] [CrossRef]
- Fardindoost, S.; Zad, A.I.; Hosseini, Z.S.; Hatamie, S. Detecting hydrogen using graphene quantum dots/WO3 thin films. Mater. Res. Express 2016, 3, 116407. [Google Scholar] [CrossRef]
- Hübner, M.; Simion, C.E.; Haensch, A.; Barsan, N.; Weimar, U. CO sensing mechanism with WO3 based gas sensors. Sens. Actuators B Chem. 2010, 151, 103–106. [Google Scholar] [CrossRef]
- Prajapati, C.S.; Bhat, N. Ppb level detection of NO2 using a WO3 thin film-based sensor: Material optimization, device fabrication and packaging. RSC Adv. 2018, 8, 6590–6599. [Google Scholar] [CrossRef]
- Shimizu, Y.; Matsunaga, N.; Hyodo, T.; Egashira, M. Improvement of SO2 sensing properties of WO3 by noble metal loading. Sens. Actuators B Chem. 2001, 77, 35–40. [Google Scholar] [CrossRef]
- Susanti, D.; Perdana, A.S.; Purwaningsih, H.; Noerochim, L.; Kusuma, G.E. Preparation of CO gas sensor from WO3 nanomaterial synthesized via sol-gel method followed by hydrothermal process. AIP Conf. Proc. 2014, 1586, 14–19. [Google Scholar]
- Kaur, N.; Zappa, D.; Poli, N.; Comini, E. Integration of VLS-Grown WO3 Nanowires into Sensing Devices for the Detection of H2S and O3. ACS Omega 2019, 4, 16336–16343. [Google Scholar] [CrossRef]
- Godbole, R.; Vedpathak, A.; Godbole, V.; Bhagwat, S. Tungsten oxide thin films: Detection and trapping of hazardous gases. Mater. Res. Express 2017, 4, 076401. [Google Scholar] [CrossRef]
- Hübert, T.; Boon-Brett, L.; Black, G.; Banach, U. Hydrogen sensors—A review. Sens. Actuators B Chem. 2011, 157, 329–352. [Google Scholar] [CrossRef]
- Haviar, S.; Čapek, J.; Batková, Š.; Kumar, N.; Dvořák, F.; Duchoň, T.; Fialová, M.; Zeman, P. Hydrogen gas sensing properties of WO3 sputter-deposited thin films enhanced by on-top deposited CuO nanoclusters. Int. J. Hydrog. Energy 2018, 43, 22756–22764. [Google Scholar] [CrossRef]
- Ghasempour, R.; Mortazavi, S.; Zad, A.I.; Rahimi, F. Hydrogen sensing properties of multi-walled carbon nanotube films sputtered by Pd. Int. J. Hydrog. Energy 2010, 35, 4445–4449. [Google Scholar] [CrossRef]
- Liang, Y.C.; Chang, C.W. Preparation of orthorhombic WO3 thin films and their crystal quality-dependent dye photodegradation ability. Coatings 2019, 9, 90. [Google Scholar] [CrossRef]
- Safi, I. Recent aspects concerning DC reactive magnetron sputtering of thin films: A review. Surf. Coat. Technol. 2000, 127, 203–218. [Google Scholar] [CrossRef]
- Vlček, J.; Rezek, J.; Houška, J.; Kozák, T.; Kohout, J. Benefits of the controlled reactive high-power impulse magnetron sputtering of stoichiometric ZrO2 films. Vacuum 2015, 114, 131–141. [Google Scholar] [CrossRef]
- Musil, J.; Baroch, P.; Vlček, J.; Nam, K.H.H.; Han, J.G.G. Reactive magnetron sputtering of thin films: Present status and trends. Thin Solid Films 2005, 475, 208–218. [Google Scholar] [CrossRef]
- Vlček, J.; Rezek, J.; Houška, J.; Čerstvý, R.; Bugyi, R. Process stabilization and a significant enhancement of the deposition rate in reactive high-power impulse magnetron sputtering of ZrO2 and Ta2O5 films. Surf. Coat. Technol. 2013, 236, 550–556. [Google Scholar] [CrossRef]
- Vlček, J.; Belosludtsev, A.; Rezek, J.; Houška, J.; Čapek, J.; Čerstvý, R.; Haviar, S. High-rate reactive high-power impulse magnetron sputtering of hard and optically transparent HfO2 films. Surf. Coat. Technol. 2016, 290, 58–64. [Google Scholar] [CrossRef]
- Rezek, J.; Houška, J.; Procházka, M.; Haviar, S.; Kozák, T.; Baroch, P. In-Ga-Zn-O thin films with tunable optical and electrical properties prepared by high-power impulse magnetron sputtering. Thin Solid Films 2018, 658, 27–32. [Google Scholar] [CrossRef]
- Hemberg, A.; Dauchot, J.-P.; Snyders, R.; Konstantinidis, S. Evaporation-assisted high-power impulse magnetron sputtering: The deposition of tungsten oxide as a case study. J. Vac. Sci. Technol. A Vac. Surf. Film. 2012, 30, 040604. [Google Scholar] [CrossRef]
- Ganesan, R.; Akhavan, B.; Partridge, J.G.; McCulloch, D.G.; McKenzie, D.R.; Bilek, M.M.M. Evolution of target condition in reactive HiPIMS as a function of duty cycle: An opportunity for refractive index grading. J. Appl. Phys. 2017, 121, 171909. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Cho, B.K.; Han, S.H.; Cho, B.K. Metal oxide composites in conductometric gas sensors: Achievements and challenges. Sens. Actuators B Chem. 2017, 244, 182–210. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, B.; Xiao, S.; Li, H.; Wang, L.; Cai, D.; Wang, D.; Liu, Y.; Li, Q.; Wang, T. High performance and negative temperature coefficient of low temperature hydrogen gas sensors using palladium decorated tungsten oxide. J. Mater. Chem. A 2015, 3, 1317–1324. [Google Scholar] [CrossRef]
- Kumar, N.; Čapek, J.; Haviar, S. Nanostructured CuWO4/WO3-films prepared by reactive magnetron sputtering for hydrogen sensing. Int. J. Hydrog. Energy 2020. submitted. [Google Scholar] [CrossRef]
- Haviar, S.; Chlupová, Š.; Kúš, P.; Gillet, M.; Matolín, V.; Matolínová, I. Micro-contacted self-assembled tungsten oxide nanorods for hydrogen gas sensing. Int. J. Hydrog. Energy 2017, 42, 1344–1352. [Google Scholar] [CrossRef]
- Hála, M.; Čapek, J.; Zabeida, O.; Klemberg-Sapieha, J.E.; Martinu, L. Pulse management in high power pulsed magnetron sputtering of niobium. Surf. Coat. Technol. 2012, 206, 4186–4193. [Google Scholar] [CrossRef]
- Kozák, T.; Vlček, J. Dynamics of processes during the deposition of ZrO2 films by controlled reactive high-power impulse magnetron sputtering: A modelling study. J. Appl. Phys. 2017, 122, 1–9. [Google Scholar] [CrossRef]
- Kozák, T.; Vlček, J. A parametric model for reactive high-power impulse magnetron sputtering of films. J. Phys. D. Appl. Phys. 2016, 49, 055202. [Google Scholar] [CrossRef]
- Szilágyi, I.M.; Fórizs, B.; Rosseler, O.; Szegedi, Á.; Németh, P.; Király, P.; Tárkányi, G.; Vajna, B.; Varga-Josepovits, K.; László, K.; et al. WO3 photocatalysts: Influence of structure and composition. J. Catal. 2012, 294, 119–127. [Google Scholar] [CrossRef]
- Kuzmin, A.; Purans, J.; Cazzanelli, E.; Vinegoni, C.; Mariotto, G. X-ray diffraction, extended X-ray absorption fine structure and Raman spectroscopy studies of WO[sub 3] powders and (1−x)WO[sub 3−y]⋅xReO[sub 2] mixtures. J. Appl. Phys. 1998, 84, 5515. [Google Scholar] [CrossRef]
- Vinh, T.C.; Thinh, N.D.; Dat, H.T.; Tuan, T.; Phuong, D.A. Survey of WO3 thin film structure built on ito/glass substrates by the Raman and xrd spectroscopies. J. Sci. Math. Phys. 2009, 25, 47–55. [Google Scholar]
- Garcia-Sanchez, R.F.; Ahmido, T.; Casimir, D.; Baliga, S.; Misra, P. Thermal effects associated with the Raman spectroscopy of WO3 gas-sensor materials. J. Phys. Chem. A 2013, 117, 13825–13831. [Google Scholar] [CrossRef] [PubMed]
- Boulova, M.; Rosman, N.; Bouvier, P.; Lucazeau, G. High-pressure Raman study of microcrystalline WO3 tungsten oxide. J. Phys. Condens. Matter 2002, 14, 5849–5863. [Google Scholar] [CrossRef]
- Cazzanelli, E. Raman study of the phase transitions sequence in pure WO3 at high temperature and in HxWO3 with variable hydrogen content. Solid State Ion. 1999, 123, 67–74. [Google Scholar] [CrossRef]
- Staerz, A.; Berthold, C.; Russ, T.; Wicker, S.; Weimar, U.; Barsan, N. The oxidizing effect of humidity on WO3 based sensors. Sens. Actuators B Chem. 2016, 237, 54–58. [Google Scholar] [CrossRef]
- Szilágyi, I.M.; Saukko, S.; Mizsei, J.; Tóth, A.L.; Madarász, J.; Pokol, G. Gas sensing selectivity of hexagonal and monoclinic WO3 to H2S. Solid State Sci. 2010, 12, 1857–1860. [Google Scholar] [CrossRef]
- Yu, W.; Shen, Z.; Peng, F.; Lu, Y.; Ge, M.; Fu, X.; Sun, Y.; Chen, X.; Dai, N. Improving gas sensing performance by oxygen vacancies in sub-stoichiometric WO3−x. RSC Adv. 2019, 9, 7723–7728. [Google Scholar] [CrossRef]
- Gali, P.; Sapkota, G.; Syllaios, A.J.; Littler, C.; Philipose, U. Stoichiometry dependent electron transport and gas sensing properties of indium oxide nanowires. Nanotechnology 2013, 24, 225704. [Google Scholar] [CrossRef]
- De Marcellis, A.; Ferri, G.; Mantenuto, P.; Giancaterini, L.; Cantalini, C. WO3 hydrogen resistive gas sensor and its wide-range current-mode electronic read-out circuit. IEEE Sens. J. 2013, 13, 2792–2798. [Google Scholar] [CrossRef]
- Boudiba, A.; Zhang, C.; Umek, P.; Bittencourt, C.; Snyders, R.; Olivier, M.G.; Debliquy, M. Sensitive and rapid hydrogen sensors based on Pd-WO3 thick films with different morphologies. Int. J. Hydrog. Energy 2013, 38, 2565–2577. [Google Scholar] [CrossRef]
- Bose, R.J.; Illyaskutty, N.; Tan, K.S.; Rawat, R.; Matham, M.V.; Kohler, H.; Pillai, V.P.M. Hydrogen sensors based on Pt-loaded WO3 sensing layers. Europhys. Lett. 2016, 114, 66002. [Google Scholar] [CrossRef]
- Vallejos, S.; Grácia, I.; Chmela, O.; Figueras, E.; Hubálek, J.; Cané, C. Chemoresistive micromachined gas sensors based on functionalized metal oxide nanowires: Performance and reliability. Sens. Actuators B Chem. 2016, 235, 525–534. [Google Scholar] [CrossRef]


| Film | Sensitivity | Crystallinity after Annealing | Crystalline Phases | Sheet Resist. (Ω/sq) | As-Deposited Stoichiometry | Response Time (s) |
|---|---|---|---|---|---|---|
| 500 µs | 60 | moderate | monoclinic (triclinic) | 3.4 × 108 | 2.76 | 10 |
| 50 µs | 17 | moderate | tetragonal + monoclinic | 7.3 × 106 | 3.01 | 38 |
| DC | 10 | moderate | tetragonal + monoclinic | 1.2 × 106 | 2.94 | 70 |
| 100 µs | 3.8 | low | tetragonal + triclinic | 1.7 × 105 | 3.07 | 113 |
| 200 µs | 2.0 | low | triclinic (monoclinic) | 2.0 × 105 | 2.92 | 106 |
| 800 µs | – | high | triclinic (monoclinic) | 6.3 × 102 | 2.15 | – |
| Material | Response | Sensitivity Definition | Temperature, °C | Concentration, ppm | References |
|---|---|---|---|---|---|
| WO3 thin film | 5 | S =Ra/Rg | 150 | 250 | [40] |
| Pd-WO3 nanocomposites | 19 | S = (Ra − Rg)/Rg | 200 | 200 | [41] |
| Pt-WO3 thin film | ~5 | S = (Ra − Rg)/Rg | 400 | 500 | [42] |
| Pd-Graphene QDs on WO3 thin film | 1.12 | S = (Ra − Rg)/Rg | 150 | 3600 | [4] |
| Pt-WO3 nanowires | 20 | S = Ra/Rg | 200 | 100 | [43] |
| Pd-WO3 nanolamellae | 69 | S = (Ra − Rg)/Rg | 180 | 200 | [3] |
| Pd-WO3 | 12 | S = Ra/Rg | 190 | 2000 | This work |
| Pd-WO3 | 60 | S = Ra/Rg | 190 | 10,000 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, N.; Haviar, S.; Rezek, J.; Baroch, P.; Zeman, P. Tuning Stoichiometry and Structure of Pd-WO3−x Thin Films for Hydrogen Gas Sensing by High-Power Impulse Magnetron Sputtering. Materials 2020, 13, 5101. https://doi.org/10.3390/ma13225101
Kumar N, Haviar S, Rezek J, Baroch P, Zeman P. Tuning Stoichiometry and Structure of Pd-WO3−x Thin Films for Hydrogen Gas Sensing by High-Power Impulse Magnetron Sputtering. Materials. 2020; 13(22):5101. https://doi.org/10.3390/ma13225101
Chicago/Turabian StyleKumar, Nirmal, Stanislav Haviar, Jiří Rezek, Pavel Baroch, and Petr Zeman. 2020. "Tuning Stoichiometry and Structure of Pd-WO3−x Thin Films for Hydrogen Gas Sensing by High-Power Impulse Magnetron Sputtering" Materials 13, no. 22: 5101. https://doi.org/10.3390/ma13225101
APA StyleKumar, N., Haviar, S., Rezek, J., Baroch, P., & Zeman, P. (2020). Tuning Stoichiometry and Structure of Pd-WO3−x Thin Films for Hydrogen Gas Sensing by High-Power Impulse Magnetron Sputtering. Materials, 13(22), 5101. https://doi.org/10.3390/ma13225101






