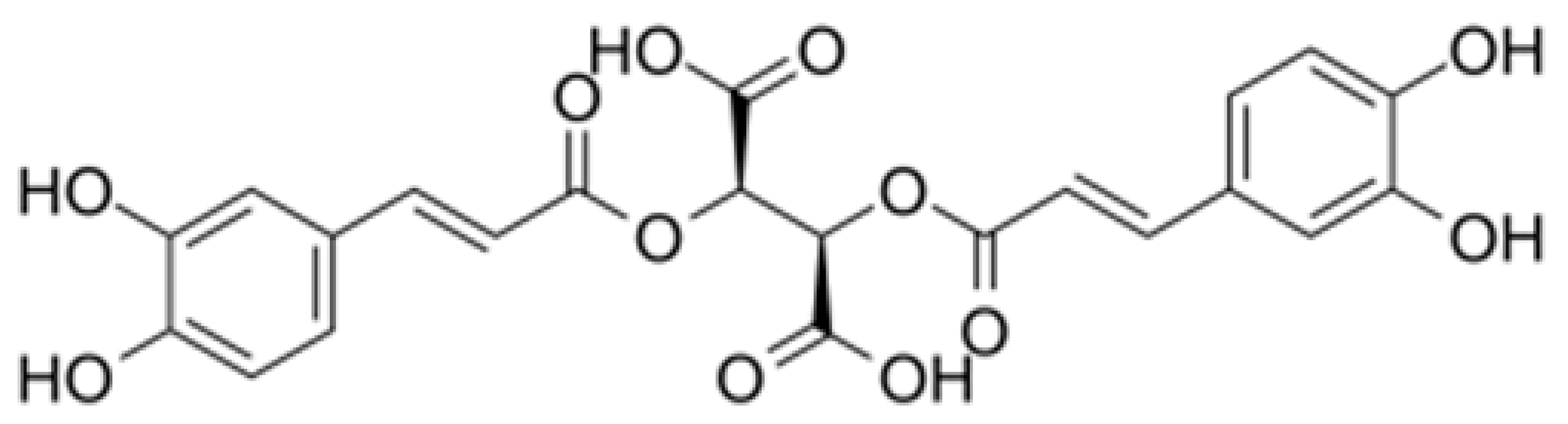
Spectroscopic, Theoretical and Antioxidant Study of 3d-Transition Metals (Co(II), Ni(II), Cu(II), Zn(II)) Complexes with Cichoric Acid
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.1.1. Synthesis of Cichoric acid Sodium Salt
2.1.2. Synthesis of Cu(II), Co(II), Zn(II) and Ni(II) Complexes with Cichoric Acid
2.1.3. Elemental Analysis Results
2.2. Measurements
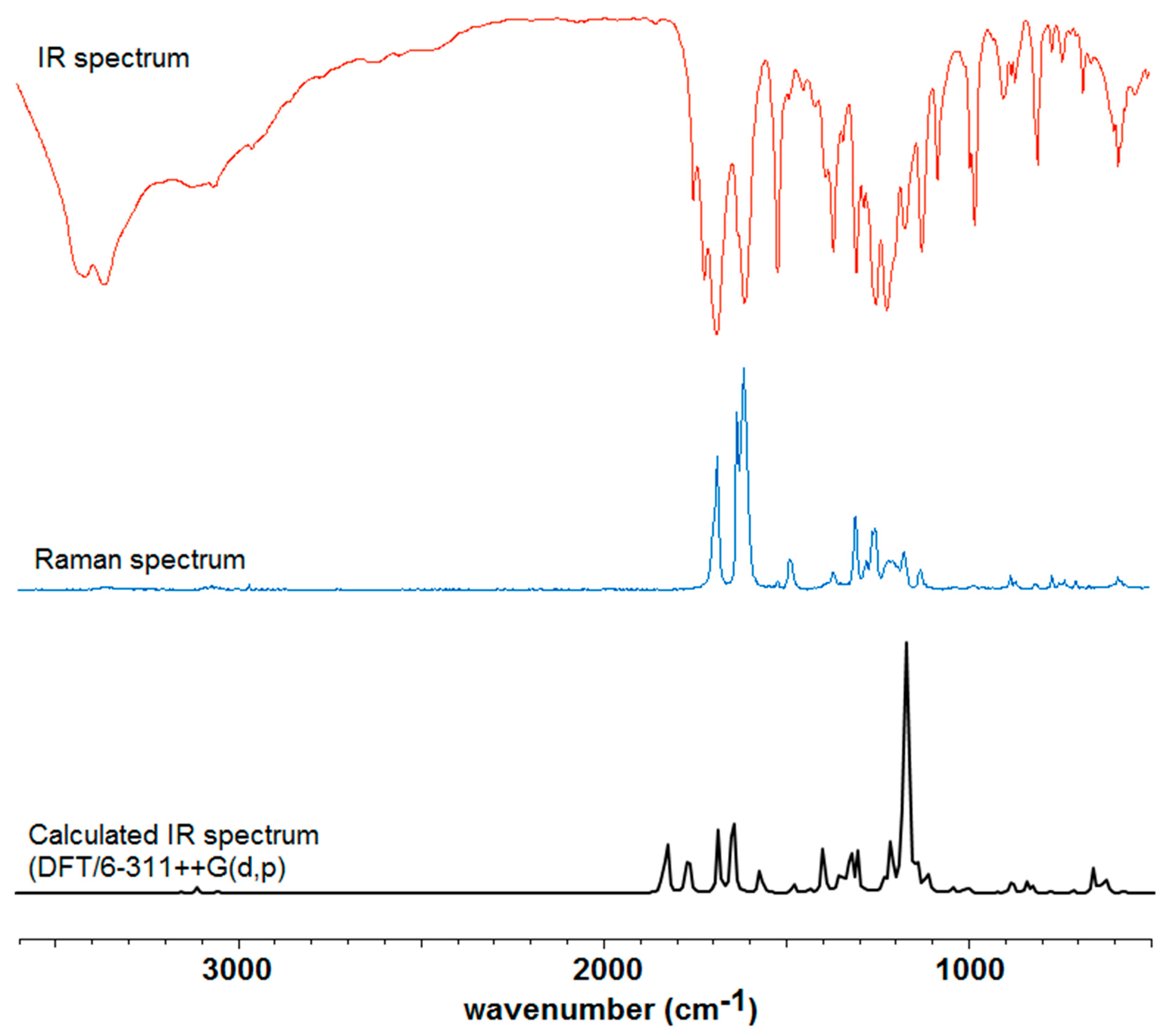
2.2.1. Infrared (IR) and Raman Study
2.2.2. Ultraviolet (UV) Study
2.2.3. Calculations
2.2.4. Antioxidant Properties
3. Results and Discussion
3.1. IR and Raman Spectra
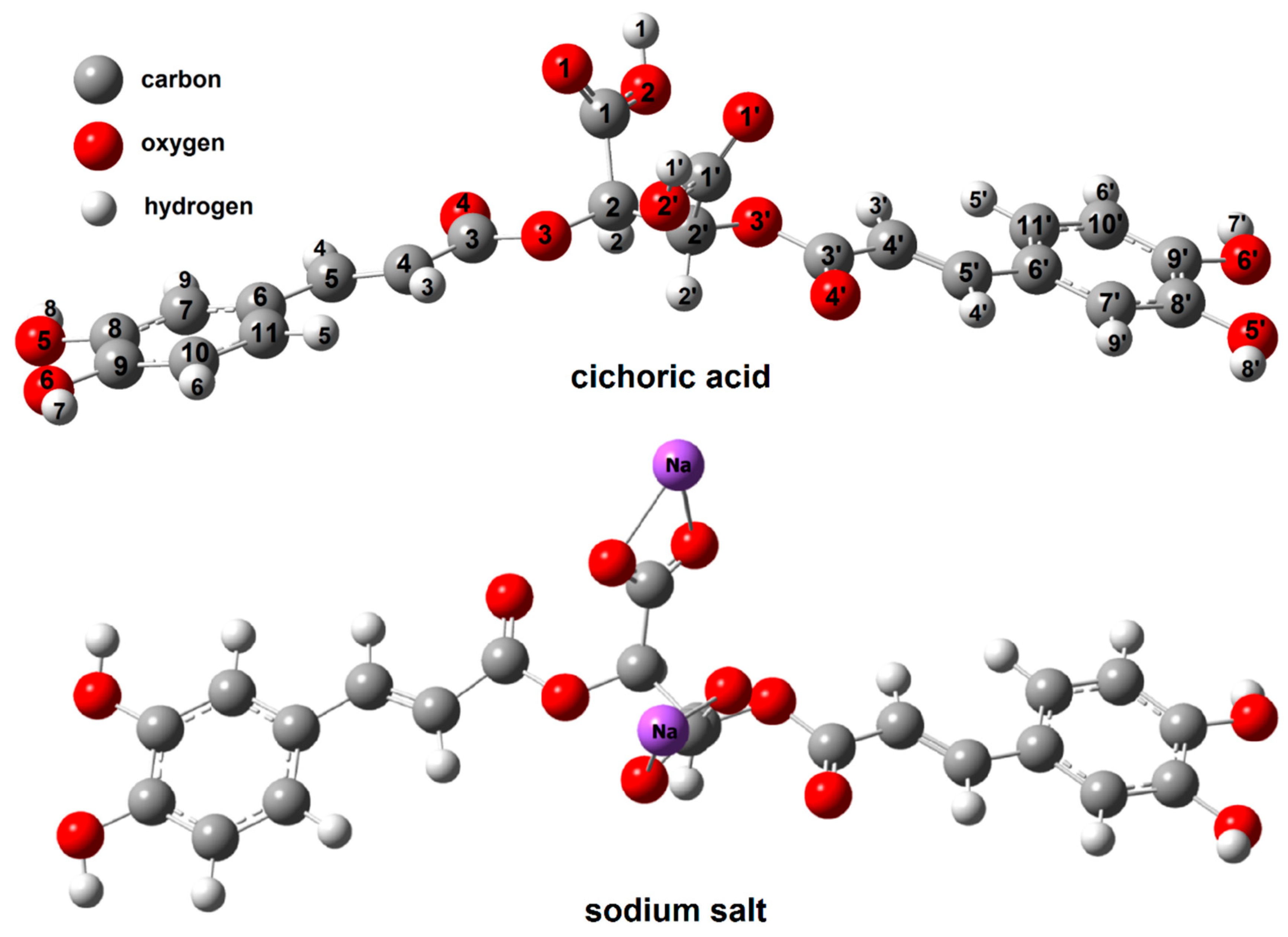
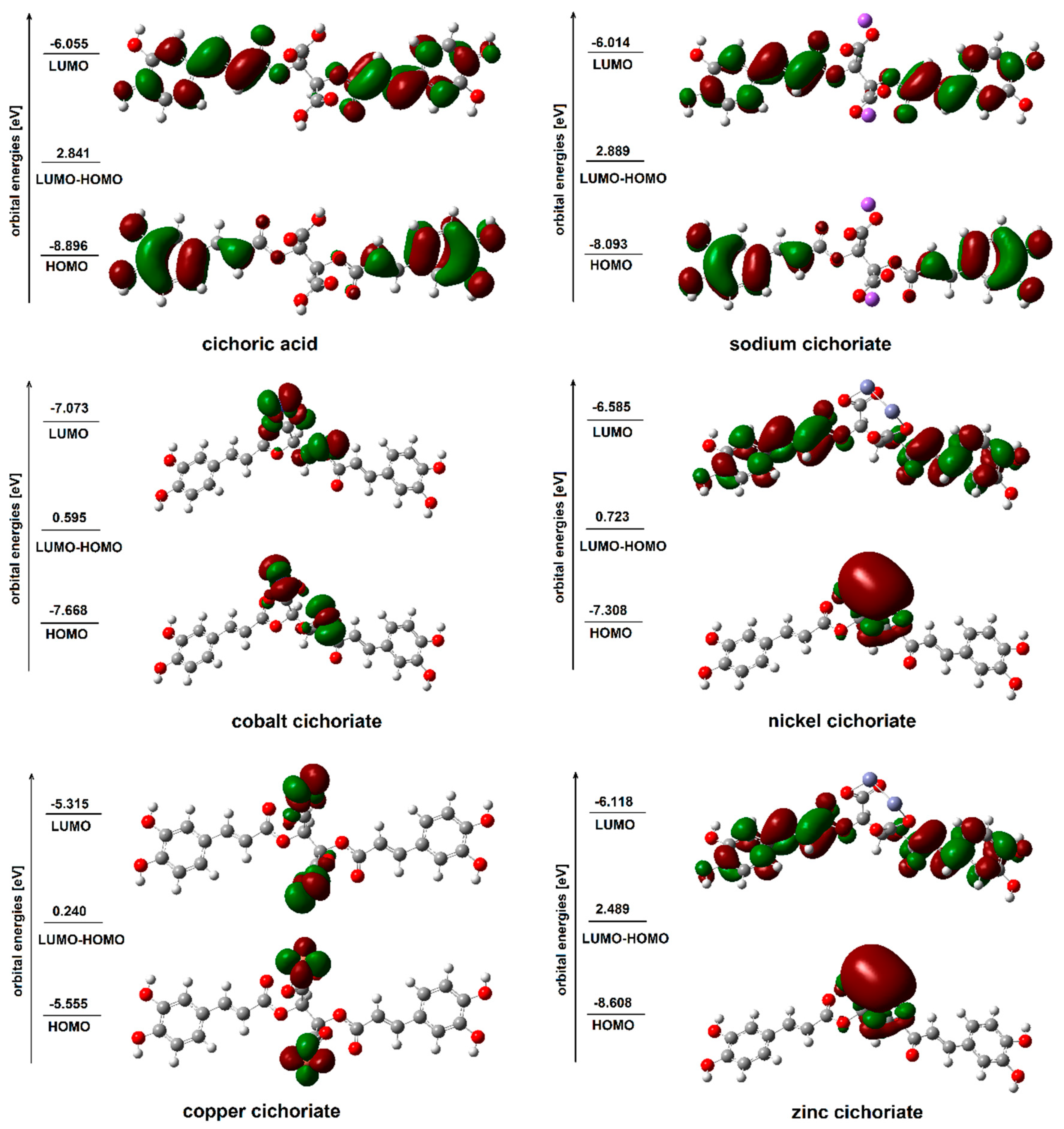
3.2. Theoretical Study
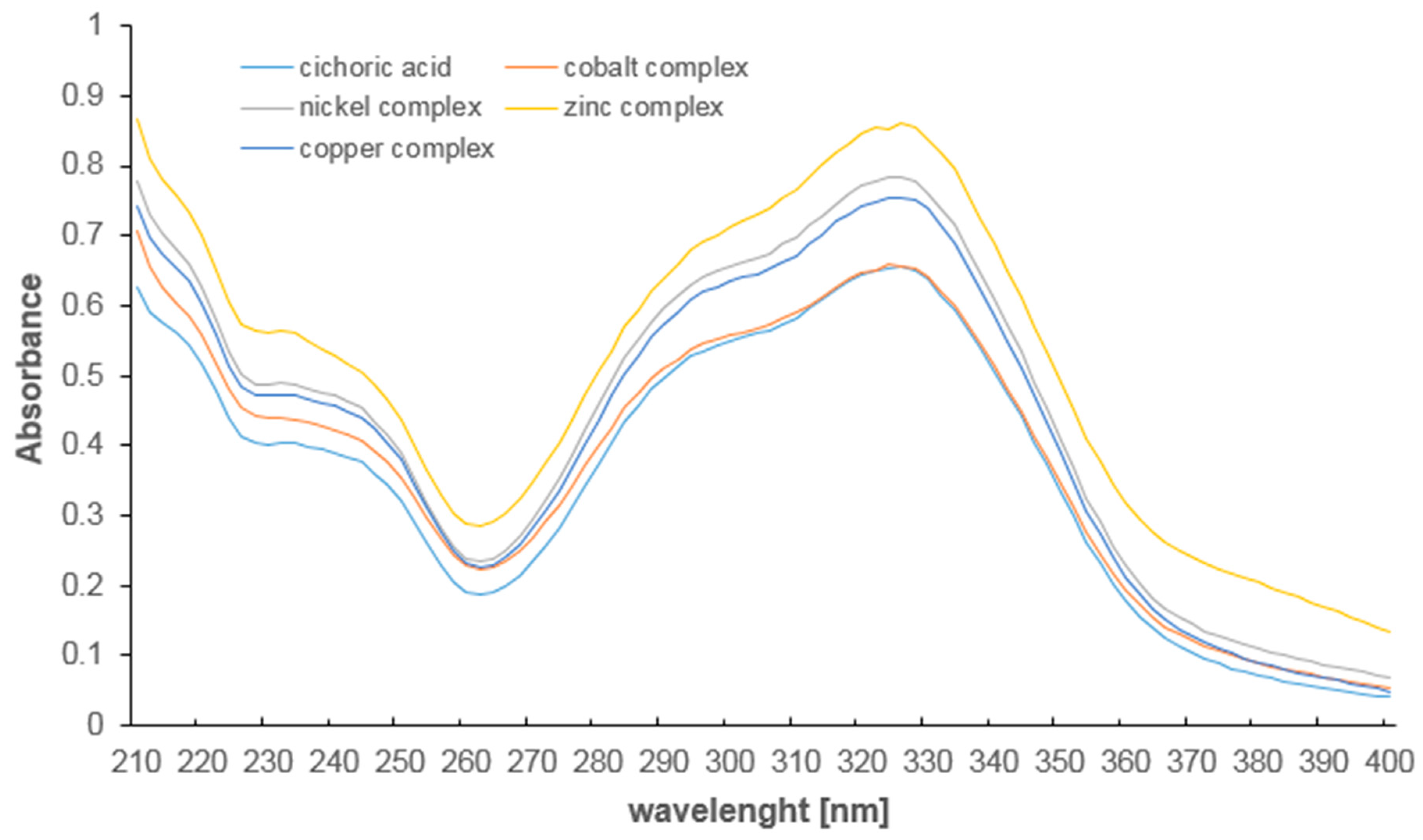
3.3. UV Study
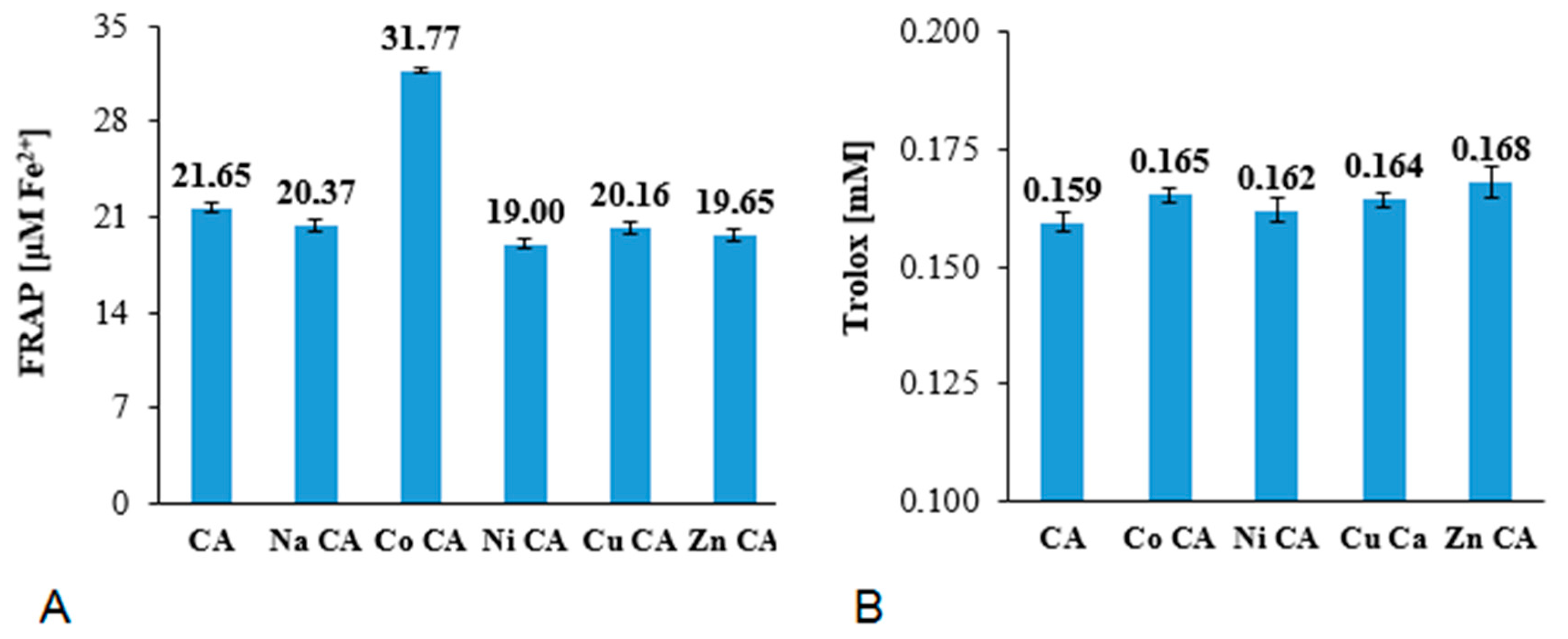
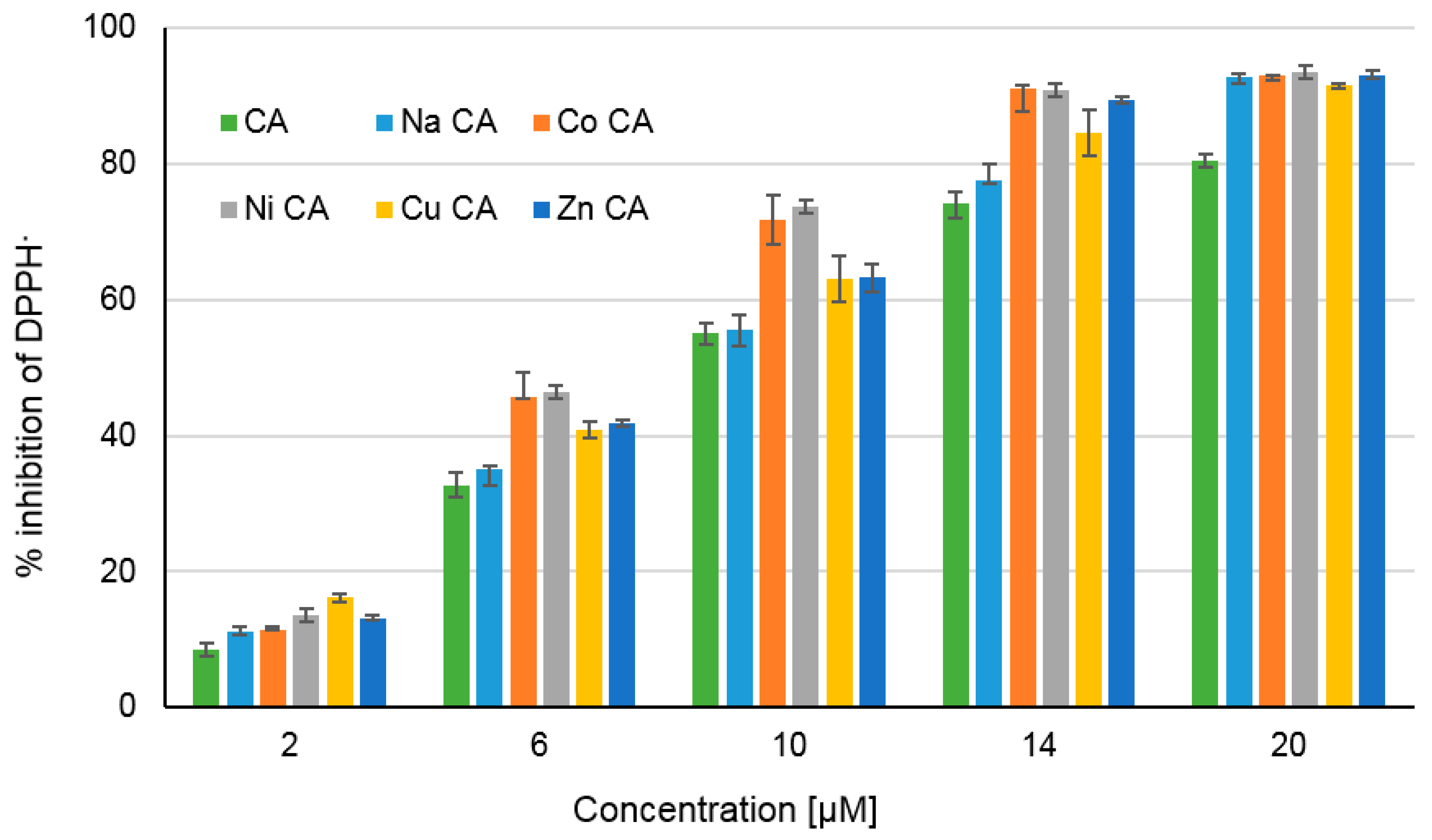
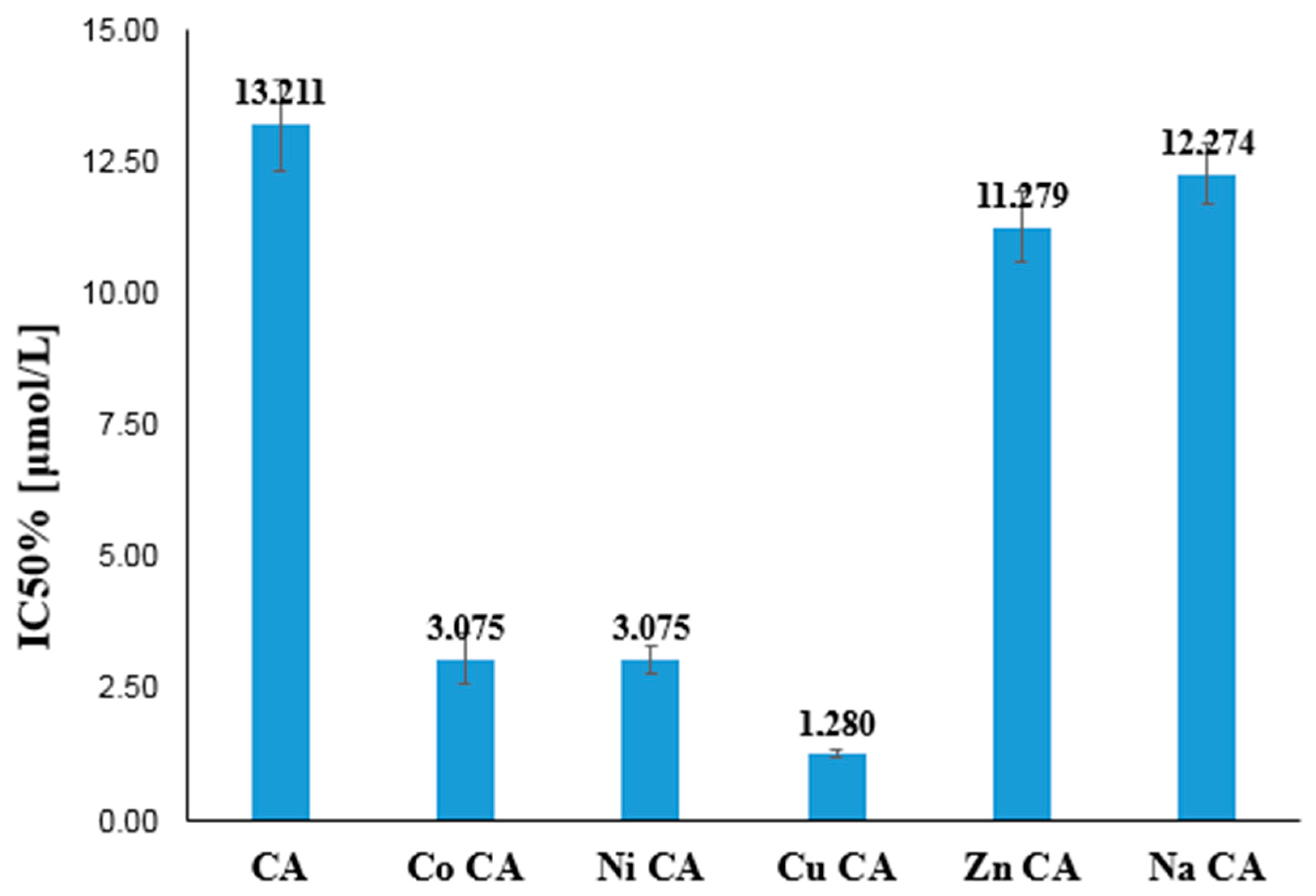
3.4. Antioxidant Study
3.4.1. Ferric-Reducing Antioxidant Power (FRAP), Cupric-Reducing Antioxidant Capacity (CUPRAC), DPPH Assays
3.4.2. SOD (Superoxide Dismutase Activity Assay)
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Herrmann, K. Occurrence and content of hydroxycinnamic acid and hydroxybenzoic acid compounds in foods. Crit. Rev. Food Sci. Nutr. 1989, 28, 315–347. [Google Scholar] [CrossRef]
- Mølgaard, P.; Johnsen, S.; Christensen, P.; Cornett, C. HPLC method validated for the simultaneous analysis of cichoric acid and alkamides in Echinacea purpurea plants and products. J. Agric. Food Chem. 2003, 51, 6922–6933. [Google Scholar] [CrossRef]
- Perry, N.B.; Burgess, E.J.; Glennie, V.I. Echinacea standardization: Analytical methods for phenolic compounds and typical levels in medicinal species. J. Sci. Food Agric. 2001, 49, 1702–1706. [Google Scholar] [CrossRef] [PubMed]
- Dalby-Brown, L.; Barsett, H.; Landbo, A.R.; Meyer, A.S.; Molgaard, P. Synergistic antioxidative effects of alkamides, caffeic acid derivatives, and polysaccharide fractions from Echinacea purpurea on in vitro oxidation of human low-density lipoproteins. J. Agric. Food Chem. 2005, 53, 9413–9423. [Google Scholar] [CrossRef] [PubMed]
- Stanisavljevic, I.; Stojicevic, S.; Velickovic, D.; Veljkovic, V.; Lazic, M. Antioxidant and antimicrobial activities of Echinacea (Echinacea purpurea L.) extracts obtained by classical and ultrasound extraction. Chin. J. Chem. Eng. 2009, 17, 478–483. [Google Scholar]
- Reinke, R.A.; Lee, D.J.; McDougall, B.R.; King, P.J.; Victoria, J.; Mao, Y.; Reinecke, M.G.; Robinson, W.E. L-Chicoric acid inhibits human immunodeficiency virus type 1 integration in vivo and is a noncompetitive but reversible inhibitor of HIV-1 integrase in vitro. Virology 2004, 326, 203–219. [Google Scholar] [CrossRef] [PubMed]
- Charvat, T.T.; Lee, D.J.; Robinson, W.E.; Chamberlin, A.R. Design, synthesis, and biological evaluation of chicoric acid analogs as inhibitors of HIVa integrase. Bioorg. Med. Chem. 2006, 14, 4552–4567. [Google Scholar] [CrossRef] [PubMed]
- Tousch, D.; Lajoix, A.D.; Hosy, E.; Azay-Milhau, J.; Ferrare, K.; Jahannault, C.; Cros, G.; Petit, P. Chicoric acid, a new compound able to enhance insulin release and glucose uptake. Biochem. Biophys. Res. Commun. 2008, 377, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Xie, G.; Wang, J.; Hou, X.; Wang, X.; Wu, W.; Liu, X. Chicoric acid prevents obesity by attenuating hepatic steatosis, inflammation and oxidative stress in high-fat diet-fed mice. Food Res. Int. 2013, 54, 345–353. [Google Scholar] [CrossRef]
- Zhu, D.; Zhang, X.; Niu, Y.; Diao, Z.; Ren, B.; Li, X.; Liu, Z.; Liu, X. Cichoric acid improved hyperglycaemia and restored muscle injury via activating antioxidant response in MLD-STZ-induced diabetic mice. Food Chem. Toxicol. 2017, 107, 138–149. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, X.; Zhai, H.; Zhang, D.; Ma, S. Chicoric acid (CA) induces autophagy in gastric cancer through promoting endoplasmic reticulum (ER) stress regulated by AMPK. Biomed. Pharmacother. 2019, 118, 109144. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.L.; Chiu, C.C.; Chen, J.Y.F.; Chan, K.C.; Lin, S.D. Cytotoxic effects of Echinacea purpurea flower extracts and cichoric acid on human colon cancer cells through induction of apoptosis. J. Ethnopharmacol. 2012, 143, 914–919. [Google Scholar] [CrossRef]
- Samsonowicz, M.; Regulska, E. Spectroscopic study of molecular structure, antioxidant activity and biological effects of metal hydroxyflavonol complexes. Spectrochim. Acta Part A 2017, 173, 757–771. [Google Scholar] [CrossRef]
- Matthias, A.; Penman, K.; Matovic, N.; Bone, K.; De Voss, J.; Lehmann, R. Bioavailability of Echinacea constituents: Caco-2 monolayers and pharmacokinetics of the alkylamides and caffeic acid conjugates. Molecules 2005, 10, 1242–1251. [Google Scholar] [CrossRef] [PubMed]
- Jabłońska-Trypuć, A.; Wydro, U.; Wołejko, E.; Świderski, G.; Lewandowski, W. Biological Activity of New Cichoric Acid–Metal Complexes in Bacterial Strains, Yeast-Like Fungi, and Human Cell Cultures In Vitro. Nutrients 2020, 12, 154. [Google Scholar] [CrossRef] [PubMed]
- Matejczyk, M.; Świsłocka, R.; Golonko, A.; Lewandowski, W.; Hawrylik, E. Cytotoxic, genotoxic and antimicrobial activity of caffeic and rosmarinic acids and their lithium, sodium and potassium salts as potential anticancer compounds. Adv. Med. Sci. 2018, 63, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Sofic, E.; Prior, R.L. Antioxidant and Prooxidant Behavior of Flavonoids: Structure-Activity Relationships. Free Radic. Biol. Med. 1997, 22, 749–760. [Google Scholar] [CrossRef]
- Moridani, M.Y.; Pourahmad, J.; Bui, H.; Siraki, A.; O’Brien, P.J. Dietary flavonoid iron complexes as cytoprotective superoxide radical scavengers. Free Radic. Biol. Med. 2003, 34, 243–253. [Google Scholar] [CrossRef]
- Gonet, B. Wolne rodniki i antyoksydanty w zdrowiu i chorobie. Czynniki Ryzyka 1998, 20–21, 11–21. [Google Scholar]
- Halliwell, B. Oxidative stress, nutrition and health. Experimental strategies for optimization of nutritional antioxidant intake in humans. Free Radic. Res. 1996, 25, 57–74. [Google Scholar] [CrossRef]
- Ziemlański, Ś.; Wartanowicz, M. Rola antyoksydantów żywieniowych w stanie zdrowia i choroby. Pediatr. Współczesna Gastroenterol. Hepatol. Żyw. Dziecka 1999, 1, 97–105. [Google Scholar]
- Grajek, W. Rola przeciwutleniaczy w zmniejszaniu ryzyka wystąpienia nowotworów i chorób układu krążenia, Żywność. Nauka Technol. Jakość 2004, 1, 3–11. [Google Scholar]
- Samsonowicz, M.; Regulska, E.; Kalinowska, M. Hydroxyflavone metal complexes-molecular structure, antioxidant activity and biological effects. Chem. Biol. Interact. 2017, 273, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Świsłocka, R.; Regulska, E.; Karpińska, J.; Świderski, G.; Lewandowski, W. Molecular structure and antioxidant properties of alkali metal salts of rosmarinic acid. experimental and DFT studies. Molecules 2019, 24, 2645. [Google Scholar] [CrossRef] [PubMed]
- Kalinowska, M.; Piekut, J.; Bruss, A.; Follet, C.; Sienkiewicz-Gromiuk, J.; Świsłocka, R.; Rzączyńska, Z.; Lewandowski, W. Spectroscopic (FT-IR, FT-Raman, 1H, 13C NMR, UV/VIS), thermogravimetric and antimicrobial studies of Ca (II), Mn (II), Cu (II), Zn (II) and Cd (II) complexes of ferulic acid. Spectrochim. Acta Part A 2014, 122, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Kalinowska, M.; Mazur, L.; Piekut, J.; Rzączyńska, Z.; Laderiere, B.; Lewandowski, W. Synthesis, crystal structure, spectroscopic properties, and antimicrobial studies of a zinc (II) complex of p-coumaric acid. J. Coord. Chem. 2013, 66, 334–344. [Google Scholar] [CrossRef]
- Kalinowska, M.; Świderski, G.; Matejczyk, M.; Lewandowski, W. Spectroscopic, thermogravimetric and biological studies of Na (I), Ni (II) and Zn (II) complexes of quercetin. J. Therm. Anal. Calorim. 2016, 126, 141–148. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision, A. 02; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Kedare, S.B.; Singh, R.P. Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Technol. 2001, 48, 412–422. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Diplock, A.T.; Symons, M.C.R. Techniques in Free Radical Research; Elsevier: Amsterdam, The Netherlands; London, UK; New York, NY, USA; Tokyo, Japan, 1991. [Google Scholar]
- Özyürek, M.; Güçlü, K.; Apak, R. The main and modified CUPRAC methods of antioxidant measurement. TrAC Trends in Anal. Chem. 2011, 30, 652–664. [Google Scholar] [CrossRef]
- Štarha, P.; Trávníček, Z.; Herchel, R.; Popa, I.; Suchý, P.; Vančo, J. Dinuclear copper (II) complexes containing 6-(benzylamino) purines as bridging ligands: Synthesis, characterization, and in vitro and in vivo antioxidant activities. J. Inorg. Biochem. 2009, 103, 432–440. [Google Scholar] [CrossRef]
- Yousef, T.A.; El-Gammal, O.A.; Ahmed, S.F.; Abu El-Reash, G.M. Synthesis, biological and comparative DFT studies on Ni(II) complexes of NO and NOS donor ligands. Spectrochim. Acta A 2015, 135, 690–703. [Google Scholar] [CrossRef] [PubMed]
- Suksrichavalit, T.; Prachayasittikul, S.; Piacham, T.; Isarankura-Na-Ayudhya, C.; Nantasenamat, C.; Prachayasittikul, V. Copper Complexes of Nicotinic-Aromatic Carboxylic Acids as Superoxide Dismutase Mimetics. Molecules 2008, 13, 3040. [Google Scholar] [CrossRef] [PubMed]
- Suksrichavalit, T.; Prachayasittikul, S.; Nantasenamat, C.; Isarankura-Na-Ayudhya, C.; Prachayasittikul, V. Copper Complexes of pyridine derivatives with superoxide scavenging and antimicrobial activities. Eur. J. Med. Chem. 2009, 44, 3259–3265. [Google Scholar] [CrossRef]
- Ji, H.F.; Zhang, H.Y. A new strategy to combat Alzheimer’s disease. Combining radical-scavenging potential with metal-protein-attenuating ability in one molecule. Bioorg. Med. Chem. Lett. 2005, 15, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Schepetkin, I.; Potapov, A.; Khlebnikov, A.; Korotkova, E.; Lukina, A.; Malovichko, G.; Kirpotina, L.; Quinn, M.T. Decomposition of reactive oxygen species by copper (II) bis (1-pyrazolyl) methane complexes. J. Biol. Inorg. Chem. 2006, 11, 499–513. [Google Scholar] [CrossRef] [PubMed]
- Kalinowska, M.; Bajko, E.; Matejczyk, M.; Kaczyński, P.; Łozowicka, B.; Lewandowski, W. The study of anti-/pro-oxidant, lipophilic, microbial and spectroscopic properties of new alkali metal salts of 5-O-caffeoylquinic acid. Int. J. Mol. Sci. 2018, 19, 463. [Google Scholar] [CrossRef] [PubMed]
- Shayeghi, M.; Latunde-Dada, G.O.; Oakhill, J.S.; Laftah, A.H.; Takeuchi, K.; Halliday, N.; Khan, Y.; Warley, A.; McCann, F.E.; Hider, R.C.; et al. Identification of an intestinal heme transporter. Cell 2005, 122, 789–801. [Google Scholar] [CrossRef]
- Andrews, N.C. Forging a field: The golden age of iron biology. Blood J. Am. Soc. Hematol. 2008, 112, 219–230. [Google Scholar] [CrossRef]
- Woźniak, M.; Czyż, M. Mimetyki dysmutazy ponadtlenkowej–potencjalne zastosowania kliniczne. Postepy Hig. Med. Dosw. 2008, 62, 613–624. [Google Scholar]
- Choudhary, M.; Patel, R.N.; Rawat Synthesis, S.P. Electrochemical, structural, spectroscopic and biological activities of mixed ligand copper (II) complexes with 2-{[(Z)-(5-bromo-2-hydroxyphenyl) methylidene]amino}benzoic acid and nitrogenous bases. J. Mol. Struct. 2014, 1060, 197–207. [Google Scholar] [CrossRef]
- Piotrowska, A.; Drzeżdżon, J.; Jacewicz, D.; Chmurzyński, L. Właściwości antyoksydacyjne, antybakteryjne i przeciwgrzybicze związków kompleksowych miedzi (II). Wiad. Chem. 2017, 71, 219–240. [Google Scholar]
- Barik, A.; Mishra, B.; Shen, L.; Mohan, H.; Kadam, R.M.; Dutta, S.; Zhang, H.Y.; Priyadarsini, K.I. Evaluation of a new copper (II)–curcumin complex as superoxide dismutase mimic and its free radical reactions. Free Radic. Biol. Med. 2005, 39, 811–822. [Google Scholar] [CrossRef] [PubMed]
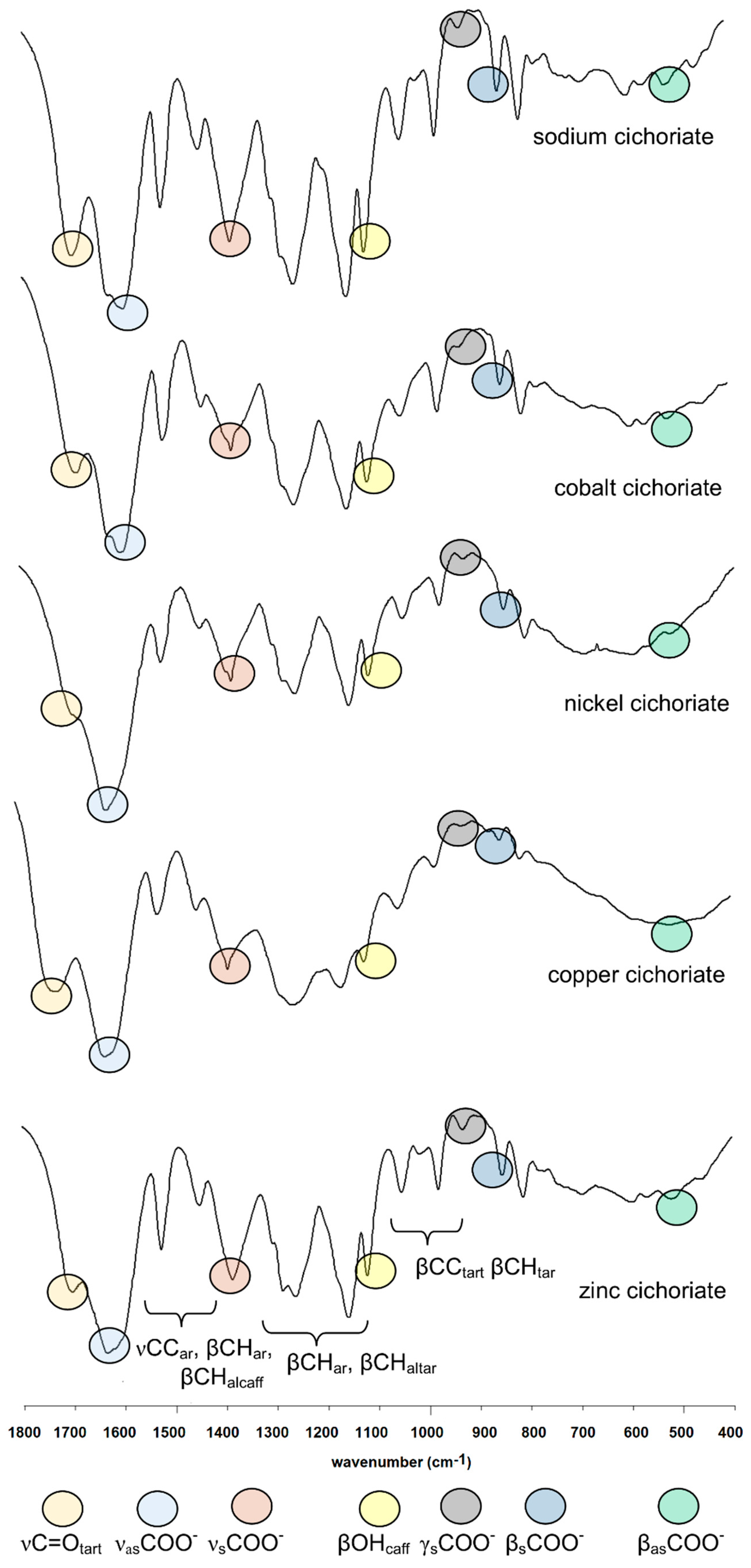
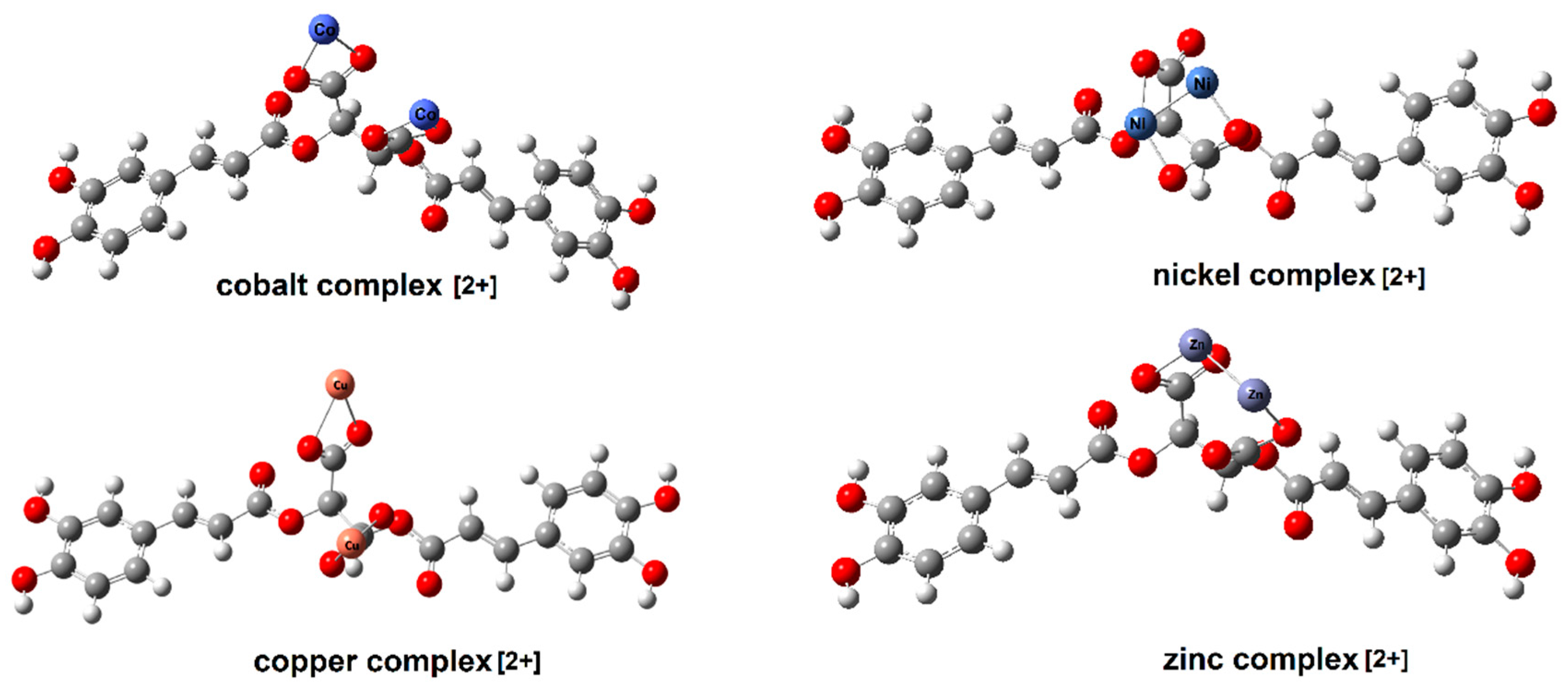
| Cichoric Acid | Sodium Salt | Complexes * | Assignments | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Copper | Nickel | Zinc | Cobalt | |||||||||||||
| Experimental | Theoretical | Experimental | Theoretical | Exp | Theoret | Exp | Exp | Exp | ||||||||
| IR | Raman | DFT | Int | HF | Int | IR | Raman | HF | Int | IR | HF | Int | IR | IR | IR | |
| 3412 s | 3758 | 109.22 | 3741 | 138.74 | 3423 | 3742 | 85.89 | 3454 vs | 3742 | 3.66 | 3441 s | 3452 vs | 3406 | νOHcaff | ||
| 1746 m | 1798 | 236.02 | 1809 | 331.95 | νasCOOHtart | |||||||||||
| 1716 s | 1788 | 352.52 | 1797 | 512.19 | νasCOOHtart | |||||||||||
| 1682 vs | 1681 s | 1732 | 6.19 | 1744 | 18.96 | 1699 s | 1703 w | 1735 | 7.47 | 1721 s | 1736 | 9.23 | 1698 s | 1692 m | νC = Ocaff, νC = Calcaff | |
| 1624 s | 1627 vs | 1730 | 495.5 | 1742 | 697.97 | 1732 | 686.64 | 1731 | 669.21 | νC = Ocaff, νC = Calcaff | ||||||
| 1627 vs | 1580 | 1255.35 | 1625 s | 1570 | 1105.71 | 1630 s | 1629 vs | 1626 s | νasCOO− | |||||||
| 1578 | 218.51 | 1568 | 184.99 | 1605 s | νasCOO− | |||||||||||
| 1606 vs | 1609 vs | 1644 | 474.78 | 1640 | 680.3 | 1599 vs | 1603 vs | 1650 | 489.32 | 1649 | 529.87 | νCCar, νC = Calcaff | ||||
| 1517 s | 1516 m | 1605 | 901.82 | 1601 | 487.27 | 1522 s | 1521 vw | 1607 | 181.58 | 1522 m | 1606 | 220.16 | 1522 m | 1524 m | 1521 m | νCCar, νC = Calcaff, βC-Har, βC-Halcaff, |
| 1484 w | 1482 m | 1600 | 106.27 | 1593 | 122.69 | 1594 | 127.39 | 1594 | 132.35 | βC-Car, βC-Har, νCCar, | ||||||
| 1446 w | 1528 | 266.54 | 1515 | 417.92 | 1449 m | 1456 vw | 1515 | 431.85 | 1446 m | 1514 | 428.92 | 1446 m | 1449 m | 1445 m | βC-Har | |
| 1385 s | 1381 vw | 1447 | 204.62 | 1385 m | 1446 | 185.54 | 1385 m | 1384 s | 1385 m | νsCOO− | ||||||
| 1430 | 139.79 | 1430 | 129.3 | νsCOO− | ||||||||||||
| 1437 | 94.71 | 1415 | 171.64 | 1414 | 120.46 | 1414 | 122.76 | νCCar, βC-Har, | ||||||||
| 1362 s | 1364 m | 1393 | 42.79 | 1412 | 41.26 | βC-Haltart | ||||||||||
| 1337 m | 1342 vw | 1386 | 1.94 | 1405 | 0.01 | βC-Haltart | ||||||||||
| 1300 s | 1303 m | 1354 | 15.64 | 1327 | 3.29 | 1304 vw | 1324 | 343.53 | 1258 s | 1325 | 329.41 | 1304 m | defring, νCCar, βC-Har, βC-Haltart | |||
| 1280 m | 1272 w | 1335 | 9.92 | 1312 | 95.46 | 1287 vw | 1312 | 54.87 | 1313 | 32.33 | 1285 m | 1285 s | βC-Har, βC-Haltart | |||
| 1246 vs | 1249 m | 1302 | 19.17 | 1259 vs | 1262 vw | 1303 | 140.18 | 1305 | 193.71 | 1260 m | 1259 s | 1260 s | βC-Haltart | |||
| 1218 vs | 1213 w | 1306 | 216.72 | 1288 | 116.76 | 1203 vw | 1286 | 67.15 | 1286 | 69.23 | defring, βC-Har, βC-Halcaff | |||||
| 1167 s | 1170 w | 1185 | 106.51 | 1164 | 72.74 | 1153 vs | 1161 vw | 1154 m | 1154 s | 1156 s | βC-Har, βC-Halcaff | |||||
| 1120 s | 1125 w | 1169 | 35.07 | 1161 | 1263.96 | 1117 s | 1120 vw | 1117 m | 1116 m | 1117 s | 1115 s | βC-Har, βO-Hcaff | ||||
| 1078 m | 1037 w | 1166 | 473.39 | 1153 | 483.48 | 1048 m | 1049 vw | 1153 | 8.67 | 1052 m | 1153 | 9.64 | 1049 w | 1051 m | 1051 m | βC-Htart,βC-Ctart |
| 990 m | 992 vw | 1067 | 215.16 | 1072 | 8.57 | 1066 | 168.31 | 1066 | 391.83 | νC(2)-O(3), νC(2′)-O(3′), | ||||||
| 976 s | 976 vw | 1055 | 10.8 | 1066 | 338.46 | 977 m | 979 vw | 1065 | 236.09 | 981 w | 1065 | 4.89 | 979 w | 979 m | 976 m | δC(2)-C(2′) |
| 976 | 1.85 | 965 | 0.25 | 967 | 0.01 | 966 | 0.01 | βC = Calcaff | ||||||||
| 961 | 41.67 | 963 | 18.6 | 965 | 43.22 | 964 | 42.67 | defring, βC = Calcaff | ||||||||
| 930 vw | 903 vw | 956 | 24.73 | 924 w | 956 | 25.84 | 930 vw | 930 w | 935 w | γsCOO− | ||||||
| 950 | 29.56 | 945 | 9.87 | 937 | 5.92 | 937 | 5.85 | defring | ||||||||
| 898 w | 896 vw | 940 | 1.08 | 936 | 5.25 | 880 vw | 929 | 19.11 | 928 | 19.28 | 896 vw | τC(2)-C(2′) | ||||
| 877 w | 868 vw | 896 | 18.1 | 895 | 19.28 | defring ou | ||||||||||
| 867 w | 862 vw | 870 | 12.29 | 885 | 17.44 | γC-Har, γC-Halcaff | ||||||||||
| 853 w | 860 vw | 870 | 18.44 | 868 w | 871 | 10.97 | 851 w | 853 w | 851 m | βsCOO− | ||||||
| 869 | 8.1 | 869 | 20.35 | βsCOO− | ||||||||||||
| 804 m | 810 vw | 835 | 66.84 | 862 | 96.44 | 810 m | 812 vw | 863 | 86.38 | 863 | 88.76 | 810 w | 812 m | 809 m | γC-Har, γC-Halcaff | |
| 826 | 52.13 | 826 | 15.3 | 813 | 80.19 | 813 | 82.51 | τC(2)-C(2′) | ||||||||
| 813 | 0.34 | 812 | 72.65 | 813 | 2.37 | 813 | 0.22 | δC(2)-H, δC(2′)-H | ||||||||
| 791 | 47.47 | 782 | 5.37 | 795 | 44.83 | 794 | 48.66 | γC-Har | ||||||||
| 765 w | 765 vw | 787 | 41.02 | 779 | 66.92 | 782 w | 787 vw | 779 w | 782 m | defring, βC(1)-C(2), βC(1′)-C(2′) | ||||||
| 736 w | 730 vw | 772 | 49.56 | 762 | 12.51 | 763 | 14.81 | 763 | 13.91 | 729 w | defring, νC-Car | |||||
| 715 vw | 731 | 5.87 | 740 | 5.32 | 715 w | 719 vw | 725 w | defring, γCOO, γC-Halcaff | ||||||||
| 698 vw | 699 vw | 723 | 5.23 | 739 | 2.12 | 746 | 3.41 | 745 | 4.05 | 698 w | 695 m | γCOOH | ||||
| 680 w | 685 vw | 703 | 1.14 | 727 | 4.36 | 688 w | 727 | 1.11 | 726 | 2.16 | 691 m | 685 m | defring, γCOOH | |||
| 659 w | 664 vw | 692 | 0.32 | 712 | 11.57 | 659 vw | 704 | 3.38 | 704 | 3.24 | 676 w | defring ou | ||||
| A596 m | 597 vw | 587 | 30.45 | 574 | 113.89 | 596 w | 573 | 16.53 | 591 m | 586 | 4.11 | 598 m | 598 m | 595 m | defring ou | |
| 583 m | 584 vw | 579 | 46.79 | 566 | 118.25 | 562 | 1.28 | 562 | 1.42 | defring | ||||||
| 576 m | 576 vw | 569 | 79.93 | 553 | 170.63 | 560 | 34.96 | 559 | 37.81 | 566 m | defring | |||||
| 565 w | 565 vw | 563 | 24.82 | 549 | 19.81 | 546 vw | 550 | 6.3 | 550 m | 550 | 7.58 | 574 w | 568 m | γO-Hcaff | ||
| 521 w | 539 | 40.51 | 521 m | 536 | 39.39 | 520 m | 520 m | 521 m | βasCOO− | |||||||
| 472 | 39.33 | 471 | 46.44 | βasCOO− | ||||||||||||
| 504 w | 504 vw | 542 | 0.55 | 517 | 7.63 | γO-Hcaff | ||||||||||
| Cichoric Acid | Sodium Cichorate * | Copper(II) Cichorate (2+) ** | Zinc (II) Cichorate (2+) | Nickel(II) Cichorate (2+) | Cobalt (II) Cichorate (2+) | |
|---|---|---|---|---|---|---|
| Energy (Hartree) | −1742.43 | −2065.08 | −5019.01 | −5296.64 | −4766.91 | −4503.67 |
| Dipole moment (D) | 1.27 | 11.19 | 8.88 | 2.21 | 3.83 | 9.02 |
| Aj | 0.995 | 0.996 | 0.996 | 0.992 | 0.989 | 0.995 |
| BAC | 0.896 | 0.907 | 0.911 | 0.876 | 0.861 | 0.903 |
| HOMA | 0.988 | 0.990 | 0.990 | 0.944 | 0.936 | 0.898 |
| EN | 0.001 | 0.001 | 0.008 | 0.338 | 0.041 | 0.001 |
| GEO | 0.011 | 0.009 | 0.001 | 0.018 | 0.041 | 0.010 |
| I6 | 94.48 | 95.04 | 95.23 | 92.90 | 92.44 | 94.83 |
| HOMO (Hartree) | −0.32693 | −0.32718 | −0.20416 | −0.31634 | −0.26857 | −0.2818 |
| LUMO (Hartree) | −0.22251 | −0.22100 | −0.19533 | −0.22485 | −0.24200 | −0.25994 |
| HOMO (eV) | −8.896 | −8.903 | −5.555 | −8.608 | −7.308 | −7.668 |
| LUMO (eV) | −6.055 | −6.014 | −5.315 | −6.118 | −6.585 | −7.073 |
| Energy gap | 2.841 | 2.889 | 0.240 | 2.489 | 0.723 | 0.595 |
| Ionization potential, I = −EHOMO | 8.896 | 8.903 | 5.555 | 8.608 | 7.308 | 7.668 |
| Electron Affinity, A = −ELUMO | 6.055 | 6.014 | 5.315 | 6.118 | 6.585 | 7.073 |
| Electronegativity, χ = (I + A)/2 | 7.475 | 7.455 | 5.440 | 7.363 | 6.947 | 7.371 |
| Chemical potential, μ = −(I + A)/2 | −7.475 | −7.455 | −5.440 | −7.363 | −6.947 | −7.371 |
| Chemical hardness, η = (I − A)/2 | 1.425 | 1.445 | 0.120 | 1.245 | 0.362 | 0.297 |
| Chemical softness, S = 1/2η | 0.351 | 0.346 | 4.167 | 0.402 | 1.383 | 1.681 |
| Electrophilicity index, ω = μ2/2η | −2.623 | −2.580 | −22.667 | 21.778 | 66.743 | 91.332 |
| Cichoric Acid | Sodium Salt | Copper(II) Complex Cation | Cobalt(II) Complex Cation | Nickel(II) Complex Cation | Zinc (II) Complex Cation | ||
|---|---|---|---|---|---|---|---|
| B3LYP/6-311++G(d,p) | HF/6-311++G(d,p) | HF/6-311++G(d,p) | HF/6-311++G(d,p) | HF/6-311++G(d,p) | HF/6-311++G(d,p) | HF/6-311++G(d,p) | |
| C1–O1 | 1.201 | 1.178 | 1.239 | 1.239 | 1.233 | 1.309 | 1.268 |
| C1–O2 | 1.344 | 1.317 | 1.236 | 1.236 | 1.247 | 1.309 | 1.275 |
| C1–C2 | 1.536 | 1.528 | 1.535 | 1.535 | 1.521 | 1.568 | 1.533 |
| O3–C2 | 1.422 | 1.398 | 1.410 | 1.410 | 1.404 | 1.416 | 1.427 |
| C3–O3 | 1.371 | 1.336 | 1.319 | 1.319 | 1.335 | 1.376 | 1.370 |
| C3–O4 | 1.210 | 1.186 | 1.188 | 1.188 | 1.184 | 1.213 | 1.216 |
| C3–C4 | 1.466 | 1.473 | 1.484 | 1.483 | 1.479 | 1.466 | 1.468 |
| C4–C5 | 1.345 | 1.327 | 1.325 | 1.325 | 1.326 | 1.348 | 1.348 |
| C5–C6 | 1.457 | 1.470 | 1.474 | 1.474 | 1.472 | 1.457 | 1.457 |
| C6–C11 | 1.402 | 1.386 | 1.386 | 1.386 | 1.386 | 1.404 | 1.404 |
| C10–C11 | 1.388 | 1.384 | 1.384 | 1.384 | 1.384 | 1.391 | 1.391 |
| C9–C10 | 1.396 | 1.382 | 1.382 | 1.382 | 1.382 | 1.397 | 1.397 |
| C8–C9 | 1.406 | 1.394 | 1.393 | 1.393 | 1.393 | 1.410 | 1.410 |
| C8–C7 | 1.388 | 1.378 | 1.379 | 1.379 | 1.378 | 1.389 | 1.389 |
| C7–C6 | 1.407 | 1.396 | 1.395 | 1.395 | 1.396 | 1.410 | 1.410 |
| C8–O5 | 1.364 | 1.348 | 1.349 | 1.349 | 1.349 | 1.365 | 1.365 |
| C9–O6 | 1.360 | 1.344 | 1.347 | 1.347 | 1.346 | 1.361 | 1.361 |
| C1′–O1′ | 1.201 | 1.178 | 1.239 | 1.239 | 1.233 | 1.214 | 1.268 |
| C1′–O2′ | 1.344 | 1.317 | 1.236 | 1.236 | 1.247 | 1.377 | 1.275 |
| C1′–C2′ | 1.536 | 1.528 | 1.535 | 1.535 | 1.521 | 1.568 | 1.533 |
| O3′–C2′ | 1.422 | 1.398 | 1.410 | 1.410 | 1.404 | 1.456 | 1.427 |
| C3′–O3′ | 1.371 | 1.336 | 1.319 | 1.319 | 1.335 | 1.400 | 1.370 |
| C3′–O4′ | 1.210 | 1.186 | 1.188 | 1.188 | 1.184 | 1.209 | 1.216 |
| C3′–C4′ | 1.466 | 1.473 | 1.483 | 1.483 | 1.479 | 1.461 | 1.468 |
| C4′–C5′ | 1.345 | 1.327 | 1.325 | 1.325 | 1.326 | 1.352 | 1.348 |
| C5′–C6′ | 1.457 | 1.470 | 1.474 | 1.474 | 1.472 | 1.453 | 1.457 |
| C6′–C11′ | 1.402 | 1.386 | 1.386 | 1.386 | 1.386 | 1.405 | 1.404 |
| C10′–C11′ | 1.388 | 1.384 | 1.384 | 1.384 | 1.384 | 1.390 | 1.391 |
| C9′–C10′ | 1.396 | 1.382 | 1.382 | 1.382 | 1.382 | 1.398 | 1.397 |
| C8′–C9′ | 1.406 | 1.394 | 1.393 | 1.393 | 1.393 | 1.411 | 1.410 |
| C8′–C7′ | 1.388 | 1.378 | 1.379 | 1.379 | 1.378 | 1.388 | 1.389 |
| C7′–C6′ | 1.407 | 1.396 | 1.395 | 1.395 | 1.396 | 1.412 | 1.410 |
| C8′–O5′’ | 1.364 | 1.348 | 1.349 | 1.349 | 1.349 | 1.362 | 1.365 |
| C9′–O6′ | 1.360 | 1.344 | 1.347 | 1.347 | 1.346 | 1.358 | 1.361 |
| Cichoric Acid | Cobalt Cichorate | Nickel Cichorate | Copper Cichorate | Zinc Cichorate | |
|---|---|---|---|---|---|
| λmax1 [nm] | 327.5 | 324.0 | 325.0 | 325.0 | 326.0 |
| λmax2 [nm] | 233.0 | 230.0 | 232.0 | 232.0 | 232.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Świderski, G.; Jabłońska-Trypuć, A.; Kalinowska, M.; Świsłocka, R.; Karpowicz, D.; Magnuszewska, M.; Lewandowski, W. Spectroscopic, Theoretical and Antioxidant Study of 3d-Transition Metals (Co(II), Ni(II), Cu(II), Zn(II)) Complexes with Cichoric Acid. Materials 2020, 13, 3102. https://doi.org/10.3390/ma13143102
Świderski G, Jabłońska-Trypuć A, Kalinowska M, Świsłocka R, Karpowicz D, Magnuszewska M, Lewandowski W. Spectroscopic, Theoretical and Antioxidant Study of 3d-Transition Metals (Co(II), Ni(II), Cu(II), Zn(II)) Complexes with Cichoric Acid. Materials. 2020; 13(14):3102. https://doi.org/10.3390/ma13143102
Chicago/Turabian StyleŚwiderski, Grzegorz, Agata Jabłońska-Trypuć, Monika Kalinowska, Renata Świsłocka, Danuta Karpowicz, Marta Magnuszewska, and Włodzimierz Lewandowski. 2020. "Spectroscopic, Theoretical and Antioxidant Study of 3d-Transition Metals (Co(II), Ni(II), Cu(II), Zn(II)) Complexes with Cichoric Acid" Materials 13, no. 14: 3102. https://doi.org/10.3390/ma13143102
APA StyleŚwiderski, G., Jabłońska-Trypuć, A., Kalinowska, M., Świsłocka, R., Karpowicz, D., Magnuszewska, M., & Lewandowski, W. (2020). Spectroscopic, Theoretical and Antioxidant Study of 3d-Transition Metals (Co(II), Ni(II), Cu(II), Zn(II)) Complexes with Cichoric Acid. Materials, 13(14), 3102. https://doi.org/10.3390/ma13143102








