Abstract
The EU’s green transition hinges on secure access to critical raw materials; vanadium is pivotal for microalloyed steels and emerging long-duration energy storage (VRFBs). Methods: We combine a market and technology review with PESTEL and Porter-5+2 analyses, complemented by a value-chain assessment and a SWOT-to-CAME strategy for the EU. Results: Vanadium supply is highly concentrated (VTM-derived, largely in CN/RU/ZA), prices are volatile, and >85% of demand remains tied to steel; yet VRFBs could shift demand shares by 2030 if costs—dominated by electrolyte—are mitigated. EU weaknesses include lack of primary mining and refining capacity; strengths include research leadership, regulatory frameworks and circularity potential (slag/catalyst recovery, electrolyte reuse). Conclusions: A resilient EU strategy should prioritize circular supply, selective upstream partnerships, battery-grade refining hubs, and targeted instruments (strategic stocks, offtake/price-stabilization, LDES-ready regulation) to de-risk vanadium for grid storage and low-carbon infrastructure. This study also discusses supply chain concentration and price volatility, and outline circular-economy pathways and decarbonization policy levers relevant to the EU’s green energy transition.
1. Introduction
In the context of the energy transition, European and US electricity markets are undergoing significant transformations as a result of the growing deployment of renewable energy sources in their respective energy mixes. One of the changes observed, especially in the European context, is the reduction in capture prices associated with the penetration of renewable technologies, specifically photovoltaic (PV) and wind power. This trend has sparked growing interest in energy storage solutions, largely driven by the reduction in the levelized cost of energy (LCOE) of renewable technologies compared to conventional generation sources [1]. In the context of the increased integration of renewable sources into modern energy systems, there is growing evidence of greater variability in power generation and, consequently, an increasing need for the implementation of storage strategies that encompass both short-duration energy storage (SDES) and long-duration energy storage (LDES). Renewable energy sources such as solar and wind are inherently variable and non-dispatchable, leading to fluctuations in power generation that challenge grid stability and energy security. The integration of large-scale and long-duration energy storage systems can mitigate these effects by balancing supply and demand over extended periods, thereby enhancing the utilization and reliability of renewable power. Among the available technologies, redox flow batteries (RFBs) [2,3]—particularly vanadium-based systems—are attracting increasing attention as scalable and durable solutions for long-duration energy storage in renewable-dominated grids [4].
Increased research into LDES has resulted in the commercialisation of various RFB technologies. Among these, vanadium redox flow batteries (VRFBs) have seen notable implementation and diffusion, despite the limited availability of vanadium [5,6,7] and its high toxicity even in low concentrations [8]. This is attributed to their stability, life cycle and scalability [9,10,11].
Furthermore, growing interest in energy storage is limited by the difficulties associated with making a homogeneous comparison of costs between the different technologies available. In a large amount of research, the analysis focuses predominantly on the investment cost (CAPEX), disregarding other critical factors that significantly influence economic models, such as efficiency, depreciation over time, and the useful life of the system [12].
However, CAPEX remains a fundamental item in the LCOE of an energy storage system, which is directly related to the cost of the raw materials used in its composition [13]. In the specific case of VRFBs, the price and availability of vanadium have a significant influence on the LCOE of this technology [6].
Crucially, securing the competitive viability of VRFBs is significantly challenged by the pronounced geopolitical concentration in key segments of the value chain. This structural vulnerability stems from the fact that vanadium supply is highly concentrated, with approximately 90% of global production originating in a limited number of nations, primarily China, Russia, and South Africa (Section 3.1). This high concentration yields a market structure quantified by a Herfindahl–Hirschman Index (HHI) of around 5000, confirming an oligopolistic market structure that amplifies price volatility (Section 3.3.8). This volatility is critical, as the cost of the electrolyte can represent between 30% and 50% of the total CAPEX (Capital Expenditure) of a VRFB system (Section 3.3.4), while price variations have historically exceeded 100% at certain intervals over recent decades (Section 3.3.8). Furthermore, the leading global deployments of this technology, such as the 800 MWh system in Dalian (Section 3.3.4), are currently concentrated in specific regions, notably China, which reinforces the global asymmetry in technological and industrial maturation.
Furthermore, European regulation provides a crucial frame of reference: the Critical Raw Materials Act (CRMA), the European Green Deal and related industrial resilience policies place secure access to raw materials at the heart of the continent’s energy and technological future. The purpose of these policies is not limited to diversifying supplies and attracting investment, but also focuses on speeding up permitting processes, incentivising recycling and promoting international partnerships [14,15].
Despite the numerous reports addressing the EU electricity market and renewable integration, there is a limited body of academic research that systematically links the strategic security of vanadium supply with the Union’s decarbonisation targets and industrial autonomy. This research aims to fill that gap by adopting a comprehensive strategic perspective that connects material criticality, industrial competitiveness, and policy design, thus providing a coherent framework to evaluate vanadium’s role in Europe’s transition toward a resilient and sustainable energy system.
The purpose of this study is to provide a systematic analysis of the vanadium market from the strategic perspective of the European Union (EU). To this end, it will offer an overview of the political, economic, socio-cultural, ecological and legal environment, as well as an internal analysis of the EU’s strengths and weaknesses in this environment. In this regard, we will seek to provide sound recommendations that contribute to strengthening European autonomy in the energy storage value chain.
2. Methodology
Key analytical tools were selected to assess the prospects for the vanadium market and the strategic sustainability of the EU in the context of the green ecological transition. The methodological design combines a comprehensive assessment of both the external competitive environment and the internal capabilities of the European Union, employing well-established instruments from the field of strategic management and industrial policy analysis.
2.1. Data Collection
The empirical foundation of the study rests on a triangulation of diverse data sources. Primary information was obtained from peer-reviewed scientific literature indexed in Scopus and Web of Science focused mainly on publications from 2020 to 2025, supplemented by earlier studies providing historical or baseline information, ensuring methodological rigor and conceptual validity. Complementary secondary and grey literature were also incorporated, including industrial and market reports from the United States Geological Survey (USGS) [16,17], IEA [18,19,20,21], and Wood Mackenzie [22], as well as official European policy documents such as the Critical Raw Materials Act [23], the Net-Zero Industry Act and communications related to the European Green Deal. This multi-source integration enables a comprehensive and up-to-date characterization of the vanadium sector and its strategic role in the EU energy transition.
To achieve the goal of providing a systematic strategic analysis of the vanadium market from the European Union’s perspective, the methodological design was structured into five integrated analytical phases (Section 2.2, Section 2.3, Section 2.4, Section 2.5 and Section 2.6). This approach systematically moves from assessing the macro-environmental factors (PESTEL) and competitive industrial structure (Porter’s 5+2 Forces) to evaluating the EU’s internal capabilities and structural weaknesses (Value Chain Analysis), culminating in the development of actionable policy recommendations through the integrated SWOT-CAME framework.
2.2. Contextual Analysis
A baseline contextualization was developed encompassing the geology, metallurgy, and industrial applications of vanadium. This stage established the conceptual and geographical boundaries of the case study and defined the key structural parameters that determine vanadium’s role as a critical raw material within the EU’s decarbonization and industrial resilience framework.
Scientifically, this phase acts as the essential empirical bridge, defining the critical structural parameters—such as the geographic concentration of supply, metallurgical complexity, and the market application split—that serve as the fundamental inputs for the formal PESTEL and Porter’s 5+2 Forces analyses.
2.3. PESTEL Analysis
A comprehensive comparative PESTEL analysis—covering Political, Economic, Social, Technological, Environmental, and Legal dimensions—was conducted to assess the macro-environmental factors shaping the European vanadium value chain. Each dimension was constructed from the previously identified data sources, systematically identifying drivers, constraints, and interdependencies that condition the EU’s strategic position in vanadium supply and innovation.
2.4. Porter’s Five + Two Forces
In a subsequent stage, Porter’s Five Forces model [24] was applied and extended to a 5+2 framework to include the influence of complementors and stakeholders such as institutional actors, NGOs focused on environmental governance, and technological alliances [25,26]. This adaptation is particularly relevant for critical raw materials, where market dynamics are strongly shaped by regulatory, environmental, and geopolitical pressures. The analysis focused on entry barriers, supplier concentration, substitutability, and bargaining power among industrial and institutional participants, providing a detailed understanding of the competitive structure of the vanadium sector.
2.5. Value Chain Analysis
For the internal dimension, Porter’s Value Chain [27] framework was adapted to the specific context of extractive industries and energy storage technologies. This adaptation allowed the mapping of European strengths and weaknesses across all stages of the vanadium lifecycle—from primary mining and refining to recycling, R&D, and industrial integration. Both tangible resources (such as deposits, processing capacity, and infrastructure) and intangible resources (including regulatory frameworks, skilled human capital, and technological know-how in metallurgy and flow batteries) were evaluated to identify systemic bottlenecks and strategic opportunities.
2.6. SWOT-CAME Integration
The external and internal analyses were subsequently integrated through a combined SWOT (Strengths, Weaknesses, Opportunities, Threats) matrix, summarizing the structural risks and competitive advantages identified in the previous stages. Building on this, the CAME (Correct, Adapt, Maintain, Exploit) framework was applied to transform diagnostic insights into actionable strategies. This stage allowed the formulation of evidence-based recommendations aligned with industrial planning, critical materials policy, and the EU’s long-term resilience objectives.
This sequential and integrative methodological design ensures transparency, consistency, and replicability, providing a rigorous analytical foundation for assessing the geopolitical, industrial, and environmental dimensions of vanadium within the European critical materials framework.
In summary, this methodological framework establishes a coherent pathway linking macro- and micro-level analytical instruments under a unified strategic lens. By combining contextual, structural, and strategic perspectives, the approach allows not only the identification of current vulnerabilities in the vanadium value chain, but also the projection of potential scenarios for its sustainable development within the European Union. The integration of well-established analytical tools—PESTEL, Porter’s extended model, Value Chain mapping, and SWOT–CAME synthesis—ensures that the resulting insights are both theoretically grounded and practically oriented. This multidimensional design ultimately provides a replicable and policy-relevant framework to support evidence-based decision-making in the governance of critical raw materials, reinforcing the analytical credibility and policy utility of the present research.
3. Results and Analysis
3.1. Main Vanadium Deposits
Vanadium is a chemical element found in the Earth’s crust in relatively high concentrations. It is found in a wide variety of materials, and its average concentration worldwide is 97 mg/kg [28]. However, the concentration of vanadium can vary significantly depending on the type of soil, as it is closely linked to its oxidation state and the pH of the medium [29].
Vanadium, a chemical element with atomic number 23, is commonly found in soils and sediments as a trace element, often replacing iron (Fe) or aluminium (Al) in primary or secondary minerals in octahedral positions in crystal structures [30]. However, due to its correlation with iron, it is more commonly found in mafic rocks than in siliceous rocks [23,31,32,33]. In line with the usual tendency of minerals to occur in nature in association with other minerals and, in exceptional circumstances, individually, most deposits are polymetallic [29,34,35,36]. Broadly speaking, four categories of vanadium deposits have been identified: vanadium titanomagnetite (VTM) deposits, tabular sandstone-hosted and surface uranium deposits, vanadium deposits associated with crude oil, coal and salts, and vanadate deposits [32,37,38,39]. In contrast to the above, some less prevalent deposits have been identified associated with graphite or in laterites, bauxites, iron ores, and phosphate ores [39].
Approximately 90% of global vanadium is extracted from VTM deposits located in Russia, South Africa, China, Finland, Canada, and Australia [36,40,41]. VTM deposits are characterised by accumulations of magnetite and ilmenite in magmatic rocks, as reported in scientific literature [37]. The minerals most commonly found in VTM deposits are magnetite (Fe3O4), ilmenite (FeTiO3), rutile, and small amounts of hematite (Fe2O3) [34,42], resulting in a close Ti-V association [39].
The origin of VTM deposits has not been conclusively elucidated in the scientific field. However, it has been observed that these deposits are commonly associated with stratified igneous intrusions in cratonic regions [38,43].
With regard to uranium–vanadium deposits, the so-called tabular sandstone-hosted deposits (Table 1) are uranium impregnations that form irregular mineralised bodies with a lenticular shape and parallel, in elevation, to the reducing sediments in which they are embedded [44,45]. These elements originate as a result of water flowing through the sediments until it reaches a sufficiently reducing formation that allows both uranium and vanadium to precipitate [46,47].
Surface uranium deposits are characterised as ‘young’ because they are predominantly located in tertiary geological formations adjacent to the topographic surface [48]. The deposits of interest, which are the subject of this study, exhibit uranium mineralisation predominantly in the form of hexavalent minerals of this element. Specifically, the presence of carnotite and tujamunite is observed [45,49,50].

Table 1.
Geometallurgical characteristics of uranium deposits in sandstones [49,50].
Table 1.
Geometallurgical characteristics of uranium deposits in sandstones [49,50].
| Type of Deposit | Mineralogy | Bargain Rocks | Notable Deposits | Metallurgical Characteristics |
|---|---|---|---|---|
| Sandstone-hosted | Pitchblende, Uraninite, Coffinite, Autunite, Fosrfouranilite. | Quartz with sulphides, carbonaceous material and ferro-magnesium mafic minerals. | Sominair, Niger; Letlhakane, Botswana; Rystkuil, South Africa; Mkuju River, Tanzania; Inkai, Kazakhstan; Khiagda, Russia | High presence of clayey materials. High-grade zones in the shape of an arrow. High carbonate content. Presence of organic matter and sulphides. Low grades. |
| Surficial | Carnotite, Tujamunite, and other complex uranyl minerals. | Carbonate rocks, clays, quartz, and gypsum. | Trekkopje, Namibia; Langer Heinrich, Namibia; Yeelirrie, Australia | Low-grade ores. Soft materials, possible mechanical stripping. High gypsum content. High chloride content. Alkaline leaching is commonly used. |
In summary, it can be stated that uranium and vanadium are transported to their final deposition location by dissolving in the region’s groundwater. This water moves laterally and vertically from the oldest geological formations to the most recent ones, which are associated with tertiary or quaternary sedimentary environments close to the surface. In the latter environment, carnotite is deposited as a result of the accumulation of uranium, potassium and vanadium in the necessary quantities and oxidation states [45,48].
In line with the above, vanadium reserves extracted as a by-product in uranium deposits are highly limited compared to vanadium titanomagnetite deposits [38]. However, as a result of uranium extraction as a primary mineral, they become a source of vanadium that, in the absence of uranium mineralisation, would not be exploited for economic reasons.
Analysis of the global reserve base (Figure 1) reveals an extreme geographical concentration of vanadium supply, as nearly all identified reserves are located within just four nations. This high concentration, with two countries alone holding well over half of the global total, establishes the critical supply risk addressed later in this study.
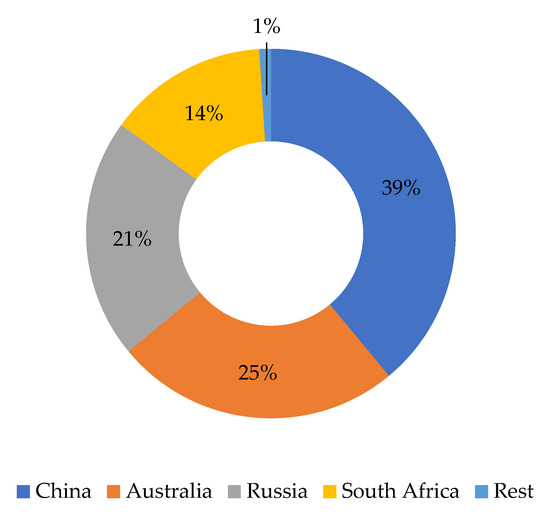
Figure 1.
Distribution of vanadium reserves by country according to reference [51].
In the specific case of surface-type uranium–vanadium deposits, despite the fact that there is still a significant concentration of deposits in Namibia and Australia, it is evident that, as a result of mining exploration and prospecting activities in the United States, specifically in the state of Texas, and in Argentina, at least two emerging uranium-bearing regions have been identified [45,48,52]. Secondly, sandstone-hosted deposits have been identified around the world, on different continents. In certain cases, the vanadium grade justifies their metallurgical processing [53].
Crucially, this concentration means that 50% of the world’s reserves are held by countries whose reliability in terms of supply is questionable. Consequently, both the European Union and Australia and the United States have considered vanadium to be a critical raw material (Table 2). That said, and in line with the above, it has been observed that, along with uncertain security of supply, the applications of vanadium have increased due to the energy transition. Therefore, it has been determined that there is likely to be an increase in demand [38,39].

Table 2.
CRM list by year.
3.1.1. Evolving EU Strategic Priorities Reflected in the CRM List
The temporal evolution of the European Union’s Critical Raw Materials (CRM) list (Table 2) provides a clear time-series analysis of the evolving strategic priorities, shifting from a focus purely on supply risk mitigation towards securing entire value chains essential for the Green and Digital Transition.
The inclusion of vanadium in the 2017 list (which continues to be relevant due to its high Supply Risk—SR of 2.3 in the 2023 assessment [62]—driven by concentrated global production in China, Russia, and South Africa) confirmed its vital role in future High-Strength Low-Alloy (HSLA) steel and emerging Long-Duration Energy Storage (LDES) applications.
However, the most recent update demonstrates a major inflection point in EU policy. For the 2023 list, materials such as Nickel and Copper were included not because they met the traditional CRM thresholds, but because they were designated as Strategic Raw Materials (SRMs). As defined under the Critical Raw Materials Act (CRMA), SRMs are essential for technologies supporting the twin green and digital transition and defense objectives. This highlights a strategic shift from simply identifying resource scarcity to actively securing the infrastructural and technological materials critical for achieving industrial resilience and autonomy.
This trend—prioritizing materials like Copper and Nickel due to their strategic end-use in electrification infrastructure—validates the EU strategy’s necessity to move beyond mere diversification (P4—Porter” and “Af1, E3, M2) and establish deep technological and financial partnerships, as defined by the CAME strategy (Af1, E3, M2—SWOT & CAME).
3.1.2. Economic Feasibility of Uranium–Vanadium Deposits: The Ivana Case Study
To illustrate the economic rationale for uranium–vanadium co-production, the Ivana deposit (Río Negro, Argentina) has been selected as a representative example. This deposit, explored by Blue Sky Uranium Corp in 2018 [63], is hosted in sandstone-surficial formations and presents a combined uranium–vanadium mineralization (mainly carnotite and tyuyamunite) with an average grade of 311 ppm U3O8 and 107 ppm V2O5, corresponding to a U/V mass ratio of approximately 2:1.
Using unit operating costs and recovery factors reported by Blue Sky Uranium Corp. (2018) [63]—mining cost ≈ 2.26 USD/t, ore processing cost ≈ 8.26 USD/t, and metallurgical recovery of 85% for uranium and 50% for vanadium and assuming current average market prices between 50 and 100 USD/lb U3O8 and 13 USD/kg V2O5, the results indicate that the cut-off grade for uranium lies between 56–112 ppm U3O8, consistent with values observed in low-grade sandstone-hosted deposits [64,65].
Conversely, the stand-alone cut-off grade for vanadium is estimated to exceed 1600 ppm V2O5, assuming the same cost structure and recovery rates [64]. Given that the actual vanadium grade in Ivana is nearly an order of magnitude lower (≈107 ppm), it is clear that vanadium extraction alone would not be economically feasible [63]. Therefore, the economic justification of uranium–vanadium deposits depends primarily on uranium revenues, with vanadium acting as a secondary product that enhances the project’s financial robustness rather than as a main driver of profitability.
3.2. Vanadium Processing and Metallurgy
The technical and economic viability of a mining operation is often conditioned by various factors, among which the mining method and extractive metallurgy stand out. In this study, a significant correlation has been found between the type of mineral present in the deposit being mined and the element in question. In the field of mining engineering, it is important to consider various geological and geographical factors when determining the mining method to be implemented or the most appropriate extraction technique for a mining operation. These factors include mineralogy, geographical location and the nature of the host rock. The case of vanadium is no exception.
Given the diversity of vanadium deposits, the extractive metallurgy techniques to be implemented will be selected based on the specific characteristics of each operation. In the case of titanomagnetite deposits, two main processing methods are identified, depending on the ore to which the metallurgical processes are applied. These methods are described in references [66,67].
Initially, vanadium was extracted directly. However, this procedure required higher purity ores and generated problems related to the management of tailings and waste [36,68]. In a second phase of research, advances in the field of extractive metallurgy led to the use of vanadium slag, a by-product of the steelmaking process, as the main material for vanadium extraction [36].
In general terms, two predominant methods for extracting vanadium have been identified. The first involves precipitating a vanadium salt by leaching a roasted ore with salt. The second method is based on leaching salt-roasted slag, obtained after smelting the ore to produce pig iron containing vanadium. This is followed by oxygen blowing in a converter, which results in the generation of vanadium-rich slag [68,69].
In contrast, alternative methods for obtaining vanadium are being explored. According to the most recent data (2021), 13.03% of the raw materials for vanadium products produced worldwide are obtained directly from vanadium-bearing titanomagnetite, while 76.20% come from vanadium slag produced from vanadium-bearing titanomagnetite [51].
Regarding U-V deposits, the procedures currently used to extract uranium from their ores can be classified into two clearly distinct categories: acid leaching and basic leaching. This distinction is determined by the inherent characteristics of the specific gangue in question [70]. As evidenced in the analysis in Table 1, minerals from various types of deposits tend to contain alkali elements in concentrations that, when exceeding 5–8% of the total, can make acid consumption prohibitive, forcing the use of basic attack strategies [71].
In the context of a uranium–vanadium deposit, where the recovery of both elements can be of great economic interest, in addition to considerations relating to gangue, the most relevant characteristic of basic treatment is selectivity. Sodium carbonate attack exhibits significantly higher selectivity compared to acid attack methods using sulphuric acid or hydrochloric acid. In certain circumstances, sodium carbonate shows greater selectivity, dissolving only silica and, occasionally, vanadium, rather than dissolving a wider variety of species in the leaching alkali liquor [72,73]. In the field of strategic metal extraction, the sodium salt roasting process for obtaining vanadium from U-V deposits exhibits a remarkable similarity to those used in titanomagnetites. However, given the need to extract two different concentrates, vanadium and uranium are separated in the liquid phase, resulting in recoveries of 70–80% for vanadium and 90–95% for uranium [34].
3.3. Vanadium Applications and Market Trends for Green Energy
3.3.1. Scope and Framing: Energy-Transition-Relevant Applications
Vanadium, chemical element number 23 in the periodic table of elements, is a versatile element with a wide range of industrial applications. However, not all technologies are directly linked to the energy transition or emissions reduction, as its most common applications are linked to the steel industry. However, it is also used in the chemical industry [74]. To properly contextualise this section, it is imperative to distinguish between traditional uses and strategic uses in the context of decarbonisation.
Currently, most of the global demand for vanadium is concentrated in the steel industry, where it is used in the form of ferrovanadium for the production of high-strength low-alloy (HSLA) steels [7]. Although this is a conventional sector, its relationship with the energy transition should not be underestimated. Vanadium steels reduce the weight and amount of material required in critical structures such as wind towers, electric transport infrastructure and sustainable urban construction. This leads to indirect savings in carbon dioxide emissions [75].
As noted in previous research, storage using lithium-ion batteries (LIB) has proven to be the most relevant emerging application in the context of the energy transition. However, high-temperature lithium-ion batteries (VRFBs) have been identified as having greater potential to improve the efficiency and sustainability of energy storage in the energy sector. These technologies are characterised by their safety, durability and storage capacity on time scales ranging from hours to days [76,77]. Consequently, they are an appropriate complement to intermittent renewable energies, such as wind and solar power. This study addresses the issue of renewable energy penetration in the market, a phenomenon which, although still in its infancy, is postulated as one of the main vectors of resilience for electrical systems in scenarios of high renewable penetration.
Other applications, such as industrial catalysts or titanium and vanadium alloys, have a more tangential relationship with the transition. While they contribute to improving the efficiency of chemical processes, reducing pollutant emissions and lightening materials, they do not have as marked a direct impact as microalloyed steel and hydrogen fuel cells (FVCF).
Consequently, Section 3.3 will focus on applications in which vanadium plays an essential role in the decarbonisation process, such as (i) microalloyed steels, which reduce the material footprint, and (ii) VRFBs, which act as a key vector in stationary storage. The rest of the applications will be briefly reviewed in order to provide a holistic view, albeit of a secondary nature. In this sense, the necessary framework is established to assess both current trends and future prospects in the energy transition.
3.3.2. Current Demand Landscape: Application Split and Material Intensity
The vanadium market shows significant concentration in certain sectors, with a notable predominance of the steel industry. The current vanadium market shows a dominant and structural dependence on the steel sector, which accounts for between 85% and 90% of global consumption (Figure 2). This imbalance is the primary source of price volatility [78]. This study addresses the use of these steels in various industrial applications. Firstly, they are used in reinforcing bars, structural profiles and oil pipelines. Secondly, there is a growing trend towards their use in infrastructure linked to the energy transition, such as wind towers, power lines and photovoltaic structures. The standard alloy ratio is in the range of 0.03–0.1% by weight of V, suggesting that minimal fluctuations in global steel production could cause significant variations in vanadium demand [79].
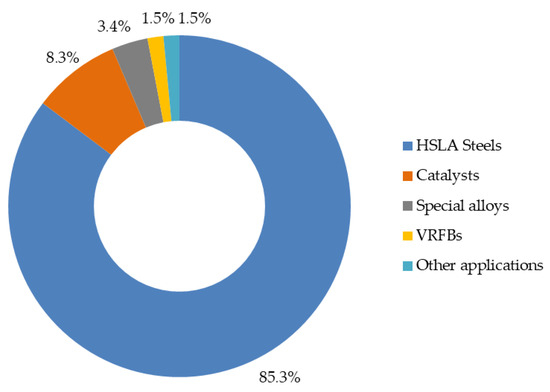
Figure 2.
Global vanadium consumption by application. The proportions have been estimated based on the value ranges reported in the literature [78] and adjusted to represent a total of 100%.
The second largest consumption block corresponds to catalysts, which represent between 7% and 10% of the market (see Figure 2) [78]. In the field of scientific research, certain catalysts have been identified that have demonstrated remarkable effectiveness in various industrial applications. These include vanadium pentoxide (V2O5) catalysts, used in the production of sulphuric acid, and catalysts used in selective catalytic reduction systems for the removal of nitrogen oxides (NOx) in power plants and industrial processes. Although these uses are more traditional, they contribute indirectly to the energy transition by improving process efficiency and reducing pollutant emissions.
Special alloys, particularly those composed of titanium and vanadium, account for approximately 3–4% of total consumption, as shown in Figure 2 [78]. These applications are particularly important in the aeronautical, biomedical and high-tech sectors, where the strength and lightness of materials are critical factors. Although their relationship with the energy transition is more peripheral, their potential contribution to weight reduction in transport and aviation components, which in turn translates into lower fuel consumption, should not be overlooked [75].
Although long-duration energy storage technologies, primarily VRFBs, currently account for less than 2% of consumption (Figure 2), this segment represents the most significant opportunity for demand diversification and future growth [78].
The remaining applications (pigments, ceramics and minor chemical products) make a marginal contribution to consumption, ranging from around 1–2%, with no prospect of substantial growth in the context of the energy transition [78].
As previous research has shown [78], current demand for vanadium is clearly linked to the steel sector, although there are signs of diversification towards the energy and technology sectors. The market thus faces a twofold complexity: profound structural interdependence with the volatile steel sector, contrasted with the rapid emergence of VRFB demand, which is projected to reconfigure the global market balance in the coming years.
3.3.3. Microalloyed Steels for Low-Carbon Infrastructure
Currently, the most widespread use of vanadium is as a microalloying element in HSLA steels. As previously mentioned, the incorporation of this element, although modest, with a variation between 0.03% and 0.10% by weight [79], has a significant impact on the mechanical properties of steel. Vanadium promotes the formation of carbides, nitrides and carbonitrides that refine grain size and increase strength and toughness without sacrificing ductility [79]. In line with recent findings, recent research on steels with high vanadium content, such as Vanadis 8 produced by Metalurgia de Polvos, has revealed that the presence of MC-type carbides (VC, >2800 HV), in conjunction with heat treatment and ion nitriding, is essential for controlling grain growth and, simultaneously, enhancing wear and corrosion resistance in aggressive environments, which translates into an increase in industrial applications [80].
These characteristics are particularly relevant in the context of the energy transition, where a high volume of steel is required for the development of low-carbon energy infrastructures. In the context of mechanical engineering and structural construction, the use of microalloyed steels in wind towers represents a significant innovation. This material allows for a reduction in section thickness without compromising the structural safety of the structure. As a result, there is a reduction in the total weight of the tower, which in turn reduces the costs associated with transport, assembly and foundations. In the field of electricity transmission and distribution networks, the use of vanadium steels has become common practice in the construction of poles, towers and reinforcements, thus contributing to the expansion of the network necessary for the integration of renewable energies. In the field of construction, the implementation of reinforcement bars with a high elastic limit allows for the optimisation of designs, which translates into a reduction in the carbon footprint associated with the material.
The impact this has is not trivial: it has been estimated that replacing conventional steels with HSLA containing vanadium can reduce steel consumption per functional unit by up to 30%, with a consequent reduction in CO2 emissions [81]. Considering that the steel industry is responsible for approximately 7–9% of global greenhouse gas emissions, these reductions take on strategic importance for international climate goals [18].
3.3.4. Vanadium Redox Flow Batteries and Long-Duration Energy Storage
VRFBs are the implementation of vanadium that is closest to the energy transition. This study addresses the distinctive principle underlying the use of vanadium in four oxidation states—V2+/V3+ in the negative electrolyte and V4+/V5+ in the positive—with the aim of avoiding cross-contamination of species, a phenomenon that can occur when redox pairs belong to different elements [76]. The chemical symmetry in question confers long-term stability and mitigates the irreversible degradation associated with cycles, positioning VRFBs as natural candidates for long-duration stationary storage in combination with wind and photovoltaic energy [82,83].
However, despite this intrinsic advantage, commercial systems still face the major challenge of vanadium ion crossover through the ion-exchange membrane separating the two half-cells. The commonly used Nafion® membrane exhibits limited selectivity between protons and multivalent vanadium ions, allowing gradual interpenetration of species that leads to capacity decay and efficiency loss during cycling. Ongoing research focuses on developing advanced membranes and composite separators with improved ion selectivity and lower permeability to vanadium ions [84,85].
In the field of architecture, power and energy are scaled independently. Specifically, power depends on the size of the electrochemical stacks, while energy depends on the volume of electrolyte stored [6]. This feature, which is not present in technologies such as lithium-ion, enables the economic estimation of systems with a duration of up to 24 h by incorporating electrolyte tanks, thus avoiding the oversizing of the associated power generation unit. In the field of network services, the observed independence has been shown to have a significant impact on reducing incremental costs per additional hour compared to chemical services, where power and energy are coupled [77,82].
Another outstanding aspect that deserves special mention is safety. In this regard, the electrolyte in VRFBs is aqueous in nature and does not have a propensity for combustion, which mitigates the risk of thermal runaway that occurs in certain lithium-ion architectures [86,87]. This fact, in conjunction with the ability to tolerate deep discharges—close to 100% without accelerated degradation— and their long service life—more than 10,000 cycles in stationary applications—leads to an optimisation of the levelized cost of storage (LCOS) when the target duration is longer and the service profile is more intensive [77,82].
Costs and intensity of vanadium use.
The main drawback associated with VRFBs is the high cost of the electrolyte, which is closely linked to the value of vanadium. As can be seen from the research carried out, the scientific literature specialising in the field of solar energy shows that the electrolyte can represent between 30% and 50% of a system’s CAPEX. It has also been determined that the typical intensity of vanadium use is in the range of 3 to 6 kg per kWh of capacity. In practical terms, storing 1 MWh of energy can require between 4.5 and 5.5 tonnes of V2O5 [88]. This phenomenon means that fluctuations in the sale price of vanadium are transferred almost directly to the investment cost of the system [89].
Although electrolyte costs represent a major share of the capital expenditure, other components included in the balance of system (BOS), such as tanks, membranes, pumps, and power conditioning equipment, also exert a significant influence on overall economics. Recent studies suggest that improvements in membrane selectivity, stack efficiency and modular plant design could significantly reduce BOS costs in the coming years [77,90].
Moreover, while vanadium-based systems are often perceived as expensive, other aqueous flow batteries, such as those based on iron or zinc chemistry, also face cost and efficiency limitations. Iron-based flow batteries generally benefit from lower electrolyte costs but exhibit lower energy density and reduced long-term stability. Conversely, VRFBs provide outstanding durability and nearly complete recyclability of the active materials, which offsets part of their higher upfront cost and supports their competitiveness in long-duration energy storage applications [91].
This dependency has promoted particular business models, such as electrolyte leasing, in which a third party finances the vanadium and the user pays for the energy service, minimising the barrier to entry and facilitating circularity (at the end of the contract, the vanadium is recovered and can be reused in another system). A recent study published by the World Bank [92] examines this approach in detail and presents it as a key element in driving commercial adoption while reducing the risks associated with price fluctuations.
Comparison with other storage technologies.
In short ranges (1–4 h), lithium-ion batteries—especially lithium iron phosphate or LFP batteries—maintain a cost advantage thanks to economies of scale and highly developed supply chains. Various analyses [93,94] place the installed cost of Li-ion below the cost of flow technologies, at least in this time range. However, when the required duration increases (ranges between 8 and 12 h), the incremental cost of VRFBs grows more slowly, since adding energy is essentially adding electrolyte and tank.
Recent technology cost assessments by the National Renewable Energy Laboratory [94] project the overnight capital cost for a complete 4 h lithium-ion battery system to average USD 326/kWh by 2030 (in 2022 USD). In comparison, the US Department of Energy [93] show reference projects for a 100 MW/10 h VRFB with total costs estimated at around USD 365–385/kWh for 2030. This price gap reinforces the strategic importance of technology differentiation: the VRFB niche relies on its competitive advantage for Long-Duration Energy Storage—specifically, for durations exceeding 8 h—where its decoupled power and energy capacity and superior cycle life (over 10,000 cycles) offset the higher upfront capital cost.
In the global market context, confirmed by the International Energy Agency [19], battery storage deployment has experienced an unprecedented surge, doubling year-on-year in 2023, with over 40 GW of new capacity added globally. This explosive growth, primarily driven by LFP chemistries for Short-Duration Energy Storage, which accounted for approximately 80% of new capacity additions in 2023, defines the competitive landscape for all battery technologies.
IEA analysis projects that to meet the COP28 (Conference of the Parties) goals—specifically tripling global renewable energy capacity—total energy storage must increase sixfold, with battery storage capacity rising 14-fold to 1200 GW by 2030 in the Net Zero Emissions Scenario. Crucially, the IEA projects that total utility-scale lithium-ion battery system costs will decline by an additional 40% by 2030 in the Stated Policies Scenario, reaching USD 175/kWh.
This intense cost pressure necessitates a clarification of forecast parameters. While the IEA stresses the definitive need for Long-Duration Energy Storage solutions to integrate higher shares of renewables without oversizing the grid, VRFBs must prove their superior Levelized Cost of Storage for durations exceeding eight hours against this backdrop of rapidly decreasing Li-ion system costs. Looking ahead to 2030, the USGS collects market estimates according to which VRFBs would absorb around 17% of vanadium consumption [16]. Such a rapid increase in the share of VRFBs will place new, intensified demands on the vanadium supply chain.
To quantify the market potential and associated risks, particularly in light of projections that VRFBs could absorb around 17% of global vanadium consumption by 2030, a clear set of quantitative assumptions is presented Table 3 which synthesizes the fundamental parameters driving the competitive scenarios for VRFB adoption, focusing on cost targets, scaling advantages, and critical mitigation strategies.

Table 3.
Key Quantitative Assumptions for VRFB Demand Scenarios.
Deployment and flagship projects.
Although the installed base of VRFBs is still modest compared to lithium-ion, in recent years a series of projects have been implemented that demonstrate the technological maturity at grid scale. In Dalian (China), the first phase of a 200 MW/800 MWh project designed for peak management and renewable integration was connected to the grid in 2022.
Outside China, Australia, the US and Europe have deployed smaller-scale projects, but with diverse use cases: microgrids and islands, photovoltaic integration with day-night storage, and deferral of transmission investments when demand grows faster than grid capacity. The IEA reports accelerated growth at the grid scale and anticipates greater technological diversity as regulators recognise the value of long-duration storage [20].
Use cases: How VRFBs fit into the electricity system.
VRFBs perform optimally in the following cases:
- Integration of intermittent renewables, allowing generation to be shifted from hours of excess to hours of deficit without severe degradation penalties [83].
- Postponement of grid reinforcements when located downstream of bottlenecks.
- VRFBs can flatten local peaks and delay reinforcement investments and grid improvements. They also have the advantage of being non-flammable and independent of power and energy, which allows for the design of customised systems [77].
- Microgrids and islands, offering operational safety, intensive cycles and the possibility of reusing the electrolyte at the end of its life [82].
Circularity: Leasing and recycling.
Throughout this paper, it has been mentioned on several occasions that a strategic advantage of VRFBs is that their electrolyte is recoverable. Unlike lithium-ion technology, where recovering lithium, nickel or cobalt with positive net economic returns remains a challenge, in VRFBs it is feasible to recirculate vanadium using relatively conventional chemical operations, regenerating the oxidation state or precipitating it for purification. Circular models such as leasing + recovery reduce initial CAPEX and vanadium price risk by partially decoupling the competitiveness of the technology from the volatility of the primary metal market [92].
Technological sensitivities and cost levers.
Although the electrolyte dominates CAPEX, other factors influence competitiveness:
- Electrodes and membranes: improvements in pressure drop, kinetics and selectivity reduce losses and plant balance costs. Advances in materials and thermal-hydraulic control increase efficiency and reduce OPEX [95].
- Vanadium concentration: increasing molarity increases energy density but requires phase stabilisation to prevent precipitation [95].
- Standardisation: scaling up production and standardising designs reduces the specific cost per kW [82].
In the medium term, gradual improvements are expected which, combined with electrolyte leasing and increased volume, will place VRFBs in competitive cost ranges for storage exceeding 8 h [1]. Some projections for 2030 place total costs (CAPEX) at around $365–385/kWh [94].
3.3.5. Circularity and Recycling in Energy Applications
The concept of circularity in the use of critical raw materials has become a priority for the energy transition. In the case of vanadium, this principle is particularly relevant not only because of the high geographical concentration of its primary production, but also because the metal itself has chemical properties that facilitate its recovery and reuse. In a scenario of recent demand—microalloyed steels today and redox flow batteries in the future—taking advantage of and scaling up vanadium recycling and revaluation routes is essential to ensure the sustainability and competitiveness of the value chain.
The economic feasibility of circularity, however, must be rigorously quantified. Despite vanadium’s intrinsic recoverability, its current contribution to meeting EU demand is marginal, highlighting an operational bottleneck. According to the 2023 EU criticality assessment, the End-of-life Recycling Input Rate (EOL-RIR) for vanadium stands at only 6%. This low figure provides quantitative evidence of the significant gap between theoretical recycling potential (O2) and industrial capacity at scale (D2). Scaling this rate is imperative to mitigate the high Supply Risk (SR = 2.3) and production concentration associated with primary supply, aligning with the strategic objective of the Raw Materials Initiative to reduce dependence on primary materials through recycling. Consequently, targeted analysis of material flows and feasibility of typical projects is required. Vanadium recovery pathways primarily concern VRFB electrolyte, spent catalysts, and steel slag [66].
As discussed in Section 3.3.4, the electrolyte is the most critical cost component in VFBRs, accounting for up to 50% of total CAPEX [89]. However, from a circularity perspective, this apparent weakness becomes an advantage, as the electrolyte in this type of battery remains chemically stable throughout its useful life [96]. In other words, after 15–20 years of operation, the electrolyte retains virtually all of the initial vanadium.
At the end of its useful life, chemical conditioning is carried out, which consists of adjusting the vanadium concentration, removing impurities and restoring operating conditions. In practice, this is equivalent to having a permanent stock of technospheric vanadium that can circulate indefinitely between successive generations of VRFBs [82].
Compared to lithium-ion batteries, where recycling processes are costly and have limited yields [97], the circularity inherent in VRFBs constitutes a differential competitive advantage.
A second relevant circularity flow is vanadium pentoxide (V2O5) catalysts. Over time, these catalysts lose their effectiveness but retain their valuable vanadium content. There are currently established processes for the recovery of spent catalysts using conventional metallurgical techniques such as leaching, selective precipitation and calcination [98,99]. The treatment of thousands of tonnes of catalysts per year can generate a stable flow of secondary vanadium that would help reduce dependence on primary mining.
Unlike VRFB electrolyte, catalysts represent a more dispersed flow with greater logistical variability, as they are generated in multiple chemical and energy plants. Even so, their vanadium content is sufficiently high—between 3 and 12% by weight, compared to less than 1% in primary ores [99]—and there are established industrial processes with yields above 80% [100]. Furthermore, as it is classified as hazardous waste, its recovery has additional environmental benefits by avoiding its disposal in landfills [18].
Steel slag is another secondary resource to consider. In regions that produce steel from titanomagnetic ores, such as China, Russia and South Africa, this slag contains significant amounts of vanadium that can be extracted using pyrometallurgical and hydrometallurgical processes [101,102].
In Europe, where vanadium mining is virtually non-existent, projects are being promoted to take advantage of this route: the Vanadium Recovery Project in Finland, which will enable vanadium to be recovered from slag and transformed into battery-grade V2O5, is a clear example of this trend, which is also in line with the objectives of the Critical Raw Materials Act by using industrial waste as a strategic source [23,103].
However, slag presents technical challenges such as variable composition, the presence of iron and titanium oxides, and energy-intensive separation processes [102]. Nevertheless, its volume and relative concentration make this source a potential pillar of secondary supply in a scenario of strong demand growth.
Challenges, prospects and opportunities.
The circularity of vanadium is still far from reaching its full potential. In the case of VRFB electrolyte, although the material is almost entirely recoverable, there is still no industrialised infrastructure for large-scale collection and reprocessing. As the first large-scale projects reach the end of their useful life, it will be necessary to develop logistics chains and regulations to ensure proper management of the electrolyte, similar to what happens with other hazardous waste.
In the field of catalysts, the main limitation is the fragmentation of the collection chain: the existence of multiple suppliers and intermediate managers increases costs and hinders traceability. As for slag, the challenge lies in the efficiency of metallurgical recovery processes and economic viability in the face of volatility in the vanadium market.
The EU, through the CRMA and the Net-Zero Industry Act, has set binding recycling capacity targets for critical raw materials, including vanadium [23]. However, turning these targets into operational chains requires investment in R&D, pilot plants and public–private partnership schemes, which are still in their infancy.
Nevertheless, developing these circular vanadium chains offers significant strategic benefits:
- Reduced dependence on imports, a primary market that is highly concentrated geographically.
- Mitigation of price volatility, by having a buffer stock of secondary supply.
- Reduced environmental footprint, by avoiding primary mining and revaluing waste.
Looking ahead to the coming years, VRFB electrolyte is expected to emerge as the main vector of this circularity as the deployment of this technology increases and secondary markets for reconditioned electrolyte appear. At the same time, spent catalysts will continue to provide a constant flow, while steel slag could become established in Europe and other regions as a strategic supply.
3.3.6. Catalysts and Emissions Control: Adjacent Contributions
Vanadium has found one of its longest-standing and most stable uses in catalysis, with a relative weight less than that of steelmaking, but with significant strategic importance. Two areas account for the bulk of this application: the production of sulphuric acid using the contact process and selective catalytic reduction systems for NOx abatement. Both examples illustrate how vanadium can contribute indirectly to the energy transition: by supporting essential chemical infrastructure for fertilisers, materials and metallurgy on the one hand, and by enabling compliance with air quality regulations in industrial sectors on the other [104].
Vanadium in the production of H2SO4.
Sulfuric acid is, in terms of tonnage, the most manufactured chemical in the world, with volumes exceeding two hundred million tonnes per year [105]. Its industrial production is based on the contact process, in which sulfur dioxide is oxidised to sulfur trioxide and then absorbed in water to form sulfuric acid. Although platinum was historically used as a catalyst, since the mid-20th century it has been replaced by systems based on V2O5 supported on silica and promoted with potassium or caesium, as these have a better cost/efficiency ratio and greater resistance to poisons such as arsenic.
This catalyst has an average life of between two and five years, although it could last up to 10 years if conditions are right. Once exhausted, it still contains a certain percentage of vanadium, which makes it a valuable waste product from a recovery perspective. Through leaching processes (acid or alkaline), followed by selective precipitation, yields of over 80–90% can be achieved and high-purity V2O5 can be obtained [98,99].
The recycling of these catalysts has a dual benefit: it avoids the disposal of hazardous waste in landfills and creates a stable secondary stream of vanadium.
Vanadium in selective catalytic reduction (SCR) of Nox.
The second major field of application is in the reference technology for the removal of NOx from combustion gases in thermal power stations, incinerators and industrial boilers. The system is based on the reaction of NO and NO2 with ammonia, in the presence of a catalyst, to produce nitrogen and water. The most common catalysts are those based on V2O5, as they offer an excellent balance between cost, activity and robustness [106].
Although the active phase is present in proportions of less than 2% by weight of V2O5, it is decisive for conversion, achieving an efficiency of more than 90% in NOx reduction [107]. At the end of its useful life, recycling is possible at rates that, under optimal conditions, can reach 80–90% [108,109].
Although the catalytic applications of vanadium do not account for volumes comparable to those of steelmaking or VRFBs, its relevance in the energy transition can be summarised in three reasons:
- This is a critical chemical infrastructure, as H2SO4 production supports key industries and, at present, there is no mature large-scale substitute for vanadium as a catalyst.
- SCR catalysts enable industrial plants to meet increasingly stringent NOx limits, reducing impacts on health and the environment.
- At the end of their life, these catalysts become a resource that can reintroduce vanadium into the value chain.
3.3.7. Titanium Alloys and Other Specialty Uses
Among titanium alloys, the most notable application is the Ti-6Al-4V alloy, also known as grade 5. This is a compound consisting of 4% vanadium, which accounts for more than 50% of the titanium used in structural applications [110]. Vanadium acts as a phase stabiliser, which allows the microstructure to be controlled, improving mechanical strength and maintaining a good combination of lightness, fatigue resistance and fracture toughness [111].
The use of these titanium alloys extends to fields as varied as [112]:
- The aeronautical and aerospace industry, where, thanks to the weight reductions achieved, savings are made in fuel and CO2 emissions. It is used in the manufacture of fuselages, turbines and anchoring systems.
- The biomedical industry, as it is a biocompatible material that is resistant to corrosion in physiological environments.
- Defence, as it can be used to manufacture lightweight armour and certain weapon components.
- Sport, where its balance between strength and lightness is very suitable.
In addition to the above, vanadium can be found in other smaller but equally strategic fields:
- Superalloys for turbines, where it helps to improve resistance to high temperatures and corrosion [113].
- Special pigments and glass [114].
- Electronics and advanced materials, such as infrared sensors or materials with thermal switching properties, thanks to its reversible metal-insulator phase transition at 68 °C [115].
- Fine chemistry and specialised catalysis for the oxidation of light hydrocarbons and other special chemical processes [37].
Although these are ‘niche’ profiles, the recovery of vanadium in these sectors could be particularly interesting in some cases. In removed implants and discarded aeronautical components, the content is high enough to justify recycling. In general, no significant growth in the use of vanadium is expected in these sectors, although this will depend on their evolution.
3.3.8. Market Structure and Price Dynamics
The global vanadium market has a unique structure characterised by (i) high geographical concentration, (ii) strong dependence on the steel industry, and (iii) marked price volatility. These characteristics not only affect supply stability but also have direct implications for the competitiveness of emerging applications such as VRFBs.
Primary vanadium production is concentrated in a few countries—in 2023, nearly 90% of global supply came from China and Russia, with a very similar figure for 2024 [107]. China is by far the leading producer, accounting for more than half of global supply.
A distinctive feature of this market is that more than 70% of the world’s vanadium is obtained as a by-product of the processing of titanomagnetic ores and steel slag. This means, as has been established throughout this paper, that the availability of vanadium is linked to the dynamics of steel especially high-strength steels [102,116]. Primary vanadium mining is currently practically marginal and, although there are mining projects in countries such as Australia and Canada, the cost of mining and processing is usually less competitive than that of vanadium obtained as a by-product [38].
Another distinctive aspect is the concentration in the processing value chain. Although the raw material can be generated in several regions, the conversion of intermediate (V2O5) or final (ferrovanadium) products is controlled by a small number of companies. This control over the refining stage gives certain players the ability to influence the global availability of vanadium products with the qualities demanded by the market [117].
In terms of products, the market is structured in three main ways:
- Ferrovanadium (FeV), mainly used in microalloyed steels.
- Vanadium pentoxide (V2O5), used as a precursor for alloys, catalysts and, more recently, in electrolytes for VRFBs.
- High-purity vanadium, with specialised applications in aeronautics, biomedicine and electronics.
The combination of these characteristics—geographical concentration, dependence on the steel industry and control of refining by a few players—makes the vanadium market structurally vulnerable to local disruptions, the effects of which are amplified on a global scale.
International trade in vanadium clearly reflects the asymmetry between a highly concentrated supply and a geographically diversified demand. The market thus faces a twofold complexity: profound structural interdependence with the volatile steel sector, contrasted with the rapid emergence of VRFB demand, which is projected to reconfigure the global market balance in the coming years (Figure 3) [17].
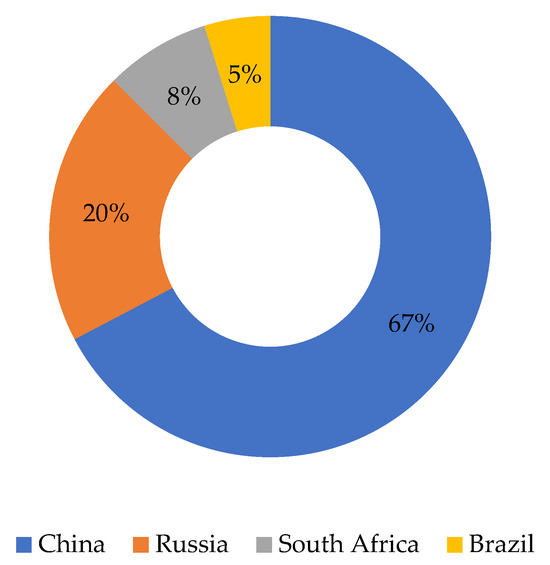
Figure 3.
Estimated annual vanadium production by country in 2024 [17]. The total production is 104,000 tonnes, and the percentages have been calculated relative to this total.
To quantitatively assess the degree of market concentration, the Herfindahl–Hirschman Index (HHI) was calculated based on 2024 production shares (China 67%, Russia 20%, South Africa 8%, and Brazil 8%). The resulting HHI value of approximately 5000 indicates a highly concentrated market structure, exceeding the 2500 thresholds commonly used by the European Commission and the U.S. Department of Justice to define oligopolistic markets. Such concentration underscores the vulnerability of the global vanadium supply chain to geopolitical and policy disruptions.
On the demand side, estimates for 2024 place consumption at around 103,000 tonnes, in balance with available supply, but with a strongly skewed sectoral distribution: around 85–90% of vanadium is used in steelmaking, while the rest is used in catalysts, special alloys and, more recently, redox flow batteries, as mentioned in previous sections.
China not only dominates mining production, but also the processing and export of V2O5 and FeV (the former is the raw material for VRFBs and the latter is used to manufacture microalloyed steels). At the same time, it is a large domestic consumer, especially in its steel industry. Russia and South Africa stand out as exporters to Europe and the United States, while Brazil is emerging as an alternative supplier [17].
This dependence creates structural vulnerability, and any disruption has immediate repercussions on global availability and prices. This situation is reinforced by the fact that vanadium supply is largely a by-product of steelmaking, so its production does not respond to the needs of the vanadium market, but rather to the dynamics of the steel industry.
Without attempting to provide an exhaustive study of price developments in this section, we would like to highlight the volatility of prices and justify, in part, the three structural factors to which they respond: (i) the high concentration of supply; (ii) the nature of vanadium as a by-product of steel; and (iii) the limited liquidity of the market, where relatively small changes in production or demand can generate significant fluctuations. The prices referred to are those of V2O5 and FeV, as the other products mentioned above do not usually have public prices and are traded in small volumes through bilateral contracts.
Demand for vanadium remains closely linked to the steel cycle (Figure 2 and Figure 4), which makes its market highly dependent on the global industrial situation. However, historical price fluctuations also respond to commercial and geopolitical factors. Between 1994 and 2003, there was a sustained increase in prices, culminating in an episode of overproduction, followed by a sharp decline. The closure of mines such as Windimura (Australia) and Vantec (South Africa) led to significant price rebounds around 2004. Conversely, periods of exploration expansion and increased production, particularly between 2010 and 2014, tended to stabilise prices [38].
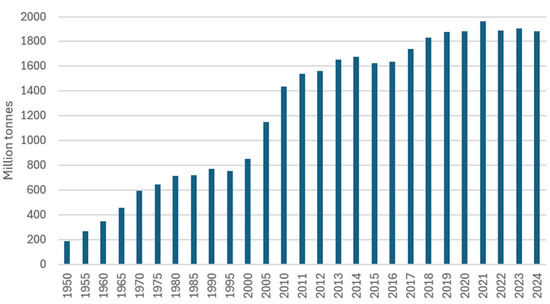
Figure 4.
World Crude Steel Production 1950 to 2024. Source from [118].
Over recent decades, price variations have exceeded 100% at certain intervals, highlighting the structural fragility of the vanadium value chain. Volatility is not a recent phenomenon: since the 1990s, exogenous events—such as the liquidation of strategic reserves in the United States—have exerted downward pressure on the price of V2O5. In contrast, industrial crises, such as the bankruptcy of EVRAZ Highveld Steel & Vanadium Ltd. in South Africa or the power cuts that affected its production in 2008, reduced supply and drove up prices [38].
Looking at a more recent time frame, other relevant events can be highlighted. Between 2017 and 2018, China decided to tighten construction standards for reinforcing steel, causing a sudden increase in demand for FeV. In just one year, the price of V2O5 rose from £10/kg to over £30/kg, while FeV in Europe reached highs of around £125/kg [119]. The situation reversed in 2019, when prices plummeted to £7–8/kg for V2O5 and £40–45/kg for FeV in Europe. The market gradually recovered in 2020–2021, against a backdrop of logistical tensions and expectations regarding the deployment of VRFBs, with V2O5 settling above £10–11/kg and FeV exceeding £60/kg. The war in Ukraine and restrictions on Russian exports added further uncertainty during 2022–2023, keeping V2O5 in a range of £9–12/kg and FeV between £50–70/kg, with peaks linked to the global energy crisis [120]. In 2024, the market entered a phase of relative stabilisation, with intermediate values of £8–9/kg for V2O5 and £45–55/kg for FeV, reflecting a temporary balance between steel demand and emerging pressure from the energy sectors (Figure 5) [17].
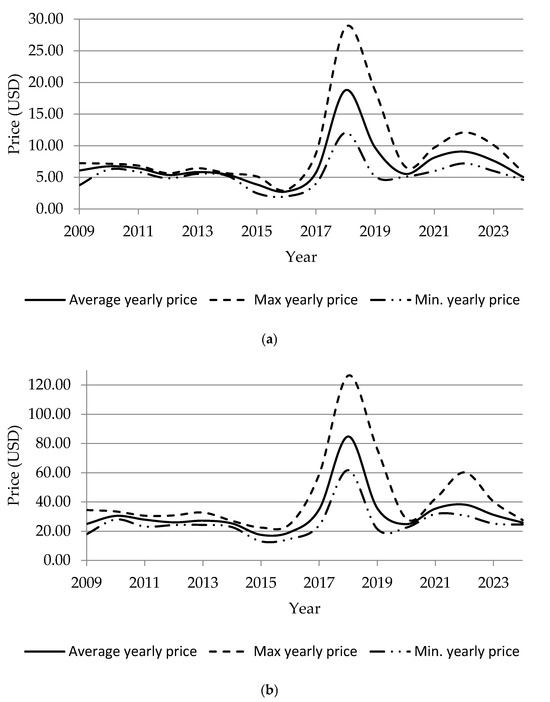
Figure 5.
Historical evolution of (a) V2O5 and (b) FeV (80%) prices in the European spot market, showing average, maximum, and minimum values. Data derived from investing.com (values in USD). Some differences with values cited in the literature are expected, as published sources may refer to different markets, purity grades or data aggregation methods. Data available up to November 2024.
The causes of these fluctuations are due to a combination of factors. The steel industry, which consumes more than 85% of vanadium, remains the main driver: any regulatory changes, such as those that occurred in China in 2018, have an immediate impact on the prices of V2O5 and FeV. The nature of vanadium as a by-product of steel production introduces rigidity into supply, as it depends on steel dynamics rather than specific demand for the metal, which amplifies volatility. In addition, the small size of the market encourages speculation and limits liquidity. Added to this are geopolitical factors, such as export restrictions in China, sanctions on Russia, disruptions in South Africa, and exposure to energy costs, which particularly affect V2O5 processing.
The future of the vanadium market will be determined by the interaction between two major vectors: traditional demand linked to steelmaking and emerging demand associated with green energy technologies, particularly VRFBs.
In the steel sector, vanadium is expected to maintain its essential role as a microalloying agent in high-strength steels. Growth in construction in emerging markets, together with policies promoting stricter safety and durability standards in infrastructure, will ensure stable and even growing demand for FeV and V2O5. China will remain the epicentre of this consumption, but other developing countries such as India and some Southeast Asian nations will increase their relative weight in the coming years [17].
However, the most significant change will come from the energy sector. Some studies [121] project that demand for vanadium for VRFBs could increase five-fold or even ten-fold by 2030, depending on the speed at which these systems are integrated into electricity grids. Therefore, if the large-scale projects planned in China, Australia or Europe materialise, they could have a significant impact.
Finally, public policies and regulatory frameworks will play a crucial role in market prospects. The inclusion of vanadium in the European Union’s list of critical raw materials (Table 2) and strategic minerals in the United States underlines its strategic importance and anticipates measures to support supply diversification and the promotion of local value chains. At the same time, energy transition policies will determine the scale of future demand.
All this serves to highlight that the vanadium market is at a turning point. The steel industry will continue to provide a solid basis for consumption, but it will be energy applications that determine the degree of expansion or pressure on supply. The extent to which diversification, recycling and price stabilisation strategies are successfully implemented will be decisive in consolidating vanadium’s role as a critical material in the transition to sustainable energy systems.
3.4. Synthesis of the Strategic Diagnosis: Integrating External Vulnerabilities and Internal Strengths for EU Policy Formulation
3.4.1. External Environment Analysis
At the European level, the Green Deal sets climate neutrality for 2050 and calls for the massive replacement of fossil fuels, as well as the deployment of enabling technologies to make the transition fair, competitive and prosperous (Table 4). This change is not insignificant: it relies on the intensive use of raw materials to manufacture renewables, networks, storage, electric mobility and low-emission industrial processes. With this premise in mind, in 2008 the European Commission launched the Raw Materials Initiative (RMI), articulated in three objectives (‘three pillars’) that remain fully valid [54]:

Table 4.
PESTEL.
- Ensure a fair and sustainable supply from global markets.
- Promote responsible supply within the EU itself (exploration, mining and processing with high standards).
- Reduce dependence on primary raw materials through resource efficiency, recycling and substitution.
The PESTEL analysis underscores the central paradox facing the EU: a high level of policy ambition coupled with persistent structural dependence on external sources of vanadium. The coincidence of high geopolitical dependence (P1) and structural economic volatility (E1, E3) represents the EU’s most significant vulnerability in the value chain. This fragility is constrained by trade tensions (P5) and the absence of domestic extraction capacity, even as EU policy (P2, P4) attempts mitigation. Economically, high CAPEX requirements (E3) and price volatility (E1) directly amplify investment risk and negatively influence the LCOS/LCOE of VRFB systems (E2). However, the growing market outlook for long-duration energy storage (LDES) (E4) provides an opportunity for stable demand growth if supported by adequate policy and financing mechanisms.
From a technological and regulatory perspective, the analysis identifies clear opportunities counterbalancing these structural weaknesses. The maturity of VRFB technology (T1) and Europe’s innovation potential in green extraction and recycling processes (T3, E4) illustrate the region’s capacity to lead in sustainable technological development. Nonetheless, the lack of large-scale industrial recycling facilities (T2) and competition from emerging chemistries (T4) pose significant barriers to scaling up vanadium-based storage systems. Social and environmental dimensions add further complexity: while public support for renewable energy and circular economy initiatives is increasing (S1, S3), social opposition to new mining projects in Europe (S2) and limited technical training in vanadium metallurgy (S5) may hinder domestic resource development. Legally, the EU benefits from a robust regulatory framework, as demonstrated by vanadium’s inclusion in the Critical Raw Materials List (L1) and the strategic provisions of the ECRMA (L2–L3), which reinforce environmental standards and supply security requirements. Despite these vulnerabilities, the macro-environment offers clear opportunities (E4, T1, T3). The EU benefits from a robust regulatory framework (L1–L3) and technological leadership in VRFB (T1) and green recycling (T3, E4). In synthesis, the central paradox is encapsulated by the coexistence of strong legal and technological foundations with persistent structural dependence on external sources of vanadium.
The threat of new entrants to the European vanadium market (Table 5) is particularly high, mainly due to a combination of financial, regulatory and geological barriers. The CAPEX associated with greenfield mining projects is extremely high, as these are metals with low concentrations and complex metallurgy, where errors in cost or reserve estimates can compromise the viability of entire projects [146,147,148]. Added to this factor are the highly restrictive environmental licences in the European Union, which slow down or even block extraction initiatives, making it very difficult for new players to enter the market without prior strategic alliances.

Table 5.
Porter.
The most critical structural feature of the global vanadium industry, confirmed by Porter’s 5+2 model, is the unprecedented bargaining power of suppliers. This is driven by extreme geographical and production concentration (2.1–2.3), granting suppliers extraordinary market influence—a dynamic reinforced by vertical integration (2.5) and state export controls (2.3). Concurrently, the threat of new entrants remains structurally low (1.1–1.3) due to high CAPEX, complex metallurgy, and stringent environmental permitting (1.2). This combination of upstream concentration and high entry barriers severely limits competition and contributes to sustained price volatility. Environmental permitting in Europe (1.2) and the lack of large-scale refining infrastructure create additional regulatory and financial barriers, meaning that new participants typically require strategic alliances or public–private partnerships to enter the market. This concentration of upstream power limits competition and contributes to sustained price volatility.
On the demand side, the bargaining power of customers is moderate and largely confined to a small number of VRFB manufacturers (3.1–3.5), whose dependence on long-term contracts and limited ability to switch chemistries constrain their negotiation leverage.
As for the threat of substitute products, the influence is moderate but growing. Although vanadium redox flow batteries (VRFBs) offer clear advantages in long-duration stationary applications—such as stability in 20,000 charge–discharge cycles and scalability of energy capacity—competing technologies such as lithium-ion batteries have dramatically reduced their levelized cost of storage (LCOS) over the last decade [1,12]. Likewise, advances in sodium-ion and new flow chemistries (Zn–Br, Zn–Cl, Fe-Cr, all-iron RFBs) reinforce competitive pressure [141,142,164,171].
Nonetheless, the EU can capitalize on the power of complementors (6.1–6.4)—particularly research institutions, component manufacturers, and energy system integrators—to stimulate innovation and reduce strategic dependency. Likewise, stakeholders and regulators (7.1–7.4), through the implementation of the Critical Raw Materials Act (CRMA), environmental standards, and ESG investment criteria, exert growing influence over market structure and corporate strategies. These dynamics underscore the need for coordinated industrial and R&D policies, aligning supply diversification with technological innovation to strengthen Europe’s resilience in the vanadium value chain.
Finally, it should be noted that the geological deposits explored to date reveal a scarcity of identified deposits in Europe and their uneven global distribution. As mentioned above, 90% of exploited reserves come from titanomagnetite deposits located in China, Russia and South Africa [39,122]. In contrast, the European Union has no active vanadium mines.
Political and legal conditions, such as CRM regulation and trade restrictions, directly affect supplier bargaining power and market entry barriers. Technological and environmental drivers influence substitution risks and rivalry intensity, while economic volatility impacts both cost competitiveness and buyer behaviour. Table 6 provides an integrated analytical matrix summarizing these interrelations and illustrating how the external context shapes the competitive structure of the vanadium industry.

Table 6.
Integrated analytical matrix combining PESTEL and Porter 5+2 dynamics.
3.4.2. Internal Analysis
Vanadium is a critical element for the energy transition in Europe, as it combines two strategic applications: its use as a microalloying agent in high-performance steels, which reduces material intensity in emission-intensive sectors, and its central role in vanadium redox flow batteries (VRFBs), considered one of the most mature long-duration energy storage (LDES) technologies for stabilising electricity systems with high renewable penetration [1,6]. Internal analysis reveals a structural gap in the EU value chain (Table 7). While the European Union is a notable leader in VRFB research, technological development and design, as well as in the deployment of green financing policies and circular economy projects [129,172], it lacks its own extractive base to ensure a stable supply of the mineral. Currently, there are no actively operating vanadium mines in the EU, which means that it is almost entirely dependent on imports from countries with high geopolitical risks [39,122].

Table 7.
Internal analysis using Value Chain tools.
The only example of vanadium ‘production’ on European soil is the Vanadium Recovery Project (VRP) in Pori, Finland, which recovers vanadium from steel slag [103]. This project not only illustrates the EU’s technological capacity for recycling and recovering industrial by-products, but also its structural limitations: the lack of direct control over primary deposits, the scarcity of primary processing centres and the absence of strategic reserves to reduce vulnerability to logistical disruptions [144,152].
Exploration in northern Europe highlights that prospecting is a strategic pillar for reducing external dependence on vanadium and other critical raw materials (CRMs) within the framework of the Critical Raw Materials Act (CRMA). In Finland, initiatives such as the Mustavaara project, a former benchmark mine in Western Europe currently undergoing industrial reactivation [183], and Soidinvaara, where geophysical campaigns and core analysis have confirmed significant concentrations of V-Ti-Fe with potential for future exploitation [184], are noteworthy. Similarly, in Norway, the stratified Bjerkreim–Sokndal complex hosts large-scale mineralisation of vanadium, titanium and phosphates, with recent results pointing to resources exceeding historical estimates [185,186]. These projects demonstrate how the favourable geology of the Fennoscandian Shield, combined with modern mapping, modelling and sampling methodologies, provides the scientific basis necessary to attract investment, develop resource estimates in accordance with international standards (NI 43-101, JORC) [146] and, ultimately, consolidate European value chains in energy storage and green steel [187].
In this sense, vanadium clearly represents the tension between the ambitions of the Green Deal and the structural weaknesses of the European supply chain. The availability of EU funds, regulatory strength and public–private cooperation are facilitating factors [23,165]. However, the success of the energy transition will depend on Europe’s ability to strengthen its position in the upstream segment, diversify sources of supply and accelerate industrial recycling on an industrial scale.
3.4.3. SWOT and CAME
The SWOT analysis (Table 8) reveals a structural paradox: while the European Union has clear strengths in R&D, advanced regulatory policies and an innovation ecosystem in energy storage, its weaknesses lie in the absence of active vanadium mines, limited primary processing capacity and dependence on imports from countries with high geopolitical risk. These limitations are amplified by external threats such as price volatility, state control of exports, and competition from substitute technologies. At the same time, opportunities are emerging linked to the circular economy, supply diversification, and European leadership in ESG regulations, which could position the EU as a benchmark in sustainable energy storage value chains.

Table 8.
SWOT.
The CAME framework (Table 9) translates the structural paradox identified in the SWOT analysis (Table 8) into a unified strategic action plan. This rigorous integration confirms that a resilient strategy must prioritize a combined approach of recycling, international diversification, and technological leadership over exclusive reliance on scarce primary mining. Specifically, the plan outlines that the EU must: (i) Correct critical Weaknesses (D1, D2) by investing in domestic processing and strategic reserves (C1, C2); (ii) Adapt to geopolitical risks (A1) and price volatility (A2) through bilateral upstream partnerships (Af1, E3); (iii) Maintain EU leadership in R&D and green standards (F1, F2, M1) as a competitive advantage; and (iv) Exploit the circular economy potential (O2) by accelerating recycling projects (E1) and focusing VRFB adoption in superior niche markets (Af3).

Table 9.
CAME.
Vanadium thus becomes not only a challenge for security of supply, but also an opportunity for the EU to demonstrate its ability to integrate sustainability, innovation and industrial sovereignty within the framework of the Green Deal.
4. Discussion & Policy Recommendations
The combination of SWOT analysis and the CAME matrix allows for the design of strategic alternatives aimed at strengthening the resilience of the vanadium value chain in the European Union, in line with the objectives of the European Green Deal and the Critical Raw Materials Act (ECRMA). The resulting proposals are structured around four key areas: (i) regulatory framework and governance, (ii) diversification of supply, (iii) capacity building and technological innovation in metallurgy and storage, and (iv) demand management.
4.1. Regulatory Framework and Governance
A first strategic option is to simplify and accelerate the implementation of the ECRMA, removing administrative redundancies that in practice delay the execution of critical projects [188]. A more agile regulatory framework would allow resources to be redirected towards vanadium exploration and processing, while also ensuring consistency with national and supranational legislation. However, legal barriers such as Law 7/2021 on Climate Change and Energy Transition, enacted by a member country such as Spain, show how environmental regulations can conflict with the exploration of surface deposits or sandstones containing uranium by prohibiting the granting of new permits for radioactive minerals. To resolve this dilemma, it is recommended to promote regulatory harmonisation that combines ecological protection guarantees with specific legal mechanisms that favour critical mineral projects, so that both environmental safety and security of supply are safeguarded.
4.2. Diversification of Supply
Reducing European vulnerability to the supply of vanadium and other critical minerals requires a two-pronged strategy: on the one hand, geographical diversification through long-term bilateral agreements with stable countries such as Australia, Canada or Brazil, reducing exposure to dominant suppliers such as China, Russia or South Africa; and on the other hand, internal regulatory harmonisation, capable of balancing green transition objectives with security of supply needs. Recent literature highlights that the acceleration of the energy transition intensifies tensions between environmental policies and the mining of critical minerals, requiring strategic priority clauses in environmental laws, integrated impact assessments that include geostrategic risks, and robust ecological compensation mechanisms [23,188,189,190]. Only a balanced framework will ensure both the protection of ecosystems and the security of vanadium supply in the EU.
To validate the feasibility of these selective upstream partnerships (Table 9 CAME Af1 and E3), specific mechanisms must be analyzed, moving beyond conceptual declarations toward verifiable policy alignment and financial commitments. The EU’s strategy of forging alliances with stable, like-minded jurisdictions is formally established through existing bilateral agreements.
For instance, the Canada-EU Strategic Partnership on Raw Materials serves as a core bilateral instrument. This partnership aims to enhance the value, security, and sustainability of trade and investment across the critical minerals value chains. These formalized agreements provide the operational and legal structure necessary for securing supply, directly addressing the reviewer’s concern regarding the lack of case support for cooperation.
The viability of this upstream cooperation is supported by the strategic interests of these allies. Canada recognizes vanadium as a mineral with “notable prospects for the future” and explicitly targets increasing exports for allies while committing significant governmental support, such as the $1.5 billion Strategic Innovation Fund, to accelerate processing and advanced manufacturing projects. This commitment to integrating upstream value chains (exploration, extraction, and processing) within allied nations provides a concrete pathway for the long-term supply agreements sought by the EU. This directly mitigates the EU’s weakness in primary processing capacity by fostering mutually beneficial investments.
Furthermore, the “selective” nature of this cooperation is defined by adherence to stringent standards, implicitly establishing criteria for comparison with global models. Canada anchors its mineral strategy in complying with the highest Environmental, Social, and Governance (ESG) standards, with explicit collaboration with the EU to advance world-class ESG criteria and standards internationally. This focus on Responsible Business Conduct and secure, ethical sourcing strengthens the resilience of the supply chain against geopolitical and ethical risks (A1—Table 8). Aligning with partners like Canada and Australia (a listed priority partner included in comparative analysis O4—Table 9) that prioritize security and high ESG performance provides the foundational support for the viability of the EU’s strategy [191].
4.3. Capacity Building and Technological Innovation in Metallurgy and Storage
Technological innovation in metallurgy and storage represents a strategic way to strengthen European autonomy in vanadium. On the one hand, the recovery of V from steel slag and fossil fuel ash is a secondary source with high potential, where the optimisation of metallurgical processes can complement the primary exploitation of titanomagnetites and multiply the competitiveness of European industry [188,192]. Funding for green metallurgy—low-energy consumption processes with a smaller environmental footprint, such as direct electrolysis or the use of alternative reducing agents—is essential for reducing costs and emissions in the production of V, Ti and Fe. On the other hand, in the field of energy storage, the recovery of vanadium from redox flow batteries (VRFBs) opens up a circular economy cycle in a growing sector. At the same time, research into alternative RFB chemistries (e.g., Fe2+/Fe3+ pairs) can diversify the technological offering, reducing exclusive dependence on V and favouring the deployment of more accessible and scalable systems in the EU [158,193]. This dual focus—waste recovery and technological diversification—would make it possible to combine security of supply, environmental sustainability and European industrial leadership in the storage value chain.
4.4. Demand Management and Controlled Stimulation
The adoption of vanadium redox flow batteries (VRFBs) in large-scale storage projects should be selectively encouraged, prioritising those applications in which their long service life, high electrochemical stability and high cycling capacity constitute differential advantages over alternative technologies, such as Li-ion, Na-ion or seasonal storage using H2. The promotion of targeted and controlled demand would not only optimise the technological fit for each use case, but also consolidate a stable domestic market capable of sustaining sustained investment in R&D, economies of scale in production and the development of specialised industrial capabilities at European level.
5. Conclusions
This study provides a comprehensive strategic analysis of the vanadium market within the framework of the European Union’s green transition. The results highlight a structural imbalance between the EU’s regulatory and technological strengths and its critical vulnerabilities in supply security.
Quantitatively, more than 85% of global vanadium demand remains concentrated in the steel sector, primarily for microalloyed steels containing 0.03–0.10% V by weight, while less than 2% currently corresponds to vanadium redox flow batteries (VRFBs). However, projections by the USGS indicate that VRFBs could represent up to 17% of global demand by 2030, implying an additional requirement of 15,000–20,000 tonnes of V2O5 per year. This prospective demand growth reinforces the need for a secure and diversified European supply base.
At the same time, the geographical concentration of production (≈90% in China, Russia, and South Africa) results in a Herfindahl–Hirschman Index (HHI) of around 5000, evidencing an oligopolistic and high-risk market structure. The EU currently lacks active vanadium mines, primary refining plants, and strategic reserves, relying almost entirely on imports of intermediate products such as V2O5 and ferrovanadium.
Nevertheless, the internal analysis identifies significant strengths: Europe leads in VRFB R&D, green metallurgy, and regulatory innovation (Critical Raw Materials Act, Net-Zero Industry Act). Projects such as the Vanadium Recovery Project in Finland demonstrate the feasibility of recovering vanadium from steel slag with yields above 70–80%, aligning industrial circularity with the objectives of the EU Green Deal. However, the End-of-Life Recycling Input Rate (EOL-RIR) of only 6% underscores the current gap between potential and industrial implementation.
The integrated SWOT–CAME assessment suggests four strategic lines of action:
- Correct the absence of primary production by reinforcing exploration in the Fennoscandian Shield and establishing strategic partnerships with Canada and Australia, which together hold over 45% of identified global reserves.
- Adapt regulatory frameworks to reconcile environmental protection with critical raw material security, accelerating permitting processes under the ECRMA.
- Maintain European leadership in VRFB innovation and green processing technologies, expanding pilot projects in electrolyte recycling and low-carbon metallurgy.
- Exploit opportunities for circularity by promoting industrial recovery of vanadium from slag, catalysts, and VRFB electrolytes, which could together supply up to 25–30% of EU demand by 2040 if properly scaled.
In conclusion, achieving strategic autonomy in vanadium requires a shift from dependence on high-risk external suppliers to a diversified, circular, and innovation-driven model. By combining recycling, upstream cooperation, and technological specialization, the EU can reduce exposure to market volatility—where V2O5 prices have historically fluctuated between 7 and 30 USD/kg—and ensure long-term competitiveness in long-duration energy storage.
Ultimately, vanadium’s strategic management represents not merely a materials policy challenge but a quantifiable opportunity to enhance Europe’s industrial resilience, climate neutrality, and technological sovereignty in the global clean energy economy.
Author Contributions
Conceptualization, I.J.S. and G.L.-C.; methodology, I.J.S.; formal analysis, I.J.S. and G.L.-C.; investigation, I.J.S. and G.L.-C.; resources, I.J.S.; data curation, I.J.S.; writing—original draft preparation, I.J.S. and G.L.-C.; writing—review and editing, I.J.S., G.L.-C. and E.G.-O.; visualization, I.J.S. and G.L.-C.; supervision, E.G.-O. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CAPEX | Capital Expenditure |
| CAME | Correct, Adapt, Maintain, Explore (strategic follow-up matrix to SWOT) |
| CRM | Critical Raw Material |
| SWOT | Strengths, Weaknesses, Opportunities, Threats |
| DOE | U.S. Department of Energy |
| EC | European Commission |
| ECHA | European Chemicals Agency |
| EIA | Energy Information Administration |
| EU | European Union |
| FeV | Ferrovanadium |
| GDP | Gross Domestic Product |
| HSLA | High-Strength Low-Alloy (steel) |
| IEA | International Energy Agency |
| IRENA | International Renewable Energy Agency |
| LCOS | Levelized Cost of Storage |
| LDES | Long-Duration Energy Storage |
| MCS | Mineral Commodity Summaries (USGS) |
| OPEX | Operating Expenditure |
| PESTEL | Political, Economic, Social, Technological, Environmental, and Legal analysis |
| R&D | Research and Development |
| SDES | Short-Duration Energy Storage |
| UNFC | United Nations Framework Classification for Resources |
| USGS | United States Geological Survey |
| V2O5 | Vanadium Pentoxide |
| VFB | Vanadium Flow Battery |
| VRFB | Vanadium Redox Flow Battery |
| VTM | Vanadiferous Titanomagnetite |
References
- Albertus, P.; Manser, J.S.; Litzelman, S. Long-duration electricity storage applications, economics, and technologies. Joule 2020, 4, 21–32. [Google Scholar] [CrossRef]
- Ciotola, A.; Fuss, M.; Colombo, S.; Poganietz, W.-R. The potential supply risk of vanadium for the renewable energy transition in Germany. J. Energy Storage 2021, 33, 102094. [Google Scholar] [CrossRef]
- Iwakiri, I.; Antunes, T.; Almeida, H.; Sousa, J.P.; Figueira, R.B.; Mendes, A. Redox Flow Batteries: Materials, Design and Prospects. Energies 2021, 14, 5643. [Google Scholar] [CrossRef]
- Chen, S.; Sun, C.; Zhang, H.; Yu, H.; Wang, W. Electrochemical Deposition of Bismuth on Graphite Felt Electrodes: Influence on Negative Half-Cell Reactions in Vanadium Redox Flow Batteries. Appl. Sci. 2024, 14, 3316. [Google Scholar] [CrossRef]
- Choi, C.; Kim, S.; Kim, R.; Choi, Y.; Kim, S.; Jung, H.; Hoon Yang, J.; Kim, H. A review of vanadium electrolytes for vanadium redox flow batteries. Renew. Sustain. Energy Rev. 2017, 69, 263–274. [Google Scholar] [CrossRef]
- Sánchez-Díez, E.; Ventosa, E.; Guarnieri, M.; Trovò, A.; Flox, C.; Marcilla, R.; Soave, F.; Mazur, P.; Aranzabe, E.; Ferret, R. Redox flow batteries: Status and perspective towards sustainable stationary energy storage. J. Power Sources 2021, 481, 228804. [Google Scholar] [CrossRef]
- Rogers, B.L.; Banerjee, S. Mine the gap: Sourcing vanadium for the energy transition. Joule 2025, 9, 102139. [Google Scholar] [CrossRef]
- Huang, Y.; Long, Z.; Wang, L.; He, P.; Zhang, G.; Hughes, S.; Yu, H.; Huang, F. Fingerprinting vanadium in soils based on speciation characteristics and isotope compositions. Sci. Total Environ. 2021, 791, 148240. [Google Scholar] [CrossRef]
- Mitali, J.; Dhinakaran, S.; Mohamad, A. Energy storage systems: A review. Energy Storage Sav. 2022, 1, 166–216. [Google Scholar] [CrossRef]
- Amir, M.; Deshmukh, R.; Khalid, H.; Said, Z.; Raza, A.; Muyeen, S.M.; Nizami, A.; Madurai Elavarasan, R.; Saidur, R.; Sopian, K. Energy storage technologies: An integrated survey of developments, global economical/environmental effects, optimal scheduling model, and sustainable adaption policies. J. Energy Storage 2023, 72, 108694. [Google Scholar] [CrossRef]
- Padamurthy, A.; Vatala, M.; Gandla, P.K.; Sheelwant, A.; Vemanaboina, H. A review on energy storage technologies: Current trends and future opportunities. J. Phys. Conf. Ser. 2024, 2765, 012007. [Google Scholar] [CrossRef]
- Schmidt, O.; Melchior, S.; Hawkes, A.; Staffell, I. Projecting the Future Levelized Cost of Electricity Storage Technologies. Joule 2019, 3, 81–100. [Google Scholar] [CrossRef]
- Mauler, L.; Lou, X.; Duffner, F.; Leker, J. Technological innovation vs. tightening raw material markets: Falling battery costs put at risk. Energy Adv. 2022, 1, 136–145. [Google Scholar] [CrossRef]
- Righetti, E.; Rizos, V. Reducing Supply Risks for Critical Raw Materials: Evidence and Policy Options. 2024. Available online: https://circulareconomy.europa.eu/platform/sites/default/files/2024-01/CEPS-InDepthAnalysis-2024-01_Reducing-supply-risks-for-critical-raw-materials.pdf (accessed on 18 April 2025).
- Schlosser, S.J.; Naegler, T. Raw material risk in clean energy technologies and the power supply system: For which materials should price fluctuations be prioritised? Energy Rep. 2025, 13, 4359–4374. [Google Scholar] [CrossRef]
- USGS. Mineral Commodity Summaries 2024; Report 2024; U.S. Geological Survey: Reston, VA, USA, 2024. [CrossRef]
- USGS. Vanadium Datasheet 2025; U.S. Geological Survey: Reston, VA, USA, 2025.
- Budinis, S.; Levi, P.; Mandová, H.; Vass, T.; Pales, A.F.; Gül, T. Iron and Steel Technology Roadmap: Towards More Sustainable Steelmaking; International Energy Agency (IEA): Paris, France, 2020; Available online: https://www.iea.org/reports/iron-and-steel-technology-roadmap (accessed on 18 April 2025).
- IEA. Batteries and Secure Energy Transitions; International Energy Agency (IEA): Paris, France, 2024; Available online: https://www.iea.org/reports/batteries-and-secure-energy-transitions (accessed on 29 April 2025).
- IEA. Energy Storage. In Annual Report 2023; International Energy Agency (IEA): Paris, France, 2023. [Google Scholar]
- International Energy Agency. The Role of Critical Minerals in Clean Energy Transitions; IEA: Paris, France, 2021; Available online: https://www.iea.org/reports/the-role-of-critical-minerals-in-clean-energy-transitions (accessed on 21 June 2025).
- Roskill-Vanadium: Outlook to 2030; Wood Mackenzie: Edinburgh, UK, 2021; Available online: https://www.woodmac.com/reports/metals-roskill-vanadium-outlook-to-2030-545713/ (accessed on 5 July 2025).
- European Commission. Critical Raw Materials Act (CRMA); European Commission: Brussels, Belgium, 2023; Available online: https://single-market-economy.ec.europa.eu/publications/critical-raw-materials-act_en (accessed on 19 July 2025).
- Porter, M.E. The structure within industries and companies’ performance. Rev. Econ. Stat. 1979, 61, 214–227. [Google Scholar] [CrossRef]
- Brandenburger, A.; Nalebuff, B. Coopetition: Kooperativ konkurrieren. Mit der Spieltheorie zum Unternehmenserfolg. In Das Summa Summarum des Management; Boersch, C., Elschen, R., Eds.; Gabler: Wiesbaden, Germany, 2007. [Google Scholar] [CrossRef]
- Freeman, R.B. Longitudinal analyses of the effects of trade unions. J. Labor. Econ. 1984, 2, 1–26. [Google Scholar] [CrossRef]
- Porter, M.E. The Competitive Advantage: Creating and Sustaining Superior Performance; Free Press: New York, NY, USA, 1985; Available online: https://www.hbs.edu/faculty/Pages/item.aspx?num=193 (accessed on 18 April 2025).
- Schlesinger, W.H.; Klein, E.M.; Vengosh, A. Global biogeochemical cycle of vanadium. Proc. Natl. Acad. Sci. USA 2017, 114, E11092–E11100. [Google Scholar] [CrossRef]
- Imtiaz, M.; Shahid Rizwan, M.; Xiong, S.; Li, H.; Ashraf, M.; Shahzad, S.; Shahzad, M.; Rizwan, M.; Tu, S. Vanadium, recent advancements and research prospects: A review. Environ. Int. 2015, 80, 79–88. [Google Scholar] [CrossRef]
- Ibrahim, A.H.; Lyu, X.; Sharafeldin, H.E.; ElDeeb, A.B. Eco-Friendly and Complex Processing of Vanadium-Bearing Waste for Effective Extraction of Valuable Metals and Other By-Products: A Critical Review. Recycling 2025, 10, 6. [Google Scholar] [CrossRef]
- Cappuyns, V.; Slabbinck, E. Occurrence of Vanadium in Belgian and European Alluvial Soils. Appl. Environ. Soil. Sci. 2012, 2012, 979501. [Google Scholar] [CrossRef]
- Brough, C.; Bowell, R.; Larkin, J. Chapter 4—The geology of vanadium deposits. In An Introduction to Vanadium: Chemistry, Occurrence and Applications; Nova Science Pub Inc.: Hauppauge, NY, USA, 2019; pp. 87–117. ISBN 978-1-53616-119-9. [Google Scholar]
- Evans, H.; White, J. The colorful vanadium minerals: A brief review and a new classification. Mineral. Rec. 1987, 18, 333–340. [Google Scholar]
- Gao, F.; Uthmon Olayiwola, A.; Liu, B.; Wang, S.; Du, H.; Li, J.; Wang, X.; Chen, D.; Zhang, Y. Review of Vanadium Production Part I: Primary Resources. Miner. Process. Extr. Metall. Rev. 2021, 43, 466–488. [Google Scholar] [CrossRef]
- Moskalyk, R.R.; Alfantazi, A.M. Processing of vanadium: A review. Miner. Eng. 2003, 16, 793–805. [Google Scholar] [CrossRef]
- Xu, B.; Jiang, W.; Dai, Z.; Yao, L.; Yang, L.; Zheng, J. Advancements and challenges in vanadium extraction processes from vanadium titano-magnetite and its derivatives. Resour. Conserv. Recycl. 2025, 216, 108178. [Google Scholar] [CrossRef]
- Kelley, K.; Scott, C.; Polyak, D.; Kimball, B. Vanadium; Report 1802U; U.S. Geological Survey: Reston, VA, USA, 2017. [CrossRef]
- Simandl, G.J.; Paradis, S. Vanadium as a critical material: Economic geology with emphasis on market and the main deposit types. Appl. Earth Sci. 2022, 131, 218–236. [Google Scholar] [CrossRef]
- Boni, M.; Bouabdellah, M.; Boukirou, W.; Putzolu, F.; Mondillo, N. Vanadium ore resources of the African continent: State of the Art. Ore Geol. Rev. 2023, 157, 105423. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, D.; Liu, W.; Sun, Z.; Zhang, B.; He, R. Global vanadium industry development report 2021. Iron Steel Vanadium Titan 2022, 43, 1–9. [Google Scholar]
- Yang, Y.; Hui, B.; Yan, S.; Chen, C.; Deng, J. Overview of global Vanadium-titanium magnetite resources and comprehensive utilization. Multipurp. Util. Miner. Resour. 2023, 4, 1–11. [Google Scholar]
- Liang, J. Discussion on Oxidation and Roasting Process of Vanadium Extraction from Vanadium Titanium Magnetite. Guangxi Chem. Technol. 1975, 4, 46–56. [Google Scholar]
- Maier, W.D.; Barnes, S.-J.; Groves, D. The Bushveld Complex, South Africa: Formation of platinum–palladium, chrome-and vanadium-rich layers via hydrodynamic sorting of a mobilized cumulate slurry in a large, relatively slowly cooling, subsiding magma chamber. Miner. Depos. 2013, 48, 1–56. [Google Scholar] [CrossRef]
- Bruneton, P.; Cuney, M. Geology of uranium deposits. In Uranium for Nuclear Power; Hore-Lacy, I., Ed.; Woodhead Publishing: Cambridge, UK, 2016; pp. 11–52. [Google Scholar] [CrossRef]
- IAEA. Unconformity-Related Uranium Deposits; IAEA: Vienna, Austria, 2018; Available online: https://public.ebookcentral.proquest.com/choice/publicfullrecord.aspx?p=5722807 (accessed on 6 January 2022).
- Hou, B.; Keeling, J.; Li, Z. Paleovalley-related uranium deposits in Australia and China: A review of geological and exploration models and methods. Ore Geol. Rev. 2017, 88, 201–234. [Google Scholar] [CrossRef]
- Hou, B.; Michaelsen, B.; Li, Z.; Keeling, J.; Fabris, A. Paleovalley-related uranium: Exploration criteria and case-studies from Australia and China. Episodes 2014, 37, 150–171. [Google Scholar] [CrossRef]
- Hall, S.M.; Van Gosen, B.S.; Paces, J.B.; Zielinski, R.A.; Breit, G.N. Calcrete uranium deposits in the Southern High Plains, USA. Ore Geol. Rev. 2019, 109, 50–78. [Google Scholar] [CrossRef]
- Bowell, R.J.; Grogan, J.; Hutton-Ashkenny, M.; Brough, C.; Penman, K.; Sapsford, D.J. Geometallurgy of uranium deposits. Miner. Eng. 2011, 24, 1305–1313. [Google Scholar] [CrossRef]
- Pownceby, M.I.; Johnson, C. Geometallurgy of Australian uranium deposits. Ore Geol. Rev. 2014, 56, 25–44. [Google Scholar] [CrossRef]
- Liu, S.; Wang, L.; Chen, J.; Ye, L.; Du, J. Research progress of vanadium extraction processes from vanadium slag: A review. Sep. Purif. Technol. 2024, 342, 127035. [Google Scholar] [CrossRef]
- NEA; IAEA. Uranium 2022: Resources, Production and Demand; OECD: Paris, France, 2023. [Google Scholar] [CrossRef]
- Dahlkamp, F.J. Uranium Deposits of the World: USA and Latin America; Springer Science & Business Media: New York, NY, USA, 2010. [Google Scholar]
- European Commission. Critical Raw Materials for the EU; European Commission: Brussels, Belgium, 2011. [Google Scholar]
- Erdmann, L.; Graedel, T.E. Criticality of non-fuel minerals: A review of major approaches and analyses. Environ. Sci. Technol. 2011, 45, 7620–7630. [Google Scholar] [CrossRef]
- European Commission. Report on Critical Raw Materials for the EU; European Comission: Brussels, Belgium, 2014. [Google Scholar]
- Blengini, G.A.; Blagoeva, D.; Dewulf, J.; Torres de Matos, C.; Nita, V.; Vidal-Legaz, B.; Latunussa, C.; Kayam, Y.; Talens Peirò, L.; Baranzelli, C.E.L.; et al. Methodology for Establishing the EU List of Critical Raw Materials-Guidelines 1; European Union: Brussels, Belgium, 2017. [Google Scholar]
- Achzet, B.; Helbig, C. How to evaluate raw material supply risks—An overview. Resour. Policy 2013, 38, 435–447. [Google Scholar] [CrossRef]
- European Commission. Communication on the 2017 List of Critical Raw Materials for the EU. COM(2017) 490 Final; European Commission: Brussels, Belgium, 2017. [Google Scholar]
- European Commission. Study on the EU’s List of Critical Raw Materials; European Commission: Brussels, Belgium, 2020. [Google Scholar]
- European Commission. Proposal for a Regulation Establishing a Framework for Ensuring a Secure and Sustainable Supply of Critical Raw Materials (Critical Raw Materials Act); European Commission: Brussels, Belgium, 2023. [Google Scholar]
- European Commission: Directorate-General for Internal Market, Entrepreneurship and SMEs; Grohol, M.; Veeh, C. Study on the Critical Raw Materials for the EU 2023—Final Report; Publications Office of the European Union: Luxembourg, 2023. [Google Scholar] [CrossRef]
- Blue Sky Uranium Corp. Initial Mineral Resource Estimate for the Ivana Uranium-Vanadium Deposit, Amarillo Grande Project; Blue Sky Uranium Corp.: Río Negro, Argentina, 2018. [Google Scholar]
- Salguero, I.J. Comparación Metodológica en la Planificación a Largo Plazo de un Yacimiento Modelizado Mediante RECMIN. Master’s Thesis, University of Oviedo, Oviedo, Spain, 2024. [Google Scholar]
- Salguero, I.J.; Martín, R.A.; Álvarez, I.D.; Fernández, C.C. Análisis y optimización mediante RecMin y modelos ARIMA de un yacimiento de uranio a cielo abierto. In Proceedings of the XV International Congress on Energy and Mineral Resources, León, Spain, 22–24 November 2023; pp. 1–8. Available online: https://www.sendaeventos.com/qrns/741428/docs/8533027082023102525.pdf (accessed on 18 April 2025).
- Guo, H.; Bai, J.; Zhang, J.; Li, H. Mechanism of Strength Improvement of Magnetite Pellet by Adding Boron-bearing Iron Concentrate. J. Iron Steel Res. Int. 2014, 21, 9–15. [Google Scholar] [CrossRef]
- Zhu, X.; Li, W.; Guan, X. Vanadium extraction from titano-magnetite by hydrofluoric acid. Int. J. Miner. Process 2016, 157, 55–59. [Google Scholar] [CrossRef]
- Taylor, P.R.; Shuey, S.A.; Vidal, E.E.; Gomez, J.C. Extractive metallurgy of vanadium-containing titaniferous magnetite ores: A review. Min. Metall. Explor. 2006, 23, 80–86. [Google Scholar] [CrossRef]
- Fischer, R.P. Vanadium Resources in Titaniferous Magnetite Deposits; US Government Printing Office: Washington, DC, USA, 1975.
- Ballester, A.; Verdeja, L.; Sancho, J. Metalurgia Extractiva. Volumen 1: Fundamentos; Editorial Síntesis: Madrid, Spain, 2000; ISBN 9788477388029. [Google Scholar]
- Boutonnet, G. Concentrés d’Uranium; Techniques de l’Ingenieur: Saint-Denis, France, 1985; Available online: http://inis.iaea.org/search/search.aspx?orig_q=RN:17046769 (accessed on 2 August 2025).
- Gajda, D.; Kiegiel, K.; Zakrzewska-Koltuniewicz, G.; Chajduk, E.; Bartosiewicz, I.; Wolkowicz, S. Mineralogy and uranium leaching of ores from Triassic Peribaltic sandstones. J. Radioanal. Nucl. Chem. 2015, 303, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Michel, P. Production de concentrés d’uranium naturel. Génie Nucl 1997. [Google Scholar] [CrossRef]
- Keller, P.; Anderson, C. The Production of Critical Materials as By Products. Asp. Min. Miner. Sci. 2018, 2, 14. [Google Scholar] [CrossRef]
- Kumar, P.P.; Santos, D.A.; Braham, E.J.; Sellers, D.G.; Banerjee, S.; Dixit, M.K. Punching above its weight: Life cycle energy accounting and environmental assessment of vanadium microalloying in reinforcement bar steel. Environ. Sci. Process. Impacts 2021, 23, 275–290. [Google Scholar] [CrossRef]
- Barzigar, A.; Ebadati, E.; Mujumdar, A.S.; Hosseinalipour, S.M. A comprehensive review of vanadium redox flow batteries: Principles, benefits, and applications. Next Res. 2025, 2, 100767. [Google Scholar] [CrossRef]
- Olabi, A.G.; Allan, M.A.; Abdelkareem, M.A.; Deepa, T.D.; Alami, A.H.; Abbas, Q.; Alkhalidi, A.; Sayer, E.T. Redox Flow Batteries: Recent Development in Main Components, Emerging Technologies, Diagnostic Techniques, Large-Scale Applications, and Challenges and Barriers. Batteries 2023, 9, 409. [Google Scholar] [CrossRef]
- SCRREEN2 Consortium. SCRREEN2 Factsheet: Vanadium—Updated 2022; European Commission, Horizon 2020 Programme: Brussels, Belgium, 2022; Available online: https://scrreen.eu (accessed on 18 September 2025).
- Villalobos, J.C.; Del-Pozo, A.; Campillo, B.; Mayen, J.; Serna, S. Microalloyed Steels through History until 2018: Review of Chemical Composition, Processing and Hydrogen Service. Metals 2018, 8, 351. [Google Scholar] [CrossRef]
- González-Pociño, A.; Alvarez-Antolin, F.; Peral-Martinez, L.B. Effects of Nitriding and Thermal Processing on Wear and Corrosion Resistance of Vanadis 8 Steel. Coatings 2024, 14, 1066. [Google Scholar] [CrossRef]
- Hasanbeigi, A.; Springer, C.; Global, E. How Clean Is the U.S. Steel Industry? An International Benchmarking of Energy and CO2 Intensities. 2019. Available online: https://www.bluegreenalliance.org/wp-content/uploads/2021/04/HowCleanistheU.S.SteelIndustry.pdf (accessed on 18 April 2025).
- Leung, P.; Li, X.; De León, C.P.; Berlouis, L.; Low, C.J.; Walsh, F.C. Progress in redox flow batteries, remaining challenges and their applications in energy storage. Rsc Adv. 2012, 2, 10125–10156. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, J.; Kintnet-Meyer, M.C.W.; Lu, X.; Choi, D.; Lemmon, J.P.; Liu, J. Electrochemical energy storage for green grid. Chem. Rev. 2011, 111, 3577–3613. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, Q.; Xiong, S.; Song, Z. Research progress on optimized membranes for vanadium redox flow batteries. Inorg. Chem. Front. 2024, 11, 4049–4079. [Google Scholar] [CrossRef]
- Gandomi, Y.A.; Aaron, D.S.; Nolan, Z.B.; Ahmadi, A.; Mench, M.M. Direct Measurement of Crossover and Interfacial Resistance of Ion-Exchange Membranes in All-Vanadium Redox Flow Batteries. Membranes 2020, 10, 126. [Google Scholar] [CrossRef]
- Ebner, S.; Spirk, S.; Stern, T.; Mair-Bauernfeind, C. How Green are Redox Flow Batteries? ChemSusChem 2023, 16, e202201818. [Google Scholar] [CrossRef]
- Viswanathan, V.V.; Crawford, A.J.; Thomsen, E.C.; Shamim, N.; Li, G.; Huang, Q.; Reed, D.M. An Overview of the Design and Optimized Operation of Vanadium Redox Flow Batteries for Durations in the Range of 4–24 Hours. Batteries 2023, 9, 221. [Google Scholar] [CrossRef]
- Ghimire, P.C.; Bhattarai, A.; Lim, T.M.; Wai, N.; Skyllas-Kazacos, M.; Yan, Q. In-Situ Tools Used in Vanadium Redox Flow Battery Research—Review. Batteries 2021, 7, 53. [Google Scholar] [CrossRef]
- Polyak, D.E. Vanadium—2022 Minerals Yearbook (Advance Release); U.S. Department of the Interior: Washington, DC, USA; U.S. Geological Survey: Reston, VA, USA, 2022. Available online: https://www.usgs.gov (accessed on 28 September 2025).
- Shoaib, M.; Vallayil, P.; Jaiswal, N.; Vaigunda Suba, P.I.; Sankararaman, S.; Ramanujam, K.; Thangadurai, V. Advances in Redox Flow Batteries—A Comprehensive Review on Inorganic and Organic Electrolytes and Engineering Perspectives. Adv. Energy Mater. 2024, 14, 2400721. [Google Scholar] [CrossRef]
- Huan, Z.; Sun, C.; Ge, M. Progress in Profitable Fe-Based Flow Batteries for Broad-Scale Energy Storage. WIREs Energy Environ. 2024, 13, e541. [Google Scholar] [CrossRef]
- Bank, T.W. Vanadium Market Outlook to 2035; International Bank for Reconstruction and Development/The World Bank: London, UK, 2023. [Google Scholar]
- Sprenkle, V.; Li, B.; Zhang, L.; Robertson, L.A. Technology Strategy Assessment Findings from Storage Innovations 2030 Flow Batteries; DOE: Petaling Jaya, Malaysia, 2023.
- Cole, W.; Karmakar, A. Cost Projections for Utility-Scale Battery Storage: 2023 Update. National Renewable Energy Laboratory, Technical Report 2023. Available online: https://docs.nrel.gov/docs/fy23osti/85332.pdf (accessed on 1 October 2025).
- Cao, L.; Skyllas-Kazacos, M.; Menictas, C.; Noack, J. A review of electrolyte additives and impurities in vanadium redox flow batteries. J. Energy Chem. 2018, 27, 1269–1291. [Google Scholar] [CrossRef]
- Lourenssen, K.; Williams, J.; Ahmadpour, F.; Clemmer, R.; Tasnim, S. Vanadium redox flow batteries: A comprehensive review. J. Energy Storage 2019, 25, 100844. [Google Scholar] [CrossRef]
- Harper, G.; Sommerville, R.; Kendrick, E.; Driscoll, L.; Slater, P.; Stolking, R.; Walton, A.; Christensen, P.; Heidrich, O.; Lmbert, S.; et al. Recycling lithium-ion batteries from electric vehicles. Nature 2019, 575, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Al Amayreh, H.H.; Khalaf, A.; Hawwari, M.I.; Hourani, M.K.; Al Bawab, A. The Recovery of Vanadium Pentoxide (V2O5 from Spent Catalyst Utilized in a Sulfuric Acid Production Plant in Jordan. Materials 2023, 16, 6503. [Google Scholar] [CrossRef] [PubMed]
- Romanovskaia, E.; Romanovski, V.; Kwapinski, W.; Kurilo, I. Selective recovery of vanadium pentoxide from spent catalysts of sulfuric acid production: Sustainable approach. Hydrometallurgy 2021, 200, 105568. [Google Scholar] [CrossRef]
- Painuly, A.S. Separation and recovery of vanadium from spent vanadium pentaoxide catalyst by Cyanex 272. Environ. Syst. Res. 2015, 4, 7. [Google Scholar] [CrossRef]
- Shyrokykh, T.; Neubert, L.; Volkova, O.; Sridhar, S. Two Potential Ways of Vanadium Extraction from Thin Film Steelmaking Slags. Processes 2023, 11, 1646. [Google Scholar] [CrossRef]
- Yang, M.; Yang, J. Vanadium extraction from steel slag: Generation, recycling and management. Environ. Pollut. 2024, 343, 123126. [Google Scholar] [CrossRef]
- EIT Raw Material. Win-Win for Europe: Mineral Processing Facility in Finland Set to Increase Vanadium Production for Europe. Available online: https://eitrawmaterials.eu/press-releases/win-win-europe-mineral-processing-facility-finland-set-increase-vanadium-production?utm_source=chatgpt.com (accessed on 1 October 2025).
- Zhang, Y.; Bo, W.; Zheng, Q.; Liu, T.; Xue, N.; Hu, P.; Liu, H. A review of processing and utilization technology for vanadium shale resources. Sep. Purif. Technol. 2025, 379, 135185. [Google Scholar] [CrossRef]
- OEC. Sulfuric Acid. Available online: https://oec.world/en/profile/hs/sulfuric-acid (accessed on 1 October 2025).
- Sorrels, J.L.; Randall, D.D.; Schaffner, K.S.; Fry, C.R. Selective Catalytic Reduction; United States Environmental Protection Agency (EPA): Research Triangle Park, NC, USA, 2019. Available online: https://www.epa.gov/sites/default/files/2017-12/documents/scrcostmanualchapter7thedition_2016revisions2017.pdf (accessed on 18 April 2025).
- Ye, B.; Jeong, B.; Lee, M.; Kim, T.H.; Park, S.; Jung, J.; Lee, S.; Kim, H. Recent trends in vanadium-based SCR catalysts for NOx reduction in industrial applications: Stationary sources. Nano Converg. 2022, 9, 51. [Google Scholar] [CrossRef]
- Ferella, F. A review on management and recycling of spent selective catalytic reduction catalysts. J. Clean. Prod. 2020, 246, 118990. [Google Scholar] [CrossRef]
- Qian, X.; Ao, W.; Ding, H.; Wang, X.; Sun, S. A Review on Resource Utilization of Spent V-W-Ti Based Selective Catalytic Reduction Catalysts. Materials 2022, 15, 7984. [Google Scholar] [CrossRef] [PubMed]
- Lütjering, G.; Williams, J. Titanium Engineering Materials and Processes; Springer: New York, NY, USA, 2007. [Google Scholar]
- Benmessaoud, F.; Cheikh, M.; Vidal, V.; Matsumoto, H.; Velay, V. Experimental study and modeling of the microstructural effects on the mechanical behavior of Ti-6Al-4V titanium alloy. Mech. Res. Commun. 2025, 148, 104473. [Google Scholar] [CrossRef]
- Lin, Y.; Yuan, S.; Sun, C.; Yuan, J.; Zhang, M.; Liu, Y.; Zhang, Z.; Chu, X. Research on additive manufacturing of Ti-6Al-4V material based on digital light processing technology. Mater. Sci. Eng. A 2025, 945, 148973. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, C.; Ruan, J.; Omori, T.; Kainuma, R.; Ishida, K.; Liu, X. High-strength Co–Al–V-base superalloys strengthened by γ′-Co3(Al,V) with high solvus temperature. Acta Mater. 2019, 170, 62–74. [Google Scholar] [CrossRef]
- Hu, Q.; Xu, Y.; Deng, X.; Hu, S.; Xu, J.; Zhou, F.; Chi, R. Effect of Potassium Salt on Swelling of Halloysite Clay Mineral during Leaching Process of Ionic Rare Earth Ore. Minerals 2023, 13, 906. [Google Scholar] [CrossRef]
- Jeong, J.; Aetukuri, N.; Graf, T.; Schladt, T.D.; Samant, M.G.; Parkin, S.S.P. Suppression of Metal-Insulator Transition in VO2 by Electric Field–Induced Oxygen Vacancy Formation. Science 2013, 339, 1402–1405. [Google Scholar] [CrossRef]
- Gilligan, R.; Nikoloski, A.N. The extraction of uranium from brannerite—A literature review. Miner. Eng. 2015, 71, 34–48. [Google Scholar] [CrossRef]
- Rodby, K.; Jaffe, R.; Olivetti, E.; Brushett, F. Materials availability and supply chain considerations for vanadium in grid-sacle redox flow batteries. J. of Power Sources 2023, 560. [Google Scholar] [CrossRef]
- World Steel Association. World Steel in Figures. 2025. Available online: https://worldsteel.org/data/world-steel-in-figures/world-steel-in-figures-2025/ (accessed on 27 August 2025).
- USGS. Vanadium Data Sheet; USGS: New York, NY, USA, 2020.
- USGS. Vanadium Datasheet 2023; USGS: New York, NY, USA, 2023.
- Hund, K.; La Porta, D.; Fabregas, T.P.; Laing, T.; Drexhage, J. Minerals for Climate Action; World Bank: Washington, DC, USA, 2020. [Google Scholar] [CrossRef]
- USGS. Mineral Commodity Summaries: Vanadium; U.S. Geological Survey: Reston, VA, USA, 2025. Available online: https://pubs.usgs.gov/periodicals/mcs2025/mcs2025-vanadium.pdf (accessed on 27 August 2025).
- Renner, S.; Friedrich-W, W. Volatility drivers on the metal market and exposure of producing countries. Miner. Econ. 2020, 33, 311–340. [Google Scholar] [CrossRef]
- Segreto, M.; Principe, L.; Desormeaux, A.; Torre, M.; Tomassetti, L.; Tratzi, P.; Paolini, V.; Petracchini, F. Trends in social acceptance of renewable energy across Europe—A literature review. Int. J. Environ. Res. Public. Health 2020, 17, 9161. [Google Scholar] [CrossRef]
- Watt, J.A.J.; Burke, I.T.; Edwards, R.A.; Malcolm, H.M.; Mayes, W.M.; Olszewska, J.P.; Pan, G.; Graham, M.C.; Heal, K.V.; Rose, N.L.; et al. Vanadium: A Re-Emerging Environmental Hazard. Environ. Sci. Technol. 2018, 52, 11973–11974. [Google Scholar] [CrossRef] [PubMed]
- Darling, R.M. Techno-economic analyses of several redox flow batteries using levelized cost of energy storage. Curr. Opin. Chem. Eng. 2022, 37, 100855. [Google Scholar] [CrossRef]
- Poli, N.; Bonaldo, C.; Moretto, M.; Guarnieri, M. Techno-economic assessment of future vanadium flow batteries based on real device/market parameters. Appl. Energy 2024, 362, 122954. [Google Scholar] [CrossRef]
- Lesser, P.; Gugerell, K.; Poelzer, G.; Hitch, M.; Tost, M. European mining and the social license to operate. Extr. Ind. Soc. 2021, 8, 100787. [Google Scholar] [CrossRef]
- Selänniemi, A.; Hellström, M.; Björklund-Sänkiaho, M. Long-Duration Energy Storage—A Literature Review on the Link between Variable Renewable Energy Penetration and Market Creation. Energies 2024, 17, 3779. [Google Scholar] [CrossRef]
- Agyekum, E.B.; Abdullah, M.; Odoi-Yorke, F.; Ameen, A.; Chowdhury, P.; Raza, M.A.; Rashid, F.L.; Hussein, A.K. A state-of-the-art review of electrolyte systems for vanadium redox flow battery—Status of the technology, and future research directions. Energy Convers. Manag. X 2025, 27, 101180. [Google Scholar] [CrossRef]
- Blume, N.; Turek, T.; Minke, C. Sustainability Development of Stationary Batteries: A Circular Economy Approach for Vanadium Flow Batteries. Batteries 2024, 10, 240. [Google Scholar] [CrossRef]
- Zuo, Y.; Fu, W.; Leung, P.; Wondimu, T.H.; Mohamend, M.R.; Flox, C.; Shah, A.A.; Xu, Q.; Liao, Q. Sustainable recycling and regeneration of redox flow battery components. Future Batter. 2025, 5, 100044. [Google Scholar] [CrossRef]
- Weber, S.; Peters, J.F.; Baumann, M.; Weil, M. Life Cycle Assessment of a Vanadium Redox Flow Battery. Environ. Sci. Technol. 2018, 52, 10864–10873. [Google Scholar] [CrossRef]
- Drobe, M.; Haubrich, F.; Gajardo, M.; Marbler, H. Processing Tests, Adjusted Cost Models and the Economies of Reprocessing Copper Mine Tailings in Chile. Metals 2021, 11, 103. [Google Scholar] [CrossRef]
- Spandagos, C.; Reaños, M.A.T.; Lynch, M.Á. Public acceptance of sustainable energy innovations in the European Union: A multidimensional comparative framework for national policy. J. Clean. Prod. 2022, 340, 130721. [Google Scholar] [CrossRef]
- Tian, W.; Du, H.; Wang, J.; Weigand, J.J.; Qu, J.; Wang, S.; Li, L. A Review of Electrolyte Additives in Vanadium Redox Flow Batteries. Materials 2023, 16, 4582. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, N.B.; Bezerra, A.K.; Neto, J.M.M.; Silva, E.A. Mining Law: In Search of Sustainable Mining. Sustainability 2021, 13, 867. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhou, T.; Wang, T.; Zhang, M.; Zhang, S.; Yang, H. Long-Duration Energy Storage: A Critical Enabler for Renewable Integration and Decarbonization. Energies 2025, 18, 466. [Google Scholar] [CrossRef]
- Brambati, F.; Ruscio, D.; Biassoni, F.; Hueting, R.; Tedeschi, A. Predicting acceptance and adoption of renewable energy community solutions: The prosumer psychology. Open Res. Eur. 2022, 2, 115. [Google Scholar] [CrossRef]
- Bača, P.; Libich, J.; Gazdošová, S.; Polkorab, J. Sodium-Ion Batteries: Applications and Properties. Batteries 2025, 11, 61. [Google Scholar] [CrossRef]
- Mahmood, A.; Zheng, Z.; Chen, Y. Zinc–Bromine Batteries: Challenges, Prospective Solutions, and Future. Adv. Sci. 2024, 11, 2305561. [Google Scholar] [CrossRef]
- Phogat, P.; Dey, S.; Wan, M. Comprehensive review of Sodium-Ion Batteries: Principles, Materials, Performance, Challenges, and future Perspectives. Mater. Sci. Eng. B 2025, 312, 117870. [Google Scholar] [CrossRef]
- Zhang, Y.-Q.; Wang, G.-X.; Liu, R.-Y.; Wang, T.-H. Operational Parameter Analysis and Performance Optimization of Zinc–Bromine Redox Flow Battery. Energies 2023, 16, 3043. [Google Scholar] [CrossRef]
- Lewicka, E.; Guzik, K.; Galos, K. On the Possibilities of Critical Raw Materials Production from the EU’s Primary Sources. Resources 2021, 10, 50. [Google Scholar] [CrossRef]
- McCaffrey, C.; Poitiers, N. Making Industrial Policy Work: A Case Study on the European Battery Alliance Academy. Bruegel Working Paper 01/2024. 2024. Available online: https://www.bruegel.org/system/files/2024-01/WP%2001%202024_0.pdf (accessed on 15 June 2025).
- Krzemień, A.; Fernández, P.R.; Sánchez, A.S.; Álvarez, I.D. Beyond the pan-european standard for reporting of exploration results, mineral resources and reserves. Resour. Policy 2016, 49, 81–91. [Google Scholar] [CrossRef]
- Nieto, L.S.; Valverde, G.F.; Krzemień, A.; Fernández, P.R.; Rodríguez, F.J.I. Economic risks in mining investments: A prospective analysis of capital cost estimation in copper mining projects. Resour. Policy 2024, 99, 105427. [Google Scholar] [CrossRef]
- Díaz, A.B.; Álvarez, I.D.; Fernández, C.C.; Krzemień, A.; Rodríguez, F.J.I. Calculating ultimate pit limits and determining pushbacks in open-pit mining projects. Resour. Policy 2021, 72, 102058. [Google Scholar] [CrossRef]
- Yang, J.; Wang, Y.; Gao, X.; Zuo, R.; Song, L.; Jin, C.; Wang, J.; Teng, Y. Vanadium: A Review of Different Extraction Methods to Evaluate Bioavailability and Speciation. Minerals 2022, 12, 642. [Google Scholar] [CrossRef]
- Cellura, S.; Mazza, A.; Bompard, E.; Corgnati, S. An Extended Approach to the Evaluation of Energy Storage Systems: A Case Study of Li-Ion Batteries. Electronics 2023, 12, 2391. [Google Scholar] [CrossRef]
- Garttan, G.; Alahakoon, S.; Emami, K.; Jayasinghe, S.G. Battery Energy Storage Systems: Energy Market Review, Challenges, and Opportunities in Frequency Control Ancillary Services. Energies 2025, 18, 4174. [Google Scholar] [CrossRef]
- Domaracká, L.; Šaffová, D.; Čulková, K.; Taušová, M.; Kowal, B.; Matušková, S. Future Development of Raw Material Policy Based on Statistical Data Analysis. Resources 2025, 14, 90. [Google Scholar] [CrossRef]
- National Minerals Information Center. U.S. Geological Survey Mineral Commodity Summaries 2024 Data Release (Ver. 2.0, March 2024); U.S. Geological Survey: Denver, CO, USA, 2024. [CrossRef]
- Wang, F.; Ai, F.; Lu, Y.-C. Ion selective membrane for redox flow battery, what’s next? Energy 2023, 1, 100053. [Google Scholar] [CrossRef]
- Pavloudakis, F.; Roumpos, C.; Spanidis, P.-M. Sustainable Mining and Processing of Mineral Resources. Sustainability 2024, 16, 8393. [Google Scholar] [CrossRef]
- Wårell, L. Mineral Deposits Safeguarding and Land Use Planning—The Importance of Creating Shared Value. Resources 2021, 10, 33. [Google Scholar] [CrossRef]
- Jia, Q.; Li, Z.; Ruan, H.; Luo, D.; Wang, J.; Ding, Z.; Chen, L. A Review of Carbon Anode Materials for Sodium-Ion Batteries: Key Materials, Sodium-Storage Mechanisms, Applications, and Large-Scale Design Principles. Molecules 2024, 29, 4331. [Google Scholar] [CrossRef]
- Li, L.; Wang, B.; Jiao, K.; Ni, M.; Du, Q.; Liu, Y.; Li, B.; Ling, G.; Wang, C. Comparative techno-economic analysis of large-scale renewable energy storage technologies. Energy AI 2023, 14, 100282. [Google Scholar] [CrossRef]
- LDES Council. Global Long Duration Energy Storage Annual Report 2024. LDES Council 2024. Available online: https://www.ldescouncil.com/ (accessed on 9 September 2025).
- Fikru, M.G.; Brodmann, J.; Eng, L.L.; Grant, J.A. ESG ratings in the mining industry: Factors and implications. Extr. Ind. Soc. 2024, 20, 101521. [Google Scholar] [CrossRef]
- Vegh, G.; Madikere Raghunatha Reddy, A.K.; Li, X.; Deng, S.; Liu, T.; Amine, K.; Zaghib, K. North America’s Potential for an Environmentally Sustainable Nickel, Manganese, and Cobalt Battery Value Chain. Batteries 2024, 10, 377. [Google Scholar] [CrossRef]
- Hu, Y.; Min, Z.; Zhu, G.; Zhang, Y.; Pei, Y.; Chen, C.; Sun, Y.; Liang, G.; Cheng, H. Predeposited lead nucleation sites enable a highly reversible zinc electrode for stable zinc-bromine flow batteries. Nat. Commun. 2025, 16, 3255. [Google Scholar] [CrossRef] [PubMed]
- Huq, N.M.L.; Mahbubul, I.M.; Lotif, G.; Ashrafi, M.R.; Himan, M. Development and Performance Analysis of a Low-Cost Redox Flow Battery. Processes 2024, 12, 1461. [Google Scholar] [CrossRef]
- Soloveichik, G.L. Flow batteries: Current status and trends. Chem. Rev. 2015, 115, 11533–11558. [Google Scholar] [CrossRef]
- Giosuè, C.; Marchese, D.; Cavalletti, M.; Isidori, R.; Conti, M.; Orcioni, S.; Ruello, M.L.; Stipa, P. An Exploratory Study of the Policies and Legislative Perspectives on the End-of-Life of Lithium-Ion Batteries from the Perspective of Producer Obligation. Sustainability 2021, 13, 11154. [Google Scholar] [CrossRef]
- Eerola, T.; Komnitsas, K. Preliminary Assessment of Social License to Operate (SLO) and Corporate Communication in Four European Lithium Projects. Mater. Proc. 2023, 15, 35. [Google Scholar] [CrossRef]
- Sim, M.-S.; Lee, J.-M.; Kim, Y.-S.; Lee, C.-H. Resilient Responses to Global Supply Chain Disruptions: Focusing on the Stock Price of Global Logistics Companies. Appl. Sci. 2024, 14, 11256. [Google Scholar] [CrossRef]
- Xiao, G.; Xu, L. Challenges and Opportunities of Maritime Transport in the Post-Epidemic Era. J. Mar. Sci. Eng. 2024, 12, 1685. [Google Scholar] [CrossRef]
- Chou, Y.-S.; Devi, N.; Lin, Y.-T.; Arpornwichanop, A.; Chen, Y.-S. CVD Grown CNTs-Modified Electrodes for Vanadium Redox Flow Batteries. Materials 2024, 17, 3232. [Google Scholar] [CrossRef] [PubMed]
- Comello, S.; Reichelstein, S. The emergence of cost effective battery storage. Nat. Commun. 2019, 10, 2038. [Google Scholar] [CrossRef] [PubMed]
- Belongia, S.; Wang, X.; Zhang, X. Progresses and Perspectives of All-Iron Aqueous Redox Flow Batteries. Adv. Funct. Mater. 2024, 34, 2302077. [Google Scholar] [CrossRef]
- Colombo, E. The Fifth Freedom: Shaping EU Innovation Policy for Renewable Energy Storage and Decarbonization. Energies 2025, 18, 3570. [Google Scholar] [CrossRef]
- Wittenberg, A.; de Oliveira, D. Critical Raw Material Resource Potentials in Europe. Mater. Proc. 2023, 15, 24. [Google Scholar] [CrossRef]
- Ratshitanga, M.; Ayeleso, A.; Krishnamurthy, S.; Rose, G.; Moussavou, A.A.A.; Adonis, M. Battery Storage Use in the Value Chain of Power Systems. Energies 2024, 17, 921. [Google Scholar] [CrossRef]
- Su, D.; Mei, Y.; Liu, T.; Amine, K. Global Regulations for Sustainable Battery Recycling: Challenges and Opportunities. Sustainability 2025, 17, 3045. [Google Scholar] [CrossRef]
- Peng, Z.; Wang, Z.; Han, Z.; Zhu, Y.; Li, Y.; Xie, K. Vanadium–Titanium Magnetite Concentrate, Calcium–Magnesium Composite Roasting and Sulfuric Acid Leach-ing for Vanadium Extraction from Pellets. Metals 2023, 13, 1135. [Google Scholar] [CrossRef]
- Shen, K.; Li, F.; Long, Y.; Yamg, Y.; Long, H.; Luo, R.; Ma, W.; Hua, J.; Yang, Z.; Zhuo, O.; et al. Sustainable Recovery of Vanadium from Stone Coal via Nitric Acid Oxygen Pressure Leaching. Materials 2025, 18, 2530. [Google Scholar] [CrossRef]
- Issa, T.B.; Van Yken, J.; Singh, P.; Nikoloski, A.N. Advancements and Applications of Redox Flow Batteries in Australia. Batteries 2025, 11, 78. [Google Scholar] [CrossRef]
- Safarzadeh, H.; Di Maria, F. Progress, Challenges and Opportunities in Recycling Electric Vehicle Batteries: A Systematic Review Article. Batteries 2025, 11, 230. [Google Scholar] [CrossRef]
- Koray, M.; Kaya, E.; Keskin, M.H. Determining Logistical Strategies to Mitigate Supply Chain Disruptions in Maritime Shipping for a Resilient and Sustainable Global Economy. Sustainability 2025, 17, 5261. [Google Scholar] [CrossRef]
- Lim, B.; Aylmore, M.; Alorro, R.D. Technospheric Mining of Critical and Strategic Metals from Non-Ferrous Slags. Metals 2024, 14, 804. [Google Scholar] [CrossRef]
- Jammulamadaka, H.; Pisupati, S.V. A Critical Review of Extraction Methods for Vanadium from Petcoke Ash. Fuels 2023, 4, 58–74. [Google Scholar] [CrossRef]
- Seppä, V.-M.; Rantala, E.; Lovén, P. NI43-101 Technical Report, Preliminary Economic Assessment on the Mustavaara Vanadium Project, Finland; AFRY Finland Oy; Strategic Resources Inc.: Washington, DC, USA, 2021. [Google Scholar]
- Critical Metals plc. Soidinvaara Vanadium Project, Finland; Critical Metals: Mala, Sweden, 2018. [Google Scholar]
- SRK Exploration Services Ltd. Competent Person’s Report on the Bømlo and Bjerkreim Projects, Norway; SRK Exploration Services Ltd.: Wales, UK, 2018. [Google Scholar]
- SRK Exploration Services Ltd. End of Phase 1 Exploration Report, Bjerkreim, Norway; SRK Exploration Services Ltd.: Wales, UK, 2019. [Google Scholar]
- Jonsson, E.; Törmänen, T.; Keiding, J.K.; Bjerkgård, T.; Eilu, P.; Pokki, J.; Gautneb, H.; Reginiussen, H.; Rosa, D.; Sadeghi, M.; et al. Critical metals and minerals in the Nordic countries of Europe: Diversity of mineralization and green energy potential. Geol. Soc. Lond. Spec. Publ. 2023, 526, 95–152. [Google Scholar] [CrossRef]
- Toves, E.Z. Estudio de la “EUROPEAN CRITICAL RAW MATERIALS ACT” y sus Posibles Consecuencias. Caso de Estudio del Proyecto Iberian West Belt. Master’s Thesis, University of Oviedo, Oviedo, Spain, 2024. [Google Scholar]
- Ali, S.H.; Giurco, D.; Arndt, N.; Nickless, E.; Brown, G.; Demetriades, A.; Durrheim, R.; Enriquez, M.A.; Kinnaird, J.; Littleboy, A.; et al. Mineral supply for sustainable development requires resource governance. Nature 2017, 543, 367–372. [Google Scholar] [CrossRef]
- Malin, S.A.; Ryder, S.; Lyra, M.G. Environmental justice and natural resource extraction: Intersections of power, equity and access. Environ. Sociol. 2019, 5, 109–116. [Google Scholar] [CrossRef]
- Minister of Natural Resources. The Canadian Critical Minerals Strategy. From Exploration to Recycling. 2022. Available online: https://www.canada.ca/en/campaign/critical-minerals-in-canada/canadian-critical-minerals-strategy.html (accessed on 15 June 2025).
- Zhang, G.; Wang, K.; Luo, M. Mechanism on the Separation of Vanadium and Titanium from Vanadium Slag by Roasting with Ammonium Sulfate. Separations 2022, 9, 196. [Google Scholar] [CrossRef]
- Noack, J.; Wietschel, L.; Gerlach, D.; Roznyatovskaya, N.; Menicta, C.; Skyllas-Kazacos, M.; Pinkwart, K. Techno-economical modeling and simulations for redox flow batteries. In Electrochemical Society Meeting Abstracts 240; The Electrochemical Society, Inc.: Pennington, NJ, USA, 2021; p. 158. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).