Preparation and Characterization of Lauric Acid/Modified Fly Ash/Graphene Composite as Low-Cost and Eco-Friendly Phase Change Materials for Thermal Energy Storage
Abstract
1. Introduction
2. Experiments
2.1. Materials
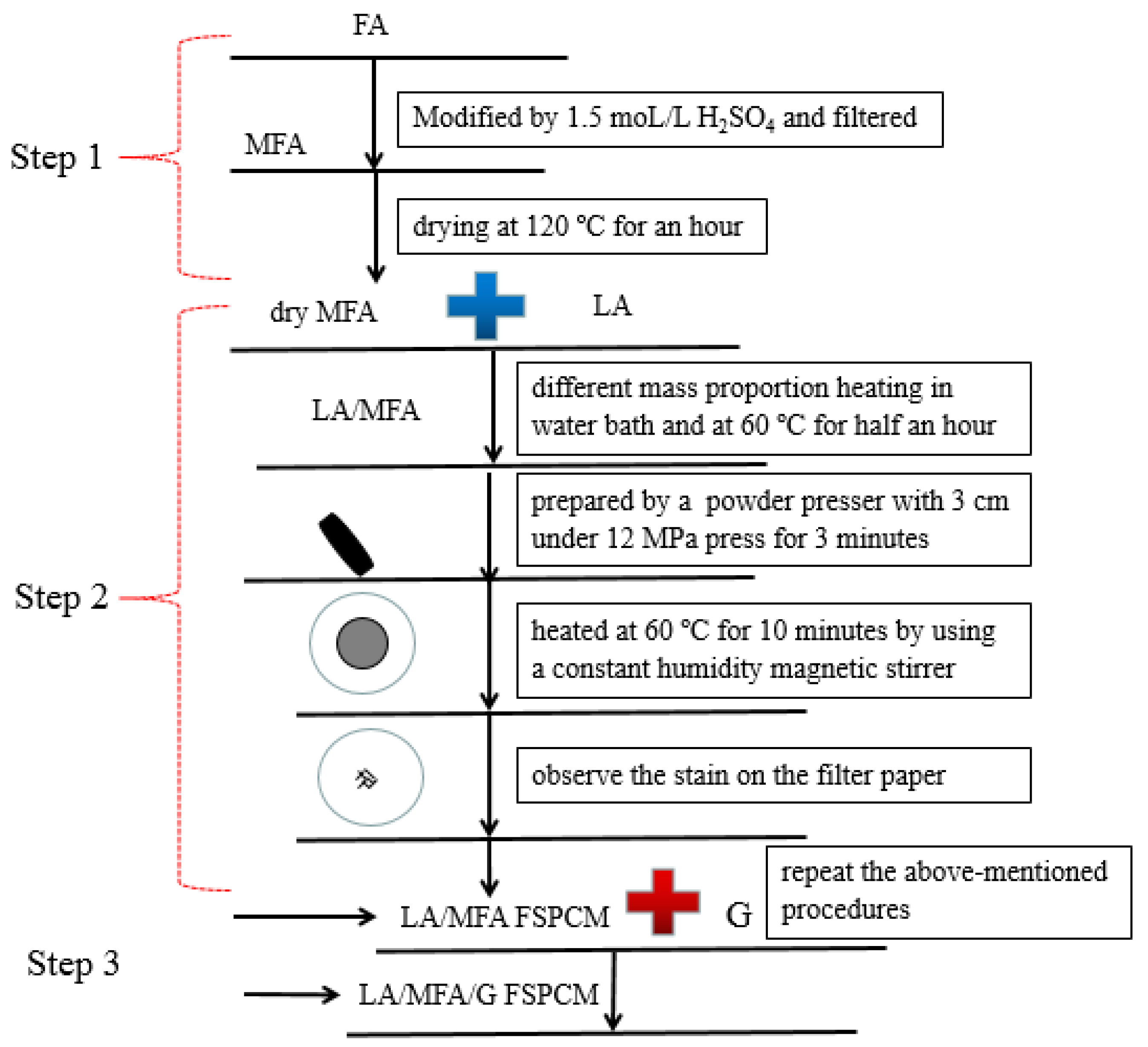
2.2. Preparation of LA/MFA/G
2.2.1. Modification of FA
2.2.2. Synthesis of LA/MFA FSPCM
2.2.3. Preparation of LA/MFA/G FSPCM
2.3. Characterization
3. Results and Discussion
3.1. Leakage Test Results of the Prepared FSPCMs
3.1.1. Leakage Test Results of LA/MFA Composites
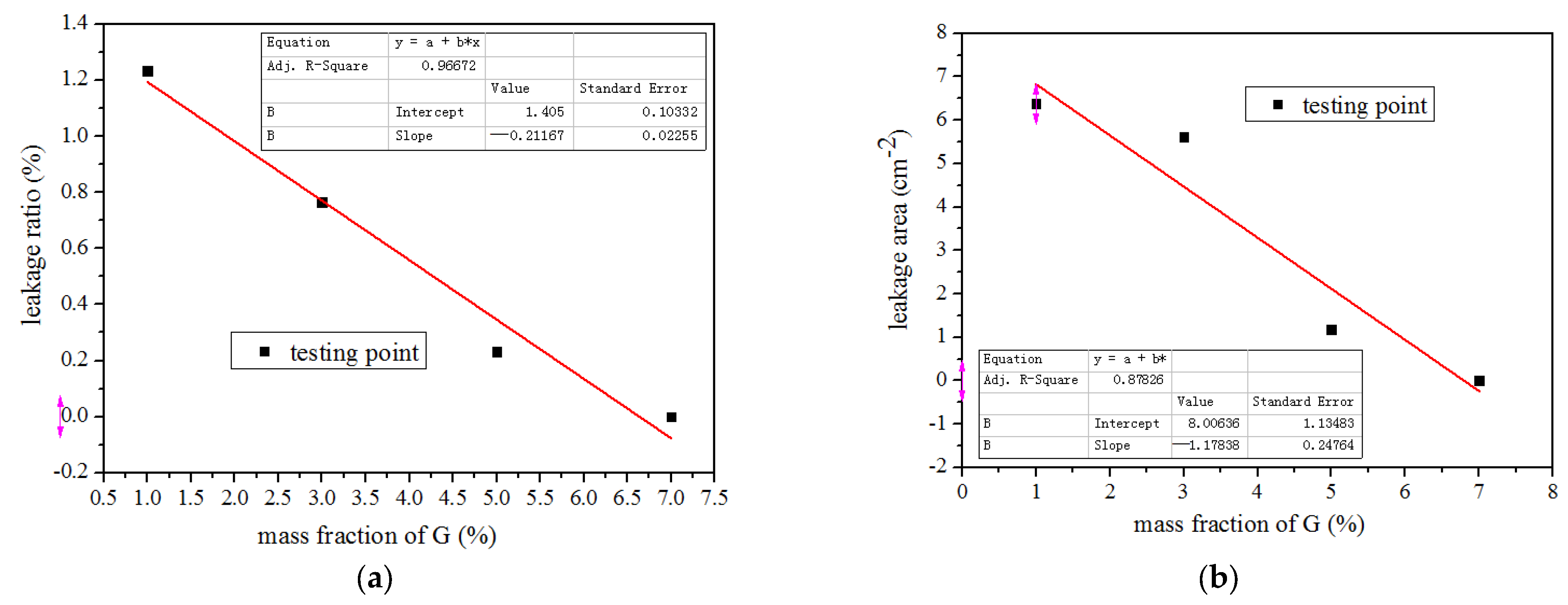
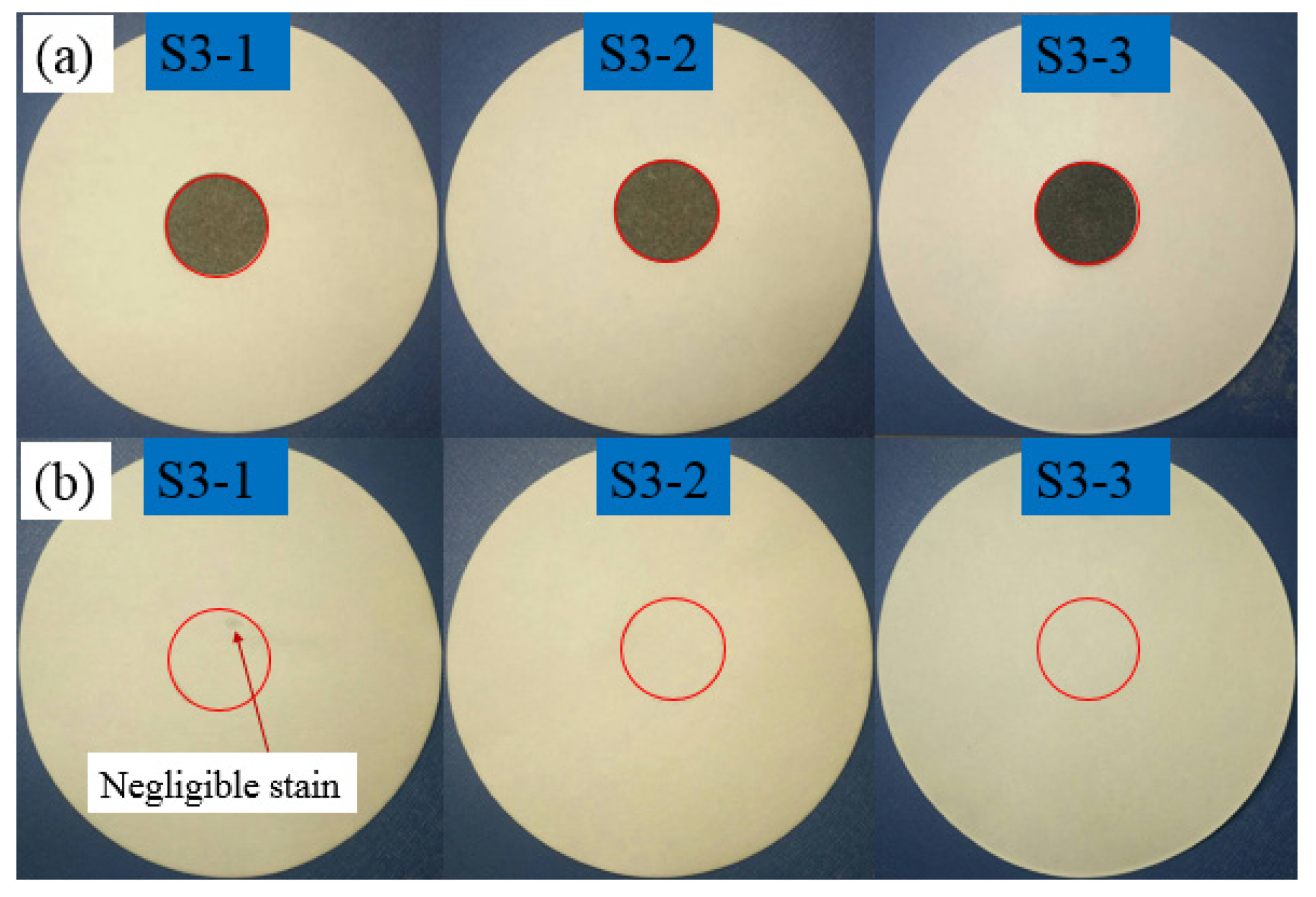
3.1.2. Leakage Test Results of LA/MFA/G Composites
3.2. Microstructure of the Prepared Composites
3.3. Chemical Compatibility of the Prepared Composites
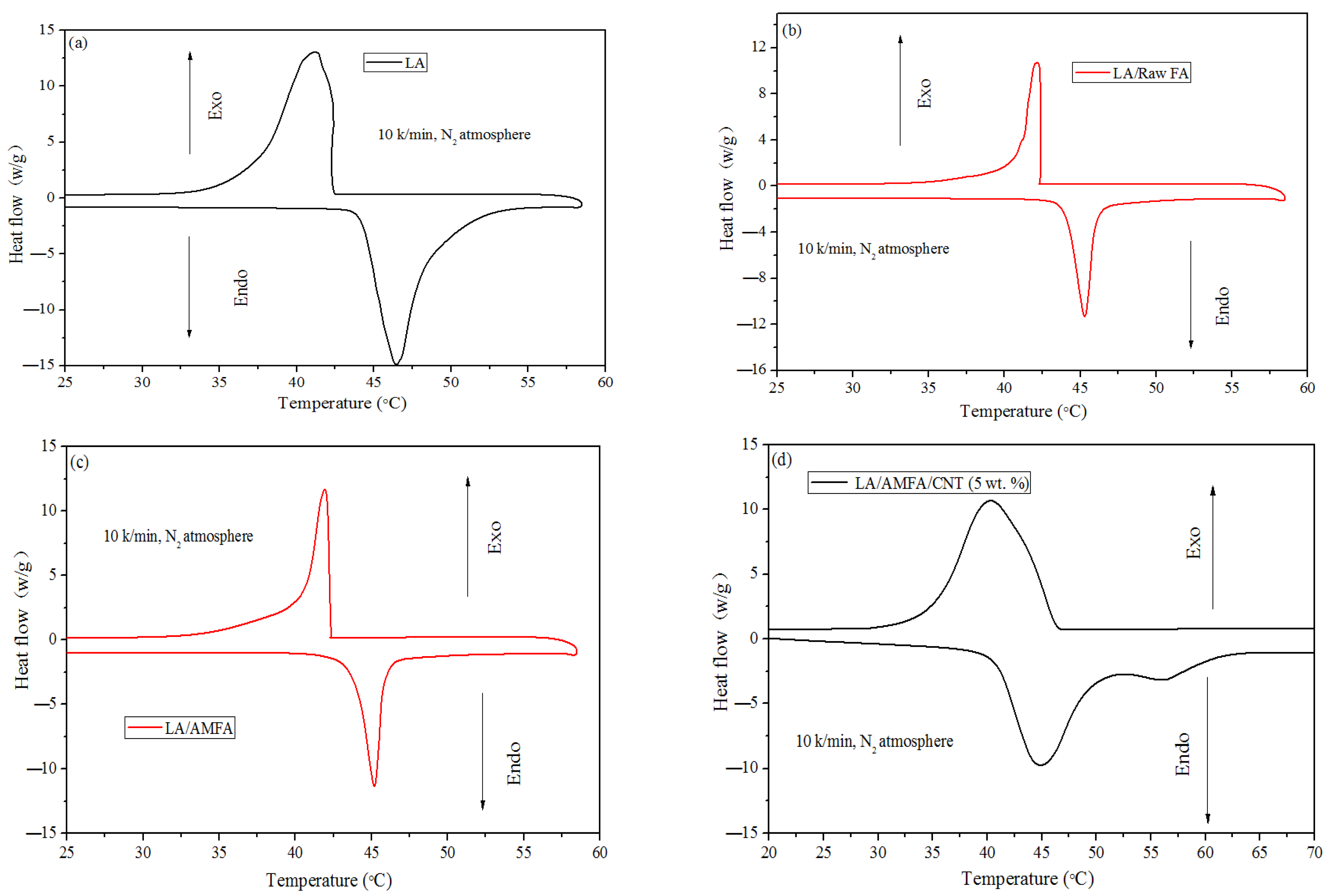
3.4. Thermal Properties of the Prepared FSPCMs
3.5. Thermal Stability of the Prepared FSPCMs
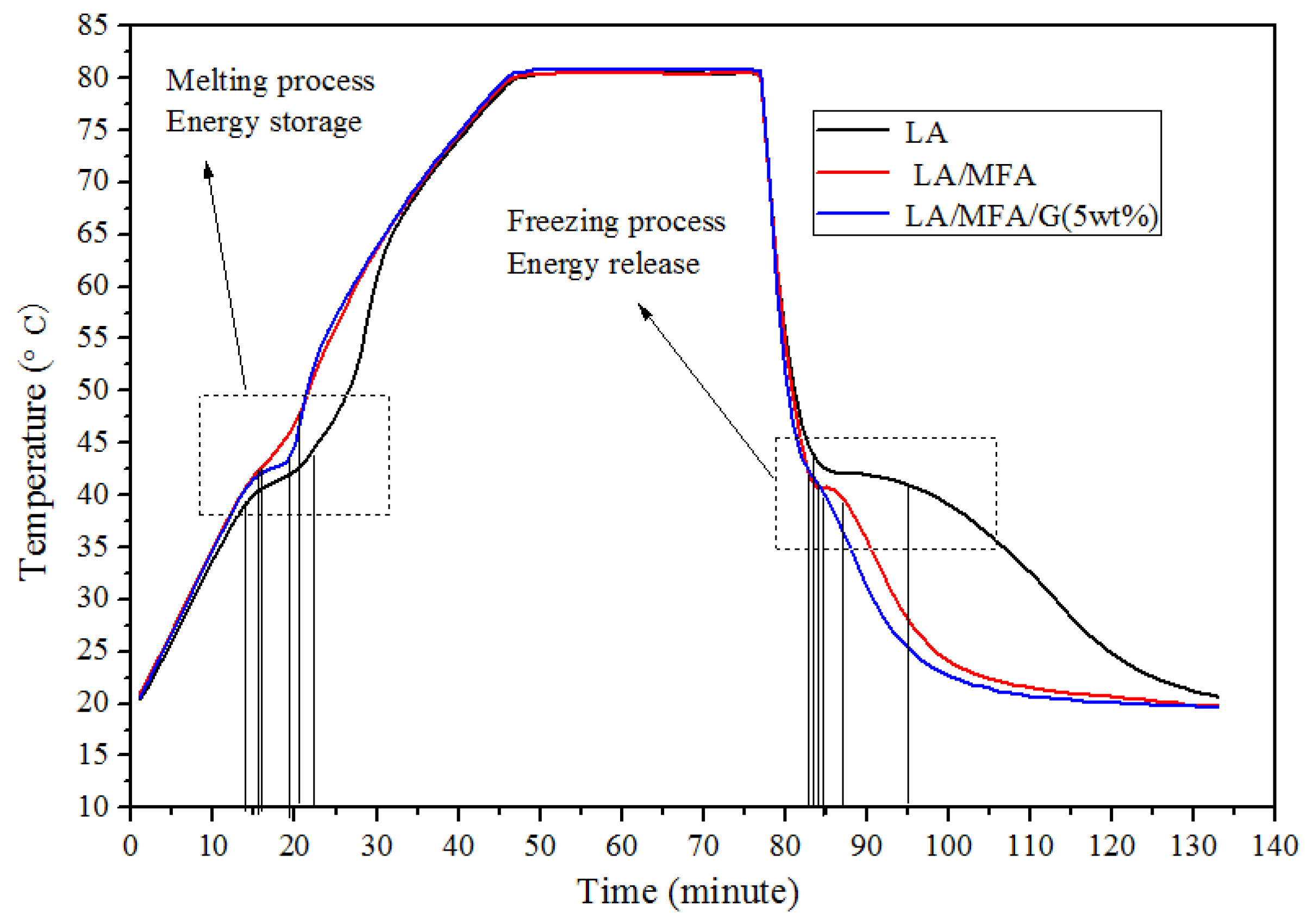
3.6. Heat Transfer Efficiency of the Prepared FSPCMs
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- He, Y.; Li, X.; Huang, P.P.; Wang, J.N. Exploring the road toward environmental sustainability: Natural resources, renewable energy consumption, economic growth, and greenhouse gas emissions. Sustainability 2022, 14, 1579. [Google Scholar] [CrossRef]
- Yong, Q.Q.; Tian, Y.P.; Qian, X.; Li, X.B. Retrofitting coal-fired power plants for grid energy storage by coupling with thermal energy storage. Appl. Therm. Eng. 2022, 215, 119048. [Google Scholar] [CrossRef]
- Montes, M.J.; Linares, J.I.; Barbero, R.; Rovira, A. Proposal of a new design of source heat exchanger for the technical feasibility of solar thermal plants coupled to supercritical power cycles. Sol. Energy 2020, 211, 1027–1041. [Google Scholar] [CrossRef]
- Tan, M.; Liu, W.D.; Shi, X.L.; Sun, Q.; Chen, Z.G. Minimization of the electrical contact resistance in thin-film thermoelectric device. Appl. Phys. Rev. 2023, 10, 021404. [Google Scholar] [CrossRef]
- Khan, M.I.; Asfand, F.; Al-Ghamdi, S.G. Progress in research and technological advancements of commercial concentrated solar thermal power plants. Sol. Energy 2023, 249, 183–226. [Google Scholar] [CrossRef]
- Ministry of Ecology and Environment of the People’s Republic of China. Annual Report on Prevention and Control of Solid Waste in China’s Large and Medium-Sized Cities, 2020; Environmental Protection in China: Beijing, China, 2020; p. 26.
- Wang, C.; Xu, G.G.; Gu, X.Y.; Gao, Y.H.; Zhao, P. High value-added applications of coal fly ash in the form of porous materials: A review. Ceram. Int. 2021, 47, 22302–22315. [Google Scholar] [CrossRef]
- Fernando, S.; Gunasekara, C.; Law, D.W.; Nasvi, M.C.M.; Setunge, S.; Dissanayake, R.; Robert, D. Environmental evaluation and economic analysis of fly ash-rice husk ash blended alkali-activated bricks. Environ. Impact Assess. Rev. 2022, 95, 106784. [Google Scholar] [CrossRef]
- Yao, Z.T.; Ji, X.S.; Sarker, P.K.; Tang, J.H.; Ge, L.Q.; Xia, M.S.; Xi, Y.Q. A comprehensive review on the applications of coal fly ash. Earth Sci. Rev. 2015, 141, 105–121. [Google Scholar] [CrossRef]
- Wang, C.Q.; Liu, K.; Huang, D.M.; Chen, Q.; Tu, M.J.; Wu, K.; Shui, Z.H. Utilization of fly ash as building material admixture: Basic properties and heavy metal leaching. Case Stud. Constr. Mater. 2022, 17, e01422. [Google Scholar] [CrossRef]
- Geng, X.Z.; Duan, Y.F.; Zhao, S.L.; Hu, J.W.; Zhao, W.M. Mechanism study of mechanochemical bromination on fly ash mercury removal adsorbent. Chemosphere 2021, 274, 129637. [Google Scholar] [CrossRef]
- Gao, K.; Iliuta, M.C. Trends and advances in the development of coal fly ash-based materials for application in hydrogen-rich gas production: A review. J. Energy Chem. 2022, 73, 485–512. [Google Scholar] [CrossRef]
- Ghosh, D.; Ghose, J.; Datta, P.; Kumari, P.; Paul, S. Strategies for phase change material application in latent heat thermal energy storage enhancement: Status and prospect. J. Energy Storage 2022, 53, 105179. [Google Scholar] [CrossRef]
- Panchal, J.M.; Modi, K.V.; Patel, V.J. Development in multiple-phase change materials cascaded low-grade thermal energy storage applications: A review. Clean. Eng. Technol. 2022, 8, 100465. [Google Scholar] [CrossRef]
- Zhang, M.; Cheng, H.F.; Wang, C.Y.; Zhou, Y. Kaolinite nanotube-stearic acid composite as a form-stable phase change material for thermal energy storage. Appl. Clay Sci. 2021, 201, 105930. [Google Scholar] [CrossRef]
- Alkhazaleh, A.H.; Almanaseer, W.; Alkhazali, A. Experimental investigation on thermal properties and fire performance of lauric acid/diphenyl phosphate/expanded perlite as a flame retardant phase change material for latent heat storage applications. Sustain. Energy Technol. 2023, 56, 103059. [Google Scholar] [CrossRef]
- Wang, T.; Zhao, S.Y.; Liu, S.; Li, J.H.; Xin, Y.X.; Lu, Q.; Chen, H.J.; Liang, C. Effect of porous carbon on thermal and physical properties of composite pure alkane/expanded vermiculite phase change energy storage materials. J. Energy Storage 2022, 54, 105220. [Google Scholar] [CrossRef]
- Ishak, S.; Mandal, S.; Lee, H.S.; Singh, J.K. Ph-controlled synthesis of sustainable lauric acid/SiO2 phase change material for scalable thermal energy storage. Sci. Rep. 2021, 11, 15012. [Google Scholar] [CrossRef]
- Wen, R.L.; Zhu, X.; Yang, C.; Sun, Z.H.; Zhang, L.Q.; Xu, Y.G.; Qiao, J.X.; Wu, X.W.; Min, X.; Huang, Z.H. A novel composite phase change material from lauric acid, nano-Cu and attapulgite: Preparation, characterization and thermal conductivity enhancement. J. Energy Storage 2022, 46, 103921–103927. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Li, C.C.; Lin, N.Z.; Xie, B.S.; Chen, J. Enhanced properties of mica-based composite phase change materials for thermal energy storage. J. Energy Storage 2021, 42, 103106. [Google Scholar] [CrossRef]
- Liu, P.; Gu, X.B.; Bian, L.; Cheng, X.F.; Peng, L.H.; He, H.C. Thermal properties and enhanced thermal conductivity of capric acid/diatomite/carbon nanotube composites as form-stable phase change materials for thermal energy storage. ACS Omega 2019, 4, 2964–2972. [Google Scholar] [CrossRef]
- Wu, S.F.; Yan, T.; Kuai, Z.H.; Pan, W.G. Thermal conductivity enhancement on phase change materials for thermal energy storage: A review. Energy Storage Mater. 2020, 25, 251–295. [Google Scholar] [CrossRef]
- Mahnaz, F.; Fathallah, K.; Keyvan, R. Preparation of paraffin/silica-graphene shape-stabilized composite phase change materials for thermal energy storage. J. Mater. Sci. Mater. Electron. 2022, 33, 12846–12856. [Google Scholar]
- Liu, Y.; Zheng, R.W.; Tian, T.; Li, J. Characteristics of thermal storage heat pipe charged with graphene nanoplatelets enhanced organic phase change material. Energy Convers. Manag. 2022, 267, 115902. [Google Scholar] [CrossRef]
- Jin, W.Z.; Jiang, L.H.; Chen, L.; Yin, T.J.; Gu, Y.; Guo, M.Z.; Yan, X.C.; Ben, X.Q. Enhancement of thermal conductivity by graphene as additive in lauric-stearic acid/treated diatomite composite phase change materials for heat storage in building envelope. Energy Build. 2021, 246, 111087. [Google Scholar] [CrossRef]
- Ren, M.; Zhao, H.; Gao, X.J. Effect of modified diatomite based shape-stabilized phase change materials on multiphysics characteristics of thermal storage mortar. Energy 2022, 241, 122823. [Google Scholar] [CrossRef]
- Hong, Y.X.; Yan, W.T.; Du, J.; Li, W.Y.; Xu, T.; Ye, W.B. Thermal performances of stearic acid/sepiolite composite form-stable phase change materials with improved thermal conductivity for thermal energy storage. J. Therm. Anal. Calorim. 2021, 143, 3317–3329. [Google Scholar] [CrossRef]
- Xie, N.; Gao, X.N.; Zhong, Y.; Ye, R.D.; Chen, S.; Ding, L.X.; Zhong, T.M. Enhanced thermal performance of Na2HPO4·12H2O composite phase change material supported by sepiolite fiber for floor radiant heating system. J. Build. Eng. 2022, 56, 104747. [Google Scholar] [CrossRef]
- Zhao, S.Y.; Li, J.H.; He, M.Y.; Song, S. Effects of Dopamine-Modified and Organic Intercalation on the Thermophysical Properties of Octadecane/Expanded Vermiculite Composite Phase Change Materials. ACS Omega 2022, 7, 13538–13545. [Google Scholar] [CrossRef]
- Deng, Y. Effect of Ag nanowires on crystallization behavior of polyethylene glycol/expanded vermiculite composite phase change material. J. Energy Storage 2021, 34, 102223. [Google Scholar] [CrossRef]
- Shi, J.B.; Li, M. Synthesis and characterization of polyethylene glycol/modified attapulgite form-stable composite phase change material for thermal energy storage. Sol. Energy 2020, 205, 62–73. [Google Scholar] [CrossRef]
- Wen, R.L.; Xu, Y.F.; Xu, Y.G.; Sun, Z.H.; Zhang, L.P.; Qiao, J.X.; Wu, X.W.; Min, X.; Huang, Z.H. Attapulgite: A promising natural mineral as carrier material for fatty acids phase change material. J. Therm. Anal. Calorim. 2022, 147, 7203–7212. [Google Scholar] [CrossRef]
- Xu, D.W.; Yang, H.M.; Ouyang, J.; Fu, L.J.; Chen, D.L. Lauric Acid Hybridizing Fly Ash Composite for Thermal Energy Storage. Minerals 2018, 8, 161. [Google Scholar] [CrossRef]
- Genc, M.; Gen, Z.K. Microencapsulated myristic acid-fly ash with TiO2 shell as a novel phase change material for building application. J. Therm. Anal. Calorim. 2018, 131, 2373–2380. [Google Scholar] [CrossRef]
- Liu, P.; Gu, X.B.; Zhang, Z.K.; Rao, J.; Shi, J.P.; Wang, B.; Bian, L. Capric Acid Hybridizing Fly Ash and Carbon Nanotubes as a Novel Shape-Stabilized Phase Change Material for Thermal Energy Storage. ACS Omega 2019, 4, 14962–14969. [Google Scholar] [CrossRef]
- Qiu, F.; Song, S.K.; Li, D.N.; Liu, Y.; Wang, Y.Q.; Dong, L.J. Experimental investigation on improvement of latent heat and thermal conductivity of shape-stable phase-change materials using modified fly ash. J. Clean. Prod. 2020, 246, 118952. [Google Scholar] [CrossRef]
- Hekimoglu, G.; Nas, M.; Ouikhalfan, M.; Sari, A.; Kurbetci, S.; Tyagi, V.V.; Sharma, R.K.; Saleh, T.A. Thermal management performance and mechanical properties of a novel cementitious composite containing fly ash/lauric acid-myristic acid as form-stable phase change material. Constr. Build. Mater. 2021, 274, 122105. [Google Scholar] [CrossRef]
- Arulselvan, S.P.; Gurusamy, S.V. Characterization of fly ash cenosphere-capric acid composite as phase change material for thermal energy storage in buildings. Energy Sources Part A 2022, 44, 2088–2102. [Google Scholar] [CrossRef]
- Naresh, R.; Parameshwaran, R.; Ram, V.V.; Srinivas, P.V. Study on thermal energy storage properties of bio-based n-dodecanoic acid/fly ash as a novel shape-stabilized phase change material. Case Stud. Therm. Eng. 2022, 30, 101707. [Google Scholar] [CrossRef]
- Liu, L.; Peng, B.; Yue, C.S.; Guo, M.; Zhang, M. Low-cost, shape-stabilized fly ash composite phase change material synthesized by using a facile process for building energy efficiency. Mater. Chem. Phys. 2019, 222, 87–95. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, Y.H.; Wu, M.; Wang, S. Fly ash/paraffin composite phase change material used to treat thermal and mechanical properties of expansive soil in cold regions. J. Renew. Mater. 2022, 10, 1153–1173. [Google Scholar] [CrossRef]
- Gu, X.B.; Liu, P.; Peng, L.H.; Zhang, Z.K.; Bian, L.; Wang, B. Low cost, eco-friendly, modified fly ash-based shape-stabilized phase change material with enhanced thermal storage capacity and heat transfer efficiency for thermal energy storage. Sol. Energy Mater. Sol. Cells 2021, 232, 111343. [Google Scholar] [CrossRef]
- Lv, P.Z.; Liu, C.Z.; Rao, Z.H. Experiment study on the thermal properties of paraffin/kaolin thermal energy storage form-stable phase change materials. Appl. Energy 2016, 182, 475–487. [Google Scholar] [CrossRef]
- Ramakrishnan, S.; Wang, X.; Sanjayan, J.; Wilson, J. Assessing the feasibility of integrating form-stable phase change material composites with cementitious composites and prevention of PCM leakage. Mater. Lett. 2017, 192, 88–91. [Google Scholar] [CrossRef]
- Feng, Y.H.; Wei, R.Z.; Huang, Z.; Zhang, X.X.; Wang, G. Thermal properties of lauric acid filled in carbon nanotubes as shape-stabilized phase change materials. Phys. Chem. Chem. Phys. 2018, 20, 7772–7780. [Google Scholar] [CrossRef]
- Shen, Q.; Ouyang, J.; Zhang, Y.; Yang, H.M. Lauric acid/modified sepiolite composite as a form-stable phase change material for thermal energy storage. Appl. Clay Sci. 2017, 146, 14–22. [Google Scholar] [CrossRef]
- Ma, F.K.; Liu, L.Q.; Ma, L.Q.; Zhang, Q.; Li, J.N.; Jing, M.; Tan, W.J. Enhanced thermal energy storage performance of hydrous salt phase change material via defective grapheme. J. Energy Storage 2022, 48, 104064. [Google Scholar] [CrossRef]
- Jin, W.Z.; Jiang, L.H.; Chen, L.; Gu, Y.; Guo, M.Z.; Han, L.; Ben, X.Q.; Yuan, H.H.; Lin, Z.X. Preparation and characterization of capric-stearic acid/montmorillonite/graphene composite phase change material for thermal energy storage in buildings. Constr. Build. Mater. 2021, 301, 124102. [Google Scholar] [CrossRef]
- Rao, Z.H.; Zhang, G.T.; Xu, T.T.; Hong, K. Experimental study on a novel form-stable phase change materials based on diatomite for solar energy storage. Sol. Energy Mater. Sol. Cells 2018, 182, 52–60. [Google Scholar] [CrossRef]
- Ma, L.Y.; Wang, Q.W.; Li, L.P. Delignified wood/capric acid-palmitic acid mixture stable-form phase change material for thermal storage. Sol. Energy Mater. Sol. Cells 2019, 194, 215–221. [Google Scholar] [CrossRef]
- Fang, G.; Li, H.; Cao, L.; Shan, F. Preparation and thermal properties of form-stable palmitic acid/active aluminum oxide composites as phase change materials for latent heat storage. Mater. Chem. Phys. 2012, 137, 558–564. [Google Scholar] [CrossRef]
- Lv, S.L.; Zhu, N.; Feng, G.H. Eutectic mixtures of capric acid and lauric acid applied in building wallboards for heat energy storage. Energy Build. 2006, 38, 708–711. [Google Scholar]
- Feldman, D.; Banu, D.; Hawes, D. Development and application of organic phase change mixtures in thermal storage gypsum wallboard. Sol. Energy Mater. Sol. Cells 1995, 36, 147–157. [Google Scholar] [CrossRef]
- Karaman, S.; Karaipekli, A.; Sari, A.; Bicer, A. Polyethylene glycol (PEG)/diatomite composite as a novel form-stable phase change material for thermal energy storage. Sol. Energy Mater. Sol. Cells 2011, 95, 1647–1653. [Google Scholar] [CrossRef]
- Sari, A.; Karaipekli, A.; Kaygusuz, K. Capric acid and myristic acid for latent heat thermal energy storage. Energy Sources Part A 2008, 30, 1498–1507. [Google Scholar] [CrossRef]
- Feldman, D.; Banu, D. DSC analysis for the evaluation of an energy storing wallboard. Thermochim. Acta 1996, 272, 243–251. [Google Scholar] [CrossRef]
- Genc, Z.K.; Canbay, C.A.; Acar, S.S.; Sekerci, M.; Genc, M. Preparation and thermal properties of heterogeneous composite phase change materials based on camphene-palmitic acid. J. Therm. Anal. Calorim. 2015, 120, 1679–1688. [Google Scholar] [CrossRef]
- Karaipekli, A.; Sari, A. Capric–myristic acid/vermiculite composite as form-stable phase change material for thermal energy storage. Sol. Energy 2009, 83, 323–332. [Google Scholar] [CrossRef]




| Item | SiO2 | Al2O3 | Fe2O3 | TiO2 | K2O | Na2O | CaO | MgO | Others |
|---|---|---|---|---|---|---|---|---|---|
| FA | 49.120 | 37.042 | 4.859 | 1.555 | 1.145 | 0.751 | 2.734 | 0.614 | 2.18 |
| MFA [37] | 52.490 | 37.433 | 4.032 | 1.564 | 1.118 | 0.784 | 0.909 | 0.534 | 1.136 |
| Step | Sample Name | Composition Ratio (wt. %) | Leakage Ratio (%) | Leakage Area (cm2) | Deformation (Yes/No) | Leakage (Yes/No) |
|---|---|---|---|---|---|---|
| 2 | S1-1 | Pure LA | 45.57 | 122.66 | Yes | Yes |
| 2 | S1-2 | 60 LA + 40MFA | 19.76 | 45.34 | Yes | Yes |
| 2 | S1-3 | 50 LA + 50MFA | 11.37 | 38.74 | Yes | Yes |
| 2 | S1-4 | 40 LA + 60MFA | 5.70 | 29.45 | Yes | Yes |
| 2 | S1-5 | 30 LA + 70MFA | 1.37 | 6.83 | No | Yes |
| 2 | S1-6 | 20 LA + 80MFA | 0 | 0 | No | No |
| 2 | S2-1 | 30 LA + 70MFA/1G | 1.23 | 6.38 | No | Yes |
| 2 | S2-2 | 30 LA + 70MFA/3G | 0.77 | 5.62 | No | Yes |
| 2 | S2-3 | 30 LA + 70MFA/5G | 0.23 | 1.18 | No | Yes |
| 2 | S2-4 | 30 LA + 70MRFA/7G | 0 | 0 | No | No |
| 3 | S3-1 | 27.5 LA + 72.5MFA/1G | 0 | 0 | No | No |
| 3 | S3-2 | 27.5 LA + 72.5MFA/3G | 0 | 0 | No | No |
| 3 | S3-3 | 27.5 LA + 72.5MFA/5G | 0 | 0 | No | No |
| Samples | Loading Rate of LA (%) | Melting Temperature (°C) | Solidifying Temperature (°C) | Measured Latent Heat of Melting (J/g) | Measured Latent Heat of Solidification (J/g) | Calculated Loading Rate of LA (%) |
|---|---|---|---|---|---|---|
| LA | 100 | 44.29 | 41.23 | 179.6 | 177.9 | 100 |
| LA/raw FA (leakage) | 25% | 45.74 | 41.39 | 44.93 | 43.60 | 24.04 |
| LA/MFA | 30 | 45.39 | 40.82 | 49.71 | 47.86 | 27.22 |
| LA/MFA/G (5 wt. %) | 27.5 | 45.38 | 40.22 | 41.08 | 39.26 | 22.87 |
| Item | Melting Temperature (°C) | Solidifying Temperature (°C) | Latent Heat of Melting (J/g) | Latent Heat of Solidification (J/g) | References |
|---|---|---|---|---|---|
| Palmitic acid (25 wt. %)/active aluminum oxide | 74.13 | 59.57 | 28.56 | 17.53 | [51] |
| Capric–lauric acid (26 wt. %)/gypsum | 19.11 | —— | 35.24 | —— | [52] |
| Propyl palmitate(25–30 wt. %)/gypsun | 19.0 | 16.0 | 40.0 | [53,54] | |
| Capric–palmitic acid (25 wt. %)/gypsum wallboard | 21.12 | 21.46 | 36.23 | 38.28 | [55] |
| Emerest 2326 (25.7 wt. %)/gypsum | 16.32 | 19.7 | 34.77 | 33.97 | [56] |
| Camphene–palmitic acid (55 wt. %)/FA | 68.32 | 60.46 | 37.08 | 34.41 | [57] |
| MA(13.11)/TiO2–FA | 52.22 | 26.68 | 23.43 | 22.57 | [34] |
| Capric–myristic acid (20 wt. %)/vermiculite +EG (2 wt. %) | 19.7 | 17.1 | 26.9 | missing | [58] |
| LA(19.3 wt. %)/RFA | 41.34 | 42.75 | 34.09 | 32.97 | [33] |
| LA(44.8 wt. %)/CNT | 35.02 | missing | 42.61 | missing | [45] |
| LA/MFA/G(5 wt. %) | 45.38 | 40.22 | 41.08 | 39.26 | This study |
| Sample | Heat Time (min) | Heat Storing Time (min) | Freezing Time (min) | Heat Releasing Time (min) | Improved Heat Rate (%) | Improved Freezing Rate (%) |
|---|---|---|---|---|---|---|
| LA | 18 | 9 | 36 | 13 | -- | -- |
| LA/MFA | 15 | 5 | 17 | 4.5 | 44.44 | 65.38 |
| LA/MFA/G (5 wt. %) | 13 | 3.5 | 12 | 2.5 | 61.11 | 80.77 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, P.; Cui, X.; Wang, Y.; Zhang, Z.; Rao, J.; Jiang, S.; Gu, X. Preparation and Characterization of Lauric Acid/Modified Fly Ash/Graphene Composite as Low-Cost and Eco-Friendly Phase Change Materials for Thermal Energy Storage. Energies 2023, 16, 5666. https://doi.org/10.3390/en16155666
Liu P, Cui X, Wang Y, Zhang Z, Rao J, Jiang S, Gu X. Preparation and Characterization of Lauric Acid/Modified Fly Ash/Graphene Composite as Low-Cost and Eco-Friendly Phase Change Materials for Thermal Energy Storage. Energies. 2023; 16(15):5666. https://doi.org/10.3390/en16155666
Chicago/Turabian StyleLiu, Peng, Xinglan Cui, Yajing Wang, Zhikai Zhang, Jun Rao, Shuai Jiang, and Xiaobin Gu. 2023. "Preparation and Characterization of Lauric Acid/Modified Fly Ash/Graphene Composite as Low-Cost and Eco-Friendly Phase Change Materials for Thermal Energy Storage" Energies 16, no. 15: 5666. https://doi.org/10.3390/en16155666
APA StyleLiu, P., Cui, X., Wang, Y., Zhang, Z., Rao, J., Jiang, S., & Gu, X. (2023). Preparation and Characterization of Lauric Acid/Modified Fly Ash/Graphene Composite as Low-Cost and Eco-Friendly Phase Change Materials for Thermal Energy Storage. Energies, 16(15), 5666. https://doi.org/10.3390/en16155666







