Inflammatory Responses in Oro-Maxillofacial Region Expanded Using Anisotropic Hydrogel Tissue Expander
Abstract
1. Introduction
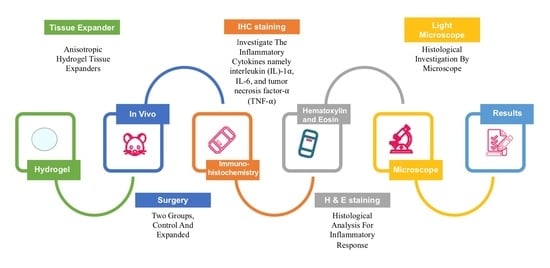
2. Materials and Methods
2.1. Ethical Approval
2.2. Study Design
2.3. Hydrogel Polymers Material
2.4. Sample Selection
2.5. Animal Preparation
2.6. Sample Preparation
2.7. Hematoxylin and Eosin (H&E) Staining
2.8. Immunohistochemistry
2.9. Visualization of the Histological Specimens
2.10. Scoring of Immunohistologically Positive Stained Samples
2.11. Statistical Analysis
3. Results
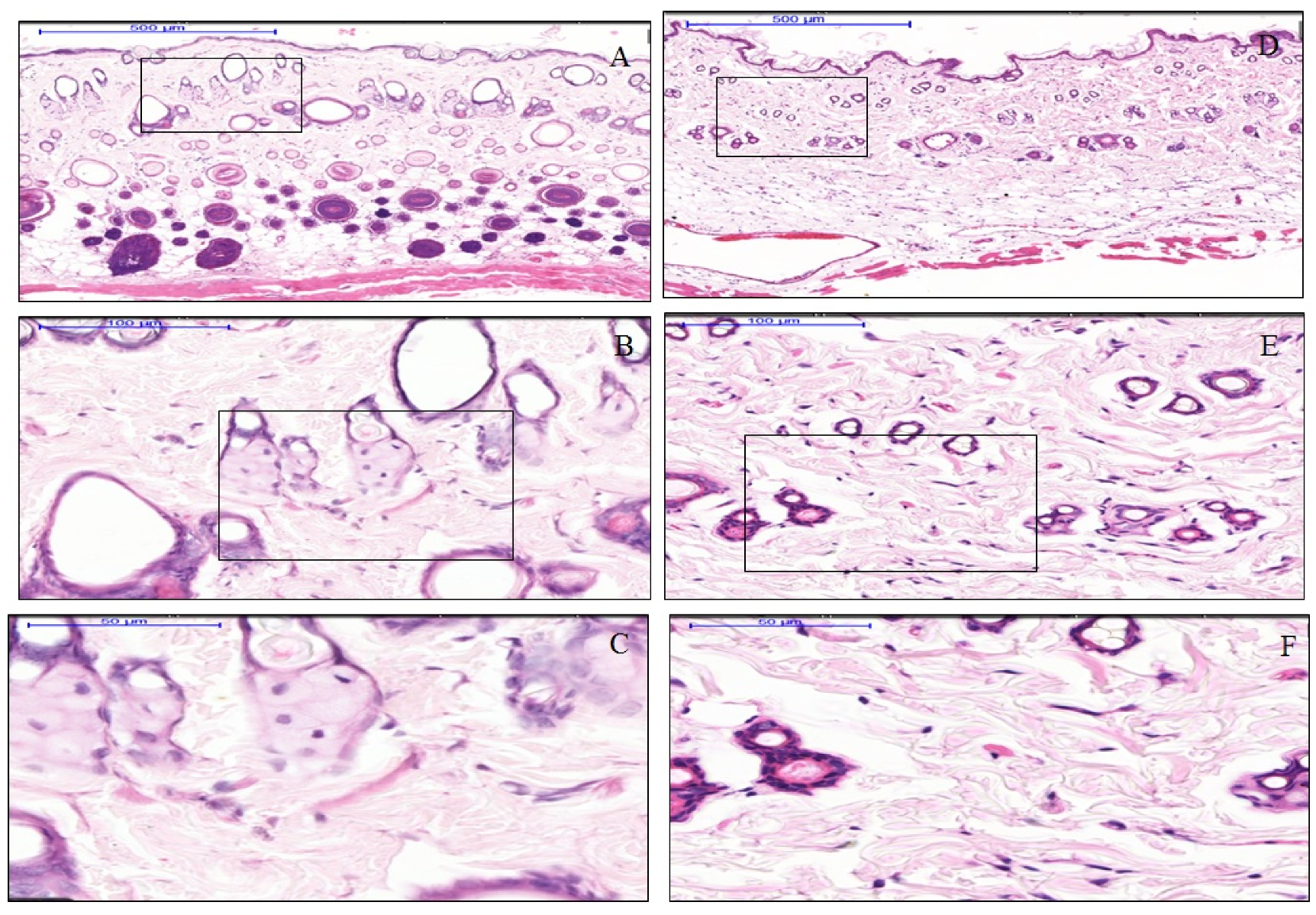
3.1. The effect of Anisotropic Hydrogel Tissue Expander on Skin Histology
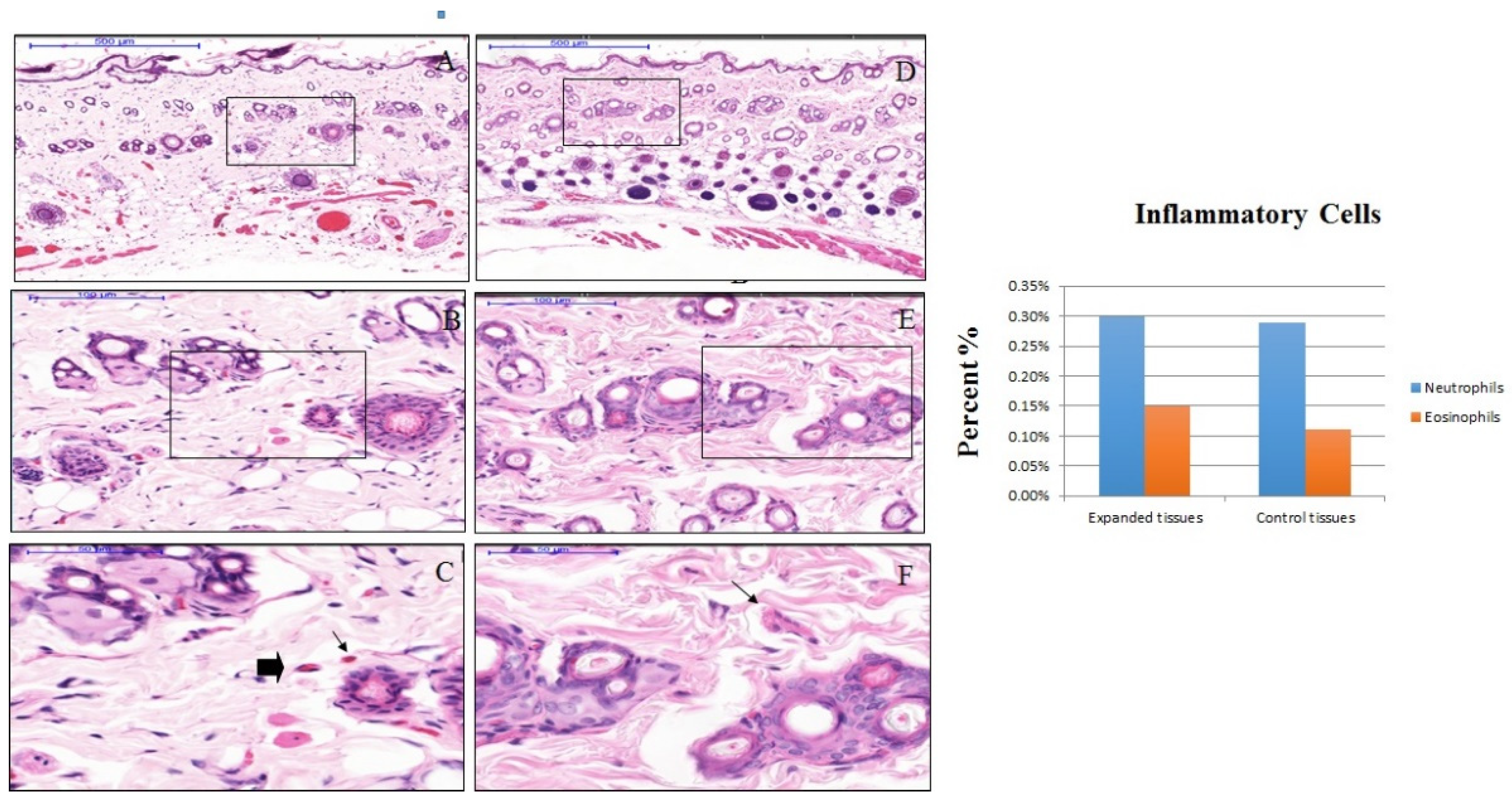
3.2. The effect of Anisotropic Hydrogel Tissue Expander on Inflammation
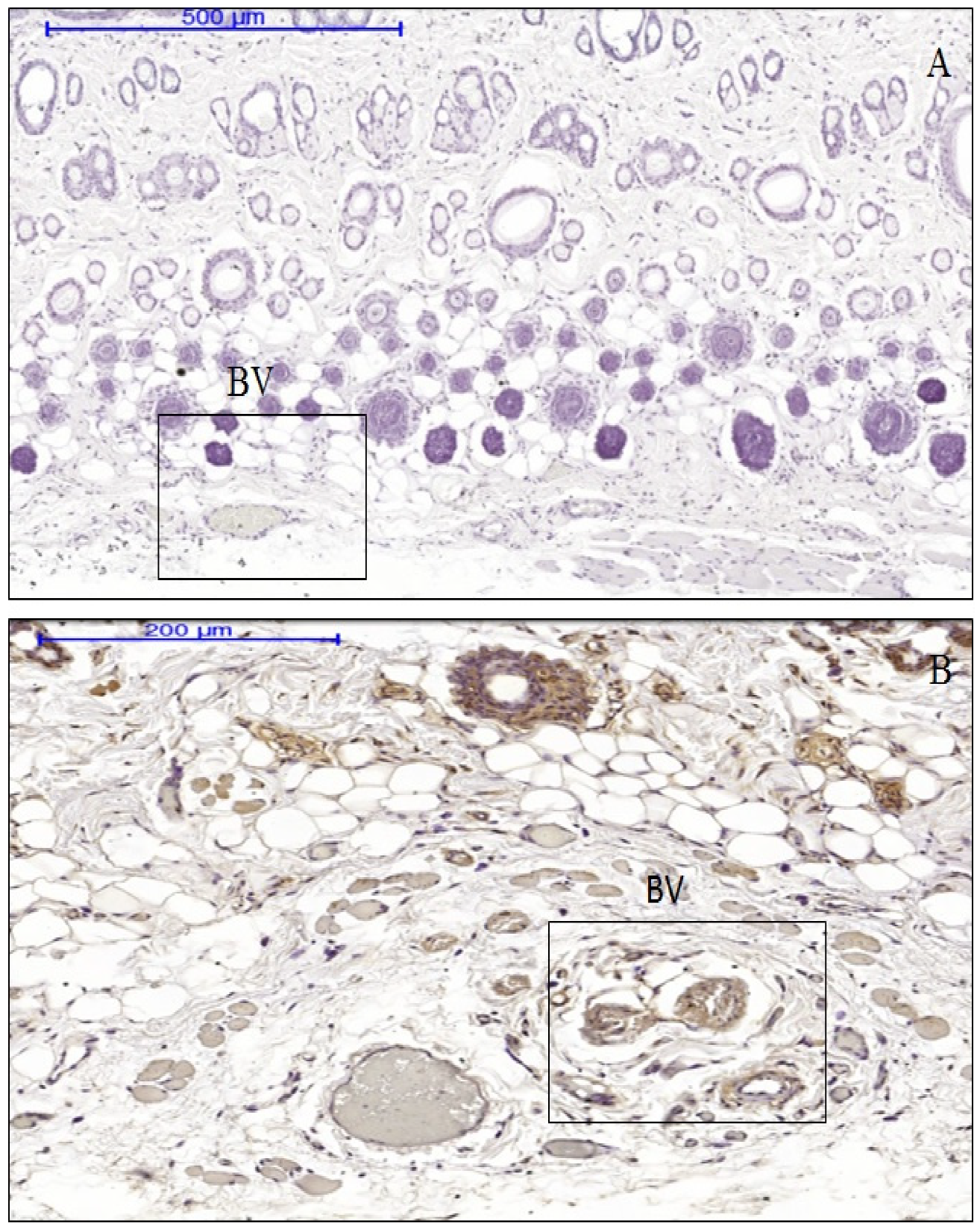
3.3. The Effect of Anisotropic Hydrogel Tissue Expansion on the Expression of IL-1, IL-6, and TNF-α
3.4. The Difference between Cytokine Production in Expanded and Non-Expanded Skin Tissue Samples
3.5. The Association between Inflammatory Cell Profiles and Cytokine Production in Immune-Positive Expanded Tissue
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nazir, M.A. Prevalence of periodontal disease, its association with systemic diseases and prevention. Int. J. Health Sci. 2017, 11, 72. [Google Scholar]
- Atsuta, A.; Ayukama, Y.; Rondo, A.; Oshiro, W.; Matsuura, Y.; Furuhashi, A.; Tsukiyama, Y.; Koyano, K. Soft tissue sealing around dental implants based on histological interpretation. J. Prosthodont. Res. 2016, 60, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, H.N.; Ozbilin, E.O.; Ustun, T. The prevalence of cleft lip and palate patients: A single-center experience for 17 years. Turk. J. Orthod. 2019, 32, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Swan, M.C.; Bucknall, D.G.; Goodacre, T.E.; Czernuszka, J.T. Synthesis and properties of a novel anisotropic self-inflating hydrogel tissue expander. Acta Biomater. 2011, 7, 11261132. [Google Scholar] [CrossRef] [PubMed]
- Nishikubo, M.; Hirahara, N.; Gomi, A.; Nozoe, E.; Norifumi, N. 3-D Analysis of Palatal Morphology Associated with Palatalized Articulation in Patients with Unilateral Cleft Lip and Palate. Oral. Sci. Int. 2009, 6, 36–45. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Ashayeri, M.; Rasouli, H.R. Comparission of local flap and skin grafts to repair cheek skin defects. J. Cutan. Aesthet. Surg. 2015, 8, 92–96. [Google Scholar] [CrossRef]
- Tepole, A.B.; Ploch, C.J.; Wong, J.E. Growing skin: A computational model for skin expansion in reconstructive surgery. J. Mech. Phys. Solids 2011, 59, 2177–2190. [Google Scholar] [CrossRef]
- Garner, J.; Davidson, D.; Eckert, G.J.; Barco, C.T.; Park, H.; Park, K. Reshapable polymeric hydrogel for controlled soft-tissue expansion: In vitro and in vivo evaluation. J. Control Release 2017, 262, 201–211. [Google Scholar] [CrossRef]
- Trubelja, A.; Bao, G. Molecular mechanisms of mechanosensing and mechanotransduction in living cells. Extrem. Mech. Lett. 2018, 20, 91–98. [Google Scholar] [CrossRef]
- Chen, G.; Cui, S.; You, L.; Li, Y.; Mei, Y.H.; Chen, X. Experimental study on multi-step creep properties of rat skins. J. Mech. Behav. Biomed. Mater. 2015, 46, 49–58. [Google Scholar] [CrossRef]
- Hsu, C.K.; Lin, H.H.; Harn, H.I.C.; Hughes, M.W.; Tang, M.J.; Yang, C.C. Mechanical forces in skin disorders. J. Dermatol. Sci. 2018, 90, 232–240. [Google Scholar] [CrossRef]
- Artola, E.A.; Trepat, X.; Cusachs, R.P. Control of Mechanotransduction by Molecular Clutch Dynamics. Trends Cell Biol. 2018, 28, 356–367. [Google Scholar] [CrossRef] [PubMed]
- Fleissner, F.; Parekh, S.H. Imaging mechanotransduction: Seeing forces from molecules to cells. Curr. Opin. Biomed. Eng. 2018, 5, 58–65. [Google Scholar] [CrossRef]
- Sethi, K.; Cram, E.J.; Bar, R.Z. Stretch-induced actomyosin contraction in epithelial tubes: Mechanotransduction pathways for tubular homeostasis. Semin. Cell Dev. Biol. 2017, 71, 146–152. [Google Scholar] [CrossRef]
- Mathieu, S.; Manneville, J.B. Intracellular mechanics: Connecting rheology and mechanotransduction. Curr. Opin. Cell Biol. 2018, 56, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Vlahakis, N.; Schroeder, M.; Limper, A.; Hubmayr, D. Stretch induces cytokine release by alveolar epithelial cells in vitro. Am. Physiol. Soc. 1999, 277, L167–L173. [Google Scholar] [CrossRef] [PubMed]
- Abuammah, A.; Maimari, N.; Towhidi, L.; Frueh, J.; Chooi, K.Y.; Warboys, C.; Krams, R. New developments in mechanotransduction: Cross talk of the Wnt, TGF-β and Notch signalling pathways in reaction to shear stress. Curr. Opin. Biomed. Eng. 2018, 5, 96–104. [Google Scholar] [CrossRef]
- Copland, I.B.; Post, M. Stretch-activated signaling pathways responsible for early response gene expression in fetal lung epithelial cells. J. Cell. Physiol. 2007, 210, 133–143. [Google Scholar] [CrossRef]
- Fedchenko, N.; Reifenrath, J. Different approaches for interpretation and reporting of immunohistochemistry analysis results in the bone tissue. Diagn. Pathol. 2014, 9, 1–12. [Google Scholar] [CrossRef]
- Milenovic, Z.M. Application of mann-whitney u test in research of professional training of primary school teachers. Metodicki obzori 2011, 6, 73–79. [Google Scholar] [CrossRef]
- Nuemann, N.D. The expansion of an area of skin by progressive distention of a subcutaneous balloon; use of the method for securing skin for subtotal reconstruction of the ear. Plast. Reconstr. Surg. 1957, 19, 124–130. [Google Scholar] [CrossRef]
- Ahmad, H.; Verma, S.; Kumar, V.L. Effect of roxithromycin on mucosal damage, oxidative stress and pro-inflammatory markers in experimental model of colitis. Inflamm. Res. 2018, 67, 147–155. [Google Scholar] [CrossRef]
- Cuomo, F.; Coppola, A.; Botti, C.; Maione, C.; Forte, A.; Scisciola, L.; Cobellis, G. Pro-inflammatory cytokines activate hypoxia-inducible factor 3alpha via epigenetic changes in mesenchymal stromal/stem cells. Sci. Rep. 2018, 8, 5842. [Google Scholar] [CrossRef] [PubMed]
- Scholz, C.C.; Taylor, C.T. Targeting the HIF pathway in inflammation and immunity. Curr. Opin. Pharmacol. 2013, 13, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Buhrmann, C.; Yazdi, M.; Popper, B.; Shayan, P.; Goel, A.; Aggarwal, B.B.; Shakibaei, M. Resveratrol Chemosensitizes TNF-beta-Induced Survival of 5-FU-Treated Colorectal Cancer Cells. Nutrients 2018, 10, 888. [Google Scholar] [CrossRef] [PubMed]
- Porta, C.; Larghi, P.; Rimoldi, M.; Totaro, M.G.; Allavena, P.; Mantovani, A.; Sica, A. Cellular and molecular pathways linking inflammation and cancer. Immunobiology 2009, 214, 761–777. [Google Scholar] [CrossRef] [PubMed]
- Chai, E.Z.; Siveen, K.S.; Shanmugam, M.K.; Arfuso, F.; Sethi, G. Analysis of the intricate relationship between chronic inflammation and cancer. Biochem. J. 2015, 468, 1–15. [Google Scholar] [CrossRef]
- Jetten, N.; Verbruggen, S.; Gijbels, M.J.; Post, M.J.; Winther, D.M.P.; Donners, M.M. Anti-inflammatory M2, but not pro-inflammatory M1 macrophages promote angiogenesis in vivo. Angiogenesis 2013, 17, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Shirley, D.; McHale, C.; Gomez, G. Resveratrol preferentially inhibits IgE-dependent PGD2 biosynthesis but enhances TNF production from human skin mast cells. Biochim. Biophys. Acta 2016, 1860, 678–685. [Google Scholar] [CrossRef]
- Scheller, J.; Chalaris, A.; Arras, D.S.; John, S.R. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim. Biophys. Acta 2011, 1813, 878–888. [Google Scholar] [CrossRef]
- Chalaris, A.; Rabe, B.; Paliga, K.; Lange, H.; Laskay, T.; Fielding, C.A.; Scheller, J. Apoptosis is a natural stimulus of IL6R shedding and contributes to the proinflammatory trans-signaling function of neutrophils. Blood 2007, 110, 1748–1755. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Vaca, E.E.; Ledwon, J.K.; Bae, H.; Topczewska, J.M.; Turin, S.Y.; Tepole, A.B. Improving tissue expansion protocols through computational modeling. J. Mech. Behav. Biomed. Mater. 2018, 82, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Aziz, J.; Ahmad, M.F.; Rahman, M.T.; Yahya, N.A.; Czernuszka, J.; Radzi, Z. AFM analysis of collagen fibrils in expanded scalp tissue after anisotropic tissue expansion. Int. J. Biol. Macromol. 2018, 107, 1030–1038. [Google Scholar] [CrossRef]
- Khavkin, J.; Ellis, D.A. Aging Skin: Histology, Physiology, and Pathology. Facial Plast. Surg. Clin. N. Am. 2011, 19, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, A.; Rizos, E.; Aggeli, A. Rheological and Morphological Investigation of Renaturated Collagen Nanogels in Physiological-like Solution Conditions. Mater. Today Proc. 2016, 3, 875–888. [Google Scholar] [CrossRef]
- Mia, M.M.; Boersema, M.; Bank, R.A. Interleukin-1β Attenuates Myofibroblast Formation and Extracellular Matrix Production in Dermal and Lung Fibroblasts Exposed to Transforming Growth Factor-β1. PLoS ONE 2014, 9, e91559. [Google Scholar] [CrossRef]
- Blum, S.R.; Baffet, G.; Théret, N. Molecular and tissue alterations of collagens in fibrosis. Matrix Biol. 2018, 68–69, 122–149. [Google Scholar] [CrossRef]
- Osei, E.T.; Noordhoek, J.A.; Hackett, T.L.; Spanjer, A.I.; Postma, D.S.; Timens, W.; Heijink, I.H. Interleukin-1alpha drives the dysfunctional cross-talk of the airway epithelium and lung fibroblasts in COPD. Eur. Respir. J. 2016, 48, 359–369. [Google Scholar] [CrossRef]
- Shimodaira, T.; Matsuda, K.; Uchibori, T.; Sugano, M.; Uehara, T.; Honda, T. Upregulation of osteopontin expression via the interaction of macrophages and fibroblasts under IL-1b stimulation. Cytokine 2018, 110, 63–69. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, J.; Zhai, T.; Li, H.; Li, H.; Huo, R.; Teng, J. CCN1 promotes IL-1β production in keratinocytes by activating p38 MAPK signaling in psoriasis. Sci. Rep. 2017, 7, 43310. [Google Scholar] [CrossRef]
- Harrison, C.A.; Gossiel, F.; Bullock, A.J.; Sun, T.; Blumsohn, A.; Neil, S.M. Investigation of keratinocyte regulation of collagen I synthesis by dermal fibroblasts in a simple in vitro model. Br. J. Dermatol. 2006, 154, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D. Interleukins as modulators of angiogenesis and anti-angiogenesis in tumors. Cytokines 2018, 118, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Gopinathan, G.; Milagre, C.; Pearce, O.M.; Reynolds, L.E.; Dilke, K.H.; Leinster, D.A.; Balkwill, F. Interleukin-6 Stimulates Defective Angiogenesis. Cancer Res. 2015, 75, 3098–3107. [Google Scholar] [CrossRef] [PubMed]
- Saharinen, P.; Eklund, L.; Alitalo, K. Therapeutic targeting of the angiopoietin-TIE pathway. Nat. Rev. Drug Discov. 2017, 16, 635–661. [Google Scholar] [CrossRef]
- Bao, P.; Kodra, A.; Canic, M.T.; Golinko, M.S.; Ehrlich, H.P.; Brem, H. The role of vascular endothelial growth factor in wound healing. J. Surg. Res. 2009, 153, 347–358. [Google Scholar] [CrossRef] [PubMed]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali Salim, K.M.; Abd Jalil, A.; Radzi, Z.; Ismail, S.M.; Czernuszka, J.T.; Rahman, M.T. Inflammatory Responses in Oro-Maxillofacial Region Expanded Using Anisotropic Hydrogel Tissue Expander. Materials 2020, 13, 4436. https://doi.org/10.3390/ma13194436
Ali Salim KM, Abd Jalil A, Radzi Z, Ismail SM, Czernuszka JT, Rahman MT. Inflammatory Responses in Oro-Maxillofacial Region Expanded Using Anisotropic Hydrogel Tissue Expander. Materials. 2020; 13(19):4436. https://doi.org/10.3390/ma13194436
Chicago/Turabian StyleAli Salim, Kholoud Mohamed, Aminah Abd Jalil, Zamri Radzi, Siti Mazlipah Ismail, Jan T. Czernuszka, and Mohammad Tariqur Rahman. 2020. "Inflammatory Responses in Oro-Maxillofacial Region Expanded Using Anisotropic Hydrogel Tissue Expander" Materials 13, no. 19: 4436. https://doi.org/10.3390/ma13194436
APA StyleAli Salim, K. M., Abd Jalil, A., Radzi, Z., Ismail, S. M., Czernuszka, J. T., & Rahman, M. T. (2020). Inflammatory Responses in Oro-Maxillofacial Region Expanded Using Anisotropic Hydrogel Tissue Expander. Materials, 13(19), 4436. https://doi.org/10.3390/ma13194436