Nanoscale Topographical Characterization of Orbital Implant Materials
Abstract
1. Introduction
2. Materials and Methods
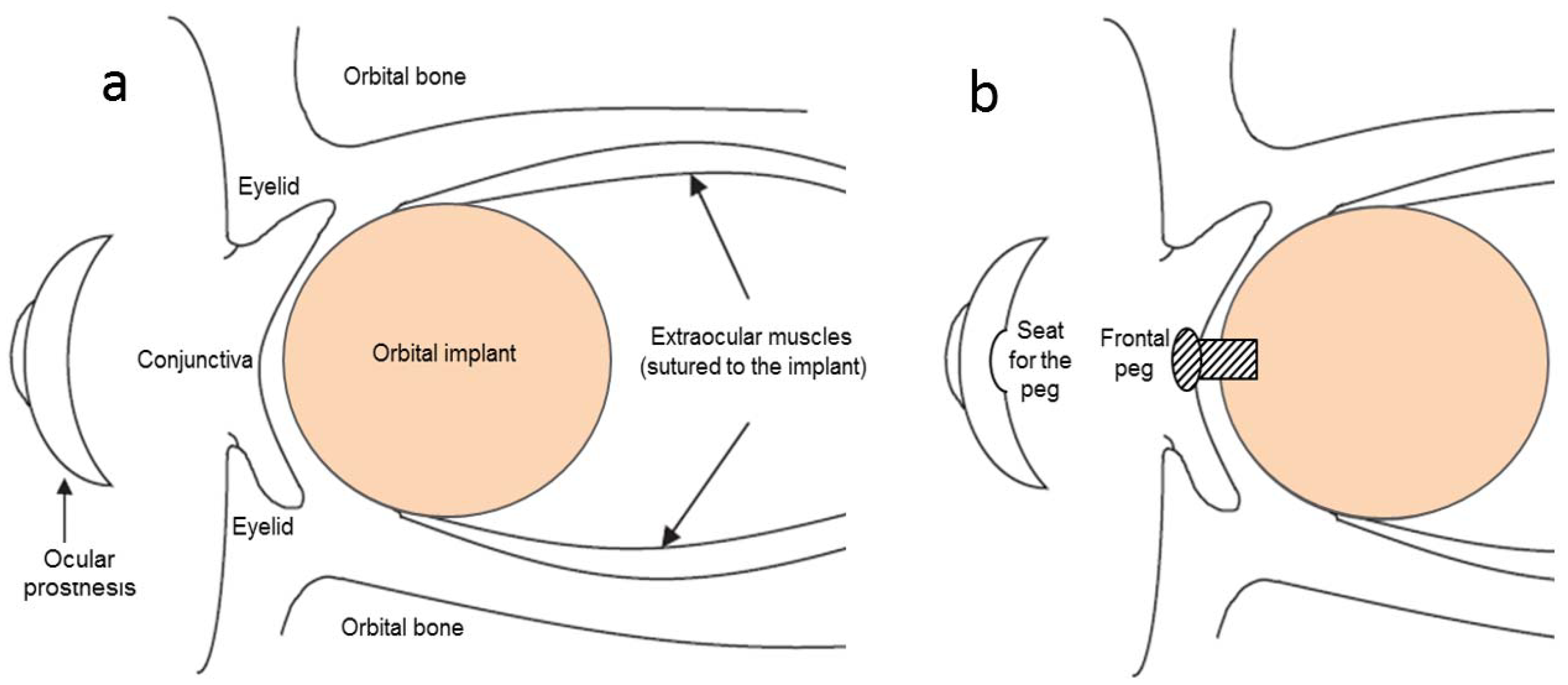
2.1. Ocular Implant Materials
2.2. Characterization
2.3. Statistical Analysis
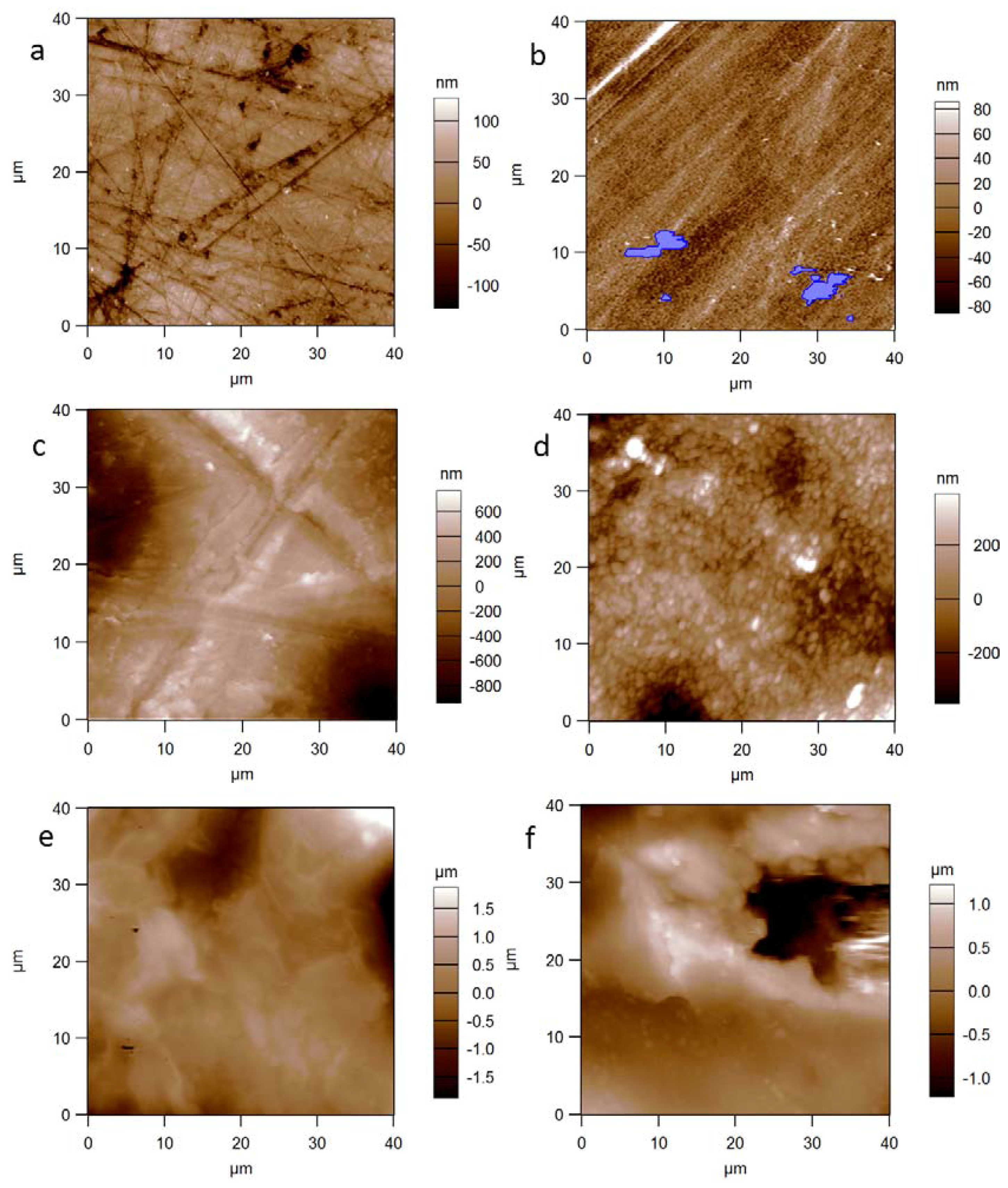
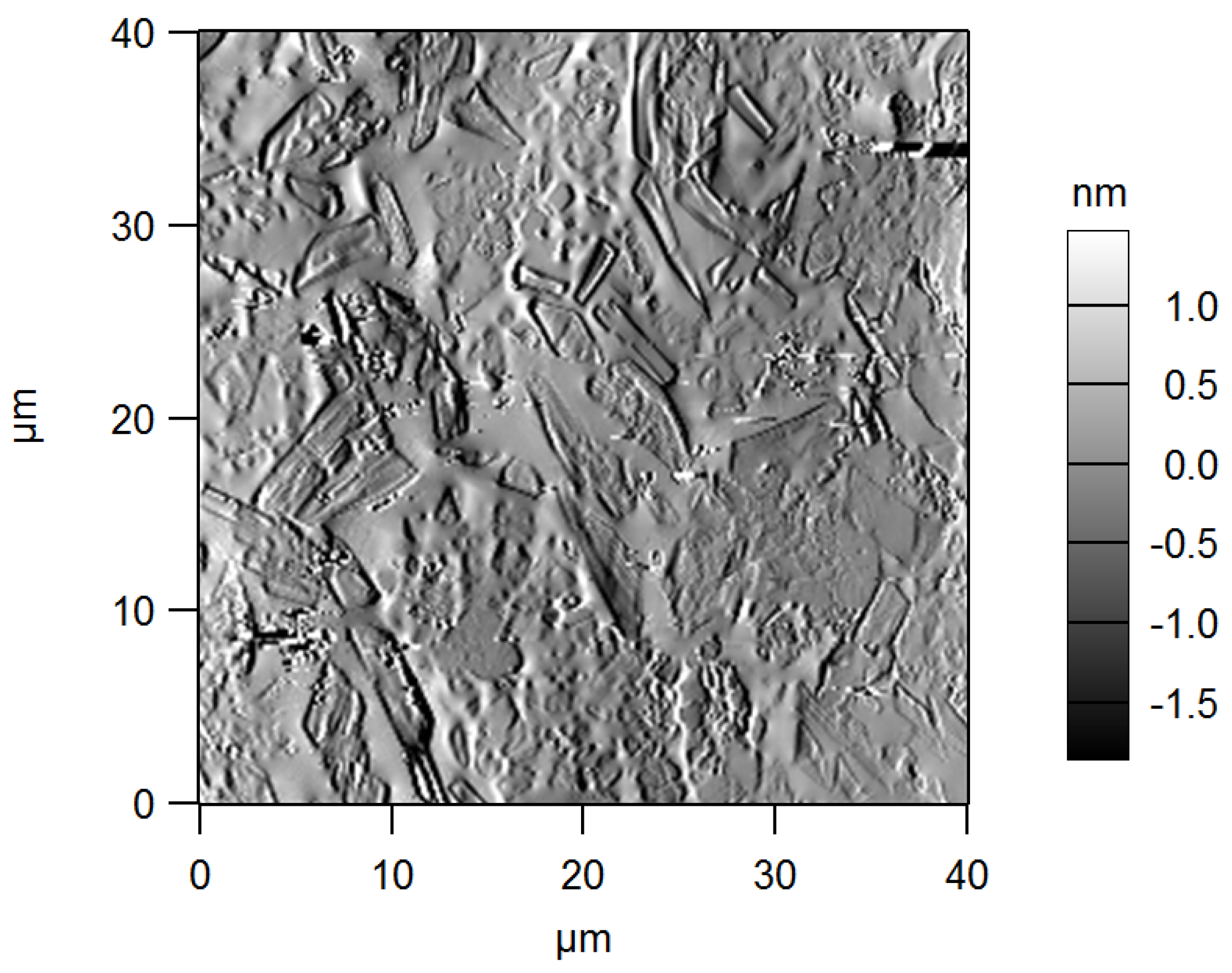
3. Results and Discussion
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Feller, L.; Jadwat, Y.; Khammissa, R.A.G.; Meyerov, R.; Schechter, I.; Lemmer, J. Cellular responses evoked by different surface characteristics of intraosseous titanium implants. Biomed. Res. Int. 2015, 2015, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wennerberg, A.; Albrektsson, T. Effects of titanium surface topography on bone integration: A systematic review. Clin. Oral Implant. Res. 2009, 20, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.M.; Jiang, Z.; Bastmeyer, M.; Lahann, J. Physical aspects of cell culture substrates: Topography, roughness, and elasticity. Small 2012, 8, 336–355. [Google Scholar] [CrossRef] [PubMed]
- Salerno, M.; Caneva-Soumetz, F.; Pastorino, L.; Patra, N.; Diaspro, A.; Ruggiero, C. Adhesion and proliferation of osteoblast-like cells on anodic porous alumina substrates with different morphology. IEEE Trans. Nanobiosci. 2013, 12, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, Z.; Boyan, B.D. Underlying mechanisms at the bone–biomaterial interface. J. Cell. Biochem. 1994, 56, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Guehennec, L. Surface treatments of titanium dental implants for rapid osseointegration. Dent. Mater. 2007, 3, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Qu, Z.; Rausch-Fan, X.; Wieland, M.; Matejka, M.; Schedle, A. The initial attachment and subsequent behavior regulation of osteoblasts by dental implant surface modification. J. Biomed. Mater. Res. Part A 2006, 82, 658–668. [Google Scholar] [CrossRef] [PubMed]
- Ventre, M.; Natale, C.F.; Rianna, C.; Netti, P.A. Topographic cell instructive patterns to control cell adhesion, polarization and migration. J. R. Soc. Intefrace 2014. [Google Scholar] [CrossRef] [PubMed]
- Courtney, J.M.; Lamba, N.M.K.; Sundaram, S.; Forbes, C.D. Biomaterials for blood-contacting applications. Biomaterials 1994, 15, 737–744. [Google Scholar] [CrossRef]
- Baino, F.; Potestio, I. Orbital implants: State-of-the-art review with emphasis on biomaterials and recent advances. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 69, 1410–1428. [Google Scholar] [CrossRef] [PubMed]
- Moshfeghi, D.M.; Moshfeghi, A.A.; Finger, P.T. Enucleation. Surv. Ophthalmol. 2000, 44, 277–301. [Google Scholar] [CrossRef]
- Patil, S.B.; Meshramkar, R.; Naveen, B.H.; Patil, N.P. Ocular prosthesis: A brief review and fabrication of an ocular prosthesis for a geriatric patient. Gerodontology 2008, 25, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, R.; Poole-Warren, L.; Conway, R.M.; Ben-Nissan, B. Porous Orbital Implants in Enucleation: A Systematic Review. Surv. Ophthalmol. 2007, 52, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Nunery, W.R.; Heinz, G.W.; Bonnin, J.M.; Martin, R.T.; Cepela, M.A. Exposure rate of hydroxyapatite spheres in the anophthalmic socket: Histopathologic correlation and comparison with silicone sphere implants. Ophthalmic Plast. Reconstr. Surg. 1993, 9, 96–104. [Google Scholar] [CrossRef]
- Dutton, J.J. Coralline Hydroxyapatite as an Ocular Implant. Ophthalmology 1991, 98, 370–377. [Google Scholar] [CrossRef]
- Gayre, G.S.; Lipham, W.; Dutton, J.J. A comparison of rates of fibrovascular ingrowth in wrapped versus unwrapped hydroxyapatite spheres in a rabbit model. Ophthalmic Plast. Reconstr. Surg. 2002, 18, 275–280. [Google Scholar] [CrossRef]
- Baino, F. Porous glass-ceramic orbital implants: A feasibility study. Mater. Lett. 2018, 212, 12–15. [Google Scholar] [CrossRef]
- Baino, F.; di Confiengo, G.G.; Faga, M.G. Fabrication and morphological characterization of glass-ceramic orbital implants. Int. J. Appl. Ceram. Technol. 2017, 1–8. [Google Scholar] [CrossRef]
- Lombardo, M.; Carbone, G.; Lombardo, G.; De Santo, M.P.; Barberi, R. Analysis of intraocular lens surface adhesiveness by atomic force microscopy. J. Cataract Refract. Surg. 2009, 35, 1266–1272. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Lee, S.J.; Shin, J.-H.; Cheong, Y.; Lee, H.-J.; Paek, J.H.; Kim, J.S.; Jin, K.-H.; Park, H.-K. Ultrastructural investigation of intact orbital implant surfaces using atomic force microscopy. Scanning 2011, 33, 211–221. [Google Scholar] [CrossRef] [PubMed]
- ISO 25178-2:2012. Geometrical Product Specifications (GPS)—SurfaceTexture: Areal—Part 2: Terms, Definitions and Surface Texture Parameters. Available online: https://www.iso.org/obp/ui/#iso:std:iso:25178:-2:ed-1:v1:en (accessed on 16 March 2018).
- Toccafondi, C.; Dante, S.; Reverberi, A.P.; Salerno, M. Biomedical Applications of Anodic Porous Alumina. Curr. Nanosci. 2015, 11, 572–580. [Google Scholar] [CrossRef]
- Toccafondi, C.; Thorat, S.; La Rocca, R.; Scarpellini, A.; Salerno, M.; Dante, S.; Das, G. Multifunctional substrates of thin porous alumina for cell biosensors. J. Mater. Sci. Mater. Med. 2014, 25, 2411–2420. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.Y.; Ding, G.Q.; Ding, J.N.; Yuan, N.Y. AFM, SEM and TEM studies on porous anodic alumina. Nanoscale Res. Lett. 2010, 5, 725–734. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Alwitry, A.; West, S.; King, J.; Foss, A.J.; Abercrombie, L.C. Long-term follow-up of porous polyethylene spherical implants after enucleation and evisceration. Ophthalmic Plast. Reconstr. Surg. 2007, 23, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, N.R.; Grant, M.P.; Iliff, N.T.; Merbs, S.L. Exposure rate of smooth surface tunnel porous polyethylene implants after enucleation. Ophthalmic Plast. Reconstr. Surg. 2014, 30, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Timoney, P.J.; Clark, J.D.; Frederick, P.A.; Krakauer, M.; Compton, C.; Horbinski, C.; Sokol, J.; Nunery, W.R. Foreign Body Granuloma Following Orbital Reconstruction with Porous Polyethylene. Ophthalmic Plast. Reconstr. Surg. 2016, 32, e137–e138. [Google Scholar] [CrossRef] [PubMed]
- Jordan, D.R.; Brownstein, S.; Dorey, M.; Yuen, V.H.; Gilberg, S. Fibrovascularization of porous polyethylene (Medpor) orbital implant in a rabbit model. Ophthalmic Plast. Reconstr. Surg. 2004, 20, 136–143. [Google Scholar] [CrossRef]
- Xu, L.-C.; Siedlecki, C.A. Effects of surface wettability and contact time on protein adhesion to biomaterial surfaces. Biomaterials 2007, 28, 3273–3283. [Google Scholar] [CrossRef] [PubMed]
- Salerno, M.; Giacomelli, L.; Derchi, G.; Patra, N.; Diaspro, A. Atomic force microscopy in vitro study of surface roughness and fractal character of a dental restoration composite after air-polishing. Biomed. Eng. Online 2010, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- Salerno, M.; Loria, P.; Matarazzo, G.; Tomè, F.; Diaspro, A.; Eggenhöffner, R. Surface Morphology and Tooth Adhesion of a Novel Nanostructured Dental Restorative Composite. Materials 2016, 9, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Derchi, G.; Vano, M.; Barone, A.; Covani, U.; Diaspro, A.; Salerno, M. Bacterial adhesion on direct and indirect dental restorative composite resins: An in vitro study on a natural biofilm. J. Prosthet. Dent. 2017, 117, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Ţəlu, Ş.; Patra, N.; Salerno, M. Micromorphological characterization of polymer-oxide nanocomposite thin films by atomic force microscopy and fractal geometry analysis. Prog. Org. Coat. 2015, 89, 50–56. [Google Scholar] [CrossRef]
- Brandão, S.M.; Schellini, S.A.; Moraes, A.D.; Padovani, C.R.; Pellizzon, C.H.; Peitl, O.; Zanotto, E.D. Biocompatibility analysis of Bioglass® 45S5 and Biosilicate® implants in the rabbit eviscerated socket. Orbit 2012, 31, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Baino, F. How can bioactive glasses be useful in ocular surgery? J. Biomed. Mater. Res. Part A 2015, 103, 1259–1275. [Google Scholar] [CrossRef] [PubMed]
- Baino, F.; Novajra, G.; Miguez-Pacheco, V.; Boccaccini, A.R.; Vitale-Brovarone, C. Bioactive glasses: Special applications outside the skeletal system. J. Non-Cryst. Solids 2016, 432, 15–30. [Google Scholar] [CrossRef]
- Crovace, M.C.; Souza, M.T.; Chinaglia, C.R.; Peitl, O.; Zanotto, E.D. Biosilicate®—A multipurpose, highly bioactive glass-ceramic. In vitro, in vivo and clinical trials. J. Non-Cryst. Solids 2016, 432, 90–110. [Google Scholar] [CrossRef]
- Chang, E.W.; Monolidis, S. Orbital floor fracture management. Facial Plast. Surg. 2005, 21, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Baino, F.; Perero, S.; Ferraris, S.; Miola, M.; Balagna, C.; Verné, E.; Vitale-Brovarone, C.; Coggiola, A.; Dolcino, D.; Ferraris, M. Biomaterials for orbital implants and ocular prostheses: Overview and future prospects. Acta Biomater. 2014, 10, 1064–1087. [Google Scholar] [CrossRef] [PubMed]
- Marano, R.; Tincani, A.J. Is there an ideal implant for orbital reconstructions? Prospective 64-case study. J. Cranio-Maxillo-Facial Surg. 2016, 44, 1682–1688. [Google Scholar] [CrossRef] [PubMed]
- Vitale-Brovarone, C.; Baino, F.; Verné, E. High strength bioactive glass-ceramic scaffolds for bone regeneration. J. Mater. Sci. Mater. Med. 2009, 20, 643–653. [Google Scholar] [CrossRef] [PubMed]
- Muzio, G.; Martinasso, G.; Baino, F.; Frairia, R.; Vitale-Brovarone, C.; Canuto, R.A. Key role of the expression of bone morphogenetic proteins in increasing the osteogenic activity of osteoblast-like cells exposed to shock waves and seeded on bioactive glass-ceramic scaffolds for bone tissue engineering. J. Biomater. Appl. 2014, 29, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Mawn, L.A.; Jordan, D.R.; Gilberg, S. Proliferation of human fibroblasts in vitro after exposure to orbital implants. Can. J. Ophthalmol. 2001, 36, 245–251. [Google Scholar] [CrossRef]
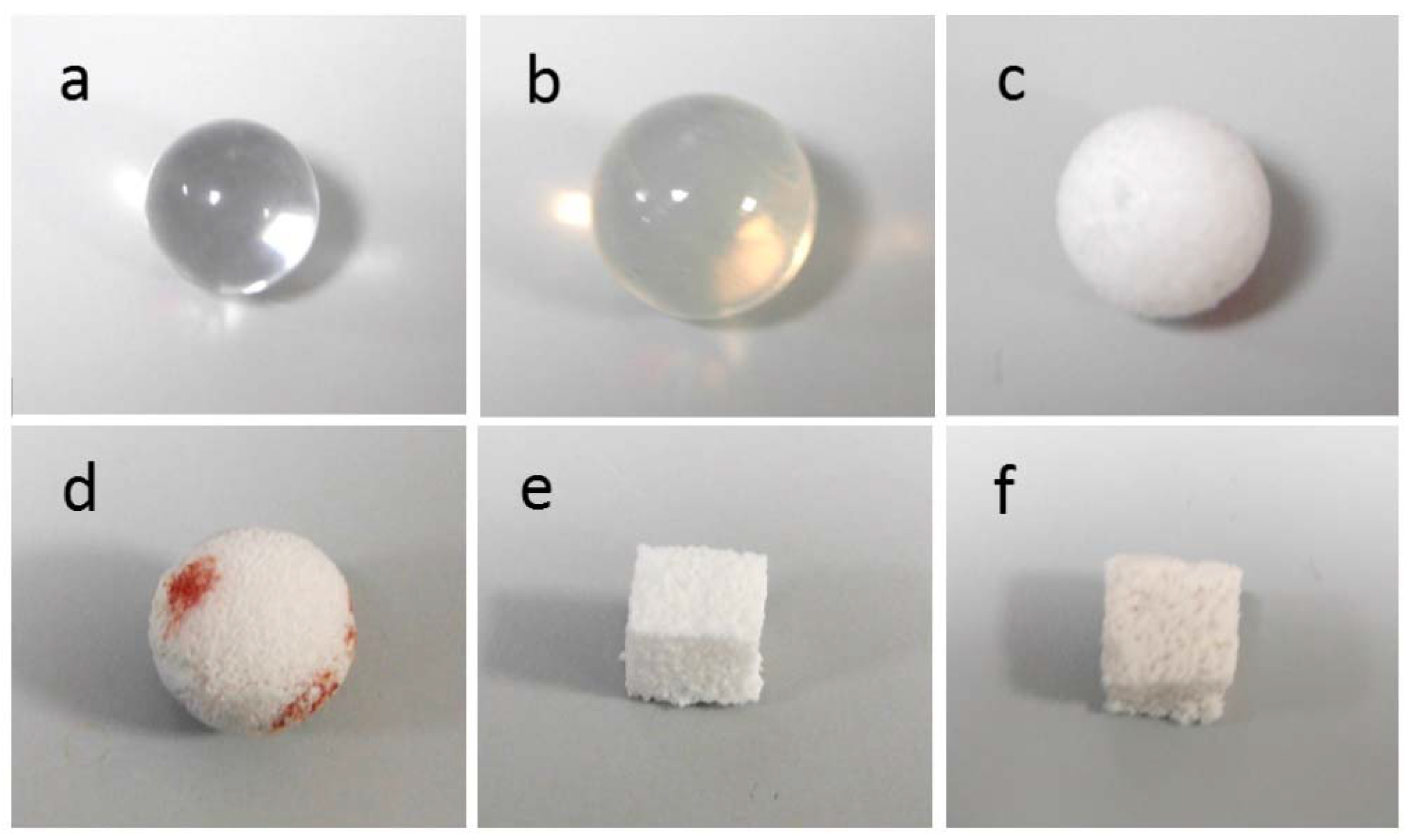
| Implant Material | Specimen Shape And Size | Crystalline Phases | Total Porosity (vol %) | Mean Macropore Size (µm) |
|---|---|---|---|---|
| PMMA | ball, ~12.6 mm diameter | none | 0 | - |
| Silicone | ball, ~15.9 mm diameter | none | 0 | - |
| PE | ball, ~14.9 mm diameter | none | 50 | 350 |
| Alumina | ball, ~13.9 mm diameter | Al2O3 | >75 | 500 |
| GCA | cuboid, ~1 cm side | CaSiO3 (wollastonite) | ~53 | 230 |
| GCB | cuboid, ~1 cm side | Na2Ca2Si3O9 (combeite), Na2Ca4(PO4)2SiO4 (silicorhenanite), Ca2Mg(Si2O7) (akermanite) | ~60 | 520 |
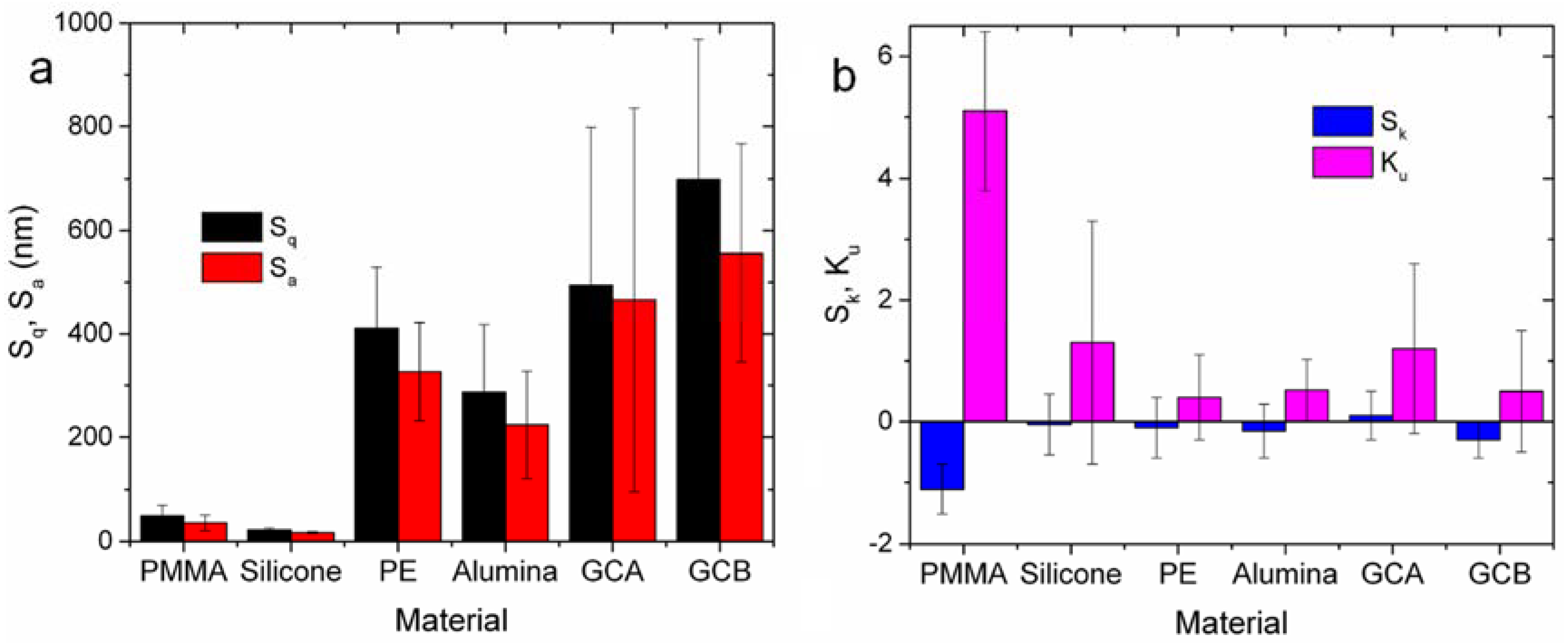
| Material | Sq (nm) | Sa (nm) | Sk | Ku |
|---|---|---|---|---|
| PMMA | 49 ± 21 | 36 ± 15 | −1.1 ± 0.4 | 5.1 ± 1.3 |
| Silicone | 22 ± 4 | 17 ± 2 | 0.1 ± 0.5 | 1.3 ± 2.0 |
| PE | 411 ± 118 | 327 ± 95 | −0.2 ± 1.0 | 0.4 ± 0.7 |
| Alumina | 287 ± 131 | 224 ± 104 | −0.2 ± 0.4 | 0.5 ± 0.5 |
| GCA | 494 ± 304 | 466 ± 370 | 0.1 ± 0.4 | 1.2 ± 1.4 |
| GCB | 699 ± 270 | 556 ± 210 | −0.3 ± 0.3 | 0.5 ± 1.0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salerno, M.; Reverberi, A.P.; Baino, F. Nanoscale Topographical Characterization of Orbital Implant Materials. Materials 2018, 11, 660. https://doi.org/10.3390/ma11050660
Salerno M, Reverberi AP, Baino F. Nanoscale Topographical Characterization of Orbital Implant Materials. Materials. 2018; 11(5):660. https://doi.org/10.3390/ma11050660
Chicago/Turabian StyleSalerno, Marco, Andrea Pietro Reverberi, and Francesco Baino. 2018. "Nanoscale Topographical Characterization of Orbital Implant Materials" Materials 11, no. 5: 660. https://doi.org/10.3390/ma11050660
APA StyleSalerno, M., Reverberi, A. P., & Baino, F. (2018). Nanoscale Topographical Characterization of Orbital Implant Materials. Materials, 11(5), 660. https://doi.org/10.3390/ma11050660







