Effect of Different Preparation Methods on the Stability of Low-Carbon Alcohol Blended Fuels
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Methods
2.2. Emulsification Curve Plotting
3. Results and Discussions
3.1. Effect of Different Preparation Methods on Microemulsions
3.1.1. Effect of Different Preparation Methods on the Amount of Co-Solvent Addition
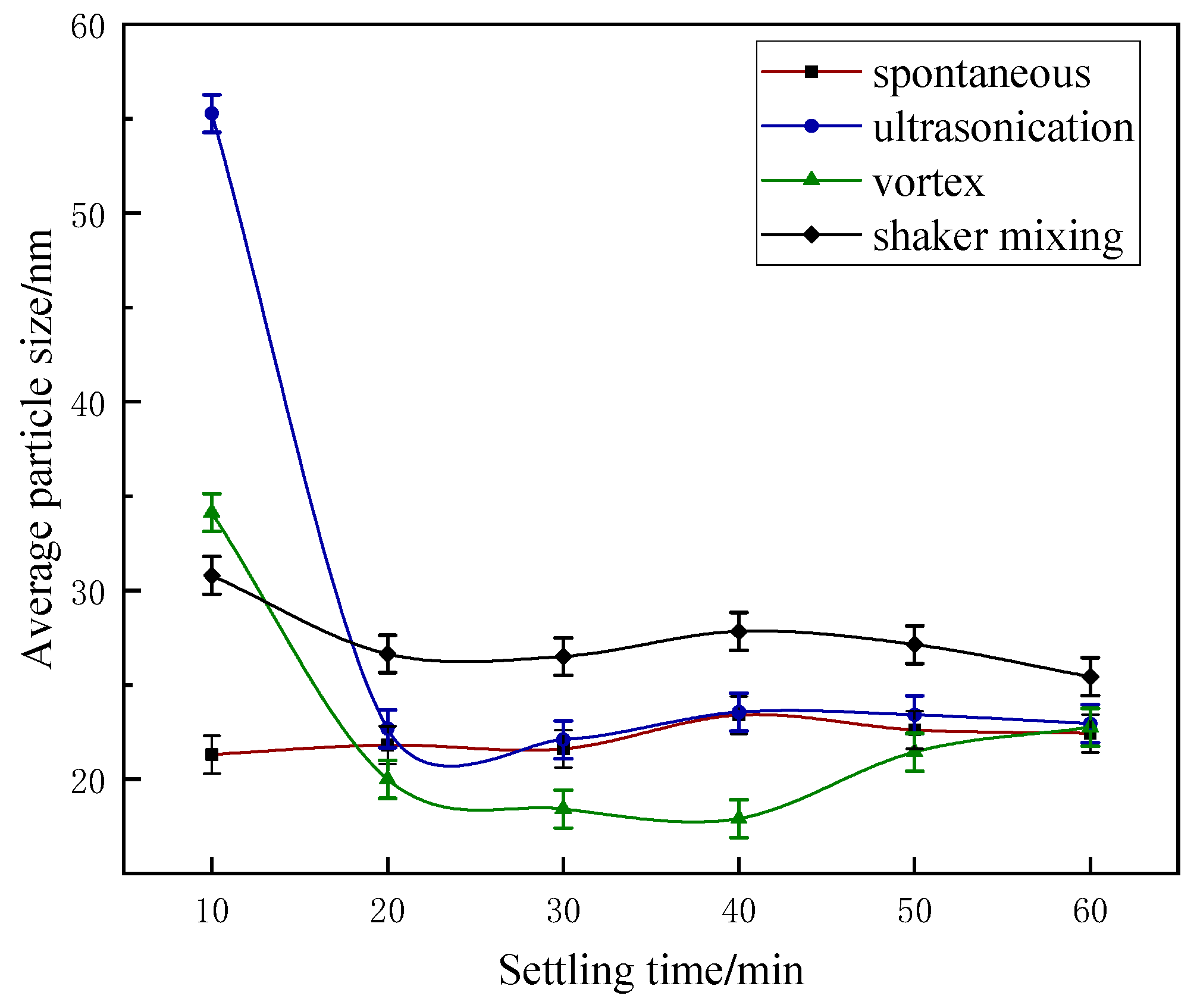
3.1.2. Effect of Different Preparation Methods on Particle Size
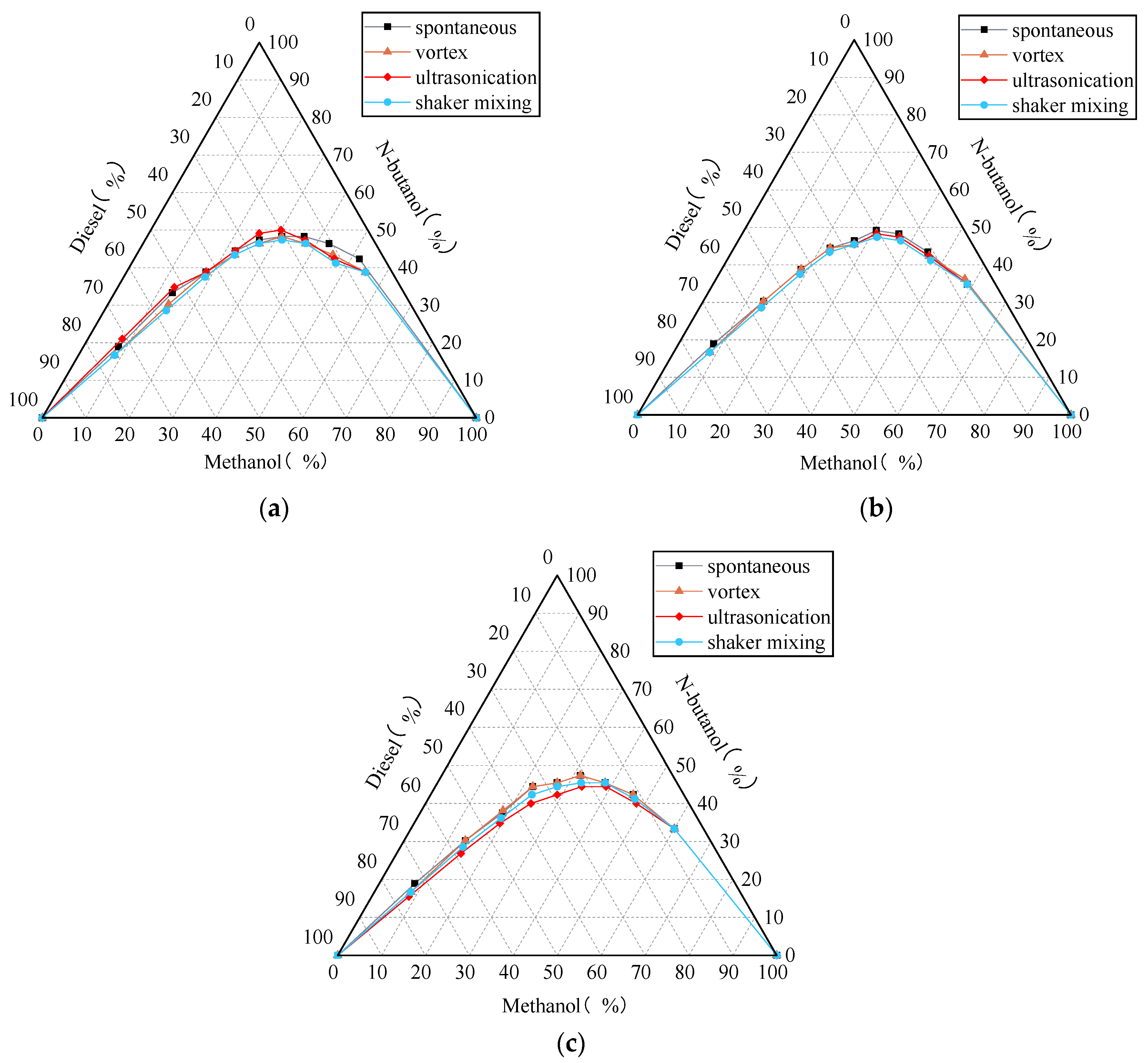
3.2. Influence of Low-Carbon Alcohol Types on Solubilising Effects
3.3. Stability of Microemulsions
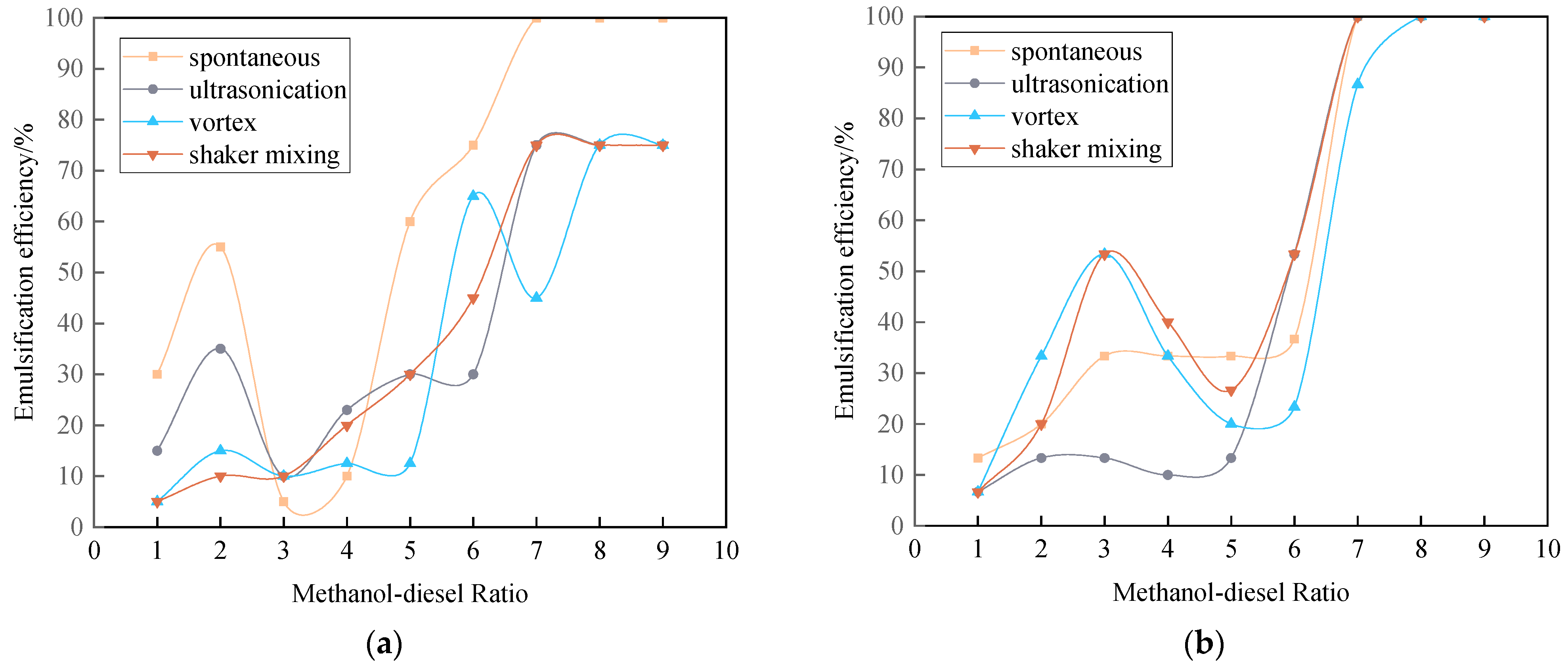
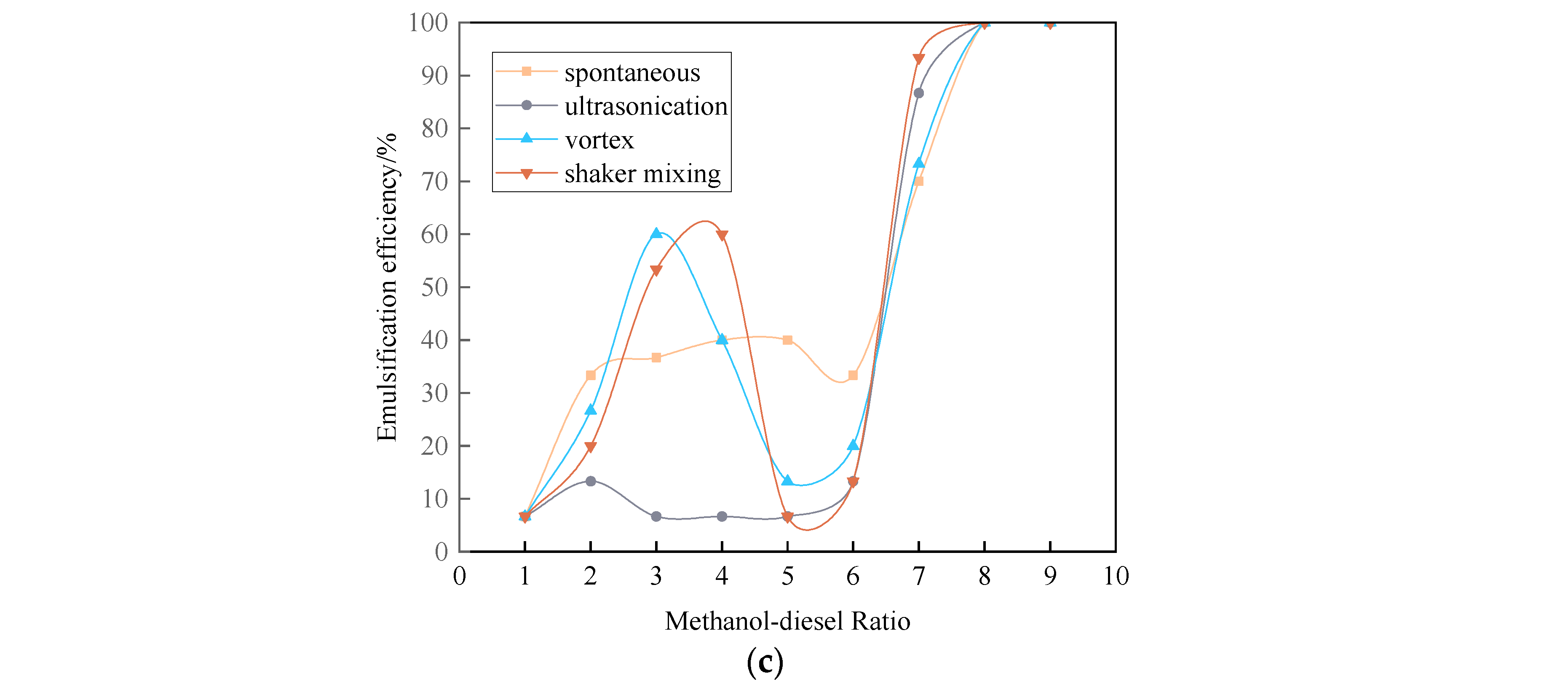
3.3.1. Emulsification Curve of Methanol Diesel Microemulsion
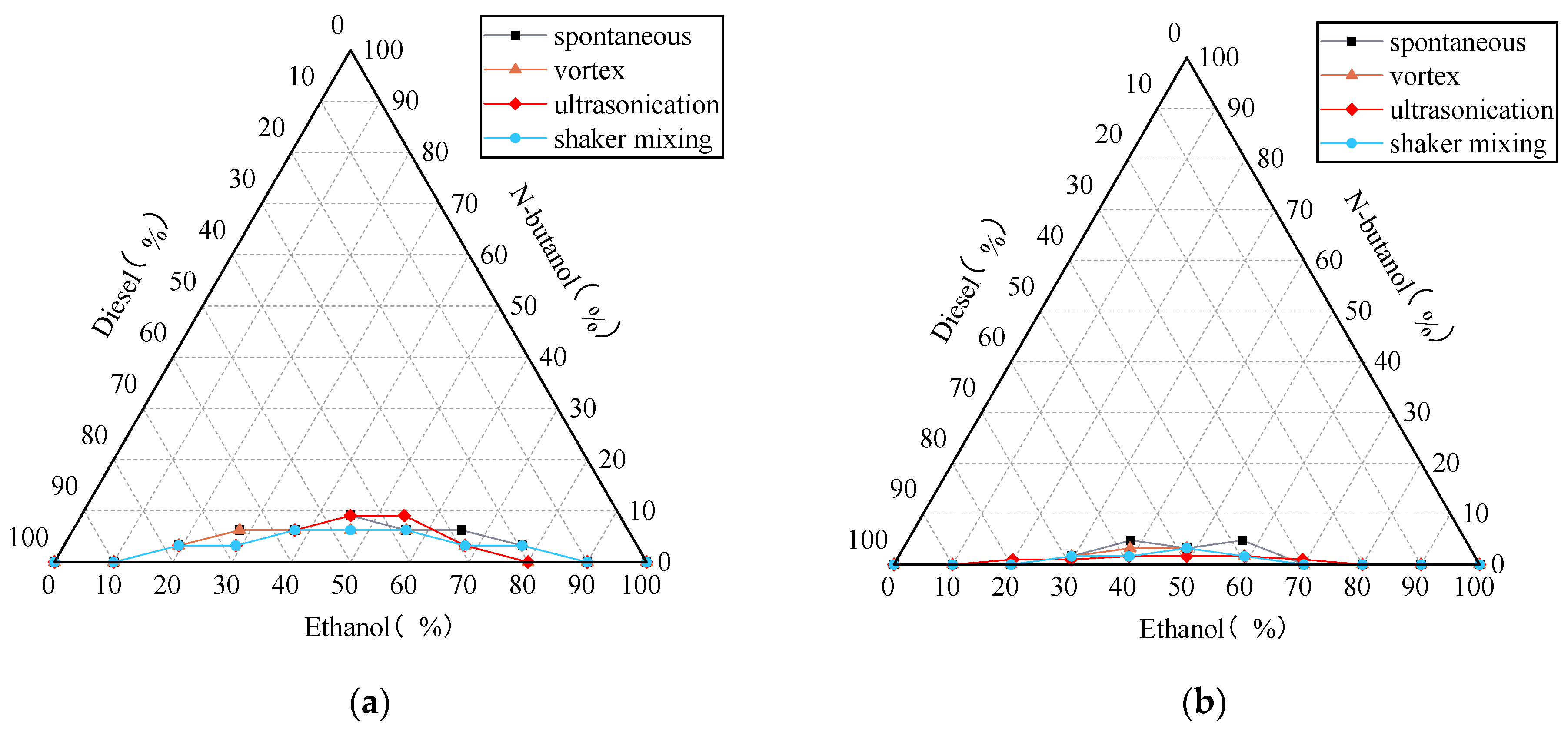
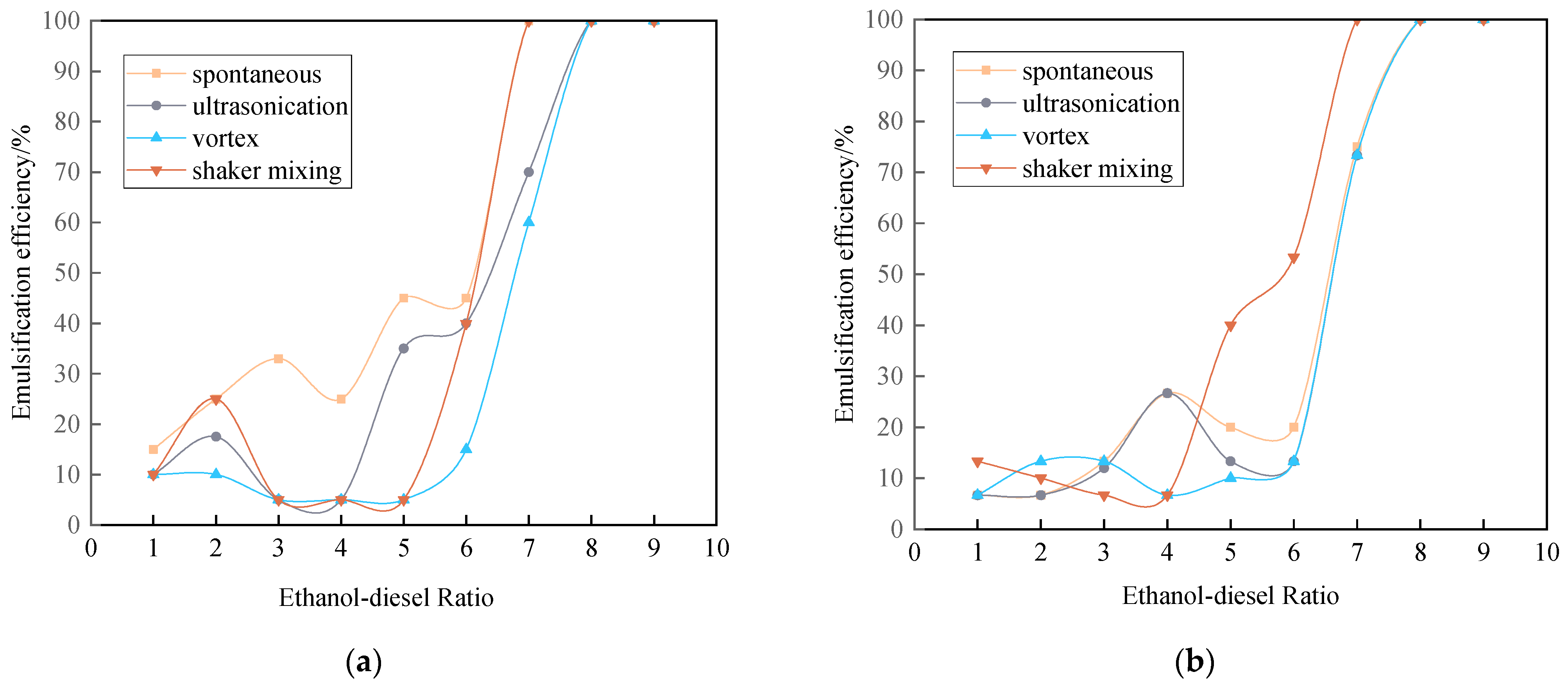
3.3.2. Emulsification Curve of Ethanol-Diesel Microemulsion
4. Conclusions
- (1)
- Temperature has an effect on the composition of microemulsions. In particular, high temperatures significantly reduce the amount of co-solvent added by the ultrasonication method by up to 25%. This is mainly due to the high-energy mode that ultrasound acts in. Except for the ultrasonication method, the addition of n-butanol was less affected by temperature in the other three preparation methods. This is mainly attributed to the combined effect of increased molecular motion and weakened hydrogen bonding during the warming process, which makes the effect of temperature on the n-butanol dosage insignificant. At 25 and 35 °C, the shaker mixing has the best co-solvent capacity.
- (2)
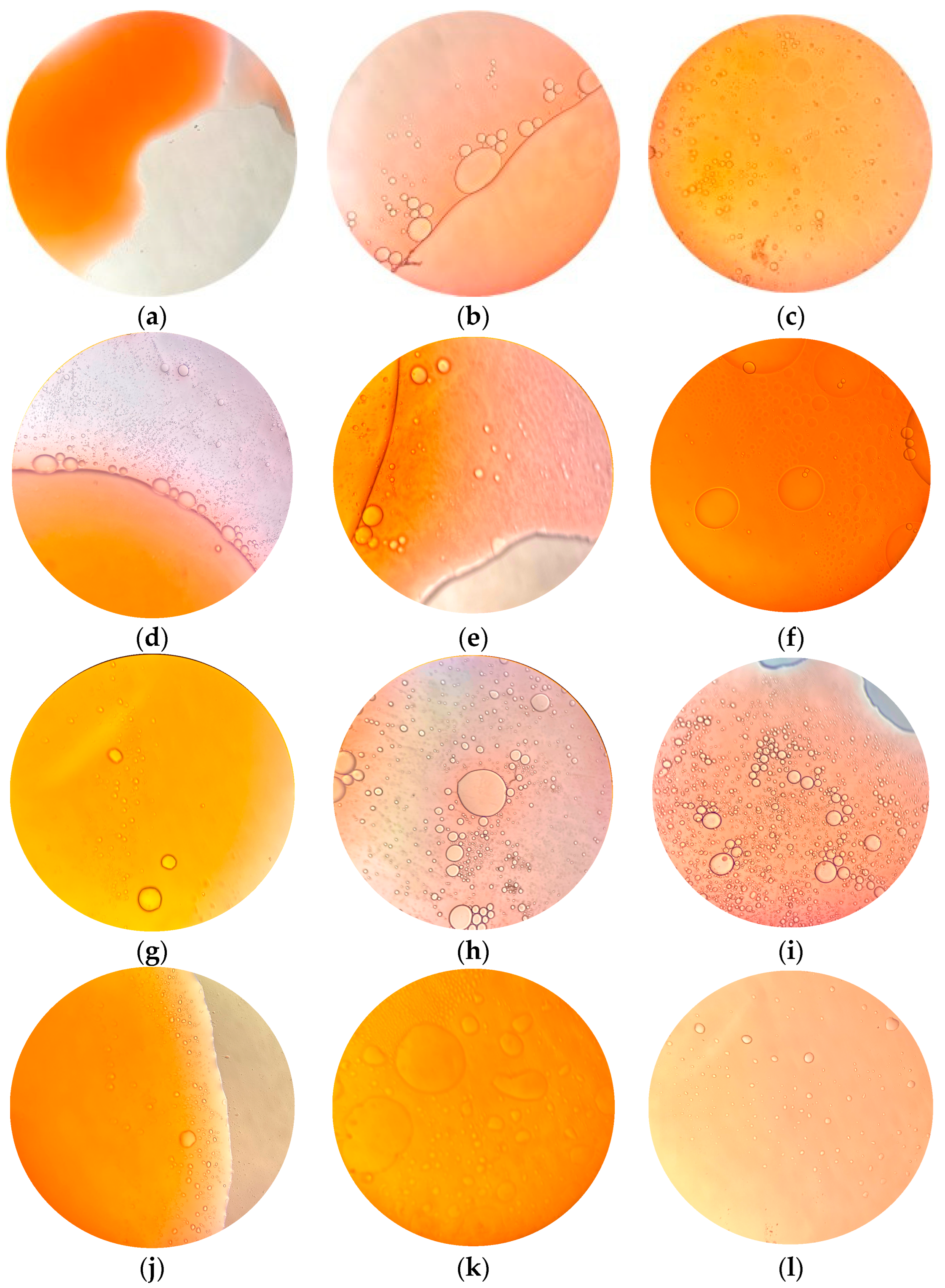
- The ultrasonication method at 45 °C has the best solubilizing capacity but the prepared microemulsion is less stable. The reasons for the differences in solubilizing ability and stability between the four microemulsion preparation methods were clearly presented by microscopic observation.
- (3)
- The stability of the microemulsion is typically enhanced when a greater proportion of low-carbon alcohol is incorporated. There exists a positive correlation between the quantity of co-solvent added and the stability of the microemulsion, and insufficient amounts of co-solvent can result in unstable microemulsions.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dey, S.; Reang, N.M.; Das, P.K.; Deb, M. A comprehensive study on prospects of economy, environment, and efficiency of palm oil biodiesel as a renewable fuel. J. Clean. Prod. 2021, 286, 124981. [Google Scholar] [CrossRef]
- Sudalaiyandi, K.; Alagar, K.; Kumar, R.V.; Manoj Praveen, V.J.; Madhu, P. Performance and emission characteristics of diesel engine fueled with ternary blends of linseed and rubber seed oil biodiesel. Fuel 2021, 285, 246–255. [Google Scholar]
- Yaqoob, H.; Teoh, Y.H.; Sher, F.; Farooq, M.U.; Jamil, M.A.; Kausar, Z.; Sabah, N.U.; Shah, M.F.; Rehman, H.Z.; Rehman, A.U. Potential of waste cooking oil biodiesel as renewable fuel in combustion engines: A Review. Energies 2021, 14, 2565. [Google Scholar] [CrossRef]
- Zhou, A.; Jin, H.; Cao, W.; Pang, M.; Li, Y.; Zhu, C. Influence of pilot injection on combustion characteristic of methanol-diesel dual-fuel engine. Energies 2022, 15, 3578. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Yin, Z.; Geng, H.; Zhu, R.; Zhen, X. Effect of injection strategy on a diesel/methanol dual-fuel direct-injection engine. Appl. Therm. Eng. 2021, 189, 116691. [Google Scholar] [CrossRef]
- Zhou, X.; Wei, L. Research on methanol-diesel blended fuel for vehicles. Henan Chem. Ind. 2015, 32, 36–38. [Google Scholar]
- Wang, X.; Shen, B.; Huang, L.; Zhang, J.; Huang, F.; Tang, Y.; Wang, M.; Wang, X. Effect of alcohol co-solvents on the inter-solubility of methanol and diesel fuel blends. J. Xi’an Pet. Univ. Nat. Sci. Ed. 2017, 32, 98–100. [Google Scholar]
- Yao, C.; Yao, A. Current status of methanol fuel application and its outlook. J. Automot. Saf. Energy Conserv. 2023, 14, 521–535. [Google Scholar]
- Hao, H.; Liu, Z.; Zhao, F.; Du, J.; Chen, Y. Coal-derived alternative fuels for vehicle use in China: A review. J. Clean. Prod. 2017, 141, 774–790. [Google Scholar] [CrossRef]
- Hulwan, D.B.; Joshi, S.V. Performance, emission and combustion characteristic of a multicylinder DI diesel engine running on diesel-ethanol-biodiesel blends of high ethanol content. Appl. Energy 2011, 88, 5042–5055. [Google Scholar] [CrossRef]
- Yusof, A.F.; Mamat, R.; Mat Yasin, M.H.; Abdullah, A.A.; Aziz, A.B. Comparative study of particulate matter (PM) emissions in diesel engine using diesel-methanol blends. In Proceedings of the 4th International Conference on Mechanical and Manufacturing Engineering (ICME 2013), Bangi-Putrajaya, Malaysia, 17–18 December 2013; pp. 1234–1277. [Google Scholar]
- Yilmaz, N. Comparative analysis of biodiesel-ethanol-diesel and biodiesel-methanol-diesel blends in a diesel engine. Energy 2012, 40, 210–213. [Google Scholar] [CrossRef]
- Yao, C.; Cheung, C.S.; Cheng, C.; Wang, Y.; Chan, T.L.; Lee, S.C. Effect of diesel/methanol compound combustion on diesel engine combustion and emissions. Energy Convers. Manag. 2008, 49, 1696–1704. [Google Scholar] [CrossRef]
- Geng, P.; Yao, C.; Wei, L.; Liu, J.; Wang, Q.; Pan, W.; Wang, J. Reduction of PM emissions from a heavy-duty diesel engine with diesel/methanol dual fuel. Fuel 2014, 123, 1–11. [Google Scholar] [CrossRef]
- Debnath, B.K.; Saha, U.K.; Sahoo, N. A comprehensive review on the application of emulsions as an alternative fuel for diesel engines. Renew. Sustain. Energy Rev. 2015, 42, 196–211. [Google Scholar] [CrossRef]
- Liu, H.F.; Hu, B.; Jin, C. Effects of different alcohols additives on solubility of hydrous ethanol/diesel fuel blends. Fuel 2016, 184, 440–448. [Google Scholar] [CrossRef]
- Gong, J. Study on preparation of methanol-diesel for automobile. Chin. J. Environ. Eng. 2009, 3, 1917–1920. [Google Scholar]
- Cao, N.; Zhao, D.; Zhang, F.; Cao, S. Examination of factors influencing the methanol blended fuel system. J. Liaoning Univ. Petrochem. Technol. 2008, 28, 27–29. [Google Scholar]
- Chen, H.P.; Lu, X.P.; Han, P.F. Preparation of microemusion of diesel oil and its properties. Chem. Eng. 2011, 39, 79–82. [Google Scholar]
- Huang, B.; Li, X.; Zhao, J.; Zhang, J.; Zhou, Y. Study of the effect of ultrasound on the stability of trap microemulsions. Coal Technol. 2014, 33, 240–243. [Google Scholar]
- Zhang, Q.Q.; Wang, X. Experimental study on the preparation and stability of methanol diesel microemulsion. Contemp. Chem. 2012, 41, 441–444. [Google Scholar]
- Waluyo, B.; Setiyo, M.; Wardana, I.N.G. The role of ethanol as a cosolvent for isooctane-methanol blend. Fuel 2020, 262, 116465. [Google Scholar] [CrossRef]
- Gao, Z.; Wu, S.; Luo, J.; Zhang, B.; Zhang, H.; Xiao, R. Optimize the co-solvent for methanol in diesel with group of oxygen-containing reagents: Molecular structure and intermolecular forces analysis. Fuel Process. Technol. 2021, 222, 106980. [Google Scholar] [CrossRef]
- Imazu, H.; Kojima, Y. Physical properties and combustion characteristics of emulsion fuels of water/diesel fuel and water/diesel fuel/vegetable oil prepared by ultrasonication. J. Jpn. Pet. Inst. 2013, 56, 52–57. [Google Scholar] [CrossRef]
- Kojima, Y.; Imazu, H.; Nishida, K. Physical and chemical characteristics of ultrasonically-prepared water-in-diesel fuel: Effects of ultrasonic horn position and water content. Ultrason. Sonochem. 2014, 21, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Khoshooei, M.A.; Scott, C.E.; Carbognani, L.; Pereira-Almao, P. Ultrasound-assisted bimetallic NiMo nanocatalyst preparation using microemulsions for in-situ upgrading application: Impact on particle size. Catal. Today 2021, 365, 132–141. [Google Scholar] [CrossRef]
- Xue, L. Formulation, Physical Measurement and Spraying Characteristics of Watery Ethanol Emulsified Fuel. Master’s Thesis, Shanghai Communication University, School of Mechanical and Power Engineering, Shanghai, China, 2015. [Google Scholar]
- Yang, C. Microemulsion study of ethanol diesel. Guangdong Chem. 2012, 39, 11–12. [Google Scholar]
- Waluyo, B.; Setiyo, M.; Wardana, I.N.G. Fuel performance for stable homogeneous gasoline-methanol-ethanol blends. Fuel 2021, 294, 120565. [Google Scholar] [CrossRef]
- Waluyo, B.; Fatimah, Y.A.; Habibi, I.; Pertiwi, F.D.; Setiyo, M.; Marlina, E. Enhancing Biodiesel-Methanol blends with 1-Butanol for stable and efficient fuels. Fuel 2024, 361, 130667. [Google Scholar] [CrossRef]
- Li, J.; Jiao, W.; Liu, Y.; Liu, W.; Xu, C.; Guo, L. Stability study of methanol-diesel emulsion. Commod. Chem. Ind. 2014, 44, 366–369. [Google Scholar]
- Jiao, W.Z.; Li, X.X.; Li, P.; He, S.Q.; Fu, M.; Wang, Y.M. Preparation and stability of methanol diesel oil emulsion underaction of ultrasonic wave. China Surfactant Deterg. Cosmet. 2015, 45, 568–571. [Google Scholar]
| Methanol volume ratio/vol% | 10 | 20 | 30 | 40 | 50 | 60 | 70 | 80 | 90 |
| Methanol volume/mL | 0.5 | 1.0 | 1.5 | 2.0 | 2.5 | 3.0 | 3.5 | 4.0 | 4.5 |
| Diesel volume/mL | 4.5 | 4.0 | 3.5 | 3.0 | 2.5 | 2.0 | 1.5 | 1.0 | 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, C.; Dong, J.; Ding, C.; Hu, J.; Geng, Z.; Li, X.; Xu, T.; Zang, G.; Liu, H. Effect of Different Preparation Methods on the Stability of Low-Carbon Alcohol Blended Fuels. Energies 2024, 17, 2796. https://doi.org/10.3390/en17112796
Jin C, Dong J, Ding C, Hu J, Geng Z, Li X, Xu T, Zang G, Liu H. Effect of Different Preparation Methods on the Stability of Low-Carbon Alcohol Blended Fuels. Energies. 2024; 17(11):2796. https://doi.org/10.3390/en17112796
Chicago/Turabian StyleJin, Chao, Juntong Dong, Chenyun Ding, Jingjing Hu, Zhenlong Geng, Xiaodan Li, Teng Xu, Guolong Zang, and Haifeng Liu. 2024. "Effect of Different Preparation Methods on the Stability of Low-Carbon Alcohol Blended Fuels" Energies 17, no. 11: 2796. https://doi.org/10.3390/en17112796
APA StyleJin, C., Dong, J., Ding, C., Hu, J., Geng, Z., Li, X., Xu, T., Zang, G., & Liu, H. (2024). Effect of Different Preparation Methods on the Stability of Low-Carbon Alcohol Blended Fuels. Energies, 17(11), 2796. https://doi.org/10.3390/en17112796







