Abstract
Biomass can be used for electricity generation, especially in developing countries, but also in developed ones, where the utilization of renewable energy sources is being integrated into a sustainable economy. There are considerable differences in the scale of biomass use and in the technology of its processing. One of the most important sources of biofuel is the biomass of grass. This research aimed to determine the long-term effects of organic fertilizers on cellulose, hemicellulose, and lignin content in the biomass of three grass species: giant miscanthus (Miscanthus × giganteus), prairie cordgrass (Spartina pectinata), and switchgrass (Panicum virgatum L.) in the first three years of growth. The experiment was established in four replications on microplots of 2 m2 in April 2018. Before planting grass rhizomes, municipal sewage sludge (SS) and spent mushroom substrate (SMS) were introduced into the soil in various combinations. Biomass is harvested in December every year. The content of structural polysaccharides in the grass species statistically significantly varied in response to organic waste. Compared to other fertilizer combinations, SS application increased the content of cellulose in the biomass of Miscanthus giganteus (43.66% of DM) and Spartina pectinata (37.69% of DM) and hemicellulose in Spartina pectinata (27.80% of DM) and Panicum virgatum (23.64% of DM). Of the three species of grass, the chemical composition of Miscanthus giganteus cell walls was the most favorable for biofuel production, with the most cellulose and hemicellulose and the least lignin compared to other grass species. The content of lignin in the biomass of Miscanthus × giganteus and Spartina pectinata was the greatest on the plot with SMS and amounted to 7.79% of DM and 12.32% of DM, respectively. In the case of Panicum virgatum, the average content of lignin was similar across all fertilized plots, with 15.42% DM.
1. Introduction
Bioenergy production has become increasingly important worldwide, with plant biomass being its main raw material [1,2]. Perennial grass species grown for energy purposes, such as giant miscanthus (Miscanthus giganteus), Chinese silvergrass (Miscanthus sinensis), prairie cordgrass (Spartina pectinata), or switchgrass (Panicum virgatum), are an important biomass source due to their high yield and content of cellulose, hemicellulose, and lignin [3]. Those three structural components of plant cells are important materials for the production of biofuel and other goods [4]. Cellulose is one of the main components of plant cell walls, providing them with mechanical strength [5]. On the other hand, hemicellulose connects with other components of cell wall structure. Lignin is a polymer compound that is difficult to process in the biofuel industry. It makes plants firm and resistant to biological decomposition [6]. The amounts of the above components in the aboveground part of plants determine the efficiency of biofuel production and other goods. Cellulose, hemicellulose, and lignin play different but complementary roles in biomass combustion. Together, they impact biomass efficiency, usefulness as an energy source, and the amount of combustion products. It is assumed that the heat of combustion of biomass with high lignin content is relatively high. In addition, compared to conventional fuels, such biomass contains high amounts of volatile substances. However, the heat of combustion also depends on other factors such as biomass humidity, density, ash content, etc. [7]. A high proportion of biomass lignin is desirable for thermal conversion, but its low content is helpful for biomass biological decomposition as that compound decomposes slower than cellulose [8]. The choice of an energy crop depends on biofuel production requirements and perennial grass processing technologies. Appropriate mineral or organic fertilizers can increase biomass yield and, consequently, the amounts of cellulose and hemicellulose. As a nutrient, nitrogen affects plant growth and protein production [9]. However, its excessive quantities provided to plants can reduce the concentration of the above compounds, as more protein is produced at their expense. The application of organic waste, especially SS and SMS, to energy crops is an effective way to improve the physicochemical properties of light soils and increase the growth of plant biomass [10]. In the present research, many factors have been considered when choosing organic materials to treat perennial grass species. SMS was selected because bisporus mushroom (Agaricus bisporus) cultivation is dynamically developing in Poland, the greatest producer in Europe, with a production of 355,000 tons. About 1–1.3 million tons of SMS is left as a by-product annually [11]. Thus, research on the agricultural use of SMS in Poland is required due to the need for its urgent utilization. SMS used in the present experiment was made from rye, wheat or triticale straw, poultry manure, waste gypsum, phosphogypsum, and calcium carbonate [12]. Landfilling this type of waste poses a threat to the natural environment, so it is necessary to utilize it in a way required by law [13,14]. According to many authors, SMS is a valuable source of plant organic matter and nutrients [10,15]. In turn, the use of SS provides biogenic elements such as nitrogen and phosphorus to crops, particularly energy crops. Thus, fertilizer treatment significantly impacts perennial grass growth and yield, which, in turn, directly impacts its value and performance as a biofuel [16,17]. Unfortunately, the literature has very few detailed studies on the impact of fertilization, species of energy plants, and their age on cell wall chemical composition.
This research aimed to assess the long-term effects of organic waste used as fertilizer on the content of cellulose, hemicellulose, and lignin in the biomass of Miscanthus × giganteus, Spartina pectinata, and Panicum virgatum L. in the first three years of growth.
2. Material and Methods
2.1. Description of the Experiment
The field experiment was conducted on anthropogenic, cultured soil of the hortisole subtype, made of heavy loamy sand with light loamy sand as subsoil [18]. The experiment was performed in a random block system and in four replications at the experimental facility of the University of Siedlce (52°17′ N, 22°28′ E), Poland. The test plants were perennial grass species of the C4 photosynthetic type—Miscanthus × giganteus (M 19), Panicum virgatum cv. Northwind, and Spartina pectinata. In April 2018, grass rhizomes of all species purchased from a plant nursery were manually planted on microplots of 2 m2 with a 1 m distance between them. The distance between the seedlings was 30 cm, and the planting rate was three rhizomes per 1 m2, i.e., 30 thousand per ha. The experiment was organized according to the following fertilization plan (Table 1).

Table 1.
Experimental design.
SS came from the sewage treatment plant in the city of Siedlce, with a capacity of approximately 24,000 m3 per day. The plant produces 1897 Mg of SS annually, an average of 5.2 Mg per day. SMS was obtained from a local mushroom farm. The substrate for growing mushrooms on the farm was produced by Unikost, and it consisted of a mixture of structure-forming materials (cereal straw, defatted soybean meal, and peat), mineral waste, and chicken manure. The peat casing was provided by Wokas. The growing cycle for white Agaricus bisporus mushroom was six weeks. Waste organic materials (SS and SMS) were introduced once in the spring of 2018 before the rhizomes were planted. On all experimental plots, the plants took root. Only mechanical weed control was used during the experiment, but in the third year, there was no need for that.
2.2. Determination of Soil and Organic Materials Properties
In air-dry soil samples collected from the 25 cm arable layer before the start of the experiment and at the end of the third year, the following were determined:
- pH value in H2O and in 1 mol L−1 KCl by the potentiometric method;
- total nitrogen (Nt) and carbon (Ct) content by the elemental analysis method with the Perkin Elmer CHNS/O Series II 2400 analyzer and thermal conductivity detector (TCD);
- total content of phosphorus, potassium, calcium, zinc, lead, cadmium, and nickel, after wet mineralization of soil samples with aqua regia, by optical emission spectrometry (ICP-OES) at Eurofins GSC Poland Ltd. in Katowice, formerly the Center for Environmental Research and Control;
- available forms of phosphorus and potassium by the Enger-Riehm method at the District Chemical-Agricultural Station in Lublin, according to the PN-R-04023:1996 and PN-R-04022:1996+Az1:2004 Polish Standards, respectively;
- available forms of magnesium by the Schachtschabel method at the District Chemical-Agricultural Station in Lublin, according to the PN-R-0420:1994+Az1:2004 Polish Standard.
In the representative sample of organic materials, the following were determined:
- dry matter content, by the drying and weighing method, after drying the sample at 105 °C until a constant weight;
- pH value in H2O and 1 mol L−1 KCl, by the potentiometric method;
- total nitrogen (Nt) content, by the modified Kjeldahl method, after mineralization in concentrated sulfuric acid (VI) in the presence of a selenium mixture [19];
- carbon of organic compounds (Corg), by the oxidation-titration method [20];
- total content of phosphorus, potassium, calcium, zinc, lead, cadmium, and nickel after wet mineralization of samples using aqua regia by optical emission spectrometry (ICP-OES).
The experiment was conducted on pH-neutral soil with high total carbon and nitrogen content (Table 2). The content of cadmium and nickel was several times lower than the limits for light soils treated with SS contained in the Regulation of the Ministry of the Environment [21]. The amounts of zinc and lead were also within the limits. The content of available forms of phosphorus, potassium, and magnesium in the top soil layer was high (mg kg−1 of soil): P2O5—117; K2O—47.5; Mg—10.04.

Table 2.
Soil chemical properties before the start of the experiment.
The content of heavy metals in SS (Table 3) was within the limits set by the Regulation of the Polish Ministry of the Environment [22]. On the other hand, it was several times lower in SMS than in SS. The plants were harvested in December 2018 (first year), December 2019 (second year), and December 2020 (third year). After weighing fresh matter from each plot to determine its yield, a representative sample with five leafy shoots was collected. Then, the dry matter content was determined after drying the sample at 105 °C until a constant weight. After its shredding and grinding, the content of neutral-detergent fiber (NFD), acid-detergent fiber (ADF), and acid-detergent lignin (ADL) was determined by near-infrared reflection spectroscopy (NIRS) using the NIRFlex N-500 device (producent Bűchl Labortechnik AG) at the Institute of Technology and Life Sciences in Falenty. On the basis of fiber content, the amounts of cellulose, hemicellulose, and lignin were calculated [23].

Table 3.
Chemical properties and dry matter content of organic waste materials.
2.3. Meteorological Conditions
The data concerning temperature and precipitation over the three years of research (2018–2020) were provided by the Institute of Meteorology and Water Management, National Research Institute in Warsaw. Sielianinov’s hydrothermal coefficient (K) was calculated to determine the temporal variability of precipitation and air temperature to interpret the results more accurately according to the following formula:
where:
P—the monthly sum of precipitation,
Σt—the sum of daily air temperature values in a given month [24].
The paper used a 9-step classification of Sielianinov’s hydrothermal coefficient (K) values:
K ≤ 0.4 extreme drought (ed),
0.4 < K ≤ 0.7 severe drought (sd),
0.7 < K ≤ 1.0 drought (d),
1.0 < K ≤ 1.3 moderate drought (md),
1.3 < K ≤ 1.6 optimal (o),
1.6 < K ≤ 2.0 moderately wet (mw),
2.0 < K ≤ 2.5 wet (w),
2.5 < K ≤ 3.0 severely wet (sw),
K > 3.0 extremely wet (ew) [25].
Optimal thermal and moisture conditions were only in June, July, and October 2018, i.e., in the first year of grass growth (Table 4). In the remaining growing seasons, they varied greatly. In June 2019 and July 2020, extremely dry conditions were observed. According to Danalatos et al. [26], June is a very important month for grass growth and development. From May to the beginning of June, the daily growth of Miscanthus giganteus shoots is usually the most intense and can amount to 3 cm. Therefore, unfavorable weather during this period may limit biomass yield. The most difficult growing conditions were in 2019, when, with the exception of May, other months ranged from extremely dry to quite dry.

Table 4.
Values of Sielianinov’s hydrothermal coefficient (K) across the months of 2018–2020 growing periods.
2.4. Statistical Processing
The results were statistically developed using the analysis of variance for a two-factor experiment. The significance of the effect of experimental factors on the value of the characteristics was checked using the Fisher–Snedecor F-test. The LSD0.05 value was calculated using Tukey’s test for a detailed comparison of means. The Statistica StatSoft 13.1 [27] program was used for calculations.
3. Results and Discussion
3.1. Biomass Cellulose Content
The three-year average cellulose content in the dry matter of Miscanthus giganteus biomass was 43.30% (Table 5), with the highest value (46.00%) recorded in the second year. On average, across the years of research, its percentage compared to control significantly increased for plants treated with all combinations of organic materials. The highest cellulose content, 43.66%, was in plants on the plot with SS, with 43.60% on that with the lowest dose of SS applied together with the highest dose of SMS (SS25 + SMS75). However, differences between plants treated with different combinations of organic waste were not statistically significant. Cellulose can constitute a fairly high percentage share (40–55%) of lignocellulosic biomass [28], but its content varies depending on plant genetic and environmental characteristics [29].

Table 5.
Cellulose content in Miscanthus giganteus (% of DM) in the first three years of growth.
In the experiment, three energy grass species were used to determine whether the same fertilizer treatment would affect the content of structural polysaccharides in a similar way. In Spartina pectinata biomass, the average cellulose content of 33.61% was lower than in Miscanthus giganteus (Table 6). Across the years of research, Spartina pectinata’s average content of cellulose increased throughout the experiment, with the highest value recorded in the third year (34.57%). The amounts of cellulose produced by that species statistically significantly varied in response to fertilizer combinations. As in the case of Miscanthus giganteus, the highest value was recorded on the plot with SS, with an average of 37.69%, and the lowest in response to SMS on its own or to the combination of SS with SMS, both applied with the same amounts of nitrogen (SS50 + SMS50). On the two above plots (SMS and SS50 + SMS50), cellulose content was 20.48% and 17.33% lower, respectively, than in response to SS. On the other hand, in control plants and in those treated with the lowest SS dose combined with the highest SMS dose (SS25 + SMS75), cellulose content was almost identical, with 34.96 and 34.74%, respectively.

Table 6.
Cellulose content in Spartina pectinata biomass (% of DM) in the first three years of growth.
According to many authors, grass cellulose content is typically around 30–50% [30,31]. However, for Spartina pectinata, it is around 35–45%. Slightly lower amounts were noted in the present experiment conducted in East-Central Poland. Gismatulina et al. [31] reported that with the age of plants, their cellulose content increased from 42 to 54%, and the total share of non-cellulosic substances decreased from 60 to 49%.
Of the three grass species used in the experiment, the lowest cellulose content was found in Panicum virgatum L. (Table 7). The average value over the first three years of growth was 31.06% of DM, with the highest content in the third year. In Panicum virgatum L., cellulose content was varied in response to fertilizer combinations. The highest amount was recorded on plots with SMS used on its own (32.02% of DM), and the lowest was recorded in response to SS on its own (29.83% of DM) and the control plot (29.86% of DM).

Table 7.
Cellulose content in Panicum virgatum L. biomass (% of DM) in the first three years of growth.
3.2. Biomass Hemicellulose Content
No statistically significant differences across treatment combinations and years of research were found in Miscanthus giganteus hemicellulose content (Table 8). As an average of the above experimental factors, it was 30.67% of DM. According to Kupryś-Caruk [32], hemicellulose share in the biomass of Miscanthus giganteus was 23.7%, while Kacprzak et al. [33] reported 24.4%. In the present experiment, its content in Miscanthus biomass ranged from 27.95 to 33.18%. According to Sun and Cheng [28], plant biomass can contain 24 to 40% of hemicellulose.

Table 8.
Hemicellulose content in Miscanthus giganteus biomass (% of DM) in the first three years of growth.
With an average value of 24.08% of DM, hemicellulose content in the biomass of Spartina pectinata was much lower than in Miscanthus giganteus (Table 9). Across years, no statistically significant differences between the first two growing periods were noted, but in the third year, it statistically significantly increased compared to the previous ones. A statistically significant difference was found between plants treated with either SS or SMS on their own and those treated with other combinations. The highest Spartina pectinata hemicellulose content (27.80% of DM) was recorded on the plot with SS, and the lowest (21.88% of DM) was recorded on that with SMS. The amount of hemicellulose produced by plants changes with their age.

Table 9.
Hemicellulose content in Spartina pectinata biomass (% of DM) in the first three years of growth.
In the biomass of Panicum virgatum L., the content of hemicellulose with an average value of 22.82% DM was slightly lower than in the biomass of Spartina pectinata (Table 10). It did not significantly vary across years of research. On average, the highest amount, with 23.64% of DM, was noted on plots with SS applied on its own and SS applied in combination with SMS (SS75 + SMS25), with 23.30% of DM. On the other hand, the lowest content was in plants treated with the combinations of SS and SMS applied in equal doses (SS50 + SMS50), with 21.75% of DM.

Table 10.
Hemicellulose content in Panicum virgatum L. biomass (% of DM) in the first three years of its growth.
3.3. Lignin Content in Grass Biomass
The amount of lignin in Miscanthus giganteus biomass was statistically significantly affected by fertilizer treatments and years of research (Table 11). As an average response to these experimental factors, its content was 7.48% in DM. The highest average content of lignin was found in the second year (8.30% of DM), while the lowest in the youngest plants was found in 2018 (6.60% of DM). On the other hand, as an experimental average, the highest amount of lignin was in miscanthus plants treated with SMS, and the lowest was in the biomass of the control plot. In their research, Turner et al. [34] recorded the share of lignin in the biomass of Miscanthus giganteus at 23.8%, while Voca et al. [35] at 13.89%. In turn, in the studies conducted by Kacprzak et al. [33], this substance was at 28.8%.

Table 11.
Lignin content in Miscanthus giganteus biomass (% of DM) in the first three years of growth.
Unlike cellulose and hemicellulose, lignin is a non-carbohydrate substance, consisting mainly of coniferyl alcohol and acting as a binder of cellulose fibers and forming a physical structure in plants [36,37]. Grass and straw contain less lignin than the biomass of coniferous trees. Lignin combustion results in the formation of methoxyphenols, which are the main component of smoke in the initial stage of combustion [37]. Hemicellulose, lignin, and cellulose respond differently to heat. The ratio of these three substances impacts the quality of volatile torrefaction products [38] and biomass energy value [39,40]. With its high content of cellulose and lignin, Miscanthus giganteus biomass can be used for other purposes besides energy production [41].
In the biomass of Spartina pectinata, the average content of lignin was higher than in Miscanthus giganteus and amounted to an average of 10.86% DM (Table 12). It did not vary in a statistically significant way across years of research, i.e., it did not change much with the age of plants. The high lignin content of perennial grass species (giant miscanthus, prairie cordgrass, and switchgrass) may be a disadvantage, as it reduces their biogas production potential [42]. In the case of esterification and transesterification, high cellulose and hemicellulose content with low lignin content is desirable. Additionally, in mechanical processes, biomass with low lignin and high cellulose and hemicellulose content is preferred. Lignin provides structural support to plants and rigidity to cell walls, but it is hard to grind and can make pellet formation difficult. Thus, low lignin content means easier formation of pellets or briquettes and ensures their uniform structure [43,44]. Cellulose and hemicellulose are sources of easily fermentable simple sugars. Cellulose is surrounded by hemicellulose and lignin fragments, which makes it difficult for bacteria responsible for methane fermentation to access it [45]. Mosier et al. [46] indicate that appropriate pre-treatment of the lignocellulosic complex can accelerate its biodegradation, increasing the efficiency of biogas production and shortening the fermentation process. In the present experiment, organic waste significantly increased lignin content in relation to the control. The highest average content of lignin was noted in grass fertilized with SMS (12.32% DM), and the lowest was noted in control plants (8.19% DM).

Table 12.
Lignin content in Spartina pectinata biomass (% DM) in the first three years of growth.
The biomass of Panicum virgatum L. contained significantly more lignin than the biomass of Miscanthus giganteus and Spartina pectinata (Table 13). The average content over the three years of the research was 15.42% DM, ranging from 14.09 to 16.54%. The content of lignin did not vary in a statistically significant way across years and fertilizer treatments. According to Grzelak et al. [40], in a grass sample dominated by sand reed (Calamagrostis epigejos), the content of lignin was 22.1% DM. The biomass of couch grass (Agropyron elongatum) was even higher and amounted to 26.5% DM [47].

Table 13.
Lignin content in Panicum virgatum L. biomass (% of DM) in the first three years of growth.
Figure 1 presents the effect of fertilizer treatment on the average content of cellulose, hemicellulose, and lignin in three perennial grass species. Only in the case of plants treated with SS on its own and with (SS25 + SMS75) was an increase in cellulose content recorded. Compared to the other fertilized plots, the content of hemicellulose was higher only after the application of SS. On the other hand, the one-off organic waste application did not affect lignin content in consecutive years. The content of structural polysaccharides in the biomass of the plants was, on average, 35.99% of cellulose and 25.86% of hemicellulose, with 11.26% of lignin.
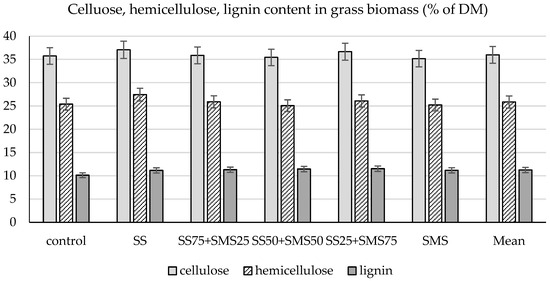
Figure 1.
The effect of fertilizer treatment on the content of cellulose, hemicellulose, and lignin (% of DM) in plant biomass (average across grass species and years). LSD0.05 for cellulose = 1.24; LSD0.05 for hemicellulose = 1.05; LSD0.05 for lignin = n.s.
In some parts of Europe, low temperatures in winter and short growing periods are the main factors limiting the growth of C4 grass species. However, they grow and develop very well in Polish climatic conditions, so their yields and chemical composition are competitive with other plants. Cell wall composition is important in crops used for biofuel production, and the development of varieties with increased polysaccharide content, reduced lignin content, and increased energy conversion efficiency is crucial for reducing energy production costs [48,49,50]. The content of these three components is the basic factor determining the biomass conversion technology [50,51].
4. Conclusions
The content of structural polysaccharides in the biomass of the three species of grass, Miscanthus giganteus, Sparitna pectinata, and Panicum virgatum L, significantly varied in response to the applied organic waste.
Compared to other fertilizer combinations, on the plots with SS, cellulose content was the highest in Miscanthus giganteus and Sparitna pectinate, while hemicellulose content was the highest in Sparitna pectinate and Panicum virgatum. Lignin content in the biomass of Miscanthus giganteus and Sparitna pectinate was the highest in response to the SMS application. In the case of Panicum virgatum, lignin content was similar on all fertilized plots. The three-year experiment indicated that of the three grass species, Miscanthus giganteus had the best chemical composition of cell walls for energy production due to its lowest content of lignin.
The present research proved that the chemical composition of grass species, especially the content of cellulose and hemicellulose, was dependent on the type of organic waste, assuming that each dose provided the same amount of nitrogen. The highest variability in cellulose, hemicellulose, and lignin content in response to fertilizer combinations was recorded for Spartina pectinata, and the lowest was recorded for Panicum virgatum L. The possibility of using organic waste may be an incentive for farmers with poor soils to grow perennial grass species with the desired chemical composition for the energy industry.
Author Contributions
Conceptualization, E.M.; methodology, E.M.; validation E.M and S.T., formal analysis E.M., writing–original draft S.T., writing—review and editing E.M., visualization S.T. All authors have read and agreed to the published version of the manuscript.
Funding
The research carried out as part of the research theme No. 161/23/B was financed by a science grant from the Ministry of Science and Higher Education.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lewandowski, I.; Scurlock, J.M.O.; Lindvall, E.; Christou, M. The development and current status of perennial rhizomatous grasses as energy crops in the US and Europe. Biomass Bioenerg. 2003, 25, 335–361. [Google Scholar] [CrossRef]
- Wang, C.; Zhong, C.; Yang, Z. Assessing bioenergy-driven agricultural land use change and biomass quantities in the US Midwest with MODIS time series. J. Appl. Remote Sens. 2014, 8, 085198. [Google Scholar] [CrossRef]
- Heaton, E.A.; Dohleman, F.G.; Long, S.P. Meeting US biofuel goals with less land: The potential of Miscanthus. BioScience 2008, 58, 946–953. [Google Scholar] [CrossRef]
- Kim, S.; Dale, B.E. Global potential bioethanol production from wasted crops and crop residues. Biomass Bioenerg. 2004, 26, 361–375. [Google Scholar] [CrossRef]
- Mora-Pale, M.; Meli, L.; Doherty, T.V.; Linhardt, R.J.; Dordick, J.S. Room temperature ionic liquids as emerging solvents for the pretreatment of lignocellulosic biomass. Biotechn. Bioeng. 2011, 108, 1229–1245. [Google Scholar] [CrossRef]
- Sanderson, M.A.; Burns, J.C. Digestibility and intake of hays from upland switchgrass cultivars. Crop Sci. 2010, 50, 2641–2648. [Google Scholar] [CrossRef]
- Adamovics, A.; Platace, R.; Kakitis, A.; Semjons, I. Evaluation of combustion properties of biomass fuel. Eng. Rural Devel. 2019, 18, 1523–1528. [Google Scholar] [CrossRef]
- Syguła, E. Research on the Impact of Pyrolysis Technological Parameters and Substrate Properties on the Release of Volatile Organic Compounds from Biochar; Uniwersytet Przyrodniczy we Wrocławiu: Wrocław, Poland, 2024; Available online: https://bip.upwr.edu.pl/ (accessed on 20 July 2024).
- Lawlor, D.W.; Lemaire, G.; Gastal, F. Nitrogen, Plant Growth and Crop Yield. In Plant Nitrogen; Lea, P.J., Morot-Gaudry, J.F., Eds.; Springer: Berlin/Heidelberg, Germany, 2001. [Google Scholar] [CrossRef]
- Malinowska, E.; Kania, P. The effects of organic waste materials on Miscanthus x giganteus yield and Zn and Ni content. Sci. Rep. 2024, 14, 16372. [Google Scholar] [CrossRef]
- Market Reports. IERiGŻ-BIP. 2023. Available online: https://ierigz.waw.pl/ (accessed on 1 September 2024).
- Sakson, N. Intensive mushroom production, green molds, substrate rotting. Biul. Prod. Pieczarek-Pieczarki 2007, 53, 13–20. ISSN 1231-0778. (In Polish) [Google Scholar]
- Council Directive 91/676/EWG of 12 December 1991 concerning the protection of waters against pollution caused by nitrates from agricultural sources. Available online: https://sip.lex.pl/akty-prawne/dzienniki-UE/dyrektywa-91-676-ewg-dotyczaca-ochrony-wod-przed-zanieczyszczeniami-67456932 (accessed on 1 September 2024).
- Regulation of the Minister of the Environment on the Waste Catalogue of 27 September 2001 (Dz.U. nr 112 poz. 1206). Available online: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=wdu20011121206 (accessed on 3 September 2024).
- Paredes, C.; Medina, E.; Moral, R.; Pérez-Murcia, M.D.; Moreno-Caselles, J.; Bustamante, M.A.; Cecilia, J.A. Characterization of the different organic matter fractions of spent mushroom substrate. Commun. Soil Sci. Plant Anal. 2009, 40, 150–161. [Google Scholar] [CrossRef]
- Guretzky, J.A.; Biermacher, J.T.; Cook, B.J.; Kering, M.K.; Mosali, J. Switchgrass for forage and bioenergy: Harvest and nitrogen rate effects on biomass yields and nutrient composition. Plant Soil 2011, 339, 69–81. [Google Scholar] [CrossRef]
- Gőnűlal, E.; Hocaoğlu, O. Bioethanol potential of switchgrass cultivars for rainfed and irrigated conditions in marginal lands. Arch. Agron. Soil Sci. 2023, 69, 3651–3669. [Google Scholar] [CrossRef]
- Taxonomy of Polish Soils; Polish Soil Science Society, Komisja Genezy Klasyfikacji i Kartografii Gleb: Warsaw, Poland, 2019.
- Kalembasa, S.; Carlson, R.W.; Kalembasa, D. A new method for the reduction in nitrates in total nitrogen determination according to the Kjeldahl method. Pol. J. Soil Sci. 1989, 22, 21–26. [Google Scholar]
- Kalembasa, S.; Kalembasa, D. A quick method for determination of C/N ratio in mineral soils. Pol. J. Soil Sci. 1992, 25, 41–46. [Google Scholar]
- Regulations of the Ministry of the Environment of September 1 on the method of assessing earth surface pollution. Dz.U. 2016, poz. 1395. Available online: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=wdu20160001395 (accessed on 1 September 2024).
- Regulations of the Ministry of Climate and the Environment of December 31 on the use of municipal sewage sludge. Dz.U. 2022, poz. 89. Available online: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20220000089 (accessed on 1 September 2024).
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animals nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Ziernicka-Wojtaszek, A. Comparison of selected indices for the assessment of atmospheric drought in the Podkarpackie Province in the years 1901–2000. Woda Środ. Obsz. Wiej. 2012, 12, 365–376. (In Polish) [Google Scholar]
- Skowera, B.; Puła, J. Pluviometric extreme conditions in spring season in Poland in the years 1971–2000. Acta Agroph. 2004, 1, 171–177. (In Polish) [Google Scholar]
- Danalatos, N.G.; Archontouli, S.V.; Mitsios, I. Potential growth and biomass productivity of Miscanthus× giganteus as affected by plant density and N-fertilization in central Greece. Biomass Bioenerg. 2007, 31, 145–152. [Google Scholar] [CrossRef]
- StatSoft, Inc. Statistica (Data Analysis Software System), version 13.1. 2021. Available online: https://www.statsoft.com (accessed on 29 May 2024).
- Sun, Y.; Cheng, J. Hydrolysis of lignocellulosic materials for ethanol production: A review. Bioresour. Technol. 2002, 83, 1–11. [Google Scholar] [CrossRef]
- Lee, J.W.; Gwak, K.S.; Park, J.Y.; Park, M.J.; Choi, D.H.; Kwon, M.; Choi, I.G. Biological pretreatment of softwood Pinus densiflora by three white rot fungi. J. Microbiol. 2007, 45, 485–491. [Google Scholar]
- Lee, W.C.; Kuan, W.C. Miscanthus as cellulosic biomass for bioethanol production. Biotech. J. 2015, 10, 840–854. [Google Scholar] [CrossRef] [PubMed]
- Gismatulina, Y.A.; Budaeva, V.V.; Veprev, S.G.; Sakovich, G.V.; Shumny, V.K. Cellulose from various parts of Soranovskii Miscanthus. Russ. J. Genet. Appl. Res. 2015, 5, 60–68. [Google Scholar] [CrossRef]
- Kupryś-Caruk, M. Effect of enzyme preparation on biogas production kinetics from giant miscanthus (Miscanthus x giganteus J.M. Greef & M. Deuter). Inż. Ap. Chem. 2017, 56, 41–42. (In Polish) [Google Scholar]
- Kacprzak, A.; Michalska, K.; Romanowska-Duda, Z.; Grzesik, M. Energy plants as a valuable raw material for biogas production. Kosmos 2012, 61, 281–293. (In Polish) [Google Scholar]
- Turner, W.; Greetham, D.; Mos, M.; Squance, M.; Kam, J.; Du, C. Exploring the bioethanol production potential of Miscanthus cultivars. Appl. Sci. 2021, 11, 9949. [Google Scholar] [CrossRef]
- Voća, N.; Leto, J.; Karažija, T.; Bilandžija, N.; Peter, A.; Kutnjak, H.; Šurić, J.; Poljak, M. Energy properties and biomass yield of Miscanthus x giganteus fertilized by municipal sewage sludge. Molecules 2021, 26, 4371. [Google Scholar] [CrossRef]
- Szufa, S. Biomass, the fuel of the 21st century. Katedra Techniki Cieplnej i Chłodnictwa, Wydz. Mechaniczny, 2015, Polit. Łódzka. Available online: https://www.bioenergiadlaregionu.eu/gfx/baza_wiedzy/163/szufa5.pdf (accessed on 4 July 2024). (In Polish).
- Mirowski, T. Utilization of biomass for energy purpose versus reduction of emission of air pollutants from municipal and households sector. Rocz. Ochr. Środ. 2016, 18, 466–477. (In Polish) [Google Scholar]
- Szufa, S. Methods of converting biomass to improve its fuel properties. Centrum Badań i Innowacji PRO-AKADEMIA, Katedra Techniki Cieplnej i Chłodnictwa, Wydz. Mechaniczny, 2010, Polit. Łódzka. Available online: https://www.proakademia.eu/gfx/baza_wiedzy/165/szufa7.pdf (accessed on 4 July 2024). (In Polish).
- Murawski, M.; Grzelak, M.; Waliszewska, B.; Knioła, A.; Czekała, W. Energy value and yield of extensively used meadows. Fragm. Agron. 2015, 32, 71–78. (In Polish) [Google Scholar]
- Grzelak, M.; Gaweł, E.; Murawski, M.; Waliszewska, B.; Knioła, A. Habitat conditions, yielding and potential energy use of biomass with dominant wood small-reed (Calamagrostis Epigejos). Fragm. Agron. 2016, 33, 38–45. (In Polish) [Google Scholar]
- Kolowca, J.; Knapik, P. Mechanical properties of miscanthus giganteus stalk. Inż. Roln. 2008, 9, 139–142. (In Polish) [Google Scholar]
- Klimiuk, E.; Pokoj, T.; Budzyński, W.; Dubis, B. Theoretical and observed biogas production from plant biomass of different fibre contents. Bioresour. Technol. 2010, 101, 9527–9535. [Google Scholar] [CrossRef] [PubMed]
- Gil, M.V.; Oulego, P.; Casal, M.D.; Pevida, C.; Pis, J.J.; Rubiera, F. Mechanical durability and combustion characteristics of pellets from biomass blends. Bioresour. Technol. 2010, 101, 8859–8867. [Google Scholar] [CrossRef] [PubMed]
- Niedziółka, I.; Szpryngiel, M.; Kachel-Jakubowska, M.; Kraszkiewicz, A.; Zawiślak, K.; Sobczak, P.; Nadulski, R. Assessment of the energetic and mechanical properties of pellets produced from agricultural biomass. Renew. Energy 2015, 76, 312–317. [Google Scholar] [CrossRef]
- Michalska, K.; Ledakowicz, S. Degradation of lignocellulosic structures and their hydrolysis products. Inż. Ap. Chem. 2012, 51, 157–159. (In Polish) [Google Scholar]
- Mosier, N.; Wyman, C.; Dale, B.; Elander, R.; Lee, Y.Y.; Holtzapple, M.; Ladisch, M. Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour. Technol. 2005, 96, 673–686. [Google Scholar] [CrossRef]
- Martyniak, D.; Fabisiak, E.; Zielewicz, W.; Martyniak, J. The biological-chemical properties of tall wheat grass (Agropyron elongatum (Host., Beauv.) in terms of potential use as biomass for energy production. Biulletyn IHAR 2011, 260/261, 375–384. (In Polish) [Google Scholar] [CrossRef]
- Wyman, C.E. What is (and is not) vital to advancing cellulosic ethanol. Trends Biotechnol. 2007, 25, 153–157. [Google Scholar] [CrossRef]
- Torres, A.F.; Slegers, P.M.; Noordam-Boot, C.M.M.; Dolstra, O.; Vlaswinkel, L.; van Boxtel, A.J.B.; Visser, R.G.F.; Trindade, L.M. Maize feedstocks with improved digestibility reduce the costs and environmental impacts of biomass pretreatment and saccharification. Biotechnol. Biofuels 2016, 9, 63. [Google Scholar] [CrossRef]
- van der Veijde, T.; Torres, A.F.; Dolstra, O.; Dechesne, A.; Visser, R.G.; Trindade, L.M. Impact of different lignin fractions on saccharification efficiency in diverse species of the bioenergy crop Miscanthus. Bioenergy Res. 2016, 9, 146–156. [Google Scholar] [CrossRef]
- Doblin, M.S.; Pettolino, F.; Bacic, A. Plant cell walls: The skeleton of the plant world. Funct. Plant Biol. 2010, 37, 357–381. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).