Abstract
Mild steel continues to be the most extensively used construction material in several industries and constructions. However, corrosion of mild steel in aggressive environments is a major concern. Under the tremendously increasing demand for improving the coatings strategies because of the environmental concerns due to some of the traditional coatings, silane pre-treatments have been emerging as one of the effective solutions, among other strategies. Different approaches, such as adding particles of metal oxide (such as SiO2, ZrO2, Al2O3, TiO2 and CeO2), incorporating plant extracts and impregnating 2D materials into the coatings, have been employed for durable corrosion resistance, including for mitigating enhanced corrosion due to the presence of bacteria. This review discusses the critical mechanistic features of silane coatings such as the role of hydrolysis and condensation in the bonding of silanes with metal surfaces. The factors that influence the performance of the silane coatings for corrosion resistance of mild steel are discussed. In particular, this review provides insight into silane coatings for mitigating microbiologically influenced corrosion (MIC) of mild steel.
1. Introduction
Organofunctional silicones, also known as silanes, are hybrid compounds of silica and organic materials related to resins; therefore, they can be used as coupling agents to improve bonding of inorganic substrates such as metals to organic resins [1,2]. The efficiency of silanes is attributed to their function as coupling agents, as they work as a chemical bridge whereby one part of the molecule attaches to the inorganic substrate (such as glass, metal or mineral) and another part ties to the adhesive, coating or polymer [1,3,4,5]. The main applications of these chemicals have been at glass/paint, metal/paint and metal/rubber interfaces [6].
The organofunctional silanes which are used as adhesion promoters or coupling agents have a general structure of X3Si(CH2)nY, where the attachment of silanes to surfaces is established through the hydrolysable alkoxy group (X), where X can be methoxy (OCH3), ethoxy (OC2H5) or acetoxy (OCOCH3) [7,8]. The enhancement of the strength of the silane–paint polymer interface is the primary function of the functional group Y through the formation of chemical bonds [9]. The most important step to form the organofunctional group of silanes is the addition of silicon hybrids to substituted olefins and acetylenes according to the reaction [10]:
The organosilanes that have an additional Si atom linked to the trialkoxy group (X3) on the other side of the functional group (Y) and have the general structure X3Si(CH2)nY(CH2)nSiX3 are called bis-silanes. The functional group Y can be, for example, an amine group or a chain of sulfur atoms. The non-functional silanes (i.e., the silanes without the functional group) have been investigated for their use as cross-linking agents, and they do not provide good paint adhesion [11,12]. For many decades, chromate conversion coatings have been used effectively as a corrosion inhibitor as well as to create an interface compatible with a wide range of paints. Cr(VI)-based treatments have historically played a very important role in the aircraft, marine, automotive and civil construction industries for corrosion resistance of different metals and alloys [13]. However, there are increasing regulatory restrictions on the use of chromate compounds because they are harmful and hazardous to health and the environment, including the serious concern of the carcinogenic effect of hexavalent Cr. Tsapakos et al. [14] have reported the chromium (III) produced as result of Cr(VI) reduction may be directly involved in mediating cross-linking of cell macromolecules, such as DNA and proteins, leading to mutagenic and carcinogenic activities of chromium. As a result, non-toxic alternatives to chromate treatments have attracted remarkable research attention. Organosilanes, which are one such alternative, are attractive for several industrial applications since they can be used not only as adhesion promoters for a wide range of paints but also as corrosion barriers for several metals and alloys [12,15]. Functional materials processing of silanes and organosilanes falls in the category of “sol-gel”. The final characteristics of the synthesized protective coatings depend on their precursor chemistry, temperature, pH of the solution, molar ratios of reactants, solvent composition, water and catalyst [16]. Depending on the silane precursors used for syntheses, different sol–gel materials are produced that possess unique functionalities for specifically modifying the surface properties required for specific applications [16]. In addition, silane treatments are an easy application process, relatively inexpensive and an environment-friendly option [17].
Mild steel is the most frequently used construction material in many industries due to its excellent mechanical properties and low cost [18]. However, it is prone to surface degradation when exposed to corrosive environments. Silane coatings are emerging as one of the environment-friendly approaches to circumvent the corrosion of metallic materials including mild steels. This review first presents an overview of the considerable knowledge of silane hydrolysis and the mechanism of their bonding with the metallic substrates as well as of the comprehensive knowledge of the silane coatings for corrosion resistance of mild steel, with a particular emphasis on the role of silane coating for the mitigation of microbiologically influenced corrosion (MIC).
2. Hydrolysis and Condensation of Organosilanes
The silanes used for improvement of adhesion and surface modification are usually alkoxysilanes, which are hydrolyzed before or during application and attachment processes. Such hydrolysis involves a complex cascade of reactions. Figure 1 illustrates a simplified view of the reaction cascade [19,20,21]. Because water and alkoxysilanes are immiscible, solvents such as alcohol are normally used as a homogenizing medium [22,23,24]. The hydrolysis reaction can be expressed as:
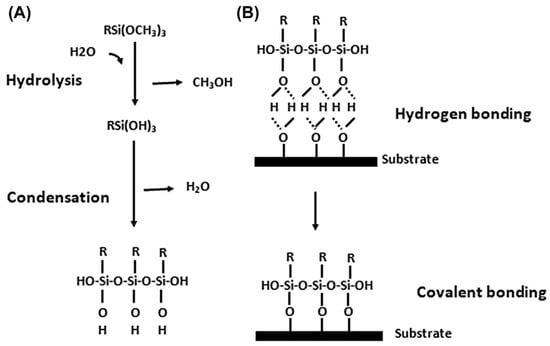
Figure 1.
Hydrolysis and condensation of alkoxysilane and bonding of silanol to the metal substrate. (A) Hydrolysis and condensation to form oligomers in the silane solution and (B) adsorption to an inorganic substrate (such as ceramics or surface oxide layers on metals) by hydrogen bonding and then covalent bonding to the substrate by a condensation reaction with hydroxyl groups [19,20,21].
The subsequent reaction is the condensation of the trihydroxysilane, as expressed in the following equation:
The majority of silanes (with the exemption of aminosilanes) are used for surface treatments under acid-catalyzed conditions, where the rate of hydrolysis is considerably greater than that under the base-catalyzed hydrolysis condition, and it is minimally affected by other carbon-bonded substituents [19]. The hydrolysis is headed by protonation of the alkoxy group [19,25]. In both acid- (Figure 2a) and base-catalyzed (Figure 2b) systems, during hydrolysis, the alkoxy group is replaced with the hydroxyl with a pentacoordinate transition state [26]. The influence of pH on the stability of the alkoxysilanes and the formed silanol’s stability are different [27]. Silanols are most stable at pH around 3, and their reactivity increases when pH is lower than 1.5 or higher than 4.5 (Figure 3a) [27]. The dependency of the hydrolysis and condensation of alkoxysilanes on the pH is different, though acids and bases catalyze both processes [28]. When the reactions are base-catalyzed, gelation is fast due to the high rate of condensation, whereas, in acid-catalyzed reactions, the rate of hydrolysis is high with slow gelation [28]. Danks et al. [26] reported that the hydrolysis step (acid/base) depends on the stability of the transition state, which is dictated by the relative electron-withdrawing or donating power of –OH versus –OR groups. As a result, the subsequent hydrolysis steps become progressively slower in acidic conditions and faster under the basic conditions.
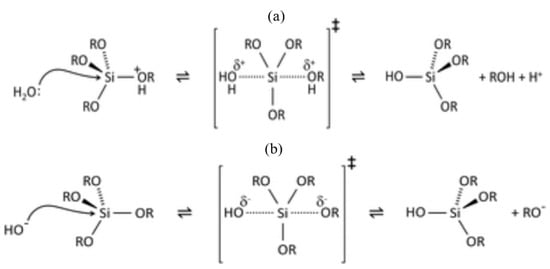
Figure 2.
(a) Acid- and (b) base-catalyzed hydrolysis of silicon alkoxides. The alkoxy group (OR) (such as methoxy (OCH3), ethoxy (OC2H5) or acetoxy (OCOCH3)) is replaced with the hydroxyl with a pentacoordinate transition state [26].
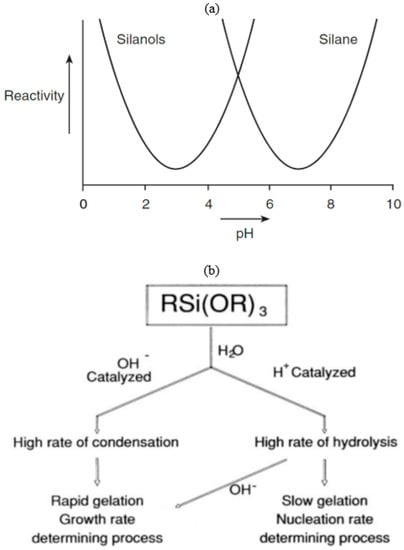
Figure 3.
(a) Reactivity of silane(s) and silanols. Silanols are most stable at pH around 3, and their reactivity increases when pH is lower than 1.5 or higher than 4.5 [27]. (b) Effect of pH on alkoxysilane hydrolysis. The gross changes in silane condensation and polymerization in an acid vs. base condition [19].
Unless the hydrolysis or the condensation is suppressed by specific conditions, both reactions will carry on simultaneously; for instance, suitable solvents can be used to prevent precipitation or gelation by slowing down or even halting the condensation [28]. Figure 3b shows the overall changes in silane condensation and polymerization in an acid versus base condition [19].
Savard et al. [29] studied the hydrolysis and condensation of γ-methacryloxypropyltrimethoxy silane in aqueous solutions and have confirmed the rates of hydrolysis and condensation to depend on the pH strongly. For example, they noted that about 25% of the initial quantity of silane was precipitated at pH = 7, whereas it was difficult to detect any insoluble in an acidic environment at pH = 2.7. For the same silane, Beari et al. [30] found that the initial concentrations of the generated silanetriol were 88% at pH 4 and 95% at pH 6 after 1.5 and 2.5 h, respectively. The concentrations decreased to below 15% at pH 4 in 74 h and to below 35% at pH 6 in 188 h. On the contrary, the concentrations of active silanol were more than 60% in both cases (i.e., on average, about two hydroxyl functions per silicon atom), confirming the reactivity of the formulation. The hydrolysis of methacryloxypropyltrimethoxy silane happened much more rapidly in acidic solution, but the yield of active silanols was higher at neutral pH, and the tendency to oligomerization was suppressed.
Cihlář [31] investigated the effect of pH and catalysts on the hydrolysis and condensation of tetraethoxysilane in a water–ethanol solution using chromatography, potentiometry and gelation tests. Both strong acids (HCl, HClO4, HNO3, H2SO4 and p-toluenesulphonic acid) and weak acids (Cl3CCOOH, (COOH)2, ClCH2COOH, CH3COOH and HCOOH) and LiOH were used as catalysts. The hydrolysis is catalyzed by acids as well as by bases. In the acidic region, the hydrolysis constant decreased as pH increased, with the minimum at a pH around 7. The condensation reaction that is catalyzed by acid as well as base is slowest at a pH of about 2.
Pu et al. [28] studied the hydrolysis and condensation kinetics of bis-triethoxysilyl ethane (BTSE) in a water–ethanol solution. The FTIR absorbance of Si-O-C group decreased with increasing duration of hydrolysis, as a result of the substitution of ethoxy groups of BTSE by OH groups. The minimum rate of hydrolysis was detected at pH = 7 (i.e., the rates increased both at pHs lower and higher than 7). They also found the hydrolysis to be much faster, in comparison with the condensation, at pH lower than 4.5, whereas the condensation was faster in the pH range of 4.5–9.0. At pH near 4.5, however, the difference between the hydrolysis and condensation rates tended to be small.
Premachandra et al. [32] investigated the dependence of hydrolysis and condensation kinetics of γ-ureidopropyltrimethoxysilane on the pH in water–methanol by FTIR spectroscopy. They found the silane to be most stable around pH 7.73, where the rate of the first hydrolysis step reached the minimum. In this water–alcohol system, the second and third hydrolysis steps were suppressed at most pHs, because the equilibrium between methanol and silane-bonded methoxy groups was quickly attained. The rates of the condensation reactions are higher at higher pH (e.g., the rates were much higher at pHs 8.97 and 9.87 than at pH 4.87). Silanols and water seemed to reach the equilibrium after sufficient time that was governed by the pH, leading to a constant number of siloxane bonds. The study also concluded that a solution of silane in the water–methanol system around pH 4.87 is rich in silanols due to the fast hydrolysis of methoxy groups and the relatively high stability of the formed silanols around this pH.
The hydrolysis of several alkyl-substituted alkoxysilanes and an antimicrobial quaternary ammonium silane was investigated in water–acetone solvents by FTIR spectroscopy [33]. FTIR spectra of trimethylmethoxysilane in 10% (w/w) water in acetone showed, at the initial stages, bands for the asymmetric stretch (1083 cm−1) and the symmetric stretch (865 cm−1) for Si-O-C methoxy and a water band. After considerable duration, which depends on the solution pH, these bands disappeared and were replaced by the C-O stretch of methanol (1031 cm−1) and the Si-OH stretch of the silanol (896 cm−1). Such behavior is attributed to the completion of hydrolysis of the methoxy group, as the silanol band is reduced and the siloxane asymmetric stretch appears. The rate of acid-catalyzed hydrolysis of trimethylmethoxysilane in 10% water–acetone was found to be much greater than the rate of condensation of siloxane bonds. A significant condensation is noticed but only after comprehensive hydrolysis. For other alkyltrimethoxysilanes (methyl-, ethyl-, propyl- or butyl-trimethoxysilane), as the alkyl group becomes larger, the acid catalysis becomes less effective. For silane containing a quaternary ammonium chloride group, the FTIR spectroscopy showed very little hydrolysis even after several hours, as the methoxy group was still detected at pH ≈ 5.5. When the pH was adjusted to 2.7, the intensity of the Si-O-C band decreased as a result of extensive hydrolysis, as evidenced by the emergence of the methanol band at 1030 cm−1 and the silanol band at 920 cm−1. Rapid and extensive condensation occurred when pH was adjusted to 7.0.
Issa et al. [34] investigated the hydrolysis mechanism of 3-cyanopropyltriethoxysilane (CTES). They found that the activation energy (∆Gǂ) of CTES hydrolysis in methanol and ethyl acetate resulted from an intermediate step, i.e., re-esterification in the case of methanol and hydrolysis of ethyl acetate at high temperatures, to produce acetic acid and ethanol and autocatalysis by acetic acid.
Paquet et al. [35] investigated the kinetics of the hydrolysis and self-condensation reactions of 3-(2-amino-ethylamino)propyl-trimethoxysilane in pure water and ethanol–water solution, using in situ 29Si NMR spectroscopy. During the early stages of the reaction, the reactivity of the silane was strongly affected by the pH of the hydrolysis environment. The silanols produced upon hydrolysis of silane water were much more stable than those produced by hydrolysis in ethanol–water; the latter was vulnerable to self-condensation, forming a siloxane bridge. They conclude that the relative ratio of silanols in water is influenced by pH that is consistent with the established role of acidic media in suppressing self-condensation.
In summary, the hydrolysis and condensation of silanes are crucial for their adhesion to metal surfaces. The pH of the environment is the most influential factor in silane hydrolysis and condensation processes. In the acidic environment (2 < pH < 4.5), stable silanols are produced. However, the condensation is facilitated in the basic conditions.
3. Mechanism of Silane Bonding with Metal
Usually, silanes are stored in a non-hydrolyzed state, and in most cases, they are hydrolyzed just before their use [36]. For bonding of silanes to a metallic substrate, the main purpose of hydrolysis is to generate active and sufficient silanols. The bonding is profoundly dictated by the pre-treatment of the metal surface. When silane is hydrolyzed in water or water + alcohol mixture, alkoxy groups convert to hydrophilic silanol groups (-SiOH) [6,37,38]. This conversion is profoundly governed by the nature of the organofunctional group on silicon and the pH of the solution [39].
The silane molecules need to be hydrolyzed to produce a sufficient number of silanol groups in each silane molecule that facilitate bonding with metal surfaces upon subsequent condensation between silanol groups (-SiOH) and metal surface hydroxyls (MeOH), as well as among silanol groups themselves [40], as shown in Figure 1. Metallo-siloxane bonds (Me-O-Si) formed as a result of the subsequent condensation (i.e., curing or drying process) of -SiOH and MeOH groups by releasing water, and a siloxane network (Si-O-Si) formed due to the reaction of silanols among themselves [11,41]. The as-formed Si-O-Si network becomes very hydrophobic if one of the substituents on the Si atom is a carbon atom [37]. For its hydrophobic nature, the formation of siloxane network -Si-O-Si- is crucial in corrosion protection. Figure 4 presents the simplified schematic of the mechanism of bonding between silane molecules and metal’s surface hydroxide layer [42].
Figure 4.
Simplified schematic of bonding mechanism between silane molecules and metal surface hydroxide layer: (a) before condensation: hydrogen-bonded interface; (b) after condensation: covalent-bonded interface [42].
Getting et al. [43] investigated the interfaces of abraded mild steel surfaces and various types of silane-based primers, using X-ray photoelectron spectroscopy (XPS) and static secondary ion mass spectroscopy (SSIMS) techniques to ascertain the bonding mechanism between primer and metal. The presence of SiO2H−, SiOH+ and SiO2 radicals was attributed to the occurrence of polymerization that produces a polysiloxane structure on the metal substrate. The presence of FeSiO+ originated from the surface iron oxide, which also provides strong evidence for the formation of a chemical bond, possibly the metallo-siloxane (Fe-O-Si) bond, as seen in Figure 1. However, no such radicals were detected when styrene-functional amine hydrochloride silane and γ-aminopropyltriethoxy silane were used. Van Ooij and Sabata [44] characterized the films of N-[2-(vinylnenzylamino)-ethyl]-3-aminopropyltrimethoxysilane and γ-aminopropyltriethoxy silane on zinc and steel substrates, respectively, by time-of-flight SIMS (TOFSIMS) and XPS techniques. For the film deposited on zinc surface and not subjected to curing, the high resolution TOFSIMS showed that typical Si peaks were subdued; instead, several high-intensity N peaks appeared. Accordingly, it was proposed that silanols are mainly absorbed by the zinc surface and the aromatic vinyl-benzyl groups are oriented away from the surface. In addition, no siloxane formation could be detected in the spectra. In fact, even after curing, there was only a slight increase in siloxane peaks. The TOFSIMS of γ-APS film deposited at pH 10.5 on an alkali cleaned mild steel showed several peaks for Si and N (without any peak for C) or peaks with Si and two N atoms. The presence of such peaks is due to the silane dimers or oligomers in which a –SiOH group of one molecule is linked with an –NH2 group of another molecule. Such internal acid–base interaction can happen in the solutions at a high pH since the –SiOH group becomes somewhat ionic. In fact, such processes involve –SiOH and NH2 groups at the metal surfaces if they are adsorbed at adjacent surface sites. When the silane was deposited from a solution of lower pH (e.g., 8.0), the peaks related to siloxane increased, and an obvious increase in the protonation of amino groups occurred. Silane molecules have been suggested to form covalent bond with iron.
Quinton et al. [45] found that the behavior of deposition of propyltrimethoxysilane (PTMS) on iron oxide is similar to that on aluminum oxide. For the silane-coated aluminum, the variation of the mass 28 peak (Si+) in SIMS was considerably different from that of the mass 71 peak (Si-O-Al). The intensity of mass 71 peak (Si-O-Al) for PTMS film on aluminum surface reached the maximum when a 0.75% silane concentration was used. Then, the intensity decreased to an asymptotic minimum level at 12% silane concentration. Because of the oscillatory nature of adsorption of PTMS on a number of metal oxide surfaces, including iron oxide, the data showed a fluctuation in the intensity of the mass 28 (Si+) peaks with increasing silane concentration. They also found that the maximum intensity of the mass 100 peak (SiOFe+) was at the same concentration as the first maximum of the mass 28 (Si+) peak. However, the rise of the mass 28 peak to an asymptotic level was at the maximum silane concentration (12%), whereas the intensity of the mass 100 peak decreased as the silane concentration increased. In another study, TOFSIMS of a non-organofunctional silane (i.e., BTSE) deposited on Al and Zn metal and Al-43.4Zn-1.6Si alloy also indicated the existence of metal–oxygen–silicon type of ion fragments which gave evidence for a chemical interaction between silane and the metal substrates [46].
Petrunin et al. [47] studied the mechanism of the formation of alkoxysilane adsorption layers from the vapor phase on a pure aluminum surface and the effect of this layer on corrosion resistance. They found that the first monolayer is adsorbed irreversibly with van der Waals forces. Subsequently, in the presence of water, covalent bonds develop between the silane and metal surface. The presence of such a silane monolayer on the metal surface decreased the water adsorption on the surface and inhibited the hydration of the metal oxide film. Moreover, formation of a negatively charged siloxane film improved the corrosion resistance while the positively charged layer enhanced the metal dissolution.
4. Silane Coatings for Corrosion Resistance of Mild Steel
Silane coating, one of the possible treatments, is found to have several attractive properties (such as being non-toxic, environment-friendly, low cost and easy to apply) to improve the corrosion resistance of different metals and alloys, including mild steel.
Jeyaram et al. [48] synthesized silane coating of 3-glycidyloxypropyl trimethoxy silane (sol A) as the precursor and (3-aminopropyl) trimethoxysilane (sol B) as the cross-linking agent on mild steel. Electrochemical impedance spectroscopy (EIS) showed that dipping five times in silane solutions improved the corrosion resistance by three times due to sol A coating and 14 times due to sol B (Figure 5a,b, respectively). The extracted electrochemical parameters from EIS suggested that the coatings developed from sol B showed higher charge transfer resistance (Rct) and lower double-layer capacitance than the coatings from sol A. The Rct of sol-B-coated mild steel increased from 65 Ω cm2 to 1370 Ω cm2, resulting in 98% protection efficiency compared to that of sol-A-coated with an efficiency of 80%. In addition, the reduction in double layer capacitance of sol B coating suggested that increasing the dipping time provides better corrosion protection. Such improvements were also reflected in polarization results, as the icorr decreased from 282 µA/cm2 for uncoated steel to 87 and 62 µA/cm2, respectively, for the steel treated with sol A and sol B (i.e., five dips in each solution), as shown, respectively, in Figure 5c,d. In salt spray test for 72 h, blistering, red rust and the corrosion products are seen on the sol-A-coated mild steel, resulting in the peeling off of coatings. However, the coatings obtained from sol B showed no delamination and no red rust, suggesting that the coatings obtained from sol B are homogeneous, compact, dense, and pin-hole free and adhere to the mild steel’s surface (Figure 5e,f).
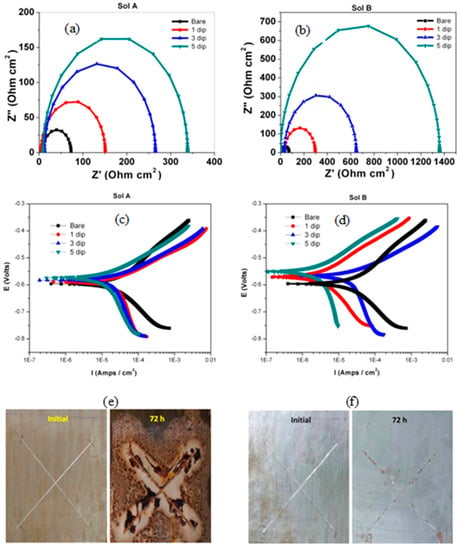
Figure 5.
EIS plot (a,b) and Tafel plot (c,d) of sol-gel A and sol-gel B coating on mild steel in 3.5% NaCl solution. Salt spray testing of samples of sol-gel A and sol-gel B coatings on mild steel (e,f) [48].
Alibakhshi et al. [49] studied the effect of hydrolysis time and concentration of silane on the corrosion protection of mild steel; tetraethylorthosilicate (TEOS) and trimethoxymethylsilane (TMOMS) were used as silane coatings. EIS results revealed that the coating of the mixture of 50% silanes (TEOS/TMOMS: 50/50 w/w) hydrolyzed for 24 h provided greater corrosion resistance in 3.5% NaCl solution, due to the hydrophobic nature of the coating and better bonding with metal surface and formation of stable film as supported by SEM. The highest corrosion resistance was observed after 1 h of exposure, after which the resistance gradually dropped (as shown in Figure 6a), presumably due to the development of conductive pathways for the electrolyte diffusion through the coating. The phase angle values were more negative in the high-frequency regime for the silane coating with 24 h of hydrolysis compared to 48 h of hydrolysis, suggesting the better protection behavior of the former.
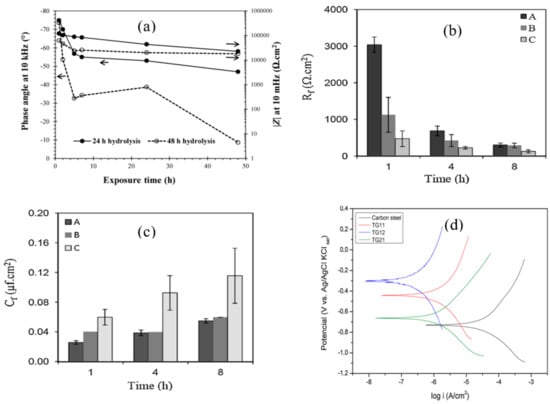
Figure 6.
(a) The impedance magnitude (|Z|) at low frequency (10 mHz) and phase angle at high frequency (10 kHz) of silane coatings with hydrolysis times of 24 and 48 h [49]. (b,c) Evolution of silane film resistance (Rf) and capacitance (Cf) (A: pH 2.8, dipping time 120 s; B: pH 2.8, dipping time 60, pH 2.8, dipping time 30 s) within 8 h of exposure to 0.1 M NaCl solution [50]. (d) Potentiodynamic polarization (PDP) of the uncoated substrate and coatings with different molar ratios of 1:1,1:2 and 2:1 of TEOS and GPTMS [51].
Other factors that influence the performance of silane coatings as a corrosion barrier are pH of hydrolysis and dipping time. Asadi et al. [50] investigated the effects of dipping duration (30, 60 and 120 s) in the silane solution and the pH of the solution (2.8–4.0) on the protection due to silane coating consisting of a mixture of tetraethoxysilane, methyltriethoxysilane and glycidyloxypropyltrimethoxysilane on mild steel. Electrochemical results showed that the coating developed by dipping the mild steel in the silane solution mixture for 120 s at pH 2.8 provided the most efficient protection with higher resistance and lower capacitance of the silane film, as shown in Figure 6b,c, which is attributed to the higher degree of cross-linking and homogeneity of the deposited coating.
Subramanian et al. [9] compared the corrosion inhibition due to silane coatings developed using two different silanes (i.e., γ-aminopropyltriethoxysilane (γ-APS) and bis-triethoxysilyl ethane (BTSE)). The silane films were developed on iron by dipping for 100 s and curing at 60 °C for 60 min in air. The films of the functional group silane (γ-APS) alone or the mixture of γ-APS and BTSE were found to have virtually no effect on the corrosion rate of iron. In contrast, the film of the non-functional silane (BTSE) alone developed at pH 4–6 decreased the corrosion rate by a factor of 15. In another related study [11], to investigate the influence of the molecular structure of silane monomers on the corrosion protection, organofunctional (γ-APS), bis-organofunctional (bis(trimethoxysilylpropyl)amine (BAS)) and bis-non-functional (BTSE) silanes were deposited on cold-rolled steel. Electrochemical tests in a relatively benign environment of 0.39 M Na2SO4 showed that the rise in the number of silanols, produced from the fully hydrolyzed silane and the absence of an amine group, would enhance the cross-linking reactions during the curing process. The greater the cross-linking density, the better is the corrosion resistance. Pepe et al. [52] studied the electrochemical corrosion of carbon steel coated with a hybrid organic–inorganic silica sol–gel. The coatings were developed by immersion of the samples in an organically modified silica sol made from hydrolysis and polycondensation of tetra-orthosilicate (TEOS) and methytriethoxisilane (MTES) by acidic catalysis. After exposure for 30 min at room temperature, the treated substrates were cured for 15 min at 400 °C and at the atmospheric pressure. The coatings thus developed showed an effective barrier against corrosive environment in the initial stages of immersion; however, the coating performance deteriorated significantly after 48 h of pre-immersion in corrosive media.
Alcantara-Garcia et al. [51] investigated the influence of different molar ratios of silanes on the chemical structure, physical properties and corrosion resistance of carbon steel. Three different molar ratios were used to prepare the organic–inorganic hybrid materials by hydrolysis and condensation of (3-glycidyloxypropyl) trimethoxysilane (GPTMS) and tetraethylorthosilane (TEOS) in an ethanol/water solution. Potentiodynamic polarization (PDP) results showed the Ecorr to significantly shift to more noble potentials, in particular for the coating prepared with a 1:2 molar ratio of TEOS to GPTMS when compared with the uncoated substrate. On the basis of the corrosion current density, the coating with a molar ratio of 1:2 showed the lowest corrosion rate, followed by coatings of 2:1 and 1:1 ratios (Figure 6d) [51].
Chico et al. [53] investigated the effect of curing time on the barrier properties of two types of silane, 3-aminopropyltriethoxysilane and bi-3-triethoxysilylpropyl amine, applied on steel substrates, with and without a subsequent application of alkyd-based paint. For samples with silane treatment alone, a benign medium (0.5 M potassium sulphate) was used as electrolyte, whereas 0.6 M NaCl solution was used for the metal/silane/paint system. The electrochemical impedance spectroscopy (EIS) showed the barrier properties and corrosion protection to be closely related to the prior curing process and the extent of curing. The individual silanes (e.g., bis-(trimethoxysilylpropyl)amine (bis-amino silane) or bis-(triethoxysilylpropyl)tetrasulphide (bis-sulfur silane)) performed poorly on cold-rolled steel [54] in hot salt soak and GM scab tests, whereas a mixture of the two silanes (2% prehydrolyzed concentration each) with a mixing ratio of 9:1 for bis-amino and bis-sulfur could provide corrosion performance as good as that due to a zinc phosphate system.
Wang et al. [55] studied the influence of cleaning solution pH on the cold-rolled steel’s surface chemistry and the corrosion resistance of silane-coated steel. In this study, a part of the as-cleaned cold-rolled steel substrates was coated with 10wt% aqueous solution of bis-amino silane and vinyltriacetoxysilane (VTAS) in a weight ratio of 5:1. Another part of the substrates was coated with a silane-containing primer (80wt% epoxy resin, 10wt% bis-sulfur silane, 9wt% amino silane/VTAS mixture and tetraethoxysilane). PDP measurements showed that the icorr decreased by half of an order of magnitude when the steel substrates were pre-treated with the cleaning solution at pH 9.5 prior to silane deposition, which was also supported by EIS data.
In a series of studies [56,57,58,59], one- and two-dimensional polymer films have been developed on iron surface by modification of a monolayer of either 11-Mercapto-1-undecanol (MUO), N,N-dimethylalkylamine or p-hydroxymethaylbenzene with different silanes. The coating procedures were carried out in deoxygenated atmospheres (i.e., under nitrogen atmosphere), and the contact time with silane solutions was varied from 30 min to many hours. Nozawa and Aramaki [56] prepared one- and two-dimensional polymer films by modification of an 11-Mercapto-1-undecanol (MUO) monolayer on iron with tetraethoxysilane (TES) for 30 min at 40 °C, octyltriethoxysilane (C8TES) for 30 min at 40 °C and/or octadecyltriethoxysilane (C18TES) for 10 h at 40 °C. The protective efficiencies of the coatings were determined by polarization and EIS tests in 0.5 M NaCl. Although, the “protective efficiency” of the one-dimensional polymer film developed by modification of the MUO monolayer with C18TES for 10 h was quite high (92%) in an aerated NaCl solution, the “protective efficiency” improved by over 93% due to the coating developed upon two-dimensional polymer films. The two-dimensional films were produced by modification of the MUO monolayer adsorbed on iron with TES and (C8TES) or (C18TES). The films were composed of a two-dimensional polymer of the thiolate attached on the surface and interconnected with siloxane bonds and small amounts of iron oxide/hydroxide and water. Aramaki [57] reported that the “protective efficiency” of ultrathin two-dimensional film of BTSE plus C8TES-modified MOU layer was not very high (91.6%). A considerably high protective efficiency (98.1%) was achieved when the MUO layer was modified with BTSE followed by C18TES treatment for 48 h. This film also protected iron from atmospheric corrosion for 60 days [57]. Tsuji et al. [58] modified N,N-dimethylalkylamine monolayer with alkylchlorosilanes for developing protective films of two-dimensional polymers against iron corrosion in 0.5 M NaCl solution. The prolonged modification treatment (more than 24 h) of monolayer with a solution of octadecyltrichlorosilane (C18TCS) was applied to provide a regular arrangement of large C18 alkyl tails within the film. The calculated “protective efficiency” from EIS and polarization tests were 92.9% and 93.1% for the layer modified with 1,2- bis(trichlorosilyl)ethane (BTCSE) plus C18TCS. The “protective efficiencies” were far lower than those obtained for the MUO layer modified with BTSE and C18TES, which has been described in reference [57]. Shimura et al. [59] attempted to introduce an additional modification of ultrathin two-dimensional polymer film of p-hydroxymethaylbenzene self-assembled monolayer. The monolayer was modified with BTSE and alkyltriethoxysilane CnH2n+1Si(OC2H5)3 (CnTES, n = 8 or 18). The protective ability of the modified coating against iron corrosion in 0.5 M NaCl solution after pre-immersion for 1.5 to 72 h was tested by polarization measurement. They found that the protective ability for the self-assembled monolayer modified twice with BTSE (for 2 h at 40 °C) followed by C8TES (for 2 h at 40 °C) did not improve markedly in comparison to that of the film without additional modification with BTSE, whereas the additional modification of the polymer films of BTSE plus C8TES (for 2 h at 40 °C) and BTSE plus C18TES (for 91 h at 40 °C) with C8TES for 2 h improved the barrier properties against corrosion. The enhancement was due to improvement of the alkyl tail arrangement and additional interconnection in polymer films.
5. Approaches for Improving Silane Coatings
5.1. Metal Oxide and Metal Oxide-Impreganated Silane Coatings
Metal oxides such as SiO2, ZrO2, Al2O3, TiO2 and CeO2 are resistant to corrosion [60]. Several studies have investigated the effect of impregnation of such metal oxides into silane coatings on the corrosion resistance of metals and alloys. This section reviews the enhancement in corrosion protection of mild steel due to silane coatings impregnated with such metal oxide.
Vivar Mora et al. [61] prepared sol–gel incorporated non-functionalized and functionalized nanoparticles of silica (SiO2) for corrosion resistance of mild steel. Three different molar ratios of tetraethylorthosilicate (TEOS) and 3-glycidoxypropyltrimethoxysilane (GPTMS) were used to develop a coating with thickness greater than a few microns to accommodate the added nanoparticles. Salt spray test results showed that the addition of non-functionalized silica nanoparticles improved corrosion resistance, though corrosion is evident after 72 h. On the other hand, the corrosion resistance improved due to incorporating GPTMS-functionalized silica nanoparticles, showing very little corrosion even after 72 h. Li et al. [62] coated Q235 carbon steel with a two-layer coating composed of a primary underlying layer impregnated with silica and titania powder as filler and pigment materials and a translucent topcoat layer containing a colloidal silica sol–gel matrix cross-linked by methyltrimethoxysilane (MTMS). They noticed no visible signs of corrosion for the first 28 days of the salt spray test. However, after 35 days, the corrosion spots started to be visible. EIS tests in 5% NaCl solution revealed that the low-frequency impedance value |Z|0.01Hz is in the range of 108–109 Ω·cm2, which is relatively high compared to other coating systems.
ZrO2 is another metal oxide that finds a variety of applications, including coatings for improving the corrosion resistance of metals and alloys [63,64,65,66,67]. Incorporating ZrO2 nanoparticles into sol–gel coatings improves coatings’ mechanical properties [68]. Claire et al. [68] reported a bi-layer sol–gel coating system, with the outer layer containing zirconia nanoparticles, to improve the corrosion and abrasion properties of carbon steel. Kiruthika et al. [69] investigated a hybrid sol–gel based nanocomposite coating composed of GPTMS with zirconium-n-propoxide on mild steel. The coating enhanced the abrasion resistance (to withstand up to 500 cycles), as well as corrosion resistance.
Among the various oxides employed in coating applications, alumina is widely used as an additive for improving the wear and corrosion resistance of coatings, since it is inexpensive, chemically inert and thermally highly stable [70]. Ruhi et al. [71] developed sol–gel alumina coatings on the zinc-phosphated mild steel, followed by sintering at 300, 400 and 500 °C. A high magnification SEM micrograph showed a rough but crack-free coating developed at 300 and 400 °C (Figure 7a for the coating developed at 400 °C). However, numerous cracks were observed in the coating sintered at 500 °C (Figure 7b) due to loss of plasticity as a result of the elimination of the organic part, as detected by FTIR analysis of the coating. As a result, PDP tests (Figure 7c) revealed that the coating sintered at 500 °C possesses the lowest corrosion resistance. However, the sol–gel coated specimen sintered at 400 °C showed a minimum corrosion current density icorr. Tiwari et al. [72] prepared a conversion coating by coating the mild steel with silica sol and dipping the dried silica sol-coated sample in aluminum oxy-hydroxide sol. Then, the coated sample was heated at 500 °C for 2 h and cleaned with ethanol after cooling. For comparison, the mild steel coated with conversion sol–gel is recoated by dipping it in alumina sol and drying at room temperature for 30 min, followed by heating at 250 °C for 15 min. This coating process is repeated five times, followed by heating at 500 °C for 1 h. AFM micrographs in Figure 7d,e showed that the average roughness (Ra), measured diagonally from a 40 μm × 40 μm area scan, is nearly 110 nm and 25 nm for conversion-coated mild steel (CcMS) and alumina-sol-coated steel (Al2O3/CcMS), respectively. The AFM micrographs also revealed that a layered structure sol–gel coating is orientated in different directions with circular nanoparticles of less than 20 nm diameters (Figure 7d,e). The cluster of particles (≈1 μm size) on the CcMS suggests continuous growth in all directions. In contrast, a uniform alumina sol coating is seen on the steel surface. Small dot-like features can be observed as approximately 300–350 nm diameter agglomerates (Figure 7f,g). Figure 7h shows the polarization behavior of CcMS, CcMS/Al2O3 and bare. The alumina coating on pre-phosphated mild steel (Phos.MSAl2O3) was also shown for comparison. Corrosion current densities (icorr) for CcMS and CcMS/Al2O3 are reduced by about two and five orders of magnitude, respectively, compared to bare MS substrate.
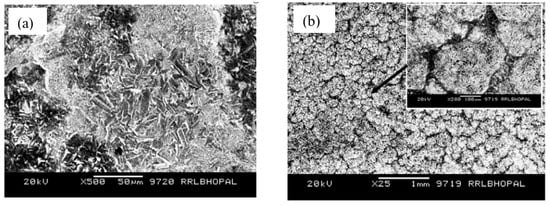
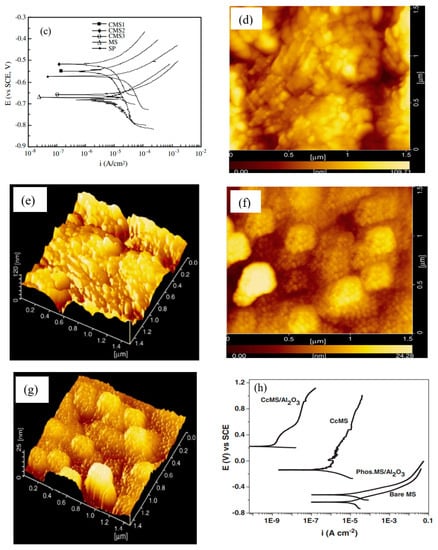
Figure 7.
Surface morphology of the sol–gel-coated specimens sintered at (a) 400 °C and (b) 500 °C [71]. (c) PDP plots for the mild steel (MS), the surface pre-treated mild steel (SP) and the sol–gel-coated specimens sintered at 300 °C (CMS1), 400 °C (CMS2) and 500 °C (CMS3) in 3.5% NaCl solution at room temperature [71]. AFM micrographs of coated surface: (d,e) conversion-coated mild steel (CcMS) and (f,g) alumina-sol-coated steel (Al2O3/CcMS). (h) PDP plots recorded at a scan rate of 0.5 mVs−1 in 3.5 wt% NaCl [72].
Due to its excellent chemical stability, heat resistance and low electron conductivity, TiO2 can be used as an excellent anti-corrosion material. It was found that applying sol–gel TiO2 coating or sol–gel SiO2 coating improved the corrosion resistance of mild steel in NaCl solution [73]. In contrast, the sol–gel film containing TiO2-SiO2 together provides the lowest corrosion protection, even lower than the bare mild steel. However, Krishna et al. [74] prepared the oxidation-resistant TiO2-SiO2 thin film coating on mild steel via the sol–gel process. They reported that a single-layer coating is sufficient to protect the substrate from oxidation with an optimized surface roughness.
CeO2, a rare earth oxide that has been extensively studied, has attracted the attention in thermocatalysis, electrocatalysis and photocatalysis applications [75]. For the corrosion protection of carbon steel, Carvalho et al. [76] studied the influence of the deposition of cerium oxide films produced from the incorporation of ceric ammonium nitrate and cerium chloride as metal precursors in sol–gel. The effect of using different temperatures and heating rates during thermal calcination was also studied. PDP results showed that cerium deposition enhanced the corrosion resistance of carbon steel, and the Ecorr shifted to the noble direction. The corrosion current densities (icorr) decreased when a 5 °C/min heating rate was used during the prior thermal calcination. The changes are more obvious when the calcination temperatures was 200 °C (Figure 8a,b). They concluded that the sol–gel coatings with ceric ammonium nitrate as a cerium precursor developed at low calcination temperatures and high heating rates are promising as barrier protection for carbon steel and other metallic surfaces.
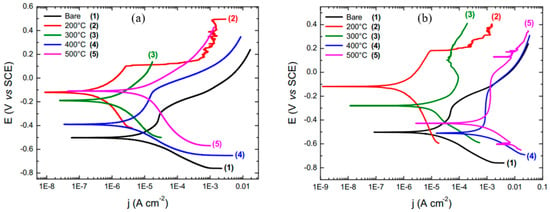
Figure 8.
PDP curves in aqueous solution of NaCl 3.5% for electrodes coated with: (a) ceric ammonium nitrate and (b) cerium chloride, calcined at different temperatures and with heating rate of 5 °C/min [76].
The epoxy–silica hybrid film of (3-glycidoxypropyl)-1,1,3,3-tetramethyldisiloxane and tetraethyl orthosilicate is synthesized for mild steel protection in a solution of 0.6 wt% NaCl and 0.6 wt% (NH4)2SO4 [77]. CeO2 pigments (0.25–2 wt%) are introduced as a filler to improve the barrier protection. EIS results revealed that using CeO2 pigments improved protection against the corrosion of mild steel compared to the unmodified silane coating, suggesting the formation of stable oxide/hydroxides of Ce within the coating network. Figure 9 compared SEM micrographs of coated mild steel before and after immersion in an aerated solution of 0.6 wt% NaCl plus 0.6 wt% (NH4)2SO4 for 14 days. White patches of silica and/or silica/CeO2 aggregates after the thermal curing were observed to be intact on the coated steel (Figure 9a–f). After the two-week immersion, some portions of the coatings are still intact, though micro-cracks and exfoliation were observed in the coating (Figure 9a1–f1). The improvement in corrosion resistance of the coated steel is due to the anticorrosive properties of CeO2 and the pigment enforcing interconnectivity.
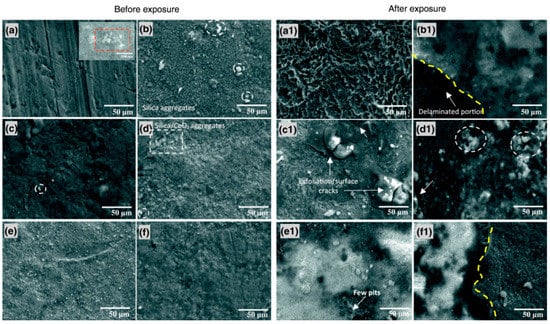
Figure 9.
SEM micrographs of the coated mild steel before (a–f) and after (a1–f1) 14 days of exposure to aerated 0.6 wt% NaCl plus 0.6 wt% (NH4)2SO4 solution: (a) the metal etched surface without and (b) with the silane coating, the coating modified with (c) 0.25, (d) 0.50, (e) 1 and (f) 2 wt% CeO2. (Inset at (a): CeO2 nanoparticle aggregates (25 nm particle size distribution)) [77].
5.2. Silane Incorporated with Plant Extracts
The hazards associated with the use of common synthetic corrosion inhibitors such as chromate triggered the need to develop nontoxic, inexpensive and environment-friendly approaches, such as the use of silane-based coatings and green inhibitors [78]. For example, the effect of incorporating caffeine (which is extracted from tea leaves) into the hybrid silane coating of TEOS and APTES to improve the corrosion resistance of mild steel in 3.5 wt% NaCl solution was investigated by Hamidon and Hussin [79]. They found that caffeine-doped silane coating improved the corrosion resistance of steel in NaCl solution, with 100 ppm caffeine providing the maximum improvement.
Ishak et al. [80] studied the influence of doping the TEOS-APTES sol–gel with curcumin in mild steel corrosion in a 0.5M HCl solution. PDP tests showed icorr to decrease with the increase in the concentration of the curcumin in the silane film; the optimum concentration was 100 ppm. Hamidon et al. [81] doped the same sol–gel system (TEOS-APTES) with different concentrations of aqueous crude extract of span tea leaves (0, 25, 50, 75 and 100 ppm). Doping the sol–gel with span tea extract improved the corrosion resistance of steel in NaCl solution, and the optimum concentration of span tea extract was 75 ppm, as suggested by PDP and EIS tests. Ghuzali et al. [82] incorporated the extracts of Clitoria ternatea (using water and ethanol as solvents) into the hybrid sol–gel of GPTMS, using TEOS as the precursor to enhance the corrosion resistance of mild steel in 0.5M HCl solution. Electrochemical impedance spectroscopy data suggested that at 75 ppm of the Clitoria ternatea extract in the sol–gel coating, the inhibition efficiencies were 89.6% and 85.6% for the coating; these green inhibitors were extracted using ethanol and water, respectively.
5.3. Silane Coatings Impregnated with Graphene Oxide
Graphene is an attractive 2D material with remarkable physical and chemical properties for diverse applications [83]. The tiny pores in the hexagonal lattice structure of graphene (i.e., 0.064 nm) are impermeable to fluid molecules; hence, the use of graphene coating is a promising tactic for attaining durable corrosion resistance of metals and alloys [83].
Haghdadeh et al. [84] functionalized the graphene oxide (GO) nanosheets with (3-glycidyloxypropyl) trimethoxysilane to be introduced into the polyurethane (PU) matrix for improving the corrosion resistance and mechanical properties of the composite coating on metals. The corrosion protection properties of the coatings were investigated using salt spray and EIS tests. In the salt spray test, blisters and corrosion spots emerged on both the GO/PU and neat PU coatings after 300 h and 600 h, respectively, that increased with the duration of salt spray. Thus, incorporating the neat GO into the PU matrix was found to be deleterious for corrosion resistance, which was attributed to the presence of many polar groups on the GO sheets and their ability to increase the hydrophilic nature of PU, thereby increasing susceptibility to corrosion. This mechanistic explanation was duly supported by the fact that when PU was added with suitably functionalized GO, i.e., fGO (instead of plain GO), corrosion resistance due to fGO/PU coating improved, which is attributed to the hydrophobic nature of fGO. With fGO/PU coating, considerably fewer corrosion spots and blisters were observed even after 900 h of salt spray. Figure 10a shows the evolution of impedance (|Z|) in the lowest frequency regime (10 mHz), i.e., representation of corrosion resistance, for the neat PU, GO/PU and fGO/PU during immersion for different durations in 3.5 wt% NaCl solution. Corrosion resistance decreased rapidly for the neat PU-coating (Figure 10a). Consistent with the salt-spray test results, the corrosion resistance of GO-containing PU coating (GO/PU) was inferior to the plain PU coating, since GO is known to suffer agglomeration that creates defects in the coating. However, suitably functionalization of GO to develop fGO/PU coating provided considerably improved and durable corrosion resistance, as seen in Figure 10a [84].
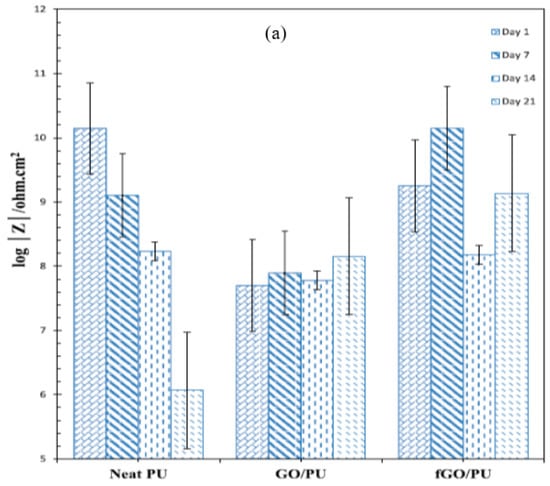
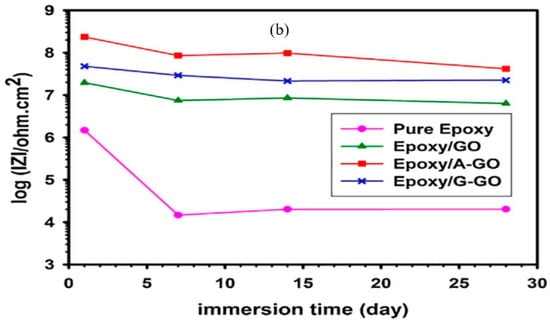
Figure 10.
(a) Impedance (|Z|) at the lowest frequency (10 mHz) extracted from Bode plots for neat PU, GO/PU and fGO/PU after immersion for different durations in 3.5 wt% NaCl [84] and (b) evolution of |Z| for pure epoxy and GO-nanocomposite-impregnated epoxy coatings during immersion in 3.5 wt% NaCl solution for 28 days [85].
Pourhashem et al. [85] modified 3-aminopropyl triethoxysilane (APTES) and 3-glysidyloxypropyl trimethoxysilane (GPTMS) silanes to create amine and epoxy end-groups and then used the modified silanes to functionalize graphene oxide (GO). The composite coating was applied onto steel. Corrosion resistance of bare, epoxy-coated and nanocomposite-epoxy-coated steel in 3.5 wt% NaCl solution was evaluated. OCP of coatings was evaluated to be positive in the order: epoxy < epoxy/GO < epoxy/G-GO < epoxy/A-GO, i.e., the addition of nanofiller improved the corrosion resistance, which is also corroborated by the corresponding impedance (|Z|) data (Figure 10b). The corrosion resistance decreased with increasing immersion time; however, the decrease is considerably less pronounced for epoxy coatings modified with GO and its derivatives. As seen in Figure 10b, the impedance (|Z|) of steel coated with unmodified (pure) epoxy decreased rapidly from ~106 to ~104 Ω cm2 within 7 days of immersion, before stabilizing. On the other hand, impedance (|Z|) stays reasonably stable in the case of epoxy coatings modified with GO and its derivatives, suggesting their ability to overcome the deleterious effect of porosity and defects of the nanocomposite coating. In another study, Pourhashem et al. [86] investigated epoxy coatings containing graphene oxide (GO) and amino-silane modified GO (A-GO), with various weight fractions (0.05, 0.1, 0.3 and 0.5 wt%), on mild steel 3.5% NaCl solution. They found the corrosion resistance due to the epoxy/A-GO coating to be significantly higher. The optimum GO content was found to be 0.1wt%; at greater contents, GO nanosheets started to suffer agglomeration.
Graphene oxide (GO)-based nano-impregnation containing silane composite coatings also provides superior corrosion resistance to mild steel in concrete solution containing chloride [87]. In the alkaline environment of concrete (pH > 10), mild steel can form a protective passivation layer; however, the passive layer can be disrupted by chloride ions, resulting in rapid corrosion of steel at the locations of disruption [88,89]. Geng et al. [87] investigated GO-modified silane coating developed on steel reinforcement bars in a simulated concrete pore solution containing 3.5% NaCl, and reported the coating to provide excellent corrosion resistance, with a maximum protection efficiency of ~99%. Sharma et al. [90] coated the mild steel rebars with an epoxy coating using reduced graphene oxide (rGO) and graphene oxide (GO) along with carbon nanotubes (CNTs) and silane agents for improving corrosion resistance in concrete. They found that the addition of rGO and GO with CNTs to pure epoxy produces a dense, impermeable and durable coating on steel, as compared to pure epoxy layer. The epoxy alone coating impedes corrosion initiation for 8 days, whereas rGO/CNT-epoxy delays it by up to 50 days. The long-term corrosion protection performance is superior for GO/CNT-epoxy coating, as compared to rGO/CNT-epoxy coating.
Table 1 summarizes the different approaches for improving silane coatings and the main findings, as reported in the literature.

Table 1.
Different approaches for improving silane coatings and the main findings, as reported in the literature.
6. Silane Coatings for Mitigation of MIC and Biofouling of Mild Steel
Biocorrosion or microbiologically influenced corrosion MIC can be defined as an electrochemical process where the microorganisms participate in initiating, facilitating or accelerating the corrosion reaction without altering its electrochemical nature [91]. MIC becomes a frequent risk factor for accidents such as fluid flooding, leakage and rupture when the equipment in different industries is used for a long time [92].
Besides their use for general corrosion protection, silanes and silanes impregnated with other environment-friendly and non-hazardous materials can mitigate microbiologically influenced corrosion (MIC) [93].
Suleiman et al. [94] synthesized a hybrid sol–gel material (HC) from allyl precursors and functionalized it into hybrid polymeric material incorporated with various organic and inorganic antibacterial agents, i.e., 1,1-dimethylbiguanide hydrochloride (HC-Bing), Irgarol (HC-Ira), MOLY-white 101 (HC-Moly), Ti nanoparticles (HC-Ti) or silver nanoparticle dispersion (HC-Ag). EIS results for the coated samples exposed to plain 3.5% NaCl solution showed that HC, HC-Ira and HC-Moly had considerably higher corrosion resistance, which was supported by icorr data in PDP tests. In contrast, it was HC-Bing and HC-Ti that showed greater resistance to biofouling. As seen in Figure 11, only two hard-shell barnacles were found to become attached to HC-Bing during 6 months of a marine biofouling test, whereas the bare steel substrate suffered heavy biofouling and corrosion. In comparison, no barnacles, polychaetes or deposits were observed on the surfaces of other coatings.
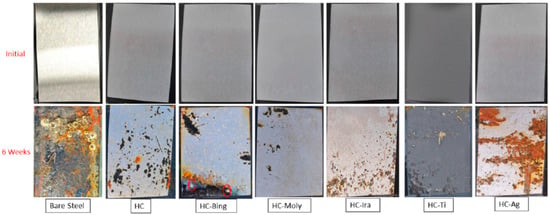
Figure 11.
Field trial samples of all coating matrices compared with bare/uncoated steel after 6 weeks of exposure to seawater in the Eastern Province of Saudi Arabia, which has minimal flow and is known for its marine macrofouling activities and high salinity [94].
Al-Saadi et al. [95] studied the influence of Bis[triethoxysilyl]ethane (BTSE) silane coating on the corrosion resistance of mild steel in an aerobic chloride medium and an anaerobic microbial medium containing sulphate-reducing bacteria (SRB). BTSE coating significantly enhanced the corrosion resistance of mild steel in 0.6M NaCl; the icorr of the uncoated steel was at least 25 times higher than that of the steel coated with BTSE (Figure 12a). EIS results in Figure 12b showed that the impedance of the BTSE-coated steel was 45 times higher than that of the uncoated mild steel. In the presence of SRB, BTSE coating provided little protection to the mild steel substrates as suggested by the more negative Ecorr and the significant increase in icorr after 14 days of exposure to marine environment inoculated with SRB (Figure 12c). They reported that the limited improvement provided by BTSE coating in the presence of SRB is due to the absence of any antimicrobial functional group in this silane.
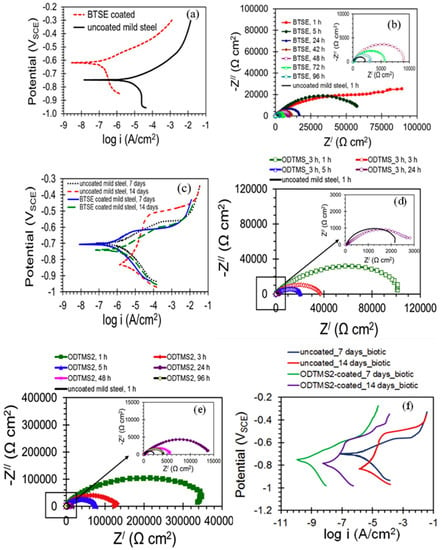
Figure 12.
(a) PDP plots of the BTSE-coated and uncoated mild steel pre-immersed for 1 h in 0.6 M NaCl solution, (b) Nyquist plots for the BTSE-coated specimens at different durations of immersion and the uncoated mild steel specimens at 1 h of immersion in 0.6 M NaCl, (c) PDP plots of the BTSE-coated and uncoated specimens after different exposure times in the marine environment with SRB [95], (d) Nyquist plots for uncoated and ODTMS-coated mild steel pre-immersed in 3.5% NaCl solution for 1, 3, 5 and 24 h, (e) Nyquist plots for the two-step coated (ODTMS2) and uncoated mild steel pre-immersed in 3.5% NaCl solution up to 96 h and (f) PDP curves of ODTMS2-coated and uncoated mild steel specimens after exposures for 7 and 14 days to marine environment inoculated with SRB [96].
Al-Saadi and Raman [96] developed octadecyltrimethoxysilane (ODTMS) coatings upon single- and two-step treatments for enhancing the corrosion resistance of mild steel in marine environments with and without SRB. The low-frequency impedance |Z| for the ODTMS-coated steel developed upon one-step treatment was ~100 kΩ cm2, which is ∼50 times higher than that for the uncoated steel (~2 kΩ cm2) after 1 h of exposure to NaCl solution, as shown in Figure 12d. For the coating deposited by two-step treatment (i.e., ODTMS2), the impedance |Z| increased by more than two orders of magnitude compared to that for the uncoated specimen. After 24 h of pre-immersion in NaCl, though the corrosion resistance of coated steel decreased by about 20 times as compared to 1 h pre-immersed coated steel, the two-step coatings still provided significant protection, as shown in Figure 12e. In the marine environment with SRB, PDP results (Figure 12f) for the uncoated mild steel revealed that the Ecorr shifted significantly towards the cathodic region (the potential moved ~128 mV more negative during the period of exposures of 7–14 days), whereas, for the mild steel coated with ODTMS2, the potential shifted only by ~35 mV towards the positive direction, suggesting considerably less susceptibility to anodic dissolution compared to the uncoated steel. The icorr values of the uncoated steel were significantly higher than those for ODTMS2-coated specimens, for exposures of 7–14 days, in the marine environment with SRB. The icorr values for uncoated steel were more than three orders of magnitude higher (for 14 days) and two-and-half orders of magnitude higher (for 7 days) than those for ODTMS2-coated steel. They reported that the improvement in corrosion resistance is attributed to the formation of the highly crystalline, densely packed, hydrophobic and orientated film due to the long aliphatic chain silane. [96].
Quaternary ammonium silanes (QAS) have shown antimicrobial and antibacterial activity. The antibacterial materials can attack the cell membrane and turn it semipermeable, causing leaking of the cell contents [91]. The electrostatic contact between the quaternary ammonium compounds and the negatively charged surfaces of bacteria facilitates interaction and penetration by the alkyl chain through the bacteria’s membrane, causing the bacterial cell’s dissolution and death [92]. Al-Saadi et al. [93] studied the inhibition action of the two-step coating of a non-functional silane, BTSE, followed by another top coating with a silane mixture of bis-[trimethoxysilylpropyl]amine (bis-amino silane) and 3-[trimethoxysilyl]-propyldimethyloctadecyl ammonium chloride (QAS) in a mixing ratio of 5:1 (v/v) for resistance of mild steel to MIC in a chloride solution with SRB. Impedance results in Figure 13a show that the corrosion resistance of the uncoated steel in the marine solution with SRB decreased with the increase in the duration of exposure, i.e., from 17.8 kΩ cm2 at 3 days to 8 kΩ cm2 at 7 days, and after 14 days, the impedance dropped down to 4 kΩ cm2. However, impedances (|Z|) of steel with the two-step silane coating incorporated with the quaternary ammonium silane were significantly higher than those for uncoated specimens, clearly evidencing the two-step silane treatment to have noticeably improved the corrosion resistance of the coated mild steel in the marine environment with SRB, as seen in Figure 13b. The most probable number (MPN) technique showed that the active SRB cells on the uncoated surface pre-exposed for 3 days to the marine environment with SRB were about 6 × 106 MPN/cm2 (Figure 13c). The number of active cells increased by more than 16 times after 7 days and remained higher by seven times after 14 days of pre-exposure. However, for silane-coated steel samples, the number of active cells adhering to the coated surfaces was much lower than those attached to the uncoated steel samples.
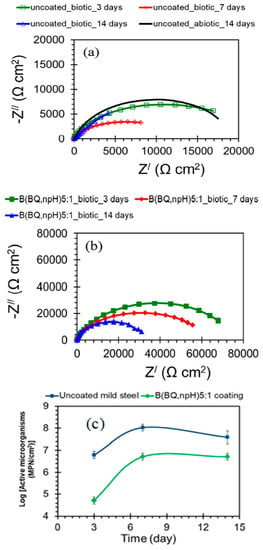
Figure 13.
(a,b) Nyquist plots of uncoated and silane-coated mild steel after exposure times of 3, 7 and 14 days in a marine environment inoculated with SRB. (c) SRB population on uncoated and two-step silane-coated mild steel surfaces with respect to the time of exposure to the marine environment with SRB [93].
Though there is some reported literature on the role of silane coatings for mitigation of MIC, this is a vastly unexplored research topic, even though the topic may have huge industrial relevance.
7. Conclusions
Due to the health risk concerns of using chromate conversion coatings for metals and alloys, silane coatings have gained significant attention as alternative surface pre-treatments for adhesion promotion and corrosion resistance enhancement. This review discussed the recent work on silane coatings for mild steel against corrosion and microbiologically influenced corrosion (MIC). The salient features of the review are:
- 1-
- The hydrolysis and condensation of silanes are among the critical factors influencing the robustness of the coatings developed on the metal surface. Most of the silanes are subjected to acid-catalyzed hydrolysis, where a high rate of hydrolysis with slow gelation is preferred. On the other hand, in the base catalyzed reaction, the rate of condensation reaction is higher with fast gelation.
- 2-
- Sufficient silanol groups generated from hydrolysis are required that facilitate the subsequent condensation/bonding between silanol groups (-SiOH) and metal surface hydroxyls (MeOH).
- 3-
- Though different studies showed the ability of silane to improve the corrosion resistance of mild steel, the durability of the developed silane coatings can be improved by incorporation of functionalized and unfunctionalized metal oxides, plant extracts and 2D materials into the coatings.
- 4-
- In the corrosive environments of chloride with the presence of sulphate-reducing bacteria, the non-functional silanes provided little improvement due to the absence of any antimicrobial functional group in such silanes. However, the long aliphatic chain silane (e.g., octadecyltrimethoxysilane (ODTMS) and quaternary ammonium silane (QAS)) improved the corrosion resistance in the microbial environment due to the formation of hydrophobic surface in case of ODTMS coating or because of the antibacterial characteristics of QAS.
Author Contributions
S.A.-S.: conceptualization, writing—review and editing; R.K.S.R.: conceptualization, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Plueddemann, E.P. General Concepts. In Silane Coupling Agents; Plueddemann, E.P., Ed.; Springer: Boston, MA, USA, 1991; pp. 1–29. [Google Scholar]
- Asadi, N.; Naderi, R. Chapter 23—Nanoparticles Incorporated in Silane Sol-Gel Coatings. In Corrosion Protection at the Nanoscale; Rajendran, S., Nguyen, T.A., Kakooei, S., Li, Y., Yeganeh, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 451–471. [Google Scholar]
- Packham, D.E. Silane adhesion promotors. In Handbook of Adhesion, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- Ebnesajjad, S. Chapter 12—Adhesion Promoters. In Surface Treatment of Materials for Adhesive Bonding, 2nd ed.; Ebnesajjad, S., Ed.; William Andrew Publishing: Oxford, UK, 2014; pp. 301–329. [Google Scholar]
- Seymour, B.R.; Deanin, R.D. History of Polymeric Composites; VSP: Rancho Cordova, CA, USA, 1987. [Google Scholar]
- Subramanian, V.; Van Ooij, W. Silane based metal pretreatments as alternatives to chromating: Shortlisted. Surf. Eng. 1999, 15, 168–172. [Google Scholar] [CrossRef]
- Al-Saadi, S.; Raman, R. Two step silane coating for corrosion resistance of steel. In Proceedings of the 18th International Corrosion Congress, Perth, Australia, 20–24 November 2011; Australasian Corrosion Association: Melbourne, Australia, 2011. [Google Scholar]
- Tan, D.; Yuan, P.; Liu, D.; Du, P. Chapter 8—Surface Modifications of Halloysite. In Developments in Clay Science; Yuan, P., Thill, A., Bergaya, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 167–201. [Google Scholar]
- Subramanian, V.; Van Ooij, W. Effect of the amine functional group on corrosion rate of iron coated with films of organofunctional silanes. Corrosion 1998, 54, 204–215. [Google Scholar] [CrossRef]
- Plueddemann, E.P. Chemistry of Silane Coupling Agents. In Silane Coupling Agents; Plueddemann, E.P., Ed.; Springer: Boston, MA, USA, 1991; pp. 31–54. [Google Scholar]
- Van Schaftinghen, T.; Le Pen, C.; Terryn, H.; Hörzenberger, F. Investigation of the barrier properties of silanes on cold rolled steel. Electrochim. Acta 2004, 49, 2997–3004. [Google Scholar] [CrossRef]
- Franquet, A.; Le Pen, C.; Terryn, H.; Vereecken, J. Effect of bath concentration and curing time on the structure of non-functional thin organosilane layers on aluminium. Electrochim. Acta 2003, 48, 1245–1255. [Google Scholar] [CrossRef]
- Wang, H.; Akid, R. Encapsulated cerium nitrate inhibitors to provide high-performance anti-corrosion sol–gel coatings on mild steel. Corros. Sci. 2008, 50, 1142–1148. [Google Scholar] [CrossRef]
- Tsapakos, M.J.; Wetterhahn, K.E. The interaction of chromium with nucleic acids. Chem. Biol. Interact. 1983, 46, 265–277. [Google Scholar] [CrossRef]
- Van Ooij, W.J.; Zhu, D. Electrochemical impedance spectroscopy of bis-[triethoxysilypropyl] tetrasulfide on Al 2024-T3 substrates. Corrosion 2001, 57, 413–427. [Google Scholar] [CrossRef]
- Eduok, U.; Faye, O.; Szpunar, J. Recent developments and applications of protective silicone coatings: A review of PDMS functional materials. Prog. Org. Coat. 2017, 111, 124–163. [Google Scholar] [CrossRef]
- Montemor, M.; Rosqvist, A.; Fagerholm, H.; Ferreira, M.G.S. The early corrosion behaviour of hot dip galvanised steel pre-treated with bis-1, 2-(triethoxysilyl) ethane. Prog. Org. Coat. 2004, 51, 188–194. [Google Scholar] [CrossRef]
- Singh, A.K.; Quraishi, M. Investigation of the effect of disulfiram on corrosion of mild steel in hydrochloric acid solution. Corros. Sci. 2011, 53, 1288–1297. [Google Scholar] [CrossRef]
- Arkles, B.; Steinmetz, J.R.; Zazyczny, J.; Mehta, P. Factors contributing to the stability of alkoxysilanes in aqueous solution. J. Adhes. Sci. Technol. 1992, 6, 193–206. [Google Scholar] [CrossRef]
- Materne, T.; De Buyl, F.; Witucki, G.L. Organosilane Technology in Coating Applications: Review and Perspectives; Dow Corning Corporation: Midland, MI, USA, 2012. [Google Scholar]
- Zhu, D.; Hu, N.; Schaefer, D.W. Chapter 1—Water-based sol-gel coatings for military coating applications. In Handbook of Waterborne Coatings; Zarras, P., Soucek, M.D., Tiwari, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–27. [Google Scholar]
- Chen, S.-L.; Dong, P.; Yang, G.H.; Yang, J.J. Kinetics of formation of monodisperse colloidal silica particles through the hydrolysis and condensation of tetraethylorthosilicate. Ind. Eng. Chem. Res. 1996, 35, 4487–4493. [Google Scholar] [CrossRef]
- Issa, A.A.; Luyt, A.S. Kinetics of alkoxysilanes and organoalkoxysilanes polymerization: A review. Polymers 2019, 11, 537. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.; Bhattacharyya, S.; Ducheyne, P. 4.428. Sol-Gel Processed Oxide Controlled Release Materials. In Comprehensive Biomaterials; Elsevier: Oxford, UK, 2011; pp. 475–495. [Google Scholar]
- Gadhave, R.V.; Gadhave, C.R.; Dhawale, P.V. Silane Terminated Prepolymers: An Alternative to Silicones and Polyurethanes. Open J. Polym. Chem. 2021, 11, 31–54. [Google Scholar] [CrossRef]
- Danks, A.E.; Hall, S.R.; Schnepp, Z. The evolution of ‘sol-gel’chemistry as a technique for materials synthesis. Mater. Horiz. 2016, 3, 91–112. [Google Scholar] [CrossRef]
- Borup, B.; Weissenbach, K. Silane Coupling Agents. In Functional Fillers for Plastics; John Wiley & Sons: Hoboken, NJ, USA, 2010; pp. 61–90. [Google Scholar]
- Pu, Z.; Van Ooij, W.; Mark, J. Hydrolysis kinetics and stability of bis (triethoxysilyl) ethane in water-ethanol solution by FTIR spectroscopy. J. Adhes. Sci. Technol. 1997, 11, 29–47. [Google Scholar]
- Savard, S.; Blanchard, L.P.; Léonard, J.; Prud’Homme, R.E. Hydrolysis and condensation of silanes in aqueous solutions. Polym. Compos. 1984, 5, 242–249. [Google Scholar] [CrossRef]
- Beari, F.; Brand, M.; Jenkner, P.; Lehnert, R.; Metternich, H.J.; Monkiewicz, J.; Siesler, H.W. Organofunctional alkoxysilanes in dilute aqueous solution: New accounts on the dynamic structural mutability. J. Organomet. Chem. 2001, 625, 208–216. [Google Scholar] [CrossRef]
- Cihlář, J. Hydrolysis and polycondensation of ethyl silicates. 1. Effect of pH and catalyst on the hydrolysis and polycondensation of tetraethoxysilane (TEOS). Colloids Surf. A Physicochem. Eng. Asp. 1993, 70, 239–251. [Google Scholar] [CrossRef]
- Premachandra, J.K.; Van Ooij, W.J.; Mark, J.E. Reaction kinetics of y-ureidopropyltrimethoxysilane in the water-methanol system studied by FTIR spectroscopy. J. Adhes. Sci. Technol. 1998, 12, 1361–1376. [Google Scholar] [CrossRef]
- Leyden, D.E.; Atwater, J.B. Hydrolysis and condensation of alkoxysilanes investigated by internal reflection FTIR spectroscopy. J. Adhes. Sci. Technol. 1991, 5, 815–829. [Google Scholar] [CrossRef]
- Issa, A.A.; El-Azazy, M.; Luyt, A.S. Kinetics of alkoxysilanes hydrolysis: An empirical approach. Sci. Rep. 2019, 9, 17624. [Google Scholar] [CrossRef] [PubMed]
- Paquet, O.; Salon, M.-C.B.; Zeno, E.; Belgacem, M.N. Hydrolysis-condensation kinetics of 3-(2-amino-ethylamino) propyl-trimethoxysilane. Mater. Sci. Eng. C 2012, 32, 487–493. [Google Scholar] [CrossRef]
- Van Ooij, W.J.; Zhu, D.; Palanivel, V.; Lamar, J.A.; Stacy, M. Overview: The potential of silanes for chromate replacement in metal finishing industries. Silicon Chem. 2006, 3, 11–30. [Google Scholar] [CrossRef]
- Zhu, D.; Van Ooij, W.J. Enhanced corrosion resistance of AA 2024-T3 and hot-dip galvanized steel using a mixture of bis-[triethoxysilylpropyl] tetrasulfide and bis-[trimethoxysilylpropyl] amine. Electrochim. Acta 2004, 49, 1113–1125. [Google Scholar] [CrossRef]
- Jayaseelan, S.K.; Van Ooij, W. Rubber-to-metal bonding by silanes. J. Adhes. Sci. Technol. 2001, 15, 967–991. [Google Scholar] [CrossRef]
- Plueddemann, E.P. Adhesion through silane coupling agents. J. Adhes. 1970, 2, 184–201. [Google Scholar] [CrossRef]
- Zhu, D.; Van Ooij, W.J. Structural characterization of bis-[triethoxysilylpropyl] tetrasulfide and bis-[trimethoxysilylpropyl] amine silanes by Fourier-transform infrared spectroscopy and electrochemical impedance spectroscopy. J. Adhes. Sci. Technol. 2002, 16, 1235–1260. [Google Scholar] [CrossRef]
- Van Ooij, W.; Zhu, D.; Stacy, M.; Seth, A.; Mugada, T.; Gandhi, J.; Puomi, P. Corrosion protection properties of organofunctional silanes—An overview. Tsinghua Sci. Technol. 2005, 10, 639–664. [Google Scholar] [CrossRef]
- Palanivel, V.; Zhu, D.; Van Ooij, W.J. Nanoparticle-filled silane films as chromate replacements for aluminum alloys. Prog. Org. Coat. 2003, 47, 384–392. [Google Scholar] [CrossRef]
- Gettings, M.; Kinloch, A. Surface analysis of polysiloxane/metal oxide interfaces. J. Mater. Sci. 1977, 12, 2511–2518. [Google Scholar] [CrossRef]
- Van Ooij, W.; Sabata, A. Characterization of films of organofunctional silanes by TOFSIMS and XPS. J. Adhes. Sci. Technol. 1991, 5, 843–863. [Google Scholar] [CrossRef]
- Quinton, J.; Dastoor, P. Characterizing the bonding mechanisms at silane-metal interfaces: A model system. J. Mater. Sci. Lett. 1999, 18, 1833–1835. [Google Scholar] [CrossRef]
- Bexell, U.; Olsson, M. Characterization of a non-organofunctional silane film deposited on Al, Zn and Al–43.4 Zn–1.6 Si alloy-coated steel: Part II. Interfacial characterization by ToF-SIMS and AES. Surf. Interface Anal. Int. J. Devoted Dev. Appl. Tech. Anal. Surf. Interfaces Thin Film. 2001, 31, 223–231. [Google Scholar]
- Petrunin, M.; Nazarov, A.; Mikhailovski, Y.N. Formation mechanism and anticorrosive properties of thin siloxane films on metal surfaces. J. Electrochem. Soc. 1996, 143, 251. [Google Scholar] [CrossRef]
- Jeyaram, R.; Elango, A.; Siva, T.; Ayeshamariam, A.; Kaviyarasu, K. Corrosion protection of silane based coatings on mild steel in an aggressive chloride ion environment. Surf. Interfaces 2020, 18, 100423. [Google Scholar] [CrossRef]
- Alibakhshi, E.; Akbarian, M.; Ramezanzadeh, M.; Ramezanzadeh, B.; Mahdavian, M. Evaluation of the corrosion protection performance of mild steel coated with hybrid sol-gel silane coating in 3.5 wt.% NaCl solution. Prog. Org. Coat. 2018, 123, 190–200. [Google Scholar] [CrossRef]
- Asadi, N.; Naderi, R.; Saremi, M.; Arman, S.; Fedel, M.; Deflorian, F. Study of corrosion protection of mild steel by eco-friendly silane sol-gel coating. J. Sol-Gel Sci. Technol. 2014, 70, 329–338. [Google Scholar] [CrossRef]
- Alcantara-Garcia, A.; Garcia-Casas, A.; Jimenez-Morales, A. The effect of the organosilane content on the barrier features of sol-gel anticorrosive coatings applied on carbon steel. Prog. Org. Coat. 2020, 139, 105418. [Google Scholar] [CrossRef]
- Pepe, A.; Galliano, P.; Aparicio, M.; Durán, A.; Ceré, S. Sol-gel coatings on carbon steel: Electrochemical evaluation. Surf. Coat. Technol. 2006, 200, 3486–3491. [Google Scholar] [CrossRef]
- Chico, B.; Galván, J.; De La Fuente, D.; Morcillo, M. Electrochemical impedance spectroscopy study of the effect of curing time on the early barrier properties of silane systems applied on steel substrates. Prog. Org. Coat. 2007, 60, 45–53. [Google Scholar] [CrossRef]
- Sundararajan, G.P.; Van Ooij, W. Silane based pretreatments for automotive steels. Surf. Eng. 2000, 16, 315–320. [Google Scholar] [CrossRef]
- Wang, Y.; Puomi, P.; Van Ooij, W.J. Effect of substrate cleaning solution pH on the corrosion performance of silane-coated cold-rolled steel. J. Adhes. Sci. Technol. 2007, 21, 935–960. [Google Scholar] [CrossRef]
- Nozawa, K.; Aramaki, K. One- and two-dimensional polymer films ofmodified alkanethiol monolayers for preventing iron fromcorrosion. Corros. Sci. 1999, 41, 57–73. [Google Scholar] [CrossRef]
- Aramaki, K. Protection of iron corrosion by ultrathin two-dimensional polymer films of an alkanethiol monolayer modified with alkylethoxysilanes. Corros. Sci. 1999, 41, 1715–1730. [Google Scholar] [CrossRef]
- Tsuji, N.; Nozawa, K.; Aramaki, K. Ultrathin protective films prepared by modification of an N, N-dimethylalkylamine monolayer with chlorosilanes for preventing corrosion of iron. Corros. Sci. 2000, 42, 1523–1538. [Google Scholar] [CrossRef]
- Shimura, T.; Aramaki, K. Additional modification to two-dimensional polymer films of a hydroxymethylbenzene self-assembled monolayer with alkyltriethoxysilanes for enhancing the protective abilities against iron corrosion. Corros. Sci. 2008, 50, 596–604. [Google Scholar] [CrossRef]
- Wang, D.; Bierwagen, G.P. Sol-gel coatings on metals for corrosion protection. Prog. Org. Coat. 2009, 64, 327–338. [Google Scholar] [CrossRef]
- Vivar Mora, L.; Naik, S.; Paul, S.; Dawson, R.; Neville, A.; Barker, R. Influence of silica nanoparticles on corrosion resistance of sol-gel based coatings on mild steel. Surf. Coat. Technol. 2017, 324, 368–375. [Google Scholar] [CrossRef]
- Li, Y.; Wu, C.; Xue, M.; Cai, J.; Huang, Y.; Yang, H. Preparation of sol-gel derived anticorrosive coating on Q235 carbon steel substrate with long-term corrosion prevention durability. Materials 2019, 12, 1960. [Google Scholar] [CrossRef]
- Battez, A.H.; González, R.; Viesca, J.; Fernández, J.; Fernández, J.D.; Machado, A.; Chou, R.; Riba, J. CuO, ZrO2 and ZnO nanoparticles as antiwear additive in oil lubricants. Wear 2008, 265, 422–428. [Google Scholar] [CrossRef]
- Liu, H.; Sun, X.; Yin, C.; Hu, C. Removal of phosphate by mesoporous ZrO2. J. Hazard. Mater. 2008, 151, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Mu, W.; Han, Y. Characterization and properties of the MgF2/ZrO2 composite coatings on magnesium prepared by micro-arc oxidation. Surf. Coat. Technol. 2008, 202, 4278–4284. [Google Scholar] [CrossRef]
- Liu, H.; Wang, G.; Wexler, D.; Wang, J.; Liu, H.-K. Electrochemical performance of LiFePO4 cathode material coated with ZrO2 nanolayer. Electrochem. Commun. 2008, 10, 165–169. [Google Scholar] [CrossRef]
- Lim, S.; Cho, J. PVP-Assisted ZrO2 coating on LiMn2O4 spinel cathode nanoparticles prepared by MnO2 nanowire templates. Electrochem. Commun. 2008, 10, 1478–1481. [Google Scholar] [CrossRef]
- Claire, L.; Marie, G.; Julien, G.; Jean-Michel, S.; Jean, R.; Marie-Joëlle, M.; Stefano, R.; Michele, F. New architectured hybrid sol-gel coatings for wear and corrosion protection of low-carbon steel. Prog. Org. Coat. 2016, 99, 337–345. [Google Scholar] [CrossRef]
- Kiruthika, P.; Subasri, R.; Jyothirmayi, A.; Sarvani, K.; Hebalkar, N. Effect of plasma surface treatment on mechanical and corrosion protection properties of UV-curable sol-gel based GPTS-ZrO2 coatings on mild steel. Surf. Coat. Technol. 2010, 204, 1270–1276. [Google Scholar] [CrossRef]
- Ruhi, G.; Modi, O.P.; Singh, I.B.; Jha, A.K.; Yegneswaran, A.H. Wear and electrochemical characterization of sol-gel alumina coating on chemically pre-treated mild steel substrate. Surf. Coat. Technol. 2006, 201, 1866–1872. [Google Scholar] [CrossRef]
- Ruhi, G.; Modi, O.; Sinha, A.; Singh, I. Effect of sintering temperatures on corrosion and wear properties of sol-gel alumina coatings on surface pre-treated mild steel. Corros. Sci. 2008, 50, 639–649. [Google Scholar] [CrossRef]
- Tiwari, S.K.; Sahu, R.K.; Pramanick, A.K.; Singh, R. Development of conversion coating on mild steel prior to sol gel nanostructured Al2O3 coating for enhancement of corrosion resistance. Surf. Coat. Technol. 2011, 205, 4960–4967. [Google Scholar] [CrossRef]
- Fallet, M.; Mahdjoub, H.; Gautier, B.; Bauer, J.-P. Electrochemical behaviour of ceramic sol-gel coatings on mild steel. J. Non Cryst. Solids 2001, 293, 527–533. [Google Scholar] [CrossRef]
- Krishna, V.; Padmapreetha, R.; Chandrasekhar, S.; Murugan, K.; Johnson, R. Oxidation resistant TiO2-SiO2 coatings on mild steel by Sol-Gel. Surf. Coat. Technol. 2019, 378, 125041. [Google Scholar] [CrossRef]
- Li, Q.; Song, L.; Liang, Z.; Sun, M.; Wu, T.; Huang, B.; Luo, F.; Du, Y.; Yan, C.-H. A Review on Ceo2-Based Electrocatalyst and Photocatalyst in Energy Conversion. Adv. Energy Sustain. Res. 2021, 2, 2000063. [Google Scholar] [CrossRef]
- Carvalho, J.B.R.; Silva, R.S.; Cesarino, I.; Machado, S.A.S.; Eguiluz, K.I.B.; Cavalcanti, E.B.; Salazar-Banda, G.R. Influence of the annealing temperature and metal salt precursor on the structural characteristics and anti-corrosion barrier effect of CeO2 sol-gel protective coatings of carbon steel. Ceram. Int. 2014, 40, 13437–13446. [Google Scholar] [CrossRef]
- Eduok, U.; Faye, O.; Tiamiyu, A.; Szpunar, J. Fabricating protective epoxy-silica/CeO2 films for steel: Correlating physical barrier properties with material content. Mater. Des. 2017, 124, 58–68. [Google Scholar] [CrossRef]
- Rani, B.E.A.; Basu, B.B.J. Green Inhibitors for Corrosion Protection of Metals and Alloys: An Overview. Int. J. Corros. 2012, 2012, 380217. [Google Scholar] [CrossRef]
- Hamidon, T.S.; Hussin, M.H. Susceptibility of hybrid sol-gel (TEOS-APTES) doped with caffeine as potent corrosion protective coatings for mild steel in 3.5 wt.% NaCl. Prog. Org. Coat. 2020, 140, 105478. [Google Scholar] [CrossRef]
- Ishak, N.A.; Hamidon, T.S.; Zi-Hui, T.; Hussin, M.H. Extracts of curcumin-incorporated hybrid sol-gel coatings for the corrosion mitigation of mild steel in 0.5 M HCl. J. Coat. Technol. Res. 2020, 17, 1515–1535. [Google Scholar] [CrossRef]
- Hamidon, T.S.; Ishak, N.A.; Hussin, M.H. Enhanced corrosion inhibition of low carbon steel in aqueous sodium chloride employing sol-gel-based hybrid silanol coatings. J. Sol-Gel Sci. Technol. 2021, 97, 556–571. [Google Scholar] [CrossRef]
- Ghuzali, N.A.M.; Noor, M.A.A.C.M.; Zakaria, F.A.; Hamidon, T.S.; Husin, M.H. Study on Clitoria ternatea extracts doped sol-gel coatings for the corrosion mitigation of mild steel. Appl. Surf. Sci. Adv. 2021, 6, 100177. [Google Scholar] [CrossRef]
- Al-Saadi, S.; Raman, R.K.S.; Anisur, M.R.; Ahmed, S.; Crosswell, J.; Alnuwaiser, M.; Panter, C. Graphene coating on a nickel-copper alloy (Monel 400) for microbial corrosion resistance: Electrochemical and surface characterizations. Corros. Sci. 2021, 182, 109299. [Google Scholar] [CrossRef]
- Haghdadeh, P.; Ghaffari, M.; Ramezanzadeh, B.; Bahlakeh, G.; Saeb, M.R. The role of functionalized graphene oxide on the mechanical and anti-corrosion properties of polyurethane coating. J. Taiwan Inst. Chem. Eng. 2018, 86, 199–212. [Google Scholar] [CrossRef]
- Pourhashem, S.; Vaezi, M.R.; Rashidi, A.; Bagherzadeh, M.R. Distinctive roles of silane coupling agents on the corrosion inhibition performance of graphene oxide in epoxy coatings. Prog. Org. Coat. 2017, 111, 47–56. [Google Scholar] [CrossRef]
- Pourhashem, S.; Rashidi, A.; Vaezi, M.R.; Bagherzadeh, M.R. Excellent corrosion protection performance of epoxy composite coatings filled with amino-silane functionalized graphene oxide. Surf. Coat. Technol. 2017, 317, 1–9. [Google Scholar] [CrossRef]
- Geng, Y.; Zhou, P.; Li, S.; Cao, J.; Zhou, Z.; Wu, Z.; Liu, A. Superior corrosion resistance of mild steel coated with graphene oxide modified silane coating in chlorinated simulated concrete solution. Prog. Org. Coat. 2022, 164, 106716. [Google Scholar] [CrossRef]
- Guo, F.; Al-Saadi, S.; Raman, R.S.; Zhao, X. Durability of fiber reinforced polymer (FRP) in simulated seawater sea sand concrete (SWSSC) environment. Corros. Sci. 2018, 141, 1–13. [Google Scholar] [CrossRef]
- Yu, X.; Al-Saadi, S.; Zhao, X.-L.; Raman, R.S. Electrochemical Investigations of Steels in Seawater Sea Sand Concrete Environments. Materials 2021, 14, 5713. [Google Scholar] [CrossRef]
- Sharma, N.; Sharma, S.; Sharma, S.K.; Mahajan, R.L.; Mehta, R. Evaluation of corrosion inhibition capability of graphene modified epoxy coatings on reinforcing bars in concrete. Constr. Build. Mater. 2022, 322, 126495. [Google Scholar] [CrossRef]
- Videla, H.A. Manual of Biocorrosion, 1st ed.; Routledge: New York, NY, USA, 1996. [Google Scholar] [CrossRef]
- Yazdi, M.; Khan, F.; Abbassi, R. Microbiologically influenced corrosion (MIC) management using Bayesian inference. Ocean. Eng. 2021, 226, 108852. [Google Scholar] [CrossRef]
- Al-Saadi, S.; Raman, R.K.S.; Panter, C. A Two-Step Silane Coating Incorporated with Quaternary Ammonium Silane for Mitigation of Microbial Corrosion of Mild Steel. ACS Omega 2021, 6, 16913–16923. [Google Scholar] [CrossRef]
- Suleiman, R.K.; Saleh, T.A.; Al Hamouz, O.C.S.; Ibrahim, M.B.; Sorour, A.A.; El Ali, B. Corrosion and fouling protection performance of biocide-embedded hybrid organosiloxane coatings on mild steel in a saline medium. Surf. Coat. Technol. 2017, 324, 526–535. [Google Scholar] [CrossRef]
- Al-Saadi, S.; Banerjee, P.C.; Raman, R.K.S. Corrosion of bare and silane-coated mild steel in chloride medium with and without sulphate reducing bacteria. Prog. Org. Coat. 2017, 111, 231–239. [Google Scholar] [CrossRef]
- Al-Saadi, S.; Raman, R.K.S. A long aliphatic chain functional silane for corrosion and microbial corrosion resistance of steel. Prog. Org. Coat. 2019, 127, 27–36. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).