Application of Rumen Microorganisms for Enhancing Biogas Production of Corn Straw and Livestock Manure in a Pilot-Scale Anaerobic Digestion System: Performance and Microbial Community Analysis
Abstract
1. Introduction
2. Results and Discussion
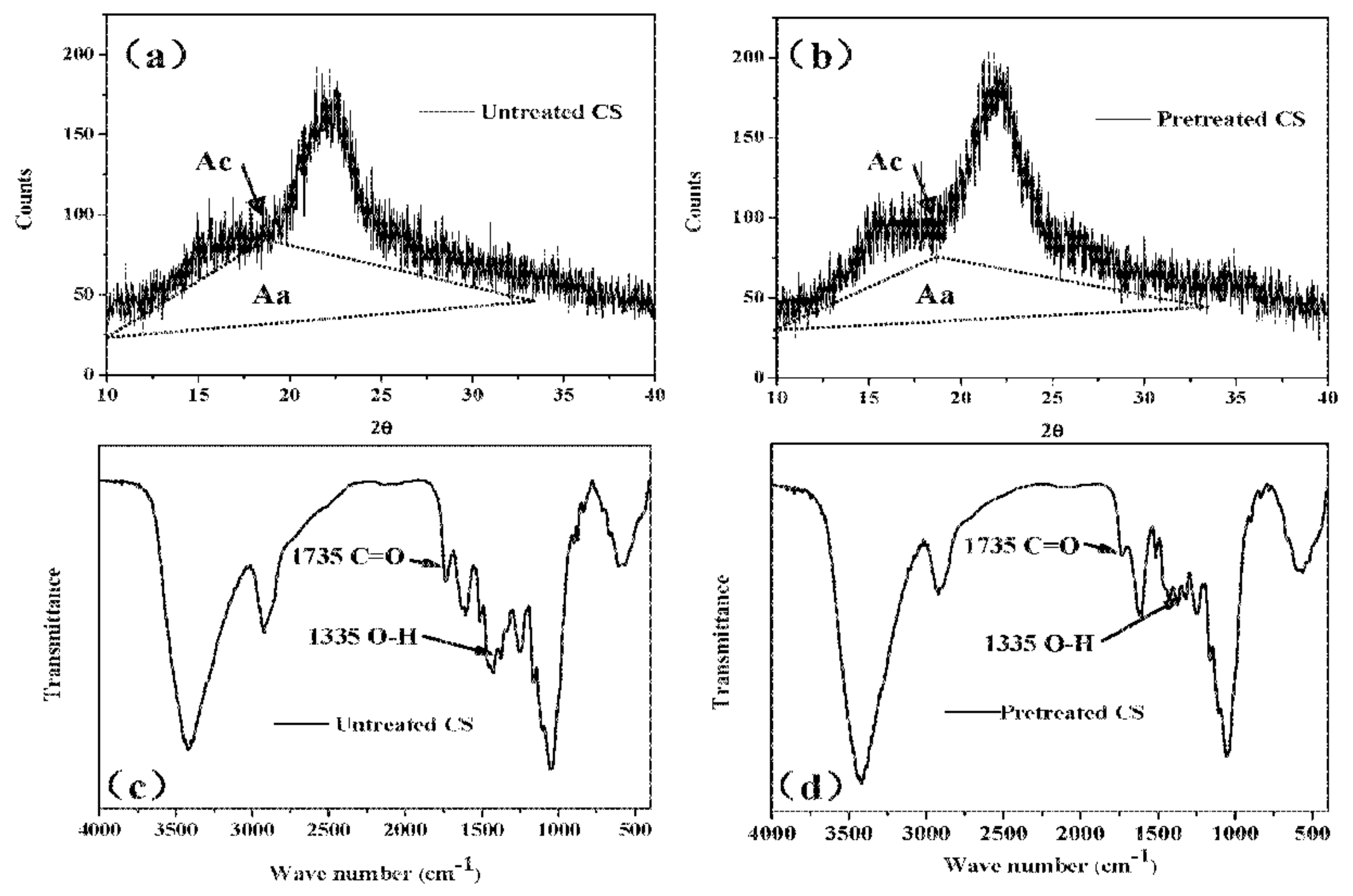
2.1. Pretreatment of CS with Biogas Liquid
2.2. Temperature and pH Value
2.3. VFAs Production
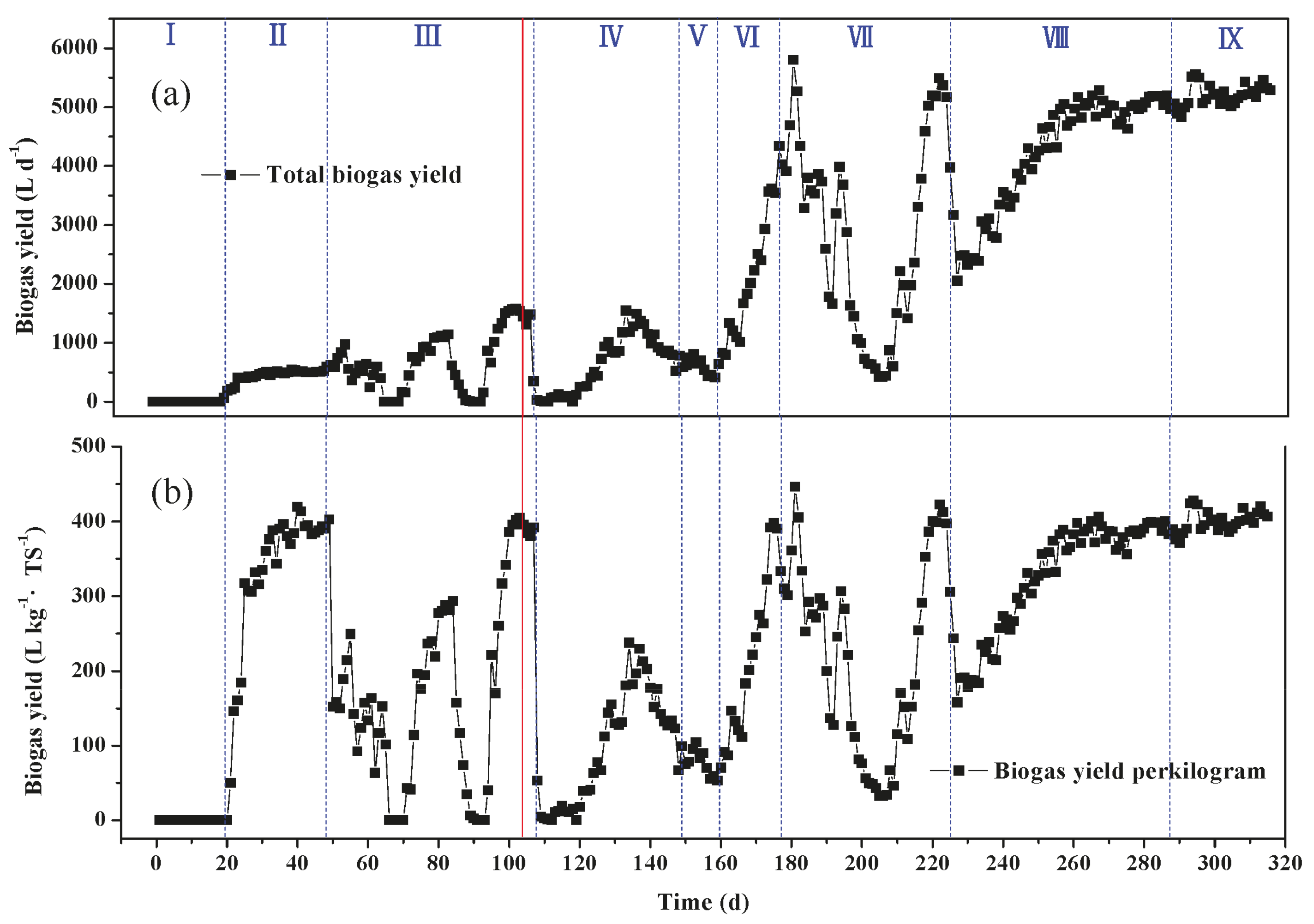
2.4. Biogas Yield
2.5. Microbial Community Analysis
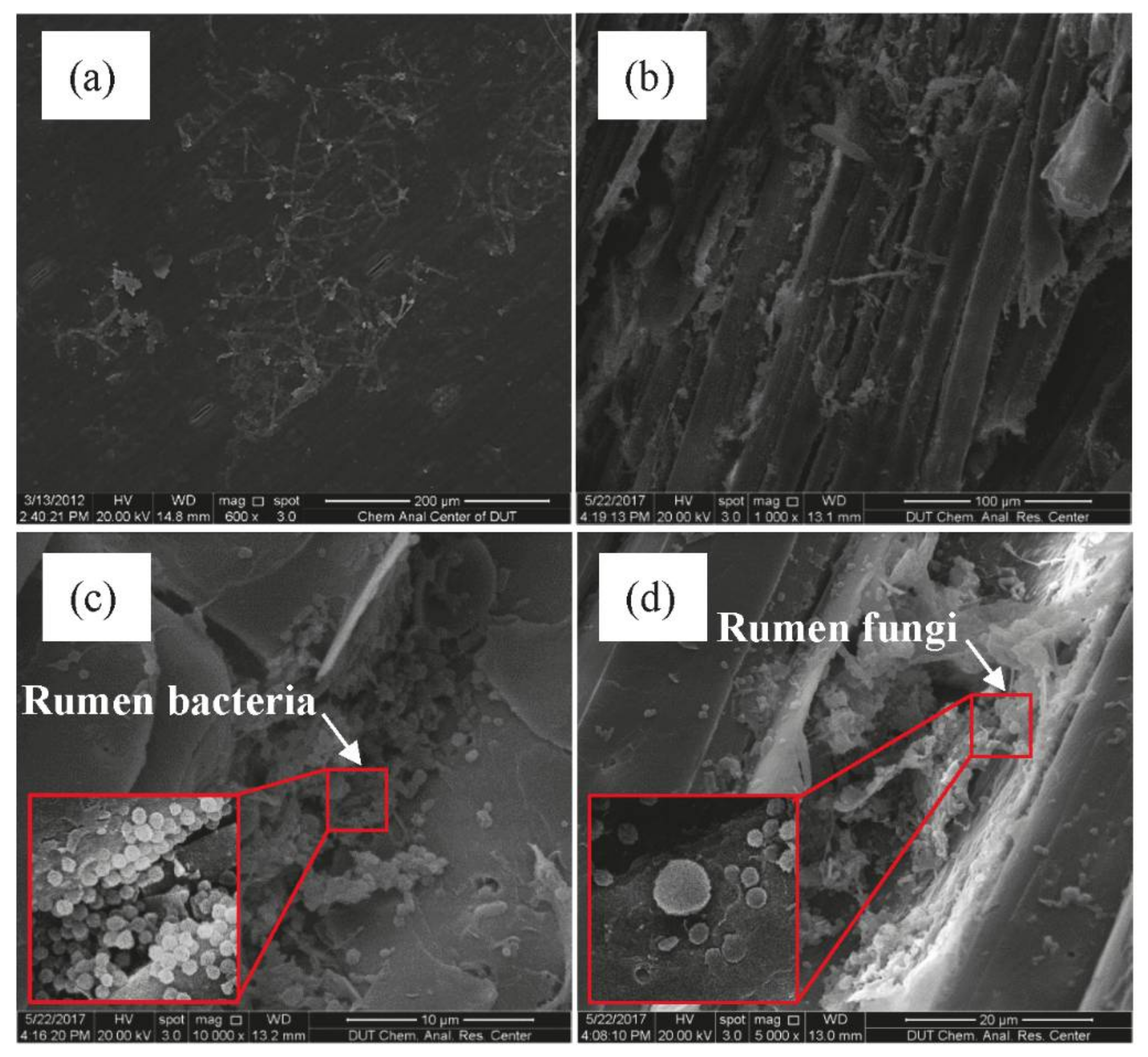
2.6. Physical Form and SEM Images of Substrates
2.7. Mass Balance
3. Materials and Methods
3.1. Inoculum and Substrates
3.2. Experimental and Incubation Conditions
3.3. Analytical Methods
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ju, X.; Zhang, F.; Bao, X.; Römheld, V.; Roelcke, M. Utilization and management of organic wastes in Chinese agriculture: past, present and perspectives. Sci. China Ser. C: Life Sci. 2005, 48, 965–979. [Google Scholar]
- Li, H.; Cao, Y.; Wang, X.; Ge, X.; Li, B.; Jin, C. Evaluation on the production of food crop straw in China from 2006 to 2014. BioEnergy Res. 2017, 10, 949–957. [Google Scholar] [CrossRef]
- Ning, Z.; Ji, M. Changes and outlook about production amount of livestock and poultry manure in China. Agric. Outlook 2014, 1, 46–48. [Google Scholar]
- Zhang, X.; Zhai, M.; Wang, Y.; Gao, Y.; Zhao, H.; Zhou, X.; Gao, J. Research on biomass energy utilization in Chinese rural area: Challenges and opportunities. Emerald Group Publ. Ltd. 2016, 257–281. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Y.; Hao, L. Contributions of open crop straw burning emissions to PM2.5 concentrations in China. Environ. Res. Lett. 2016, 11. [Google Scholar] [CrossRef]
- Prapinagsorn, W.; Sittijunda, S.; Reungsang, A.; Sciubba, E. Co-digestion of napier grass and its silage with cow dung for methane production. Energies 2017, 10, 1654. [Google Scholar] [CrossRef]
- Powlson, D.S.; Riche, A.B.; Coleman, K.; Glendining, M.J.; Whitmore, A.P. Carbon sequestration in European soils through straw incorporation: Limitations and alternatives. Waste Manag. 2008, 28, 741–746. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, H.; Strong, P.J.; Xu, S.; Liu, S.; Lu, K.; Sheng, K.; Guo, J.; Che, L.; He, Z.; et al. Thermal properties of biochars derived from waste biomass generated by agricultural and forestry sectors. Energies 2017, 10, 469. [Google Scholar] [CrossRef]
- Mao, C.; Feng, Y.; Wang, X.; Ren, G. Review on research achievements of biogas from anaerobic digestion. Renew. Sustain. Energy Rev. 2015, 45, 540–555. [Google Scholar] [CrossRef]
- Mata-Alvarez, J.; Dosta, J.; Romero-Güiza, M.S.; Fonoll, X.; Peces, M.; Astals, S. A critical review on anaerobic co-digestion achievements between 2010 and 2013. Renew. Sustain. Energy Rev. 2014, 36, 412–427. [Google Scholar] [CrossRef]
- Brodeur, G.; Yau, E.; Badal, K.; Collier, J.; Ramachandran, K.B.; Ramakrishnan, S. Chemical and physicochemical pretreatment of lignocellulosic biomass: A review. Enzym. Res. 2011, 4999, 787532. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.Y.; Xu, X.C.; Gao, Y.; Yang, F.L.; Wang, G. Anaerobic fermentation of biogas liquid pretreated maize straw by rumen microorganisms in vitro. Bioresour. Technol. 2014, 153, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Hungate, R.E. The Rumen and Its Microbes; Academic Press Inc.: London, UK, 1966; pp. 466–525. [Google Scholar]
- Li, N.; Yang, F.; Xiao, H.; Zhang, J.; Ping, Q. Effect of feedstock concentration on biogas production by inoculating rumen microorganisms in biomass solid waste. Appl. Biochem. Biotech. 2017, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Yue, Z.; Wang, J.; Liu, X.; Yu, H. Comparison of rumen microorganism and digester sludge dominated anaerobic digestion processes for aquatic plants. Renew. Energy 2012, 46, 255–258. [Google Scholar] [CrossRef]
- Yue, Z.; Yu, H.; Wang, Z. Anaerobic digestion of cattail with rumen culture in the presence of heavy metals. Bioresour. Technol. 2007, 98, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.H.; Yu, H.Q. Application of rumen microorganisms for enhanced anaerobic fermentation of corn stover. Process Biochem. 2005, 40, 2371–2377. [Google Scholar] [CrossRef]
- Alrawi, R.A.; Ahmad, A.; Ismail, N.; Kadir, M.O.A. Anaerobic co-digestion of palm oil mill effluent with rumen fluid as a co-substrate. Desalination 2011, 269, 50–57. [Google Scholar] [CrossRef]
- Gijzen, H.J.; Derikx, P.J.; Vogels, G.D. Application of rumen microorganisms for a high rate anaerobic digestion of papermill sludge. Biol. Wastes 1990, 32, 169–179. [Google Scholar] [CrossRef]
- Change, O.C. Intergovernmental Panel on Climate Change. World Meteorological Organization, 2007. Available online: http://www.ipcc.ch/pdf/assessment-report/ar4/syr/ar4_syr_full_report.pdf (accessed on 12–17 November 2007).
- Mosoni, P.; Martin, C.; Forano, E.; Morgavi, D.P. Long-term defaunation increases the abundance of cellulolytic ruminococci and methanogens but does not affect the bacterial and methanogen diversity in the rumen of sheep. J. Anim. Sci. 2011, 89, 783. [Google Scholar] [CrossRef] [PubMed]
- Van, T.E.; Hundley, V. The importance of pilot studies. Nurs. Stand. 2010, 16, 33. [Google Scholar]
- Chundawat, S.P.S.; Balan, V.; Sousa, L.D.C.; Dale, B.E. Bioalcohol Production: Biochemical Conversion of Lignocellulosic Biomass; Woodhead Publishing Limited: Cambridge, UK, 2010. [Google Scholar]
- Carrillo, F.; Colom, X.; Sunol, J.J.; Saurina, J. Structural FTIR analysis and thermal characterisation of lyocell and viscose-type fibres. Eur. Polym. J. 2004, 40, 2229–2234. [Google Scholar] [CrossRef]
- Phillipson, K.; Hay, J.N.; Jenkins, M.J. Thermal analysis FTIR spectroscopy of poly (ε-caprolactone). Thermochim. Acta 2014, 595, 74–82. [Google Scholar] [CrossRef]
- Mezzullo, W.G.; Mcmanus, M.C.; Hammond, G.P. Life cycle assessment of a small-scale anaerobic digestion plant from cattle waste. Appl. Energy 2013, 102, 657–664. [Google Scholar] [CrossRef]
- Lindorfer, H.; Braun, R.; Kirchmayr, R. Self-heating of anaerobic digesters using energy crops. Water Sci. Technol. 2006, 53, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Yu, H.; Zhu, R. Influence of particle size and pH on anaerobic degradation of cellulose by ruminal microbes. Int. Biodeter. Biodeg. 2005, 55, 233–238. [Google Scholar] [CrossRef]
- Dixon, R.M.; Dixon, R.M. Effects of addition of urea to a low nitrogen diet on the rumen digestion of a range of roughages. Aust. J. Agric. Res. 1999, 50, 1091–1098. [Google Scholar] [CrossRef]
- Zhou, J.; Yang, J.; Yu, Q.; Yong, X.; Xie, X.; Zhang, L.; Wei, P.; Jia, H. Different organic loading rates on the biogas production during the anaerobic digestion of rice straw: A pilot study. Bioresour. Technol. 2017, 244, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Behera, S.K.; Kim, J.W.; Park, H.S. Methane production potential of leachate generated from Korean food waste recycling facilities: A lab-scale study. Waste Manag. 2009, 29, 876–882. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Chen, Y.; Zhou, Q. Waste activated sludge hydrolysis and short-chain fatty acids accumulation under mesophilic and thermophilic conditions: Effect of pH. Water Res. 2009, 43, 3735–3742. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wang, C.; Pei, C.X.; Li, H.Y.; Wang, Y.X.; Zhang, S.L.; Zhang, Y.L.; He, J.P.; Wang, H.; Yang, W.Z. Effects of isovalerate supplementation on microbial status and rumen enzyme profile in steers fed on corn stover based diet. Livest. Sci. 2014, 161, 60–68. [Google Scholar] [CrossRef]
- Schmidt, J.E.; Ahring, B.K. Effects of hydrogen and formate on the degradation of propionate and butyrate in thermophilic granules from an upflow anaerobic sludge blanket reactor. Appl. Environ. Microbiol. 1993, 59, 2546–2551. [Google Scholar]
- Baek, G.; Kim, J.; Kim, J.; Lee, C. Role and potential of direct interspecies electron transfer in anaerobic digestion. Energies 2018, 11, 107. [Google Scholar] [CrossRef]
- Yangin-Gomec, C.; Ozturk, I. Effect of maize silage addition on biomethane recovery from mesophilic co-digestion of chicken and cattle manure to suppress ammonia inhibition. Energy Convers. Manag. 2013, 71, 92–100. [Google Scholar] [CrossRef]
- Kim, M.; Ahn, Y.H.; Speece, R.E. Comparative process stability and efficiency of anaerobic digestion: Mesophilic vs. Thermophilic. Water Res. 2002, 36, 4369. [Google Scholar] [CrossRef]
- Russell, J.B. The importance of pH in the regulation of ruminal acetate to propionate ratio and methane production in vitro. J. Dairy Sci. 1998, 81, 3222–3230. [Google Scholar] [CrossRef]
- Hegarty, R.S.; Gerdes, R. Hydrogen production and transfer in the rumen. Recent Adv. Anim. Nutr. Aust. 1999, 12, 37–44. [Google Scholar]
- Zhao, B.; Yue, Z.; Ni, B.; Mu, Y.; Yu, H.; Harada, H. Modeling anaerobic digestion of aquatic plants by rumen cultures: Cattail as an example. Water Res. 2009, 43, 2047–2055. [Google Scholar] [CrossRef] [PubMed]
- Chandra, R.; Takeuchi, H.; Hasegawa, T. Methane production from lignocellulosic agricultural crop wastes: A review in context to second generation of biofuel production. Renew. Sustain. Energy Rev. 2012, 16, 1462–1476. [Google Scholar] [CrossRef]
- Moestedt, J.; Påledal, S.N.; Schnürer, A.; Nordell, E. Biogas production from thin stillage on an industrial scale—Experience and optimisation. Energies 2013, 6, 5642–5655. [Google Scholar] [CrossRef]
- Fernandes, T.V.; Keesman, K.J.; Zeeman, G.; van Lier, J.B. Effect of ammonia on the anaerobic hydrolysis of cellulose and tributyrin. Biomass Bioenergy 2012, 47, 316–323. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Wang, J.; Meng, L. Effects of volatile fatty acid concentrations on methane yield and methanogenic bacteria. Biomass Bioenergy 2009, 33, 848–853. [Google Scholar] [CrossRef]
- Yu, G.; Chen, X.; Liu, Z.; Zhou, X.; Zhang, Y. Effect of inoculum sources on the anaerobic digestion of rice straw. Bioresour. Technol. 2014, 158, 149–155. [Google Scholar]
- Muller, Z.O. Feed from Animal Wastes: Feeding Manual; Food and Agriculture Organization of the United Nations: Rome, Italy, 1982. [Google Scholar]
- Bhattacharya, A.N.; Taylor, J.C. Recycling animal waste as a feedstuff: A review. J. Anim. Sci. 1975, 41, 1438–1457. [Google Scholar] [CrossRef]
- Xie, S.; Lawlor, P.G.; Frost, P.; Dennehy, C.D.; Hu, Z.; Zhan, X. A pilot scale study on synergistic effects of co-digestion of pig manure and grass silage. Int. Biodeter. Biodeg. 2017, 123, 244–250. [Google Scholar] [CrossRef]
- Miller, T.L. Methanobrevibacter; William, B.W., Fred, R., Eds.; Wiley Online Library: Hoboken, NJ, USA, 2015. [Google Scholar]
- Henderson, C. The effects of fatty acids on pure cultures of rumen bacteria. J. Agr. Sci. 1973, 81, 107–112. [Google Scholar] [CrossRef]
- David, R.B.; Richard, W.C. Bergey’s Manual of Systematic Bacteriology: Volume One: Archaea and the Deeply Branching and Phototrophic Bacteria; Springer Science & Business Media: Berlin, Germany, 2010. [Google Scholar]
- Goberna, M.; Gadermaier, M.; García, C.; Wett, B.; Insam, H. Adaptation of methanogenic communities to the cofermentation of cattle excreta and olive mill wastes at 37 °C and 55 °C. Appl. Environ. Microb. 2010, 76, 6564–6571. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Song, Z.; Li, D.; Yuan, Y.; Liu, X.; Zheng, T. The effects of initial substrate concentration, C/N ratio, and temperature on solid-state anaerobic digestion from composting rice straw. Bioresour. Technol. 2015, 177, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Ziganshin, A.M.; Liebetrau, J.; Pröter, J.; Kleinsteuber, S. Microbial community structure and dynamics during anaerobic digestion of various agricultural waste materials. Appl. Microbiol. Biotechnol. 2013, 97, 5161–5174. [Google Scholar] [CrossRef] [PubMed]
- St-Pierre, B.; Wright, A.D.G. Comparative metagenomic analysis of bacterial populations in three full-scale mesophilic anaerobic manure digesters. Appl. Microbiol. Biotechnol. 2014, 98, 2709–2717. [Google Scholar] [CrossRef] [PubMed]
- Demirel, B.; Scherer, P. The roles of acetotrophic and hydrogenotrophic methanogens during anaerobic conversion of biomass to methane: A review. Rev. Environ. Sci. Biotechnol. 2008, 7, 173–190. [Google Scholar] [CrossRef]
- Karakashev, D.; Batstone, D.J.; Angelidaki, I. Influence of environmental conditions on methanogenic compositions in anaerobic biogas reactors. Appl. Environ. Microbiol. 2005, 71, 331–338. [Google Scholar] [CrossRef] [PubMed]
- De Vrieze, J.; Hennebel, T.; Boon, N.; Verstraete, W. Methanosarcina: the rediscovered methanogen for heavy duty biomethanation. Bioresour. Technol. 2012, 112, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Akin, D.E. Ultrastructure of rumen bacterial attachment to forage cell walls. Appl. Environ. Microbiol. 1976, 31, 562–568. [Google Scholar] [PubMed]
- Rezaeian, M.; Beakes, G.W.; Parker, D.S. Methods for the isolation, culture and assessment of the status of anaerobic rumen chytrids in both in vitro and in vivo systems. Mycol. Res. 2004, 108, 1215–1226. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Segal, L.; Creely, J.J.; Martin, A.E., Jr.; Conrad, C.M. An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Apha, A.W. Standard Methods for the Examination of Water and Wastewater, 19th ed.; American Public Health Association: Washington, DC, USA, 1995. [Google Scholar]




 ), digestate (
), digestate ( ), pH adjustment (
), pH adjustment ( ), biogas (
), biogas ( ), biogas liquid (
), biogas liquid ( ), and water-heating (
), and water-heating ( ).
).
 ), digestate (
), digestate ( ), pH adjustment (
), pH adjustment ( ), biogas (
), biogas ( ), biogas liquid (
), biogas liquid ( ), and water-heating (
), and water-heating ( ).
).
| Sample | OTU | ACE | Chao1 | Coverage | Shannon |
|---|---|---|---|---|---|
| Original rumen archaea | 44 | 46 | 45 | 0.999608 | 0.97 |
| Days 100 (solely CS) | 74 | 82 | 83 | 0.999356 | 1.38 |
| Days 220 (CS with pig manure (PM)) | 100 | 104 | 102 | 0.999335 | 2.4 |
| Parameter | CS b | PM b | CM b |
|---|---|---|---|
| pH | - | 7.89 ± 0.03 | 7.73 ± 0.01 |
| Moisture content (%) | 15 ± 2.2% | 79.32 ± 0.05 | 73.36 ± 0.03 |
| Volatile solid a (%) | 92.90 ± 0.32 | 85.62 ± 0.08 | 80.67 ± 0.12 |
| Total organic carbon a (%) | 51.88 ± 0.82 | 43.57 ± 0.38 | 39.9 ± 0.29 |
| NH4+–N (%) | - | 0.85 ± 0.02 | 1.39 ± 0.05 |
| Kjeldahlnitrogen a (%) | 0.61 ± 0.13 | 3.28 ± 0.34 | 5.33 ± 0.42 |
| C/N ratio | 85.05 ± 0.48 | 13.28 ± 0.28 | 7.49 ± 0.36 |
| Experimental Procedure | Experimental Stage | Solid Content | Remarks | ||
|---|---|---|---|---|---|
| CS | PM | CM | |||
| Part I | Stage I | 1% | - | - | Inoculating and starting up |
| Part II | Stage II | 1% | - | - | Anaerobic digestion of the CS |
| Stage III | 3% | - | - | CS associating with urea addition | |
| Stage IV | 5% | - | - | Limiting solid content of solely carbon-rich substrate | |
| Part III | Stage V | 5% | 1% | - | Anaerobic co-digestion of CM with livestock manures |
| Stage VI | 5% | 2% | - | ||
| Stage VII | 5% | 5% | - | ||
| Stage VIII | 5% | - | 5% | ||
| Stage IX | 3% | - | 7% | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, W.; Xu, X.; Yang, F. Application of Rumen Microorganisms for Enhancing Biogas Production of Corn Straw and Livestock Manure in a Pilot-Scale Anaerobic Digestion System: Performance and Microbial Community Analysis. Energies 2018, 11, 920. https://doi.org/10.3390/en11040920
Jin W, Xu X, Yang F. Application of Rumen Microorganisms for Enhancing Biogas Production of Corn Straw and Livestock Manure in a Pilot-Scale Anaerobic Digestion System: Performance and Microbial Community Analysis. Energies. 2018; 11(4):920. https://doi.org/10.3390/en11040920
Chicago/Turabian StyleJin, Wenyao, Xiaochen Xu, and Fenglin Yang. 2018. "Application of Rumen Microorganisms for Enhancing Biogas Production of Corn Straw and Livestock Manure in a Pilot-Scale Anaerobic Digestion System: Performance and Microbial Community Analysis" Energies 11, no. 4: 920. https://doi.org/10.3390/en11040920
APA StyleJin, W., Xu, X., & Yang, F. (2018). Application of Rumen Microorganisms for Enhancing Biogas Production of Corn Straw and Livestock Manure in a Pilot-Scale Anaerobic Digestion System: Performance and Microbial Community Analysis. Energies, 11(4), 920. https://doi.org/10.3390/en11040920




