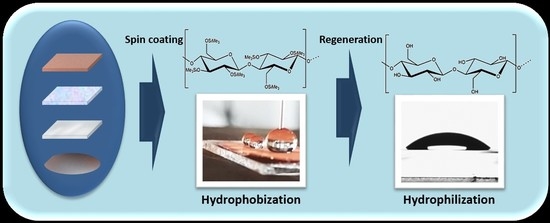
Deposition of Cellulose-Based Thin Films on Flexible Substrates
Abstract
1. Introduction
2. Materials and Methods
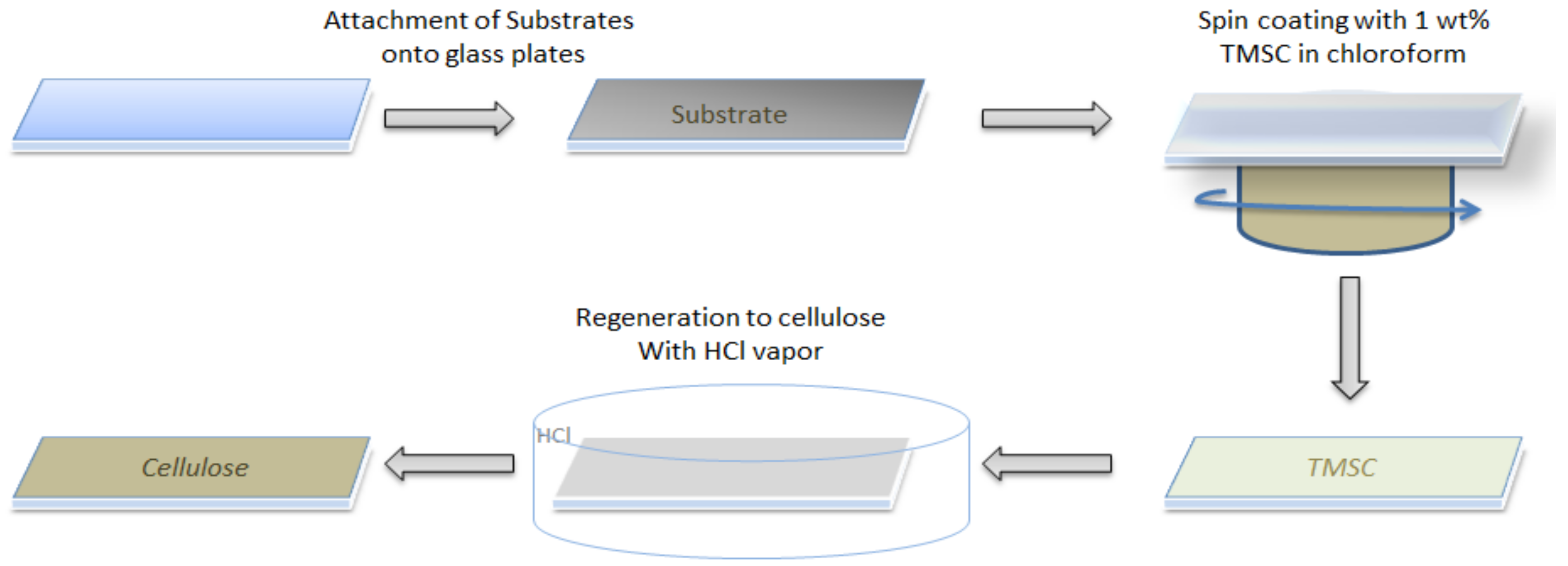
2.1. Substrate Cleaning and Film Preparation
2.2. Stylus Profilometry
2.3. Contact Angle Measurements and Surface Free Energy
2.4. Infrared Spectroscopy
2.5. Light Microscopy
2.6. Atomic Force Microscopy Measurements
2.7. Scanning Electron Microscopy
3. Results
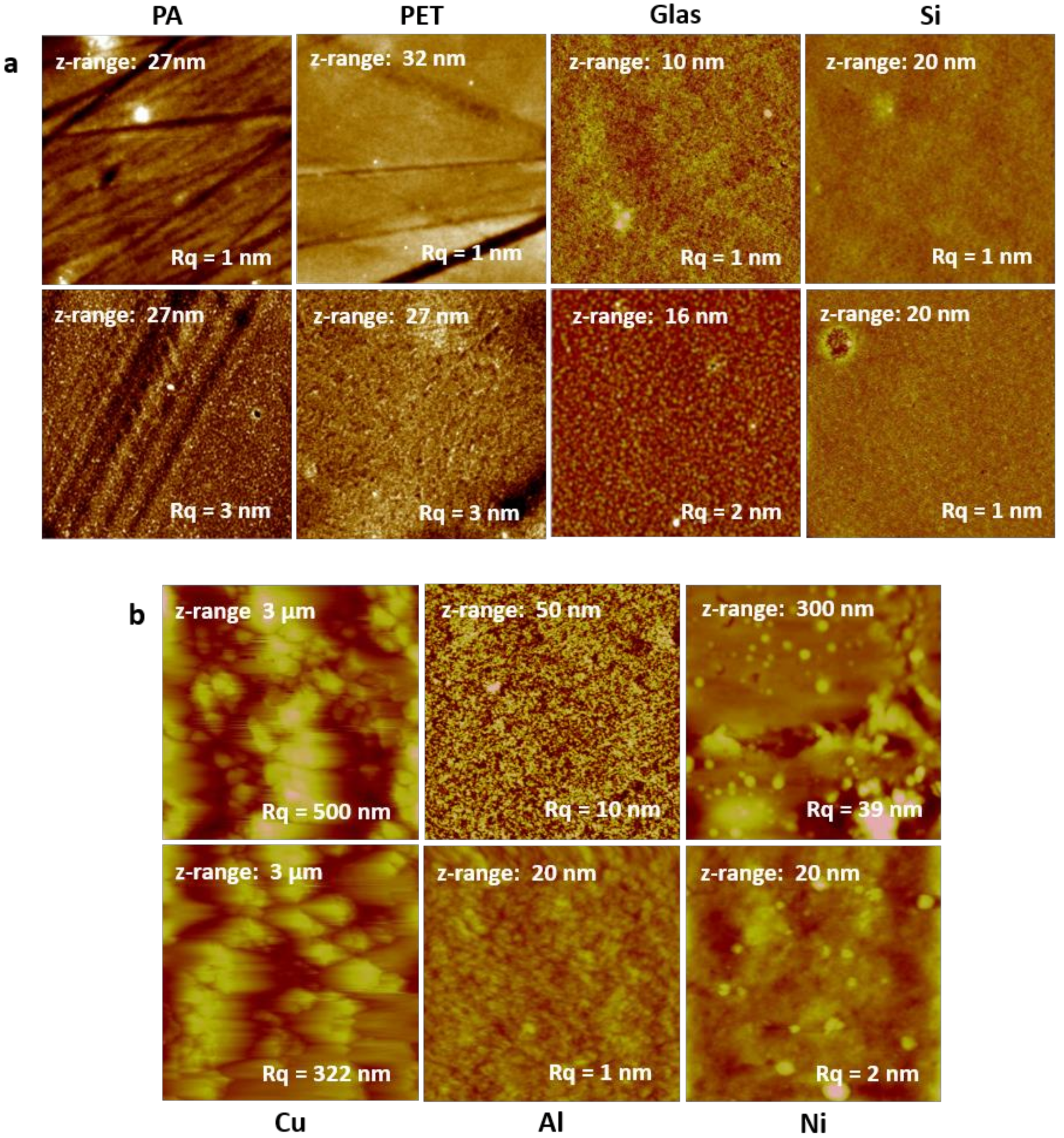
3.1. Surface Morphology—Atomic Force Microscopy
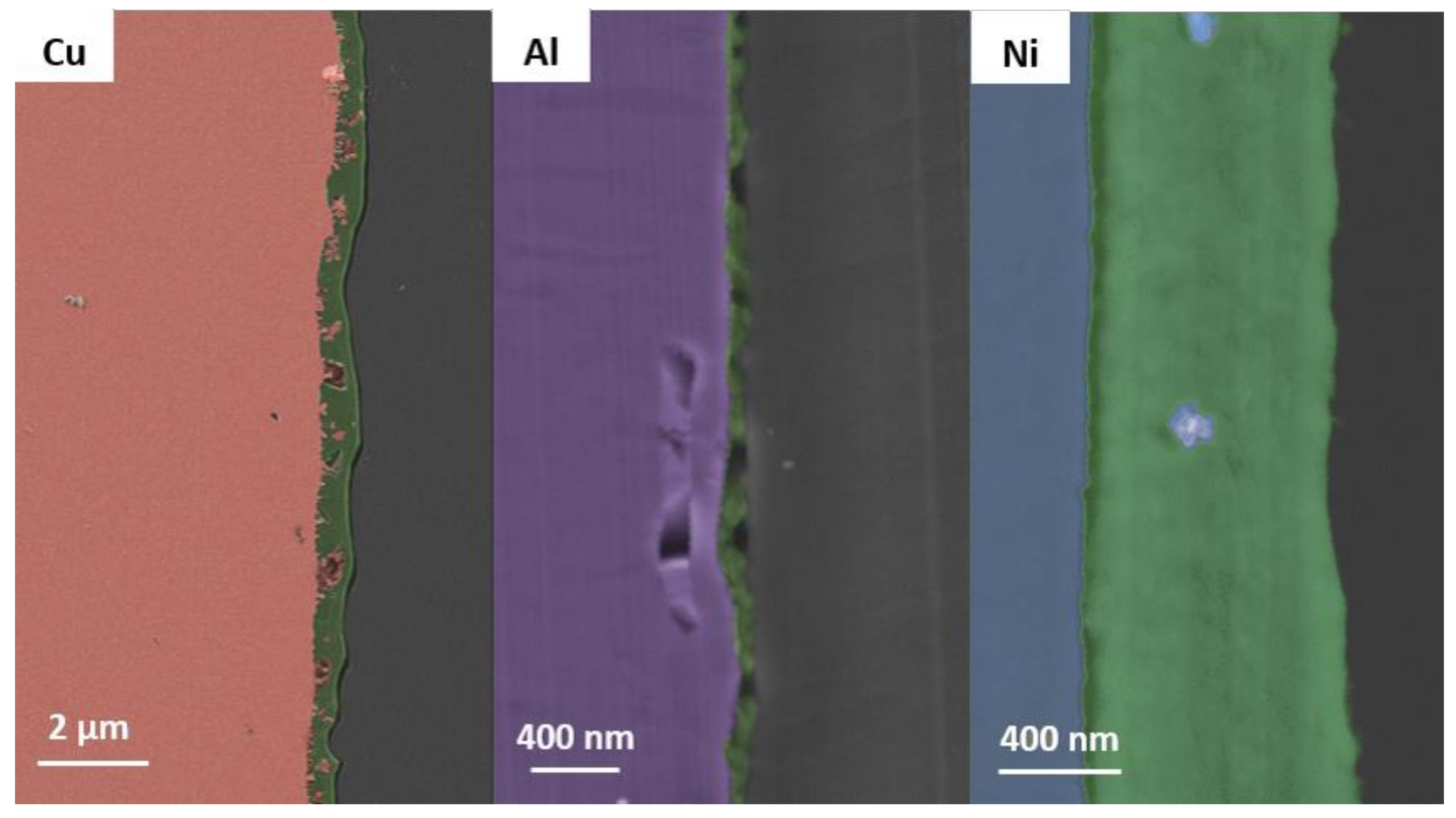
3.2. Scanning Electron Microscopy
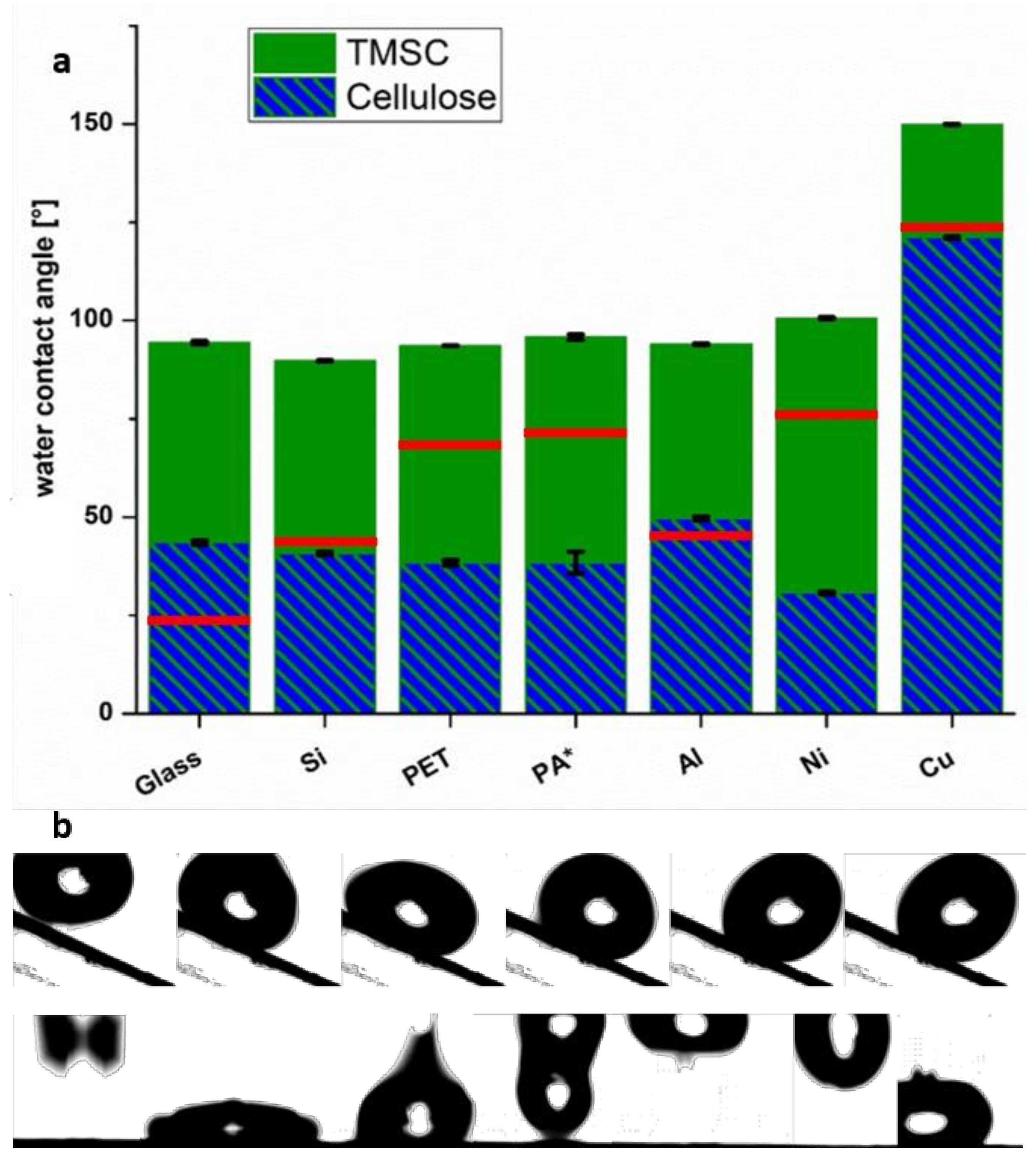
3.3. Wettability of TMSC Coatings with Water
3.4. Surface Free Energy (SFE)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Baumert, B.; Stratmann, M.; Rohwerder, M. The deformation response of ultra-thin polymer films on steel sheet in a tensile straining test: The role of slip bands emerging at the polymer/metal interface. Z. Metallk. 2004, 95, 447–455. [Google Scholar] [CrossRef]
- Russell, T.P.; Chai, Y. 50th anniversary perspective: Putting the squeeze on polymers: a perspective on polymer thin films and interfaces. Macromolecules 2017, 50, 4597–4609. [Google Scholar] [CrossRef]
- Mautner, A.; Lee, K.Y.; Tammelin, T.; Mathew, A.P.; Nedoma, A.J.; Li, K.; Bismarck, A. Cellulose nanopapers as tight aqueous ultra-filtration membranes. React. Funct. Polym. 2015, 86, 209–214. [Google Scholar] [CrossRef]
- Veigel, S.; Grüll, G.; Pinkl, S.; Obersriebnig, M.; Müller, U.; Gindl-Altmutter, W. Improving the mechanical resistance of waterborne wood coatings by adding cellulose nanofibres. React. Funct. Polym. 2014, 85, 214–220. [Google Scholar] [CrossRef]
- Niinivaara, E.; Wilson, B.P.; King, A.W.T.; Kontturi, E. Parameters affecting monolayer organisation of substituted polysaccharides on solid substrates upon Langmuir–Schaefer deposition. React. Funct. Polym. 2016, 99, 100–106. [Google Scholar] [CrossRef]
- Hossain, K.M.Z.; Felfel, R.M.; Rudd, C.D.; Thielemans, W.; Ahmed, I. The effect of cellulose nanowhiskers on the flexural properties of self-reinforced polylactic acid composites. React. Funct. Polym. 2014, 85, 193–200. [Google Scholar] [CrossRef]
- Lucenius, J.; Parikka, K.; Österberg, M. Nanocomposite films based on cellulose nanofibrils and water-soluble polysaccharides. React. Funct. Polym. 2014, 85, 167–174. [Google Scholar] [CrossRef]
- Kargl, R.; Mohan, T.; Ribitsch, V.; Saake, B.; Puls, J.; Kleinschek, K.S. Cellulose thin films from ionic liquid solutions. Nord. Pulp Pap. Res. J. 2015, 30, 6–13. [Google Scholar] [CrossRef]
- Kontturi, E.; Tammelin, T.; Österberg, M. Cellulose model films and the fundamental approach. Chem. Soc. Rev. 2006, 35, 1287–1304. [Google Scholar] [CrossRef] [PubMed]
- Edgar, C.D.; Gray, D.G. Smooth model cellulose I surfaces from nanocrystal suspensions. Cellulose 2003, 10, 299–306. [Google Scholar] [CrossRef]
- Enarsson, L.E.; Wågberg, L. Polyelectrolyte adsorption on thin cellulose films studied with reflectometry and quartz crystal microgravimetry with dissipation. Biomacromolecules 2009, 10, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Weißl, M.; Niegelhell, K.; Reishofer, D.; Zankel, A.; Innerlohinger, J.; Spirk, S. Homogeneous cellulose thin films by regeneration of cellulose xanthate: properties and characterization. Cellulose 2018, 25, 711–721. [Google Scholar] [CrossRef]
- Schaub, M.; Wenz, G.; Wegner, G.; Stein, A.; Klemm, D. Ultrathin films of cellulose on silicon wafers. Adv. Mater. 1993, 5, 919–922. [Google Scholar] [CrossRef]
- Mohan, T.; Kargl, R.; Doliška, A.; Vesel, A.; Köstler, S.; Ribitsch, V.; Stana-Kleinschek, K. Wettability and surface composition of partly and fully regenerated cellulose thin films from trimethylsilyl cellulose. J. Colloid Interface Sci. 2011, 358, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Buchholz, V.; Adler, P.; Bäcker, M.; Hölle, W.; Simon, A.; Wegner, G. Regeneration and hydroxyl accessibility of cellulose in ultrathin films. Langmuir 1997, 13, 3206–3209. [Google Scholar] [CrossRef]
- Kontturi, E.; Thüne, P.C.; Niemantsverdriet, J.W. Cellulose model surfaces simplified preparation by spin coating and characterization by x-ray photoelectron spectroscopy, infrared spectroscopy, and atomic force microscopy. Langmuir 2003, 19, 5735–5741. [Google Scholar] [CrossRef]
- Niegelhell, K.; Süßenbacher, M.; Sattelkow, J.; Plank, H.; Wang, Y.; Zhang, K.; Spirk, S. How bound and free fatty acids in cellulose films impact nonspecific protein adsorption. Biomacromolecules 2017, 18, 4224–4231. [Google Scholar] [CrossRef] [PubMed]
- Niegelhell, K.; Chemelli, A.; Hobisch, J.; Griesser, T.; Reiter, H.; Hirn, U.; Spirk, S. Interaction of industrially relevant cationic starches with cellulose. Carbohydr. Polym. 2018, 179, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Thomas, L.H.; Trevor Forsyth, V.; Šturcová, A.; Kennedy, C.J.; May, R.P.; Altaner, C.M.; Apperley, D.C.; Wess, T.J.; Jarvis, M.C. Structure of cellulose microfibrils in primary cell walls from collenchyma. Plant Physiol. 2013, 161, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Abe, K.; Yamamoto, H. Mechanical interaction between cellulose microfibril and matrix substance in wood cell wall determined by X-ray diffraction. J. Wood Sci. 2005, 51, 334–338. [Google Scholar] [CrossRef]
- Kittle, J.D.; Du, X.; Jiang, F.; Qian, C.; Heinze, T.; Roman, M.; Esker, A.R. Equilibrium water contents of cellulose films determined via solvent exchange and quartz crystal microbalance with dissipation monitoring. Biomacromolecules 2011, 12, 2881–2887. [Google Scholar] [CrossRef] [PubMed]
- Rossetti, F.F.; Panagiotou, P.; Rehfeldta, F.; Schneck, E.; Dommach, M.; Funari, S.S.; Timmann, A.; Müller-Buschbaum, P.; Tanaka, M. Structures of regenerated cellulose films revealed by grazing incidence small-angle x-ray scattering. Biointerphases 2008, 3, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Fujisaki, Y.; Koga, H.; Nakajima, Y.; Nakata, M.; Tsuji, H.; Yamamoto, T.; Kurita, T.; Nogi, M.; Shimidzu, N. Transparent nanopaper-based flexible organic thin-film transistor array. Adv. Funct. Mater. 2014, 24, 1657–1663. [Google Scholar] [CrossRef]
- Valentini, L.; Bittolo Bon, S.; Cardinali, M.; Fortunati, E.; Kenny, J.M. Cellulose nanocrystals thin films as gate dielectric for flexible organic field-effect transistors. Mater. Lett. 2014, 126, 55–58. [Google Scholar] [CrossRef]
- Ebner, M.; Schennach, R.; Chien, H.T.; Mayrhofer, C.; Zankel, A.; Friedel, B. Regenerated cellulose fiber solar cell. Flex. Printed Electron. 2017, 2, 014002. [Google Scholar] [CrossRef]
- Kargl, R.; Mohan, T.; Köstler, S.; Spirk, S.; Doliška, A.; Stana-Kleinschek, K.; Ribitsch, V. Functional patterning of biopolymer thin films using enzymes and lithographic methods. Adv. Funct. Mater. 2013, 23, 308–315. [Google Scholar] [CrossRef]
- Feiler, A.A.; Stiernstedt, J.; Theander, K.; Jenkins, P.; Rutland, M.W. Effect of capillary condensation on friction force and adhesion. Langmuir 2007, 23, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Mohan, T.; Zarth, C.; Doliska, A.; Kargl, R.; Grießer, T.; Spirk, S.; Heinze, T.; Stana-Kleinschek, K. Interactions of a cationic cellulose derivative with an ultrathin cellulose support. Carbohydr. Polym. 2013, 92, 1046–1053. [Google Scholar] [CrossRef] [PubMed]
- Wolfberger, A.; Petritz, A.; Fian, A.; Herka, J.; Schmidt, V.; Stadlober, B.; Kargl, R.; Spirk, S.; Griesser, T. Photolithographic patterning of cellulose: A versatile dual-tone photoresist for advanced applications. Cellulose 2015, 22, 717–727. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Rabel, W. Einige Aspekte der benetzungstheorie und ihre anwendung auf die untersuchung und veränderung der oberflächeneigenschaften von polymeren. Farbe und Lack 1971, 77, 997–1005. [Google Scholar]
- Kaelble, D.H. Dispersion-polar surface tension properties of organic solids. J. Adhes. 1970, 2, 66–81. [Google Scholar] [CrossRef]
- Marc, K.; Sarah, K.; Tim, L.; Thomas, H. Pure cellulose nanoparticles from trimethylsilyl cellulose. Macromol. Symp. 2010, 294, 96–106. [Google Scholar]
- Ngo, H.T.; Bechtold, T. Surface modification of textile material through deposition of regenerated silk fibroin. J. Appl. Polym. Sci. 2017, 134, 45098. [Google Scholar] [CrossRef]
- Mohan, T.; Spirk, S.; Kargl, R.; Doliška, A.; Ehmann, H.M.A.; Köstler, S.; Ribitsch, V.; Stana-Kleinschek, K. Watching cellulose grow—Kinetic investigations on cellulose thin film formation at the gas-solid interface using a quartz crystal microbalance with dissipation (QCM-D). Colloid Surf. A 2012, 400, 67–72. [Google Scholar] [CrossRef]
- Woods, D.A.; Petkov, J.; Bain, C.D. Surfactant adsorption by total internal reflection Raman spectroscopy. Part III: Adsorption onto cellulose. Colloid Surf. A 2011, 391, 10–18. [Google Scholar] [CrossRef]
- Kontturi, E.; Lankinen, A. Following the kinetics of a chemical reaction in ultrathin supported polymer films by reliable mass density determination with X-ray reflectivity. J. Am. Chem. Soc. 2010, 132, 3678–3679. [Google Scholar] [CrossRef] [PubMed]
- Ganner, T.; Sattelkow, J.; Rumpf, B.; Eibinger, M.; Reishofer, D.; Winkler, R.; Nidetzky, B.; Spirk, S.; Plank, H. Direct-write fabrication of cellulose nano-structures via focused electron beam induced nanosynthesis. Sci. Rep. 2016, 6, 32451. [Google Scholar] [CrossRef] [PubMed]
- Korhonen, J.T.; Huhtamäki, T.; Ikkala, O.; Ras, R.H.A. Reliable measurement of the receding contact angle. Langmuir 2013, 29, 3858–3863. [Google Scholar] [CrossRef] [PubMed]
- Hubbe, M.A.; Gardner, D.J.; Shen, W. Contact angles and wettability of cellulosic surfaces: A review of proposed mechanisms and test strategies. BioResources 2015, 10, 8657–8749. [Google Scholar] [CrossRef]
- Wang, S.; Jiang, L. Definition of superhydrophobic states. Adv. Mater. 2007, 19, 3423–3424. [Google Scholar] [CrossRef]
- Lafuma, A.; Quéré, D. Superhydrophobic states. Nat. Mater. 2003, 2, 457–460. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Masood, M.T.; Heredia-Guerrero, J.A.; Ceseracciu, L.; Palazon, F.; Athanassiou, A.; Bayer, I.S. Superhydrophobic high impact polystyrene (HIPS) nanocomposites with wear abrasion resistance. Chem. Eng. J. 2017, 322, 10–21. [Google Scholar] [CrossRef]
- Gao, L.; McCarthy, T.J. Wetting 101°. Langmuir 2009, 25, 14105–14115. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Gu, W.; Fu, J.; Liu, Y.; Chen, S. Preparation of superhydrophobic/oleophilic copper mesh for oil-water separation. Appl. Surf. Sci. 2017, 412, 599–605. [Google Scholar] [CrossRef]
- Wen, Q.; Guo, F.; Yang, F.; Guo, Z. Green fabrication of coloured superhydrophobic paper from native cotton cellulose. J. Colloid Interface Sci. 2017, 497, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Pietschnig, R.; Spirk, S. The chemistry of organo silanetriols. Coord. Chem. Rev. 2016, 323, 87–106. [Google Scholar] [CrossRef]
- Si, Y.; Yang, F.; Guo, Z. Bio-inspired one-pot route to prepare robust and repairable micro-nanoscale superhydrophobic coatings. J. Colloid Interface Sci. 2017, 498, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Taajamaa, L.; Kontturi, E.; Laine, J.; Rojas, O.J. Bicomponent fibre mats with adhesive ultra-hydrophobicity tailored with cellulose derivatives. J. Mater. Chem. 2012, 22, 12072–12082. [Google Scholar] [CrossRef]
- Gao, L.; McCarthy, T.J. How Wenzel and Cassie were wrong. Langmuir 2007, 23, 3762–3765. [Google Scholar] [CrossRef] [PubMed]
- McHale, G. Cassie and Wenzel: Were they really so wrong? Langmuir 2007, 23, 8200–8205. [Google Scholar] [CrossRef] [PubMed]
| Substrate | TMSC (nm) | Cellulose (nm) | Regen. Time (min) |
|---|---|---|---|
| GlassA | 140 ± 1 | 50 ± 1 | 15 |
| SiA | 132 ± 1 | 46 ± 1 | 15 |
| PETA | 125 ± 5 | 43 ± 2 | 15 |
| PAA | 131 ± 7 | 44 ± 4 | 15 |
| AlB | 96 ± 16 | n.d. | 30 |
| CuB | 545 ± 73 | n.d. | 30 |
| NiB | 812 ± 29 | n.d. | 30 |
| Film/Type of CA | Glass (°) | Si (°) | PET (°) | Cu (°) | Ni (°) | Al (°) |
|---|---|---|---|---|---|---|
| TMSC/ACA | 100 ± 1 | 100 ± 1 | 99 ± 1 | 151 ± 1 | 107 ± 1 | 98 ± 1 |
| TMSC/RCA | 88 ± 1 | 80 ± 1 | 88 ± 1 | 149 ± 1 | 94 ± 1 | 90 ± 1 |
| Cell ACA | 51 ± 1 | 50 ± 1 | 44 ± 1 | 138 ± 1 | 33 ± 1 | 56 ± 1 |
| Cell RCA | 36 ± 1 | 31 ± 1 | 32 ± 1 | 105 ± 1 | 28 ± 1 | 44 ± 1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schlemmer, W.; Zankel, A.; Niegelhell, K.; Hobisch, M.; Süssenbacher, M.; Zajki-Zechmeister, K.; Weissl, M.; Reishofer, D.; Plank, H.; Spirk, S. Deposition of Cellulose-Based Thin Films on Flexible Substrates. Materials 2018, 11, 2433. https://doi.org/10.3390/ma11122433
Schlemmer W, Zankel A, Niegelhell K, Hobisch M, Süssenbacher M, Zajki-Zechmeister K, Weissl M, Reishofer D, Plank H, Spirk S. Deposition of Cellulose-Based Thin Films on Flexible Substrates. Materials. 2018; 11(12):2433. https://doi.org/10.3390/ma11122433
Chicago/Turabian StyleSchlemmer, Werner, Armin Zankel, Katrin Niegelhell, Mathias Hobisch, Michael Süssenbacher, Krisztina Zajki-Zechmeister, Michael Weissl, David Reishofer, Harald Plank, and Stefan Spirk. 2018. "Deposition of Cellulose-Based Thin Films on Flexible Substrates" Materials 11, no. 12: 2433. https://doi.org/10.3390/ma11122433
APA StyleSchlemmer, W., Zankel, A., Niegelhell, K., Hobisch, M., Süssenbacher, M., Zajki-Zechmeister, K., Weissl, M., Reishofer, D., Plank, H., & Spirk, S. (2018). Deposition of Cellulose-Based Thin Films on Flexible Substrates. Materials, 11(12), 2433. https://doi.org/10.3390/ma11122433