Anti-Oxidative Activity of Alcohol-Water Extracts from Field Horsetail (Equisteum arvense) in Elastomer Vulcanizates Subjected to Accelerated Aging Processes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Elastomer
2.1.2. Cross-Linking System
2.1.3. Natural Extracts
2.1.4. Elastomer Vulcanizates
2.2. Methods
2.2.1. Thermogravimetric Analysis (TGA)
2.2.2. UV–Vis Diffuse Reflectance Spectroscopy
2.2.3. Fourier Transform Infrared Spectroscopy (FTIR)
2.2.4. Total Phenolic Content
2.2.5. Antioxidant Activity
2.2.6. Rubber Mixtures’ Preparation
2.2.7. Vulcanization
2.2.8. Atomic Absorption Spectrometry (AAS)
2.2.9. Aging Processes (UV, Thermo-Oxidative and Solar)
2.2.10. Mechanical Properties
2.2.11. Color Stability
2.2.12. The Cross-Linking Density of Vulcanizates
3. Results and Discussion
3.1. Thermogravimetric Analysis (TGA)
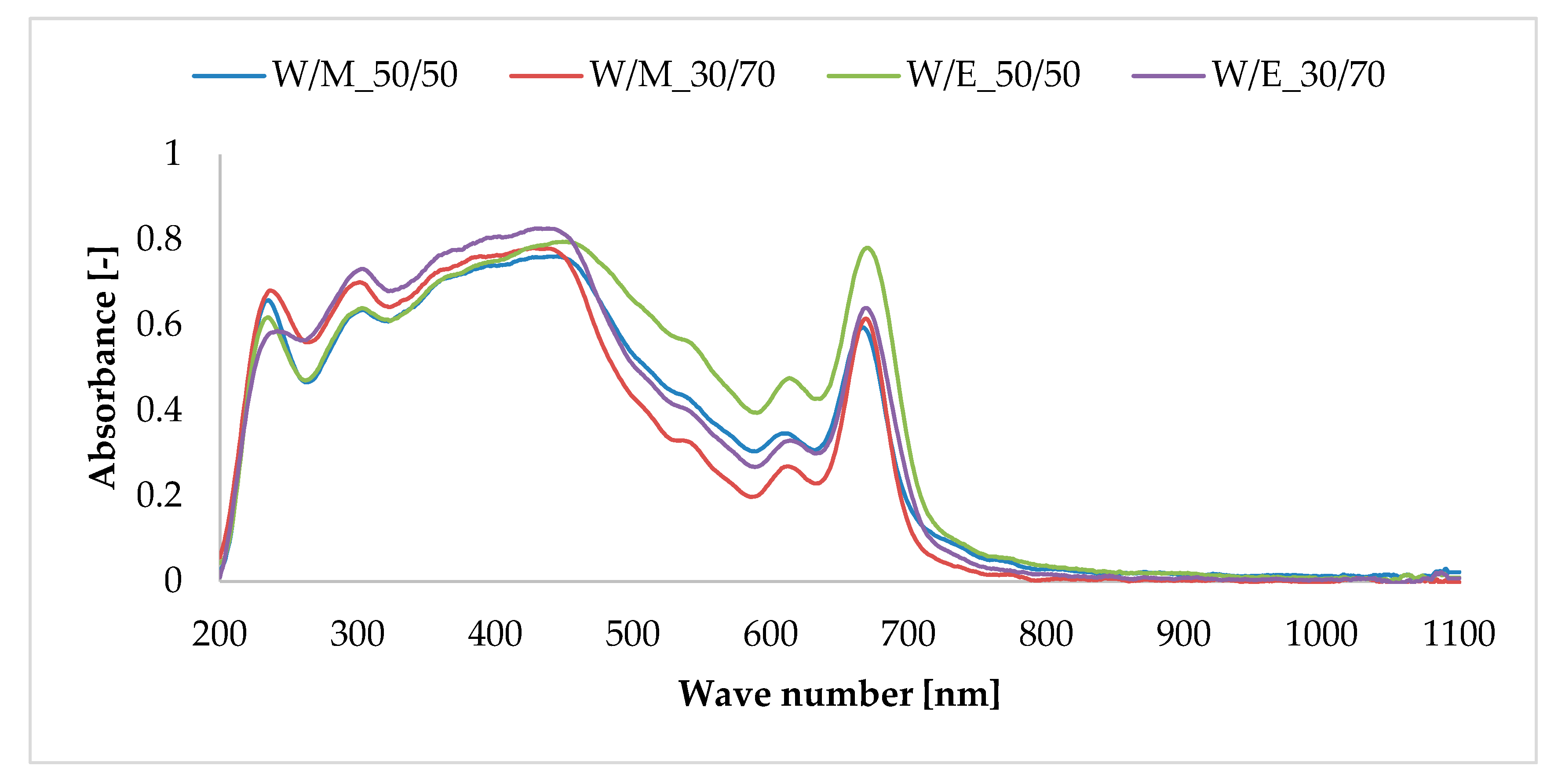
3.2. UV–Vis Diffuse Reflectance Spectroscopy
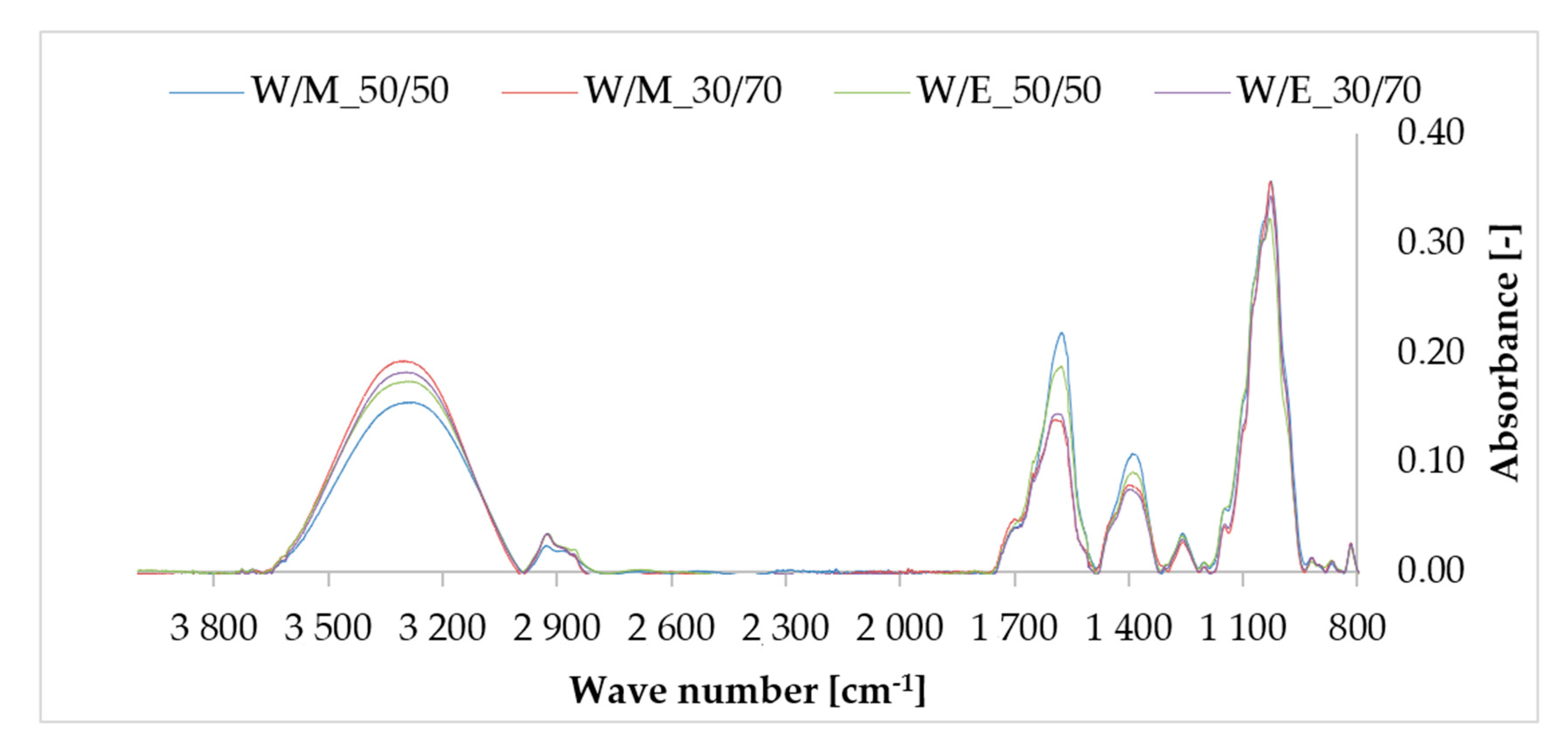
3.3. Fourier Transform Infrared Spectroscopy (FTIR)
3.4. Total Phenolic Content (TPC) and Antioxidant Activity of Horsetail Extracts
3.5. Atomic Absorption Spectrometry (AAS)
3.6. Influences of Aging Processes on the Properties of Vulcanizates
- Spatial structure (cross-linking density);
- Functional properties (mechanical properties);
- Physical properties (color).
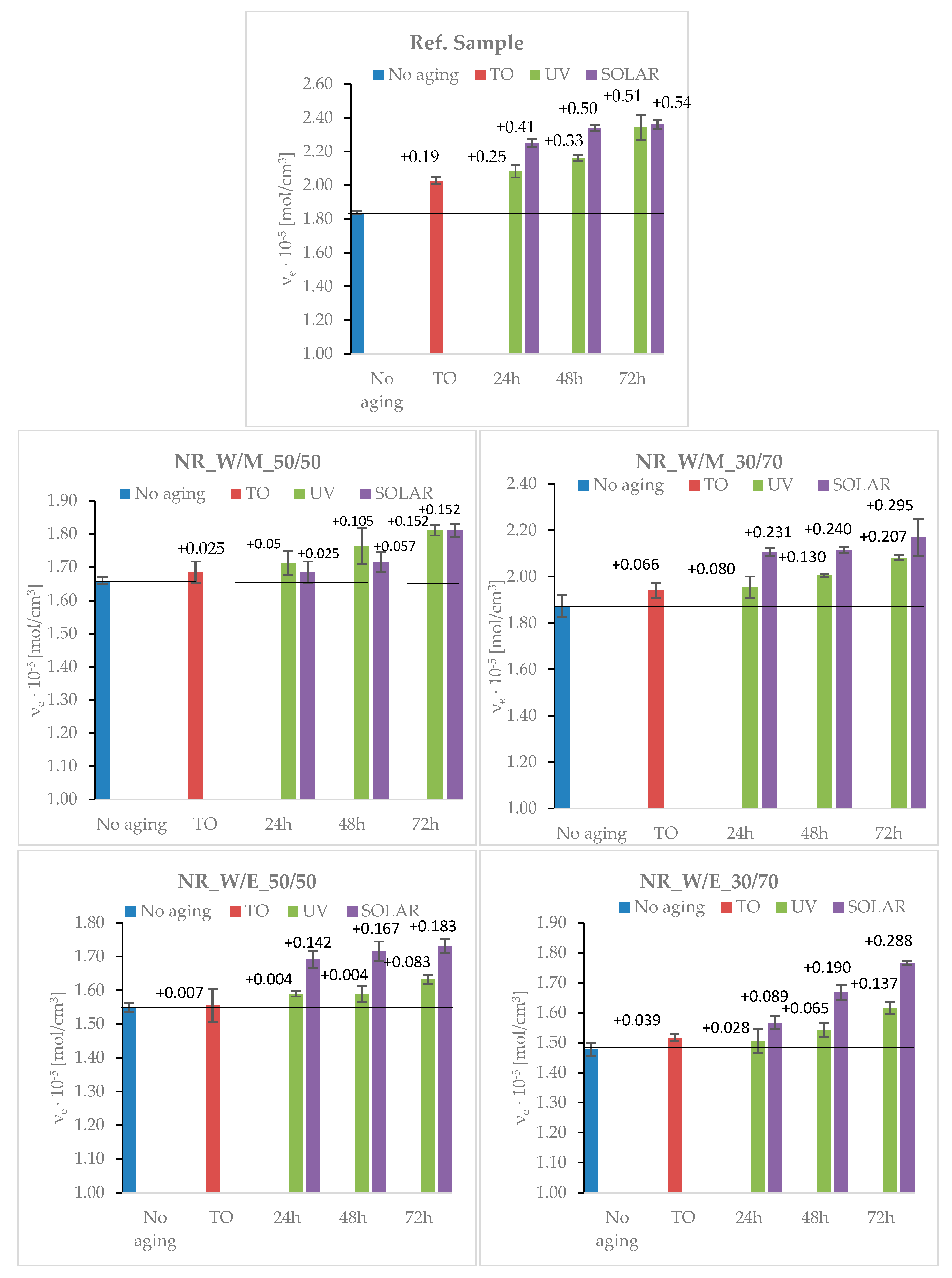
3.6.1. The Aging Effects on the Changes in Cross-Linking density
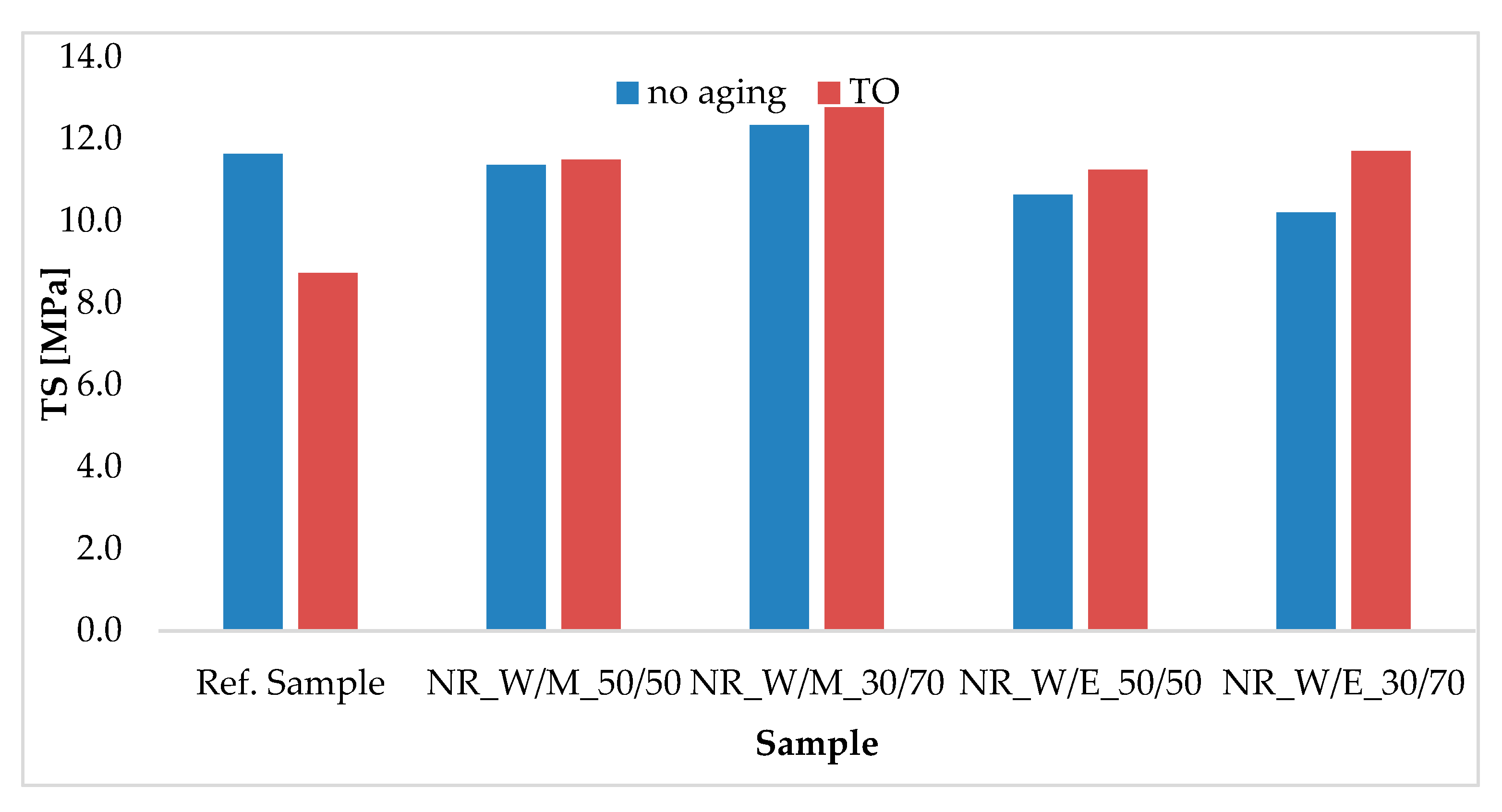
3.6.2. The Aging Effect on the Changes in Mechanical Properties
3.6.3. The Aging Effect on the Changes in Color
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Al-Snafi, P.D.A.E. The pharmacology of Equisetum arvense—A review. IOSR J. Pharm. 2017, 7, 31–42. [Google Scholar] [CrossRef]
- Dillard, C.J.; German, J.B. Phytochemicals: Nutraceuticals and human health. J. Sci. Food Agric. 2000, 80, 1744–1756. [Google Scholar] [CrossRef]
- Jayaprakasha, G.K.; Selvi, T.; Sakariah, K.K. Antibacterial and antioxidant activities of grape (Vitis vinifera) seed extracts. Food Res. Int. 2003, 36, 117–122. [Google Scholar] [CrossRef]
- Wu, L.; Hsu, H.-W.; Chen, Y.-C.; Chiu, C.-C.; Lin, Y.-I.; Ho, J.A. Antioxidant and antiproliferative activities of red pitaya. Food Chem. 2006, 95, 319–327. [Google Scholar] [CrossRef]
- Rice-Evans, C.; Miller, N.; Paganga, G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Čanadanović-Brunet, J.M.; Ćetković, G.S.; Djilas, S.M.; Tumbas, V.T.; Savatović, S.S.; Mandić, A.I.; Markov, S.L.; Cvetković, D.D. Radical scavenging and antimicrobial activity of horsetail (Equisetum arvense L.) extracts. Int. J. Food Sci. Technol. 2009, 44, 269–278. [Google Scholar] [CrossRef]
- Cao, G.; Sofic, E.; Prior, R.L. Antioxidant and prooxidant behavior of flavonoids: Structure-activity relationships. Free Radic. Biol. Med. 1997, 22, 749–760. [Google Scholar] [CrossRef]
- Dinu, C.; Vasile, G.-G.; Buleandra, M.; Popa, D.E.; Gheorghe, S.; Ungureanu, E.-M. Translocation and accumulation of heavy metals in Ocimum basilicum L. plants grown in a mining-contaminated soil. J. Soils Sediments 2020, 20, 2141–2154. [Google Scholar] [CrossRef]
- Cao, G.; Sofic, E.; Prior, R.L. Antioxidant Capacity of Tea and Common Vegetables. J. Agric. Food Chem. 1996, 44, 3426–3431. [Google Scholar] [CrossRef]
- Fu, L.; Xu, B.-T.; Xu, X.-R.; Gan, R.-Y.; Zhang, Y.; Xia, E.-Q.; Li, H.-B. Antioxidant capacities and total phenolic contents of 62 fruits. Food Chem. 2011, 129, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Nagai, T.; Myoda, T.; Nagashima, T. Antioxidative activities of water extract and ethanol extract from field horsetail (tsukushi) Equisetum arvense L. Food Chem. 2005, 91, 389–394. [Google Scholar] [CrossRef]
- Ito, N.; Fukushima, S.; Tsuda, H. Carcinogenicity and Modification of the Carcinogenic Response by bha, Bht, and Other Antioxidants. CRC Crit. Rev. Toxicol. 1985, 15, 109–150. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Ng, T.B. Antioxidative and free radical scavenging activities of selected medicinal herbs. Life Sci. 2000, 66, 725–735. [Google Scholar] [CrossRef]
- Wang, K.-H.; Lin, R.-D.; Hsu, F.-L.; Huang, Y.-H.; Chang, H.-C.; Huang, C.-Y.; Lee, M.-H. Cosmetic applications of selected traditional Chinese herbal medicines. J. Ethnopharmacol. 2006, 106, 353–359. [Google Scholar] [CrossRef]
- Visioli, F. Diet and prevention of coronary heart disease: The potential role of phytochemicals. Cardiovasc. Res. 2000, 47, 419–425. [Google Scholar] [CrossRef]
- Četojević-Simin, D.D.; Čanadanović-Brunet, J.M.; Bogdanović, G.M.; Djilas, S.M.; Ćetković, G.S.; Tumbas, V.T.; Stojiljković, B.T. Antioxidative and Antiproliferative Activities of Different Horsetail (Equisetum arvense L.) Extracts. J. Med. Food 2010, 13, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, J.G.; Blanco, M.M.; Do Monte, F.H.M.; Russi, M.; Lanziotti, V.M.N.B.; Leal, L.K.A.M.; Cunha, G.M. Sedative and anticonvulsant effects of hydroalcoholic extract of Equisetum arvense. Fitoterapia 2005, 76, 508–513. [Google Scholar] [CrossRef]
- Milutinović, M.; Radovanović, N.; Rajilić-Stojanović, M.; Šiler-Marinković, S.; Dimitrijević, S.; Dimitrijević-Branković, S. Microwave-assisted extraction for the recovery of antioxidants from waste Equisetum arvense. Ind. Crops Prod. 2014, 61, 388–397. [Google Scholar] [CrossRef]
- Sandhu, N.S.; Kaur, S.A.R.A.B.J.I.T.; Chopra, D.I.V.N.E.E.T. Equietum arvense: Pharmacology and phytochemistry—A review. Asian J. Pharm. Clin. Res. 2010, 3, 146–150. [Google Scholar]
- Mimica-Dukic, N.; Simin, N.; Cvejic, J.; Jovin, E.; Orcic, D.; Bozin, B. Phenolic Compounds in Field Horsetail (Equisetum arvense L.) as Natural Antioxidants. Molecules 2008, 13, 1455–1464. [Google Scholar] [CrossRef]
- D’Agostino, M.; Dini, A.; Pizza, C.; Senatore, F.; Aquino, R. Sterols from Equisetum arvense. Boll. Soc. Ital. Biol. Sper. 1984, 60, 2241–2245. [Google Scholar]
- Francescato, L.N.; Debenedetti, S.L.; Schwanz, T.G.; Bassani, V.L.; Henriques, A.T. Identification of phenolic compounds in Equisetum giganteum by LC–ESI-MS/MS and a new approach to total flavonoid quantification. Talanta 2013, 105, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Beckert, C.; Horn, C.; Schnitzler, J.-P.; Lehning, A.; Heller, W.; Veit, M. Styrylpyrone biosynthesis in Equisetum arvense. Phytochemistry 1997, 44, 275–283. [Google Scholar] [CrossRef]
- Pittler, M. Herbal Drugs and Phytopharmaceuticals. Focus Altern. Complement. Ther. 2010. [Google Scholar] [CrossRef]
- Saleh, N.A.M.; Majak, W.; Towers, G.H.N. Flavonoids of Equisetum species. Phytochemistry 1972, 11, 1095–1099. [Google Scholar] [CrossRef]
- Masłowski, M.; Miedzianowska, J.; Czylkowska, A.; Strzelec, K. Horsetail (Equisetum Arvense) as a Functional Filler for Natural Rubber Biocomposites. Materials 2020, 13, 2526. [Google Scholar] [CrossRef]
- Košíková, B.; Gregorová, A.; Osvald, A.; Krajčovičová, J. Role of lignin filler in stabilization of natural rubber-based composites. J. Appl. Polym. Sci. 2007. [Google Scholar] [CrossRef]
- Gregorová, A.; Košíková, B.; Moravčík, R. Stabilization effect of lignin in natural rubber. Polym. Degrad. Stab. 2006, 91, 229–233. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A.J. Colorimetry to total phenolics with phosphomolybdic acid reagents. Am. J. Enol. Vinic. 1965, 16, 144–158. [Google Scholar]
- Meda, A.; Lamien, C.E.; Romito, M.; Millogo, J.; Nacoulma, O.G. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 2005, 91, 571–577. [Google Scholar] [CrossRef]
- Al, M.L.; Daniel, D.; Moise, A.; Bobis, O.; Laslo, L.; Bogdanov, S. Physico-chemical and bioactive properties of different floral origin honeys from Romania. Food Chem. 2009, 112, 863–867. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Sowińska, A.; Maciejewska, M.; Guo, L.; Delebecq, E. Effect of SILPs on the Vulcanization and Properties of Ethylene–Propylene–Diene Elastomer. Polymers 2020, 12, 1220. [Google Scholar] [CrossRef]
- Szadkowski, B.; Kuśmierek, M.; Rybiński, P.; Żukowski, W.; Marzec, A. Application of Earth Pigments in Cycloolefin Copolymer: Protection against Combustion and Accelerated Aging in the Full Sunlight Spectrum. Materials 2020, 13, 3381. [Google Scholar] [CrossRef] [PubMed]
- Flory, P.J.; Rehner, J. Statistical Mechanics of Cross-Linked Polymer Networks I. Rubberlike Elasticity. J. Chem. Phys. 1943, 11, 512–520. [Google Scholar] [CrossRef]
- Roovers, J.; Toporowski, P.M. Characteristic Ratio and Plateau Modulus of 1,2-Polybutadiene. A Comparison with Other Rubbers. Rubber Chem. Technol. 1990, 63, 734–746. [Google Scholar] [CrossRef]
- Ahmed, K.; Nizami, S.S.; Raza, N.Z.; Habib, F. The effect of silica on the properties of marble sludge filled hybrid natural rubber composites. J. King Saud Univ.-Sci. 2013, 25, 331–339. [Google Scholar] [CrossRef]
- Fernandes, F.H.A.; Santana, C.P.; Santos, R.L.; Correia, L.P.; Conceição, M.M.; MacÊdo, R.O.; Medeiros, A.C.D. Thermal characterization of dried extract of medicinal plant by DSC and analytical techniques. J. Therm. Anal. Calorim. 2013, 113, 443–447. [Google Scholar] [CrossRef]
- Cheng, Y.; Xu, Q.; Liu, J.; Zhao, C.; Xue, F.; Zhao, Y. Decomposition of Five Phenolic Compounds in High Temperature Water. J. Braz. Chem. Soc. 2014, 25, 2102–2107. [Google Scholar] [CrossRef]
- Abdelrazek, E.M.; El Damrawi, G.; Elashmawi, I.S.; El-Shahawy, A. The influence of γ-irradiation on some physical properties of chlorophyll/PMMA films. Appl. Surf. Sci. 2010, 256, 2711–2718. [Google Scholar] [CrossRef]
- Samide, A.; Tutunaru, B. Thermal behavior of the chlorophyll extract from a mixture of plants and seaweed. J. Therm. Anal. Calorim. 2017, 127, 597–604. [Google Scholar] [CrossRef]
- Patle, T.K.; Shrivas, K.; Kurrey, R.; Upadhyay, S.; Jangde, R.; Chauhan, R. Phytochemical screening and determination of phenolics and flavonoids in Dillenia pentagyna using UV–vis and FTIR spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 242, 118717. [Google Scholar] [CrossRef] [PubMed]
- Czeczuga, B. Carotenoids in sixty-six representatives of the Pteridophyta. Biochem. Syst. Ecol. 1985, 13, 221–230. [Google Scholar] [CrossRef]
- Song, H.; Chen, C.; Zhao, S.; Ge, F.; Liu, D.; Shi, D.; Zhang, T. Interaction of gallic acid with trypsin analyzed by spectroscopy. J. Food Drug Anal. 2015, 23, 234–242. [Google Scholar] [CrossRef]
- Telange, D.R.; Patil, A.T.; Tatode, A.; Bhoyar, B. Development and Validation of UV Spectrophotometric Method for the Estimation of Kaempferol in Kaempferol: Hydrogenated Soy PhosphatidylCholine (HSPC) Complex. Pharm. Methods 2014, 5, 34–38. [Google Scholar] [CrossRef]
- Buchweitz, M.; Kroon, P.A.; Rich, G.T.; Wilde, P.J. Quercetin solubilisation in bile salts: A comparison with sodium dodecyl sulphate. Food Chem. 2016, 211, 356–364. [Google Scholar] [CrossRef]
- Živanović, S.C.; Nikolić, R.S.; Nikolić, G.M. The Influence of Mg(II) and Ca(II) Ions on Rutin Autoxidation in Weakly Alkaline Aqueous Solutions. Acta Fac. Med. Naissensis 2016, 33, 163–171. [Google Scholar] [CrossRef]
- Heneczkowski, M.; Kopacz, M.; Nowak, D.; Kuźniar, A. Infrared spectrum analysis of some flavonoids. Acta Pol. Pharm. 2001, 58, 415–420. [Google Scholar]
- Cao, J.; Peng, L.-Q.; Du, L.-J.; Zhang, Q.-D.; Xu, J.-J. Ultrasound-assisted ionic liquid-based micellar extraction combined with microcrystalline cellulose as sorbent in dispersive microextraction for the determination of phenolic compounds in propolis. Anal. Chim. Acta 2017, 963, 24–32. [Google Scholar] [CrossRef]
- Cardoso, E.D.O.; Conti, B.J.; Santiago, K.B.; Conte, F.L.; Oliveira, L.P.G.; Hernandes, R.T.; Golim, M.D.A.; Sforcin, J.M. Phenolic compounds alone or in combination may be involved in propolis effects on human monocytes. J. Pharm. Pharmacol. 2017, 69, 99–108. [Google Scholar] [CrossRef]
- Efenberger-Szmechtyk, M.; Nowak, A.; Czyzowska, A. Plant extracts rich in polyphenols: Antibacterial agents and natural preservatives for meat and meat products. Crit. Rev. Food Sci. Nutr. 2020, 1–30. [Google Scholar] [CrossRef]
- Dent, M.; Dragović-Uzelac, V.; Penić, M.; Brñić, M.; Bosiljkov, T.; Levaj, B. The effect of extraction solvents, temperature and time on the composition and mass fraction of polyphenols in dalmatian wild sage (Salvia officinalis L.) extracts. Food Technol. Biotechnol. 2013, 51, 84–91. [Google Scholar]
- Fu, Z.; Tu, Z.; Zhang, L.; Wang, H.; Wen, Q.; Huang, T. Antioxidant activities and polyphenols of sweet potato (Ipomoea batatas L.) leaves extracted with solvents of various polarities. Food Biosci. 2016, 15, 11–18. [Google Scholar] [CrossRef]
- Goyanes, S.; Lopez, C.C.; Rubiolo, G.H.; Quasso, F.; Marzocca, A.J. Thermal properties in cured natural rubber/styrene butadiene rubber blends. Eur. Polym. J. 2008, 44, 1525–1534. [Google Scholar] [CrossRef]
- Chandrasekaran, V.C. Rubber Compounding. In Rubber as a Construction Material for Corrosion Protection; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; pp. 7–26. [Google Scholar]
- Manaila, E.; Stelescu, M.; Craciun, G. Degradation Studies Realized on Natural Rubber and Plasticized Potato Starch Based Eco-Composites Obtained by Peroxide Cross-Linking. Int. J. Mol. Sci. 2018, 19, 2862. [Google Scholar] [CrossRef]
- Komethi, M.; Othman, N.; Ismail, H.; Sasidharan, S. Comparative study on natural antioxidant as an aging retardant for natural rubber vulcanizates. J. Appl. Polym. Sci. 2012, 124, 1490–1500. [Google Scholar] [CrossRef]
- Zeb, A. Concept, mechanism, and applications of phenolic antioxidants in foods. J. Food Biochem. 2020, 44, e13394. [Google Scholar] [CrossRef]

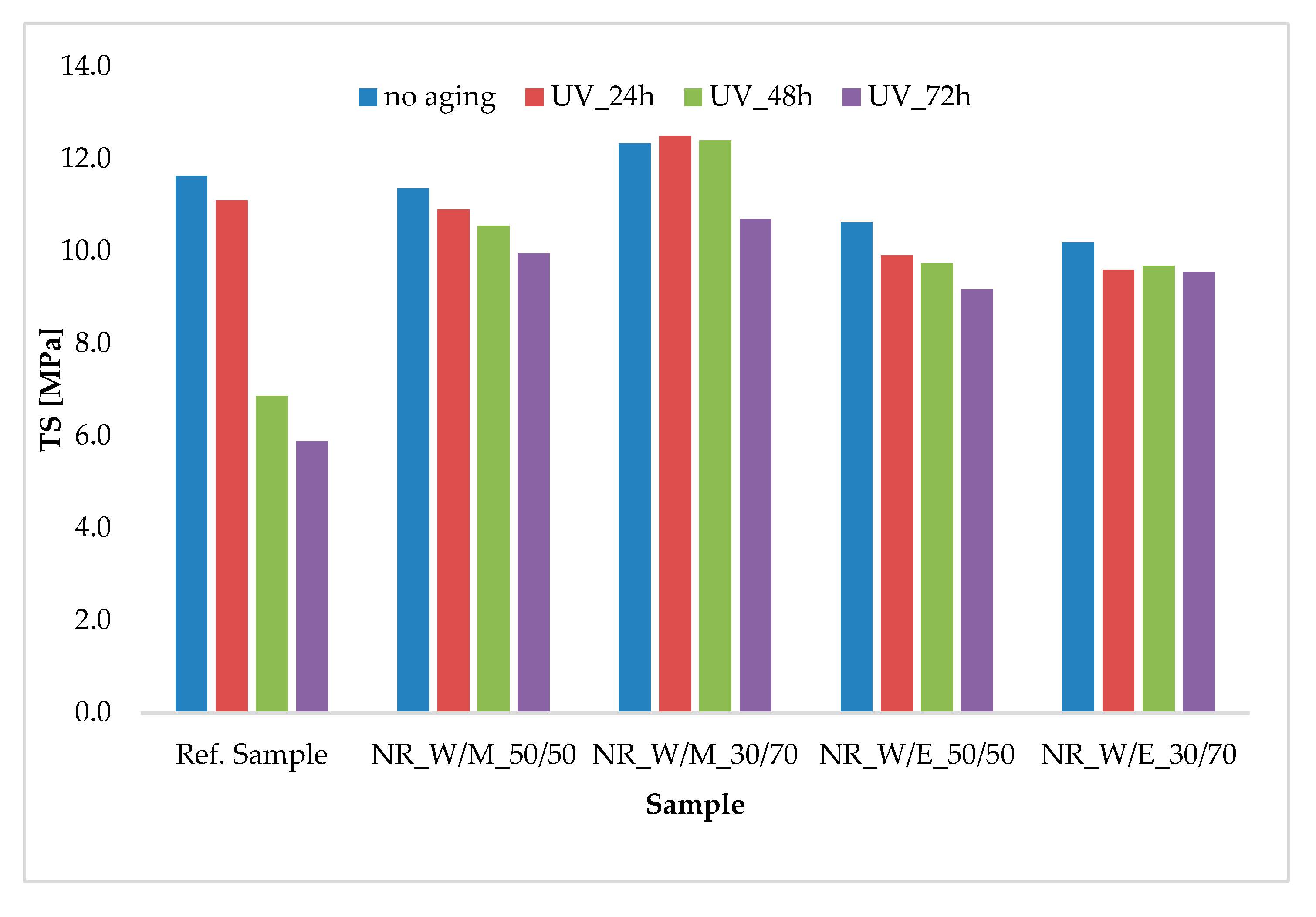
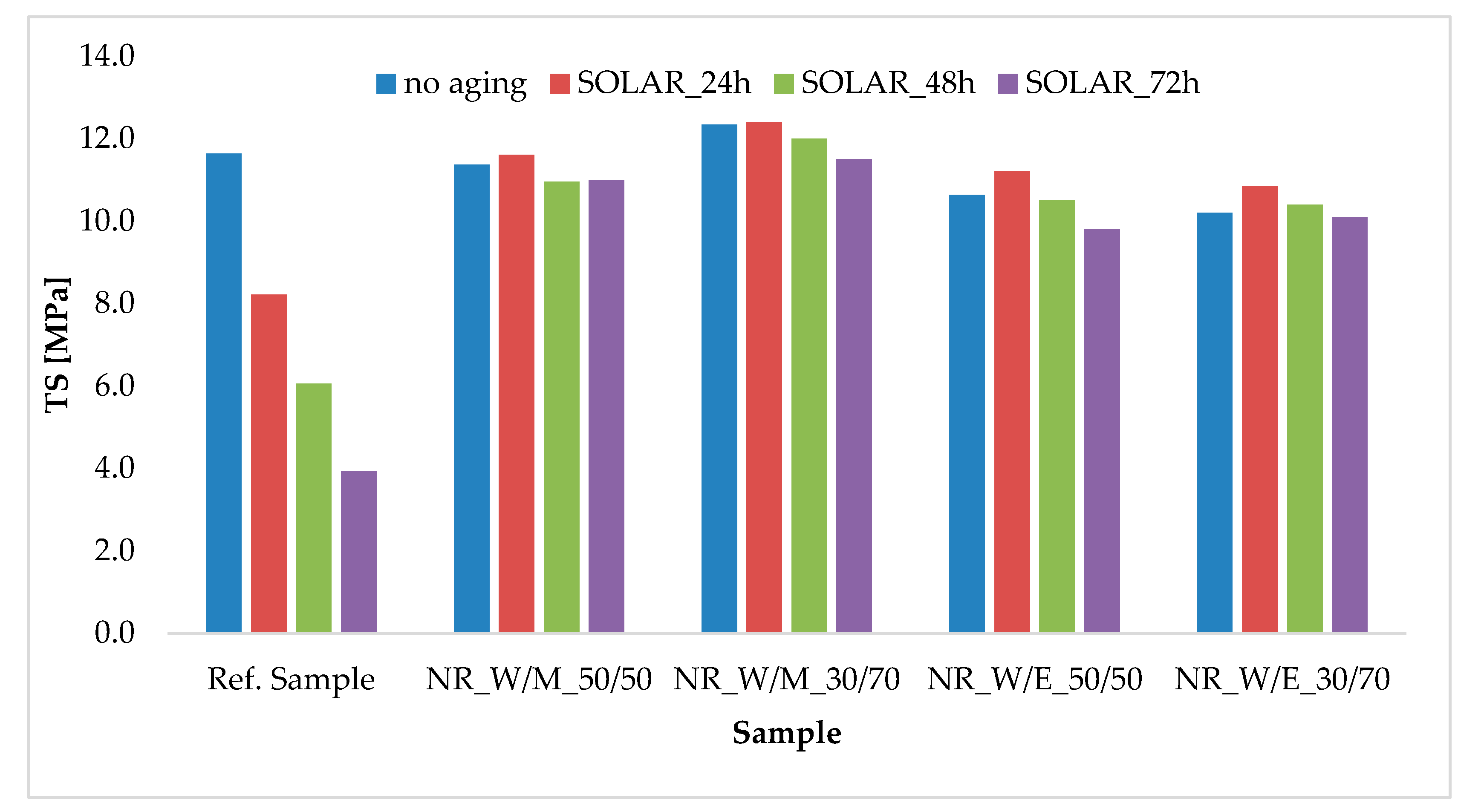
| Component | Ref. Sample | NR_ W/M_50/50 | NR_ W/M_30/70 | NR_ W/E_50/50 | NR_ W/E_30/70 |
|---|---|---|---|---|---|
| NR (phr) | 100 | ||||
| W/M_50/50 (phr) | - | 5 | - | - | - |
| W/M_30/70 (phr) | - | - | 5 | - | - |
| W/E_50/50 (phr) | - | - | - | 5 | - |
| W/E_30/70 (phr) | - | - | - | - | 5 |
| Zinc oxide (phr) | 5 | ||||
| 2-mercaptobenzothiazole (phr) | 2 | ||||
| Stearic acid (phr) | 1 | ||||
| Sulphur (phr) | 2 | ||||
| Sample | Δm25–140 °C [%] | Δm140–350 °C [%] | Δm350–600 °C [%] | R600 [%] |
|---|---|---|---|---|
| W/M_50/50 | 7.7 | 36.0 | 13.5 | 42.8 |
| W/M_30/70 | 11.3 | 40.1 | 14.4 | 34.2 |
| W/E_50/50 | 8.7 | 33.8 | 15.8 | 41.6 |
| W/E_30/70 | 7.0 | 39.9 | 13.9 | 39.3 |
| Solvent Type | TPC [µgGAE/mL] | Antioxidant Activity [µgTE/mL] | |
|---|---|---|---|
| ABTS | DPPH | ||
| W/M_50/50 | 2355.94 ± 81.21 a | 606.18 ± 0.53 a | 932.71 ± 3.90 a.b |
| W/M_30/70 | 2211.38 ± 9.05 b | 615.67 ± 1.40 a | 937.31 ± 4.16 a.b |
| W/E_50/50 | 1923.12 ± 44.44 c | 609.80 ± 16.35 a | 940.25 ± 4.16 a |
| W/E_30/70 | 2120.81 ± 56.30 b | 618.70 ± 9.49 a | 924.44 ± 1.56 b |
| Sample | Co [mg/kg] | Ni [mg/kg] | Cd [mg/kg] | Pb [mg/kg] |
|---|---|---|---|---|
| W/M_50/50 | 0.71 | 5.20 | 0.03 | 0.06 |
| W/M_30/70 | 0.65 | 3.52 | 0.03 | 0.07 |
| W/E_50/50 | 0.73 | 4.74 | 0.03 | 0.07 |
| W/E_30/70 | 0.51 | 3.79 | --- | 0.08 |
| Sample | Co [mg/kg] | Ni [mg/kg] | Cd [mg/kg] | Pb [mg/kg] |
|---|---|---|---|---|
| Ref. sample | - | 0.63 | 5.53 | 55.77 |
| NR_W/M_50/50 | - | 0.58 | 4.79 | 47.43 |
| NR_W/M_30/70 | - | 0.56 | 4.89 | 48.85 |
| NR_W/E_50/50 | - | 0.57 | 4.83 | 48.35 |
| NR_W/E_30/70 | - | 0.55 | 4.78 | 49.24 |
| Sample | Before Aging | TO | |
|---|---|---|---|
| After Aging | K[-] | ||
| Eb [%] | Eb [%] | ||
| Ref. Sample | 734 | 670 | 0.69 |
| NR_W/M_50/50 | 797 | 758 | 0.96 |
| NR_W/M_30/70 | 781 | 699 | 0.93 |
| NR_W/E_50/50 | 748 | 678 | 0.96 |
| NR_W/E_30/70 | 773 | 721 | 1.07 |
| Sample | Before Aging | UV_24h | UV_48h | UV_72h | |||
|---|---|---|---|---|---|---|---|
| After Aging | K[-] | After Aging | K[-] | After Aging | K[-] | ||
| Eb [%] | Eb [%] | Eb [%] | Eb [%] | ||||
| Ref. Sample | 734 | 721 | 0.94 | 679 | 0.55 | 623 | 0.43 |
| NR_W/M_50/50 | 797 | 790 | 0.95 | 783 | 0.91 | 774 | 0.85 |
| NR_W/M_30/70 | 781 | 780 | 1.01 | 769 | 0.99 | 739 | 0.82 |
| NR_W/E_50/50 | 748 | 760 | 0.95 | 775 | 0.95 | 777 | 0.90 |
| NR_W/E_30/70 | 773 | 778 | 0.95 | 782 | 0.96 | 756 | 0.92 |
| Sample | Before Aging | SOLAR_24h | SOLAR_48h | SOLAR_72h | |||
|---|---|---|---|---|---|---|---|
| After Aging | K[-] | After Aging | K[-] | After Aging | K[-] | ||
| Eb [%] | Eb [%] | Eb [%] | Eb [%] | ||||
| Ref. Sample | 734 | 644 | 0.62 | 543 | 0.38 | 524 | 0.24 |
| NR_W/M_50/50 | 797 | 778 | 1.00 | 789 | 0.95 | 759 | 0.92 |
| NR_W/M_30/70 | 781 | 725 | 0.93 | 732 | 0.91 | 731 | 0.87 |
| NR_W/E_50/50 | 748 | 732 | 1.03 | 736 | 0.97 | 730 | 0.90 |
| NR_W/E_30/70 | 773 | 775 | 1.07 | 744 | 0.98 | 704 | 0.90 |
| ΔE [-] | |||||||
|---|---|---|---|---|---|---|---|
| Sample | TO | UV | SOLAR | ||||
| 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | ||
| Ref. Sample | 5.51 | 5.02 | 8.58 | 9.26 | 7.13 | 8.92 | 10.85 |
| NR_W/M_50/50 | 3.20 | 3.60 | 5.51 | 5.60 | 5.51 | 5.51 | 6.50 |
| NR_W/M_30/70 | 3.11 | 4.23 | 6.57 | 7.28 | 2.35 | 3.72 | 3.74 |
| NR_W/E_50/50 | 2.92 | 2.85 | 3.67 | 4.77 | 2.42 | 4.09 | 4.21 |
| NR_W/E_30/70 | 2.79 | 3.85 | 4.29 | 5.24 | 1.21 | 2.46 | 2.75 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masłowski, M.; Miedzianowska, J.; Czylkowska, A.; Efenberger-Szmechtyk, M.; Nowak, A.; Strzelec, K. Anti-Oxidative Activity of Alcohol-Water Extracts from Field Horsetail (Equisteum arvense) in Elastomer Vulcanizates Subjected to Accelerated Aging Processes. Materials 2020, 13, 4903. https://doi.org/10.3390/ma13214903
Masłowski M, Miedzianowska J, Czylkowska A, Efenberger-Szmechtyk M, Nowak A, Strzelec K. Anti-Oxidative Activity of Alcohol-Water Extracts from Field Horsetail (Equisteum arvense) in Elastomer Vulcanizates Subjected to Accelerated Aging Processes. Materials. 2020; 13(21):4903. https://doi.org/10.3390/ma13214903
Chicago/Turabian StyleMasłowski, Marcin, Justyna Miedzianowska, Agnieszka Czylkowska, Magdalena Efenberger-Szmechtyk, Agnieszka Nowak, and Krzysztof Strzelec. 2020. "Anti-Oxidative Activity of Alcohol-Water Extracts from Field Horsetail (Equisteum arvense) in Elastomer Vulcanizates Subjected to Accelerated Aging Processes" Materials 13, no. 21: 4903. https://doi.org/10.3390/ma13214903
APA StyleMasłowski, M., Miedzianowska, J., Czylkowska, A., Efenberger-Szmechtyk, M., Nowak, A., & Strzelec, K. (2020). Anti-Oxidative Activity of Alcohol-Water Extracts from Field Horsetail (Equisteum arvense) in Elastomer Vulcanizates Subjected to Accelerated Aging Processes. Materials, 13(21), 4903. https://doi.org/10.3390/ma13214903









