Abstract
This work is aimed at solving the problem of converting diesel power drives to diesel–hydrogen fuels, which are more environmentally friendly and less expensive alternatives to diesel fuel. The method of increasing the energy efficiency of diesel fuels has been improved. The thermochemical essence of using methanol as an alternative fuel to increase energy efficiency based on the provisions of thermotechnics is considered. Alternative methanol fuel has been chosen as the initial product for the hydrogen conversion process, and its energy value, cost, and temperature conditions have been taken into account. Calculations showed that the caloric effect from the combustion of the converted mixture of hydrogen H2 and carbon monoxide CO exceeds the effect from the combustion of the same amount of methanol fuel. Engine power and fuel energy were increased due to the thermochemical regeneration of engine exhaust gas heat. An experimental setup was created to study the operation of a converted diesel engine on diesel–hydrogen products. Experimental studies of power and environmental parameters of a diesel engine converted for diesel–hydrogen products were performed. The studies showed that the conversion of diesel engines to operate using diesel–hydrogen products is technically feasible. A reduction in energy consumption was accompanied by an improvement in the environmental performance of the diesel–hydrogen engine working together with a chemical methanol conversion thermoreactor. The formation of carbon monoxide occurred in the range of 52–62%; nitrogen oxides in the exhaust gases decreased by 53–60% according to the crankshaft speed and loading on the experimental engine. In addition, soot emissions were reduced by 17% for the engine fueled with the diesel–hydrogen fuel. The conversion of diesel engines for diesel–hydrogen products is very profitable because the price of methanol is, on average, 10–20% of the cost of petroleum fuel.
1. Introduction
Reducing the cost of fuels, increasing energy, and improving the environmental safety of cars are the main problems in the field of motor transport operation today. Methods for solving these problems are varied in terms of their technical and economic results [1,2,3].
One of the most promising ways to solve the problems of energy supply and improve the environmental safety of automobile transport is the development of transport hydrogen energy [4].
Using hydrogen as the main fuel or as an active additive are two possibilities with this method. The lack of an economically viable, flexible-to-use set of hydrogen storage facilities is the most significant impediment to implementing this technology in motor vehicles. This refers to methods and devices for storing hydrogen as an individual substance and environments in which hydrogen is stored in a chemically bound state [5].
In all cases, the processes of hydrogen accumulation are accompanied by energy spending and require investments in the creation of an accumulation environment and a storage system as a whole. Therefore, the implementation of any scheme for hydrogen use depends on the choice of an economically justified and efficient method of onboard storage of this gas [6].
The following methods of onboard hydrogen storage are fundamentally possible at present [7,8]:
- In the solid-phase bound state in metal hydrides;
- In a gaseous state under pressure in containers of various types;
- In a liquid state in cryogenic tanks;
- In a chemically bound state in liquid media.
There has not been a unified approach to choosing an acceptable method for storing hydrogen in cars. The following ways have been most adapted: as compressed gas in high-pressure cylinders, in a liquefied state in cryogenic tanks, and in a bound state in metal hydride batteries (Table 1).

Table 1.
Modern methods of hydrogen storage in automobiles.
Let us analyze modern hydrogen storage methods onboard automobiles in more detail.
Storage of hydrogen in metal hydride batteries
The experimental, industrial, and commercial samples of created hydrogen metal hydride batteries let us make conclusions about the advisability of their use in automobiles as well as about their drawbacks. The most important advantages of metal hydride batteries are their ability to use the low potential heat of the engine exhaust gases to ensure their operation and the high safety of hydrogen storage. There is no evidence of an enormous mass of gas, only the slow decomposition of hydrides on cooling with a slight release of hydrogen in the case of an accidental depressurization of the battery. The main difficulties holding back the widespread introduction of metal hydride batteries at present are: the relatively significant mass of the storage devices, the necessity for the compression of hydrogen to compression ratios of 50; and the high cost of hydrogen battery alloys [9,10].
Numerous modern studies and developments are directed at overcoming these difficulties. One of the most promising areas of development is the creation of storage devices based on composite alloys. Composite systems have high thermal conductivity, low hydraulic resistance, and high resistance to structural failure in the process of multicycle reversible hydrogen sorption. The latter factor is especially important for automotive hydrogen storage systems, since it excludes the removal of fine fractions by a hydrogen flow, which is typical for metal hydride batteries.
For these systems, the consumption characteristics for hydrogen correspond to the engine characteristics in the main modes of the driving cycle. It is possible to maintain the hydrogen pressure in the system at a level of 0.3–0.5 MPa for 90% of the battery discharge time by changing the flow rate of exhaust gases. The safety of battery operation is ensured because the temperature on the element surface at the end of the discharge does not exceed 100 °C [11].
Hydrogen storage in high-pressure cylinders
Using metal–plastic cylinders designed for natural gas to accumulate hydrogen up to 2.7% of the natural gas mass and store it at high pressure (up to 20 MPa) is a possibility. Therefore, a new generation of cylinders will appear. The pressure will approach 80–100 MPa, with higher pressures possible. The transition from steel cylinders to more advanced plastic and metal–plastic ones, already mass-produced by the industry, increases the specific energy consumption by up to three times [12,13].
However, it should be noted that with increasing pressure, the energy costs for compressing the gas increase. The work required to compress hydrogen, referred to its lower calorific value (120 MJ/kg), is almost four times higher than that for natural gas at the same compression ratios. For currently existing compressors, the energy costs for compressing hydrogen from 0.1 to 20 MPa reach 9–10% of its net calorific value [14].
Liquid hydrogen storage
If cryogenic systems for storing liquid hydrogen are used, the energy intensity of a storage unit will be at its maximum limit. Liquid hydrogen has a boiling point of 252 °C and a density of 71 kg/m3, while under a pressure of 20 MPa, its density is only 16.7 kg/m3. Therefore, liquid hydrogen is more compact than compressed hydrogen. However, the large volume of the system requires a serious strengthening of the car body and reduces its usable volume. When using cylinders with multilayer insulation, the density of hydrogen at a pressure of 0.5 MPa is 130–150 kg/m3. The creation of comparatively small tanks for storing liquid hydrogen onboard automobiles s, as well as their refueling systems, is a self-sustained and laborious task [15,16].
At the same time, the leakage of hydrogen from cylinders remains a significant problem. Even for cylinders with multilayer insulation, the loss of hydrogen due to evaporation is 0.5–1.0% per day. Therefore, the efficiency and cost of accumulating hydrogen in a liquefied state for use in automobiles are determined by the entire technological chain from refueling to the engine combustion chamber. It should be noted that even the cryogenic method, which is the best in terms of energy density, is several times inferior to petroleum fuels in this indicator, not to mention storage systems. It is also necessary to solve the problem of safe storage of hydrogen onboard the car.
Accumulation of hydrogen in a chemically bound state in liquid agent
It follows from the analysis that the currently available methods of accumulating hydrogen onboard an automobile have several serious shortcomings.
Therefore, it is worthwhile to consider a safer and more usable raw material, from which it would be possible to obtain gaseous products with a high hydrogen content directly onboard a car by thermochemical transformation.
A large group of methods for accumulating and transporting hydrogen, which has been intensively studied recently, refers to hydrogen in a chemically bound state in the form of liquid chemical compounds (ammonia, methanol, ethanol, etc.). These hydrogen-carrying compounds allow simple hydrogenation reactions at moderate temperatures. When accumulating hydrogen in the form of ammonia, methanol, ethanol, etc., the production of these liquid hydrogen carriers is conducted at large enterprises, and the cost of such hydrogen accumulation is determined by the final costs of their production and the processes of consumption of the produced hydrogen [8,17,18].
For the transport complex, systems for accumulating and transporting hydrogen in the form of liquid chemical compounds may turn out to be more efficient than existing methods for storing and transporting gaseous and liquid hydrogen.
2. Materials and Methods
2.1. Hydrogen Generation in Thermochemical Reactors of the Onboard Automobile System
In automobile technology, studies of liquid agents as carriers of hydrogen have been reflected relatively recently. Nevertheless, some research has already been accumulated in such agent elaborations and systems for onboard hydrogen generation together with them [19,20,21].
In principle, both gaseous and liquid hydrocarbons can be used to produce hydrogen. Hydrogen-containing gas can be obtained from any petroleum product, including crude oil. A liquid as a carrier of hydrogen in automobiles is preferable from the point of view of ensuring the autonomy of a car. In addition, the mass index of current automobile gas tanks that could be converted to store liquid hydrogen, is sufficiently high and amounts to about 15%.
However, heavy oil products (diesel fuel, fuel oil, etc.) contain relatively little hydrogen and increased sulfur content and coking capacity [22], greatly complicating the technology of their gasification. It is more advisable to orient hydrogen generators to use light gasoline fractions as a raw material. Significant limitations are imposed by the conversion temperature i.e., 1200 °C and higher without a catalyst.
Therefore, several serious problems arise during the creation of hydrogen production systems, which hinder hydrogen’s introduction into the industry. The high-temperature level of conversion of this type of liquid hydrocarbons necessitates additional expenditures of thermal energy for the conversion process. In addition, the presence of sulfur in motor fuel excludes the possibility of using catalysts, and the high relative content of inert components in the composition of the target conversion products creates additional difficulties in their use [23].
The noted difficulties associated with the organization of the conversion process of traditional motor fuels necessitate the search for other sources of raw materials for the production of hydrogen-containing gases.
Hydrocarbon compounds have a simpler molecular structure than motor fuels and a lower dissociation temperature. At the same time, compounds with a temperature of dissociation and thermal effects in endothermic decomposition reactions that are compatible with the temperature and energy of engine exhaust gases are preferable [24]. In this case, there is a real possibility of using the free thermal energy of the exhaust gases to organize the conversion process without an additional heat source.
Alcohols and a number of other compounds have similar properties [25]. Methyl alcohol has long been used in automotive engines as a partial substitute for conventional fuels. The use of liquid methanol as a carrier of hydrogen in automobiles is considered the most appropriate, due to the increased content of hydrogen in this carrier. The molecular fraction of hydrogen in methanol is 2/3. The mass index of the hydrogen storage medium in the form of liquid methanol is about 8.5 kg/kg; that is, 8.5 kg of methanol contains 1 kg of hydrogen [26]. The mass content of hydrogen per unit volume of liquid methanol is almost 1.5 times higher than the density of liquid cryogenic hydrogen. For the operating conditions of automobiles, it is also important that the storage of hydrogen in a chemically bound state in a liquid medium ensures high safety in emergency situations.
In addition, complete conversion of methanol can be achieved at operating temperatures of about 300 °C [27], which determines the minimum possible temperature limit for the coolant (engine exhaust gases), below which the implementation of an efficient conversion process is impossible (Table 2). Such conditions predetermine the need to place the reactor close to the exhaust manifold of the engine.

Table 2.
Conversion temperature of the main alternative fuels that can be used in internal combustion engines.
The conversion of methanol into a hydrogen-containing gas using a small-sized thermochemical reactor is fundamentally feasible on any transport internal combustion engine type. The greatest depth of chemical reactions, which determines the maximum degree of methanol conversion, can be achieved at a certain level of energy balance and temperature regime of the reactor installed in the exhaust system of the engine.
Therefore, for the efficient implementation of the conversion process, it is required that the heating coolant (exhaust gases) have the potentially required levels of thermal power and temperature. The need for an intensive heat supply to the reaction zone is caused, first of all, by the manifestation of a high endothermic effect of the methanol conversion reaction. Additional thermal power is also required for organizing other stages of the conversion process: for preheating methanol to the boiling point, for its evaporation, and for raising the vapor temperature to the level of the working conversion temperature. The total cost of thermal energy for the organization of a fully completed conversion process of 1 kg of methanol reaches approximately 7 MJ.
A significant feature of the onboard synthesis of a hydrogen-containing product from methanol is the manifestation of an energy-saving effect. As a result of endothermic transformation in the reactor, the chemical energy of the converted product relative to the initial product (methanol) is equal to the amount of the recovered energy of the exhaust gases. The method of thermal energy utilization removed with exhaust gases is called the thermochemical recovery of exhaust gas heat since it is based on the principle of thermochemical conversion of the energy of the original fuel to a higher energy level using the heat of exhaust gases.
The thermochemical essentiality of the increasing energy of the initial fuel represents the basic provisions of thermotechnics. This will be shown on the basis of the study of the thermal effects from the combustion of methanol (CH3OH), which is based on two methods. According to the first and second methods of CH3OH oxidation, the final and initial state of the system are the same: initial–1 kmol CH3OH, final–1 kmol CO2 and 2 kmol H2O.
According to the first method, CH3OH is burned directly in the combustion chamber of an engine [28]
where QM–exothermic thermal effect from the combustion of air-methanol mixture, QM = 629,440 kJ.
As a result of Equation (1) by the first method, 3 kmol of combustion products are formed.
According to the second two-stage method of CH3OH conversion, alcohol decomposed at first CH3OH + 3/2O2 → CO2 + 2H2O − QM
CH3OH → CO + 2H2 − QC,
It produces 1 kmol CO and 2 kmol H2 with endothermic heat conversion QC.
Then burn in air 3 kmol obtained by Equation (2) of conversion products of CH3OH:
The total thermal effect:
Q∑ = 481,500 + 282,600 = 764,100 kJ/kmol,
The thermal effects for Equations (3) and (4) are given according to the data [29]. Then, according to Hess’s law, the total thermal effects for different routes of CH3OH oxidation must coincide
629,440 kJ/kmol = 764,100 kJ/kmol − QC.
Hence, the thermal endothermic effect of the conversion (CH3OH dissociation reaction) will be QC = 134,660 kJ/kmol.
Therefore, calculation showed that the caloric effect from the combustion of the converted mixture of H2 and CO exceeds the effect from the combustion of the same amount of non-convertible CH3OH (source fuel) by QC = 134,660 kJ/kmol (i.e., 21.4%), and it corresponds to the decomposition of methanol energy.
Thus, the onboard hydrogen synthesis implementation process from methanol contributes to an increase in the efficiency of using the energy of its initial carrier (methanol) and as a result, the efficiency of the engine operating cycle.
2.2. Improvement of Chemical Kinetics of Fuel Oxidation Processes in Diesel Engines
The synthesis of hydrogen from methanol seems to be the most appropriate and relevant method for diesel engines.
Modern diesel engines, along with well-known advantages, also have significant disadvantages, primarily related to the problem of meeting new environmental requirements [30]. Enforcing environmental demands in modern diesel engines is so expensive that most automakers have announced they are phasing out diesel engines. The use of hydrogen from methanol is a practical alternative concept for the development and improvement of the working cycle of a diesel engine without a significant change in its basic design based on methods of physicochemical influence on intra-cylinder processes.
According to the modern provisions of the theory of combustion, the process of hydrocarbon oxidation is a set of chain-thermal reactions. In this case, a number of sequential and parallel chain reactions proceed at higher rates than the direct reaction according to the stoichiometric equation. This is explained by the low activation energy of the components formed during the multistage reaction.
Thus, it is possible to effectively influence the chemical kinetics of fuel oxidation processes by changing the reactivity of the reaction medium, i.e., by purposefully increasing the active particles in it. Increasing the concentration of active particles in the reacting hydrocarbon-but-air medium in the combustion chamber seems to be possible by introducing products with reactive components into its volume. The introduction of even small doses of reactive products into the fuel–air mixture will significantly expand the limits of optimal control of the processes of the diesel engine operating cycle, which determine its environmental and economic indicators.
Hydrogen occupies a special place among the activating agents used in engines. The high efficiency of its impact on the engine working processes is associated, first of all, with the unusually high rate of combustion of this gas. Regarding diesel engines, it is possible to consider using hydrogen both as an independent type of fuel, when diesel fuel is used only as a pilot fuel, and as an additive to the base fuel.
The efficiency of the fuel–air mixture activation by hydrogen or gas mixtures containing hydrogen depends on the relative hydrogen content in the composition of the working fluid and the gas-dynamic state of the reacting agent. They determine the temporal and spatial extent of the activated combustion chamber zone, the total effect, and the activation effect on the output indicators of the diesel engine, which determines its environmental and economic qualities. As a result of the action of the activating components, the activation energy of the base mass of the fuel–air mixture, which characterizes its reactivity, decreases. This is due to a decrease in the participation in the total chemical process of spontaneous generation reactions that require high activation energies.
The influence of hydrogen on the processes of nitrogen oxidation and soot emission in a diesel engine is manifested at various stages of the operating cycle. Its reactive activity makes it possible to expand the flammability limits of the mixture, thereby contributing to the effective burnout of zones with both rich and lean fuel–air mixtures. For diesel engine operating cycle conditions, ignition is multi-focal. The presence of reactive products in the mixture reduces the thermodynamic inhomogeneity of the reacting medium in the volume of the combustion chamber due to the increase in ignition zones. At the same time, the number of zones with a local maximum temperature level, which is the “suppliers” of NOx, decreases [31].
The activation of pre-flame hydrogen reactions and subsidence in the duration of the induction period contribute to the fuel’s portion reduction. This fuel takes part in the ignition, reducing the amount of released heat in the volumetric stage of rapid combustion. As a result, it reduces the maximum cycle temperature and, consequently, the rate of nitrogen oxidation.
The presence of an additional amount of hydrogen in the reaction zone leads to a decrease in the output of NOx with exhaust gases since during the combustion of the fuel–air mixture, in addition to oxidative reactions, nitrogen reduction takes place.
2H2 + 2NO → 2H2O + N2
At the same time, the participation of chemically unbound hydrogen in the diffusion combustion of fuel counteracts the process of soot emission. Free hydrogen exerts its influence on the mechanism of soot release in all phases of the formation and burnout of carbon particles. Hydrogen intensifies the processes of soot burning due to the forming water, which acts as a carbon oxidizer in the wet gasification reaction. The reduction in soot emission in the fuel combustion processes contributes to a decrease in heat losses per cycle and a corresponding increase in the fuel efficiency of a diesel engine [32].
The above analysis of the mechanisms of the influence of hydrogen-containing additives on the in-cylinder processes of a diesel engine is not exhaustive. However, it should be emphasized, with all the advantages, the use of hydrogen in diesel engines is associated with several significant difficulties. The main problem of using hydrogen as a motor fuel is the lack of infrastructure for its production in the required quantities. In addition, to date, the industry has not mastered the production of small-sized energy-intensive means of storing hydrogen onboard automobiles.
It follows from the above that participation in the hydrogen-working cycle contributes to a qualitative improvement in the environmental performance of a diesel engine, at least in terms of two main components of exhaust gases, nitrogen oxide and soot, which, as you know, together determine the total toxicity of diesel exhaust. Naturally, the extent of this improvement can be quantified by direct experiments on a diesel engine.
The processes of conversion of mono-hydrocarbons (methane, methanol, etc.), which have a relatively simple chemical structure, have been studied in the most detail in the literature [33]. In contrast, gasoline or diesel fuel are not mono-component compounds, and their composition consists of a mixture of several saturated hydrocarbons CnH2n+1 with a carbon chain length n = 7–10. None of the components has a predominant content, and for each grade of gasoline or diesel fuel, there is a particular chemical formula. Therefore, the process of conversion of petroleum fuel, containing in its composition a large number of hydrocarbon fractions with different physicochemical properties, is a complex chemical process characterized by multi-stage chemical transformations of hydrocarbons [34]. The final efficiency of the conversion process is determined by the energy costs associated with its implementation and the content of the target product, hydrogen, in the conversion products.
Despite the noted difficulties, using the accumulated experience, the authors of this article developed an experimental version of the onboard system for producing hydrogen. The main functional link of this system is a thermochemical reactor, where fuel is converted into a hydrogen-containing gas at high temperatures.
2.3. Experimental Onboard Hydrogen Generation System Based on Methanol Conversion
The aim of this experimental study is using alcohol products in alternative hydrogen mixtures in existing diesel engines to improve their environmental performance and save petroleum fuel.
An evaluation of the thermochemical method of hydrogen production in the operating cycle of the diesel type D21A was performed on a stand in the laboratory. A brief technical description of the experimental diesel–hydrogen engine D21A1 is shown in Table 3.

Table 3.
Brief technical characteristics of the experimental diesel–hydrogen engine D21A1.
The scheme of the experimental stand based on the diesel engine D21A1 for research of indicators on the products of methanol conversion is shown in Figure 1 and Figure 2.

Figure 1.
Scheme for the study parameters of the work on the experimental diesel–hydrogen engine D21A1.

Figure 2.
Appearance of the experimental stand based on the engine D21A1 for the study of parameters on diesel–hydrogen fuel mixtures: 1—tank for diesel fuel; 2—autonomous heater; 3—load compressor; 4—gearbox; 5—D21A1 diesel engine; 6—tank for methanol; 7—an exhaust manifold of the engine; 8—thermochemical reactor; 9—thermal insulation of the exhaust manifold; 10—gas meter.
The diesel engine/5/ (Figure 2) was connected using a gearbox/4/ to a load compressor/3/. The stand intake system included an air receiver equipped with a gas meter/10/ with an air filter. A methanol conversion reactor/8/ was installed in the exhaust system of the stand, connected to the diesel exhaust manifold and the exhaust gas removal system/7/ of the engine. The system for supplying the stand with diesel fuel included a tank/1/ and equipment installed on the diesel engine: fuel filter, high-pressure fuel pump, and fuel injectors.
The exhaust gas thermal energy of the engine from the exhaust manifold/7/ was used to maintain the required thermal regime of the heated engine conversion in the thermochemical reactor/8/. The experimental engine could start in two ways in a cold start. According to the first method, the engine could start on 100% diesel fuel. According to the second method, an autonomous diesel heater/2/ was used to preheat the thermochemical reactor for a cold engine running on the diesel–hydrogen mixture.
The hydrogen supply system (Figure 3) was arranged in such away. Liquid methanol was supplied by a fuel pump through a filter/3/ to a reactor/6/ from the tank/1/ (alloyed steel). The heat transferred to the reactor from the exhaust gases of exhaust tract/2/. The temperature was measured by thermocouples inside. Next, methanol in the gas phase fed into the gas reducer/11/ through the heat exchange module of the heating coolant of the engine cooling system. The control and regulation of the gaseous methanol conversion products supply to the diesel inlet was implemented by a throttle device. In the exhaust manifold behind the reactor, gas sampling units/4/ were mounted for connecting gas analysis equipment.

Figure 3.
Appearance of the hydrogen supply system for the study of the experimental stand performance based on the diesel engine D21A1: 1—tank for liquefied methanol; 2—exhaust manifold of the engine; 3—filter; 4—gas collector; 5—thermal insulation of the exhaust manifold; 6—thermochemical reactor (thermal insulation is partially dismantled); 7—D21A1 diesel engine; 8—gas meter; 9—viewing window in the supply tube of gaseous methanol; 10—high-pressure diesel pump; 11—gas reducer.
The thermochemical reactor was a copper tube with a diameter of 16 mm, which was wound on the exhaust pipe. To reduce heat loss, an asbestos thread/5/ was wound on top of the reactor. To visually control the transition of methanol from a liquid to a gas state, a window/9/ of thick-walled glass was mounted at the reactor outlet.
The conditions for the complete conversion of methanol were evaluated as follows. The methanol flow rate through the reactor/6/ was reduced until traces of condensate appeared in the window of the quartz viewing tube/9/ according to the steady-state operating parameters of the engine. After that, the methanol flow rate was reduced until the traces disappeared. Then, the flow of methanol through the reactor was fixed and maintained unchanged for a while. The absence of condensate in the quartz viewing tube gave the possibility to consider the obtained flow rate as corresponding to the condition of complete dissociation of methanol since there were no vapors of unreacted alcohol.
During the experiment, the diesel–hydrogen engine worked with a full load at engine speeds of 800, 900, 1000, 1100, 1200, 1300, 1400, 1500, 1600, 1700, and 1800 rpm. The measurements were carried out according to the methods developed based on GOST 14846-91 on the stationary operation modes of the diesel–hydrogen engine. The preliminary determination of the number of toxic components in the exhaust gases was carried out using the gas analyzer Autotest 02.03P by direct measurement. Graphing and final calculation of the number of nitrogen oxides and carbon oxides in the exhaust gases were carried out with a chromatograph NeoCHROM class C using gas chromatography. The same instrument was used to measure the content of H2 and CO generated during the breakdown of methanol. The measurement of soot concentration was performed using the smoke meter Bosch EFAW 65/68. Measurement of the number of nitrogen oxides and carbon oxide components in the exhaust gases was carried out by taking samples into sampling bags. An adsorption-type infrared non-dispersive detector was used for the analysis of carbon oxide. A chemiluminescence detector was used to analyze nitrogen oxides. Exhaust gas samples were installed at a distance of 1.2 m from the outlet of the exhaust system. Tanks with diesel and methanol fuel were placed on electronic scales, and the mass of consumed fuel was determined using electronic scales. The weight of air on the inlet was calculated on the measured temperature, volume, and pressure of air.
3. Results and Discussion
Analysis of the prototype thermochemical reactor operation for methanol conversion included checking its performance, determining the zone of stable operation in terms of the yield of a hydrogen-containing product, assessing its productivity in terms of the main components of the synthesized gas, and taking operating characteristics that establish the relationship between the composition of the products obtained in the reactor conversion, the degree of methanol conversion, and the temperature regime of the conversion process.
One of the most significant problems for the normal functioning of the conversion system is providing it with the necessary amount of thermal energy and a given level of operating temperatures in the reaction space to achieve the complete methanol thermochemical conversion process and the maximum degree of conversion (Conversion Rate-CR).
At CR < 100%, methanol conversion products at their outlet from the reactor, along with CO and H2, contain vapors of unreacted methanol. In this case, the reaction properties of the gas–vapor mixture entering the engine and the nature of their impact on the combustion process will differ from the performance of synthesis gas.
Heat losses were minimized during experimental studies by using highly efficient thermal insulation of the exhaust duct and reactor based on an asbestos sheet (in Figure 3, for clarity, the heat insulator is partially removed).
Table 4 shows the temperature field of the experimental methanol conversion reactor.

Table 4.
Hydrogen output for different engine operating modes.
The most important characteristics of a catalytic methanol conversion reactor include the dependence of the degree of conversion on the temperature in the reactor. Such characteristics can only be obtained on the basis of the experiment (Figure 4). It was found experimentally that almost complete conversion of methanol (CR > 90%) is achieved in the experimental reactor at 550 K and higher.
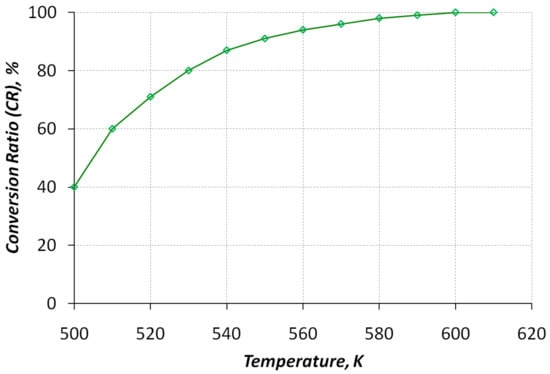
Figure 4.
Dependence of the methanol conversion degree with respect to the temperature of the synthesis gas at the reactor outlet.
At the same time, the chosen medium already contributes to the beginning of the process of methanol decomposition at a temperature of 510–520 K, which makes it possible to partially convert methanol with the release of a hydrogen component at low-load operating modes of the engine with a temperature deficit.
Figure 5 shows the dependence of the hydrogen and carbon monoxide efficiency on the temperature in the reactor. Experiments have shown that the maximum possible hydrogen content is 65%, and the maximum CO content is close to 35%. In general, analyzing the results of a preliminary experimental study of the characteristics of the methanol conversion reactor, the minimum allowable temperature level, above which the methanol decomposition reaction can be considered complete, corresponds to 570–580 K.
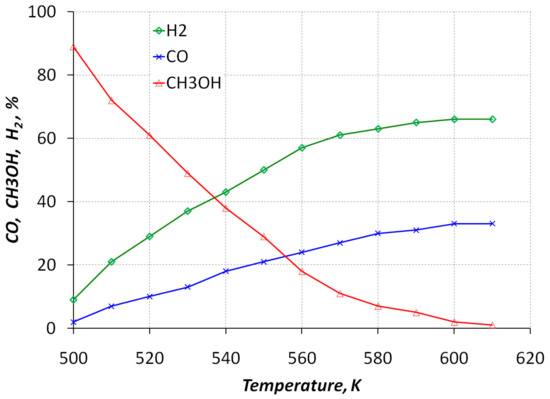
Figure 5.
Dependence of the efficiency of the process of formation of hydrogen and carbon monoxide on the temperature in the reactor.
Experimental dependences of the external speed characteristics of the diesel–hydrogen engine D21A1 converted for diesel fuel, methanol conversion products (diesel–hydrogen fuel), and methanol fuel are shown in Figure 6. Analyzing the experimental power values, it was found that at nominal speed (n = 1800 rpm), the effective power for diesel fuel was 18.1 kW.
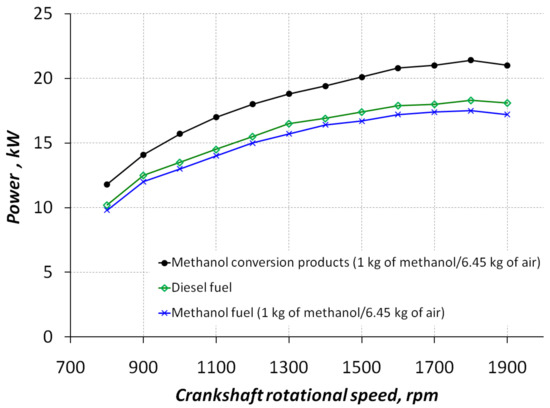
Figure 6.
Experimental dependences of the effective power on the diesel–hydrogen engine crankshaft speed for different motor fuels.
Further analysis of the experimental power values showed that at nominal speed (n = 1800 rpm), the effective power for methanol fuel (1 kg of CH3OH/6.45 kg of air) was 17.1 kW. However, on the products of 100% methanol conversion (1 kg of CH3OH/6.45 kg of air), the effective power was 21.2 kW (1 kg of CH3OH/6.45 kg of air). On average, the value of the effective power of the engine in the entire frequency range of the crankshaft working on methanol fuel (1 kg of CH3OH/6.45 kg of air) in comparison to diesel fuel decreased by 5%. For methanol conversion products, (1 kg of CH3OH/6.45 kg of air) it increased by 14%.
Thus, the increase in the calorific value of gaseous methanol conversion products compared to liquid methanol and diesel fuel resulted in an increase in power compared to the base engine.
Above, the analysis and justification of the mechanisms of action of hydrogen-containing products of methanol conversion on the processes of the diesel engine operating cycle, which determine its environmental qualities, was given. The experimental studies described below fully confirmed the results of this analysis. Environmental indicators characterizing the qualitative change in the working process of a diesel engine during its operation on mixed fuel, which includes hydrogen, are shown in Figure 7 and Figure 8.
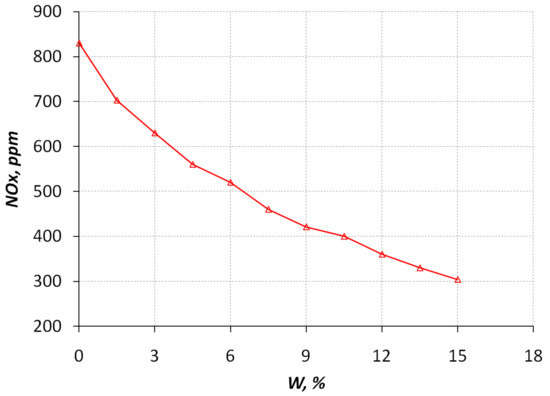
Figure 7.
Dependence of the nitrogen oxide NOx content in the exhaust gases with respect to the percentage of methanol conversion products in a mixed diesel–hydrogen fuel at an engine crankshaft speed of 1800 rpm.
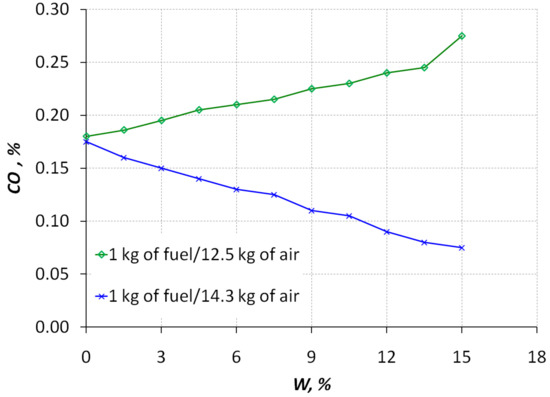
Figure 8.
Dependence of the carbon monoxide CO content in the exhaust gases with respect to the percentage of methanol conversion products in a mixed diesel–hydrogen fuel at engine crankshaft speed 1600 rpm.
Emissions of toxic components were estimated from the relative content of the methanol conversion products in the mixture
where -mass of methanol conversion products in the mixture; -mass of diesel fuel in the mixture.
From Figure 7 and Figure 8, it follows that as the relative content of the conversion products in the mixed fuel increases, the content of nitrogen oxides NOx and carbon monoxide CO in the exhaust gases decreases at a stoichiometric ratio for diesel fuel (1 kg of fuel/14.3 kg of air). For 100% methanol, the stoichiometric ratio is 1 kg of fuel/6.45 kg of air. Therefore, experiments were carried out on the content of the carbon monoxide CO in the exhaust gases with a slight decrease in the amount of air in the mixture-1 kg of fuel/12.5 kg of air. At the same time, the content of the carbon monoxide CO increases slightly (Figure 8), which is explained by the fact that the methanol conversion products contain this gas component, which, with a lack of air and incomplete combustion in the engine combustion chamber, is present in the composition of the exhaust gases at the diesel engine outlet.
Further work of the reactor was carried out at temperatures of 500 K (Figure 5), adding 9–10% water instead. For such a minimum supply of water from the reactor, CO conversion was carried out in the range of 52–62% compared to the amount of CO when operating on pure diesel fuel ( = 0%). For such a minimum supply of water from the reactor, the release of NO was in the range of 40–47% of the amount of NO when operating on clean diesel fuel. An increase of hydrogen content in the fuel leads to reduced emissions of nitrogen oxides (Figure 7). If the amount of air in the mixture decreases, the content of nitrogen oxides falls even more. The way this interacts is similar to the way the EGR system works in diesel engines. With increasing hydrogen content in the fuel, carbon monoxide emission is reduced (Figure 8). If the amount of air in the mixture decreases, the content of carbon oxides begins to increase. For comparison, Figure 8 shows a graph of the growth of carbon oxides in the reduction of the fuel/air ratio from 1 kg of fuel/14.3 kg of air to 1 kg of fuel/12.5 kg of air.
In Figure 9, the soot concentration expressed by a Filter Soot Number (FSN) unit is shown for the exhaust gases concerning the percentage of methanol conversion products in a mixed diesel–hydrogen fuel at an engine crankshaft speed of 1800 rpm. The measurement was performed using the smoke meter Bosch EFAW 65/68. Increasing the hydrogen content in the fuel led to a reduction in soot emissions (Figure 9). Such a significant reduction is because smoke methanol has 50% oxygen in its molecule which ensures intensive burnout of soot particles in diesel. For example, for pure diesel fuel, the soot content was 3.31 FSN (187 mg/m3); at W = 15% the soot content was 2.71 FSN (127 mg/m3).
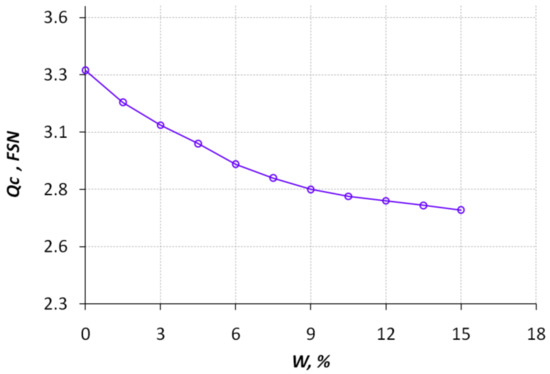
Figure 9.
Dependence of the soot concentration in the exhaust gases with respect to the percentage of methanol conversion products in a mixed diesel–hydrogen fuel at an engine crankshaft speed of 1800 rpm.
The experiment confirmed the theoretical analysis. Thus, the efficiency of the conversion process (including methanol consumption) will increase according to the increase in the load and speed modes due to the increase of the temperature of the exhaust gases and their flow through the reactor for a particular diesel engine. It follows from this that for diesel engines with forced working processes (for example, with gas turbine supercharging) and for an engine with close to nominal operating modes, using the studied method of engine power supply provides the most tangible fuel savings and higher environmental performance.
The environmental and power indicators of the diesel engine increase with increasing the amount of converted methanol, which depend on the available temperature and energy potential of the diesel exhaust gases. The higher this potential, the more methanol can be converted into a hydrogen-containing converted product. This leads to higher efficiency that determines the environmental and fuel-economic qualities of the engine.
4. Conclusions
Further improvement of internal combustion engine efficiency is the subject of this research. This paper presents the possibility of using a thermoreactor to power an internal combustion engine with the products of methanol decomposition. Methanol is a widely available and cheap fuel. Therefore, it can be used to power spark-ignition and diesel engines in a dual-fuel system. In this paper, it has been shown that the use of such a system in a diesel engine significantly improves its operational efficiency. It is possible due to the utilization of heat in the exhaust gas stream, which has been lost so far. The performed research confirmed the effectiveness of the thermoreactor. Its use allowed the operating efficiency of a dual-fuel diesel engine to improve in comparison with a conventional power supply, i.e., diesel fuel. Moreover, it has been shown that the internal combustion engine fueled with the products of methanol decomposition according to the concept presented in this paper achieves more favourable parameters with respect to reducing the emission of harmful components of exhaust gases into the environment. In particular, the emission of nitrogen oxides, carbon monoxide, and exhaust smoke were lower. The obtained results encourage continued work on the thermoreactor enabling the use of the heat in the exhaust system for the methanol decomposition. The products of this decomposition can then be burned in the internal combustion engine, which allows us to obtain more favorable parameters of its operation in comparison with conventionally powered engines.
Author Contributions
Conceptualization, S.K. and K.G.; methodology, R.L.; software, L.K. and J.M.; validation, R.S. and R.L.; formal analysis, S.K.; investigation, R.L.; resources, S.K. and R.S.; data curation, K.G.; writing—original draft preparation, L.K., S.K.,and R.S.; writing—review and editing, J.M., K.G. and R.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Panchuk, M.; Kryshtopa, S.; Sładkowski, A.; Panchuk, A. Environmental Aspects of the Production and Use of Biofuels in Transport; Lecture Notes in Networks and Systems: Book Chapter; Springer International Publishing AG: Cham, Switzerland, 2020; Volume 124, pp. 115–168. [Google Scholar]
- Yakovlieva, A.; Boichenko, S. Energy Efficient Renewable Feedstock for Alternative Motor Fuels Production: Solutions for Ukraine. Stud. Syst. Decis. Control 2020, 298, 247–259. [Google Scholar]
- Panchuk, M.; Kryshtopa, S.; Sladkowski, A.; Panchuk, A.; Mandryk, I. Efficiency of production of motor biofuels for water and land transport. Nase More 2019, 66, 6–12. [Google Scholar] [CrossRef]
- Biernat, K.; Samson-Bręk, I.A. Wodór—Paliwo przyszłości. Studia Ecol. Bioethicae 2008, 6, 331–344. [Google Scholar] [CrossRef]
- Akinyele, D.O.; Rayudu, R.K. Review of energy storage technologies for sustainable power networks. Sustain. Energy Technol. Assess. 2014, 8, 74–91. [Google Scholar] [CrossRef]
- Møller, K.T.; Jensen, T.R.; Akiba, E.; Li, H.-W. Hydrogen—A sustainable energy carrier. Prog. Nat. Sci. Mater. Int. 2017, 27, 34–40. [Google Scholar] [CrossRef]
- Pistidda, C. Solid-State Hydrogen Storage for a Decarbonized Society. Hydrogen 2021, 2, 428–443. [Google Scholar] [CrossRef]
- Chen, S.C.; Kao, Y.L.; Yeh, G.T.; Rei, M.H. An onboard hydrogen generator for hydrogen enhanced combustion with internal combustion engine. Int. J. Hydrogen Energy 2017, 42, 21334–21342. [Google Scholar] [CrossRef]
- Lototskyy, M.V.; Yartys, V.A.; Pollet, B.G.; Bowman, R.C., Jr. Metal hydride hydrogen compressors: A review. Int. J. Hydrogen Energy 2014, 39, 5818–5851. [Google Scholar] [CrossRef] [Green Version]
- Davids, M.W.; Tolj, I.; Jao, T.-C.; Lototskyy, M.; Pasupathi, S.; Sita, C. Development of a portable polymer electrolyte membrane fuel cell system using metal hydride as the hydrogen storage medium. ECS Trans. 2016, 75, 553–562. [Google Scholar] [CrossRef]
- Lototskyy, M.V.; Tolj, I.; Pickering, L.; Sita, C.; Barbir, F.; Yartys, V. The use of metal hydrides in fuel cell applications. Prog. Nat. Sci. Mater. Int. 2017, 27, 3–20. [Google Scholar] [CrossRef] [Green Version]
- Castañeda, M.; Cano, A.; Jurado, F.; Sánchez, H.; Fernández, L.M. Sizing optimization, dynamic modeling and energy management strategies of a stand-alone PV/hydrogen/battery-based hybrid system. Int. J. Hydrogen Energy 2013, 38, 3830–3845. [Google Scholar] [CrossRef]
- Hirscher, M.; Yartys, V.A.; Baricco, M.; Bellosta von Colbe, J.; Blanchard, D.; Bowman, R.C.; Broom, D.P.; Zlotea, C. Materials for hydrogen-based energy storage—Past, recent progress and future outlook. J. Alloys Compd. 2020, 827, 153548. [Google Scholar] [CrossRef]
- Modi, P.; Aguey-Zinsou, K.-F. Titanium-iron-manganese (TiFe0.85Mn0.15) alloy for hydrogen storage: Reactivation upon oxidation. Int. J. Hydrogen Energy 2019, 44, 16757–16764. [Google Scholar] [CrossRef]
- Marocco, P.; Ferrero, D.; Gandiglio, M.; Ortiz, M.M.; Sundseth, K.; Lanzini, A.; Santarelli, M. A study of the techno-economic feasibility of H2-based energy storage systems in remote areas. Energy Convers. Manag. 2020, 211, 112768. [Google Scholar] [CrossRef]
- Koohi-Fayegh, S.; Rosen, M.A. A review of energy storage types, applications and recent developments. J. Energy Storage 2020, 27, 101047. [Google Scholar] [CrossRef]
- Korohodskyi, V.; Voronkov, O.; Rogovyi, A.; Kryshtopa, S.; Bezridnyi, V.; Rudenko, N. Influence of the stratified fuel-air charge pattern on economic and environmental indicators of a two-stroke engine with spark ignition. AIP Conf. Proc. Am. Inst. Phys. 2021, 2439, 020011. [Google Scholar]
- Bazhinov, O.; Gerlici, J.; Kravchenko, O.; Zaverukha, R.; Kravchenko, K. Development of a method for evaluating the technical condition of a car’s hybrid powertrain. Symmetry 2021, 13, 2356. [Google Scholar] [CrossRef]
- Chumakov, V.L.; Devyanin, S.N.; Bijaev, A.V. Nitrogen oxide formation with nonuniform fuel distribution in diesel engine. J. Phys. Conf. Ser. 2020, 1679, 052089. [Google Scholar] [CrossRef]
- Tselischev, O.; Kudryavtsev, S.; Loriya, M.; Boychenko, S.; Lanetsky, V.; Matveeva, I.; Leonenko, S.; Tselishcheva, M. Modification of motor gasoline with bioethanol in the cavitation field. Vopr. Khimii I Khimicheskoi Tekhnologii 2020, 6, 171–178. [Google Scholar]
- Liu, Z. Economic Analysis of Methanol Production from Coal/Biomass Upgrading. Energy Sources Part B-Econ. Plan. Policy 2018, 13, 66–71. [Google Scholar] [CrossRef]
- Kryshtopa, S.; Melnyk, V.; Dolishnii, B.; Zakhara, I.; Voitsekhivska, T. Improvement of the model of forecasting heavy metals of exhaust gases of motor vehicles in the soil. East.-Eur. J. Enterp. Technol. 2019, 4, 44–51. [Google Scholar] [CrossRef]
- Shamsul, N.S.; Kamarudin, S.K.; Rahman, N.A.; Kofli, N.T. An overview on the production of bio-methanol as potential renewable energy. Renew. Sustain. Energy Rev. 2014, 33, 578–588. [Google Scholar] [CrossRef]
- Górski, K.; Smigins, R.; Longwic, R. Research on physico-chemical properties of diethyl ether/linseed oil blends for the use as fuel in diesel engines. Energies 2020, 13, 6564. [Google Scholar] [CrossRef]
- Shuliak, M.; Klets, D.; Kalinin, Y.; Kholodov, A. Selecting a rational operation mode of mobile power unit using measuring and control complex. CEUR Workshop Proc. 2019, 2387, 141–151. [Google Scholar]
- Klymenko, O.; Gorytski, V.; Gutarevych, Y.; Shchelkunov, A.; Kyrychenko, R. Requirements for a unified system of road vehicles environmental labelling and low emission zones. East.-Eur. J. Enterp. Technol. 2020, 6, 53–84. [Google Scholar] [CrossRef]
- Gerasidi, V.V.; Lisachenko, A.V.; Nikolaev, N.I. Thermotechnical tests of an electronically controlled main high-speed engine of a marine vessel. J. Phys. Conf. Ser. 2021, 2061, 012055. [Google Scholar] [CrossRef]
- Kryshtopa, S.; Górski, K.; Longwic, R.; Smigins, R.; Kryshtopa, L. Increasing parameters of diesel engines by their transformation for methanol conversion products. Energies 2021, 14, 1710. [Google Scholar] [CrossRef]
- Šmigins, R.; Kryshtopa, S.; Pajak, M. Impact of Low Level N-butanol and Gasoline Blends on Engine Performance and Emission Reduction. In Proceedings of the Transport Means—Proceedings of the International Conference, Kaunas University of Technology, Kaunas, Lithuania, 6–8 October 2021; pp. 481–486. [Google Scholar]
- Valeika, G.; Matijošius, J.; Górski, K.; Rimkus, A.; Smigins, R. A study of energy and environmental parameters of a diesel engine running on hydrogenated vegetable oil (HVO) with addition of biobutanol and castor oil. Energies 2021, 14, 3939. [Google Scholar] [CrossRef]
- Kryshtopa, S.; Panchuk, M.; Dolishnii, B.; Hnyp, M.; Skalatska, O. Research into emissions of nitrogen oxides when converting the diesel engines to alternative fuels. East.-Eur. J. Enterp. Technol. 2018, 1, 16–22. [Google Scholar] [CrossRef] [Green Version]
- Li, Y. Numerical Study on the Combustion and Emission Characteristics of a Methanol/Diesel Reactivity Controlled Compression Ignition (RCCI) Engine. Appl. Energy 2013, 106, 184–197. [Google Scholar] [CrossRef]
- Gerasidi, V.V.; Lisachenko, A.V. Analysis of fuel consumption of modern electronically controlled high-speed marine engines. J. Phys. Conf. Ser. 2021, 2061, 012057. [Google Scholar] [CrossRef]
- Mäyrä, O.; Leiviskä, K. Modeling in Methanol Synthesis, Methanol; Elsevier: Amsterdam, The Netherlands, 2018; pp. 475–492. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).