Abstract
In this study, a diarrhea model was established by exposing rats to appropriate antibiotics and Salmonella. After an in vitro screening of prebiotics, fructo-oligosaccharide and galacto-oligosaccharide (GOS) were selected; their synbiotic potential and ability to ameliorate diarrhea symptoms and intestinal inflammation with Bacillus coagulans BACO-17 were evaluated in vivo. After a 27-day feeding experiment including antibiotic intervention and Salmonella infection, it was found that using B. coagulans BACO-17 alone and in combination with GOS as a synbiotic could render a better recovery by lowering diarrhea indexes by 26.9% and 18.7%, respectively. Compared with the negative control, the administration of this synbiotic mixture resulted in the most significant increase in fecal concentrations of total short-chain fatty acids (about 2-fold higher), with a promising improvement in disrupted gut microbial balance. It was worth noting that the administration of B. coagulans BACO-17 alone or in combination with GOS effectively reduced intestinal inflammation (27–31%) and mucosal necrosis (82%) over the negative control. These results suggested that B. coagulans BACO-17 and GOS could be exploited as a promising synbiotic mixture to relieve intestinal inflammatory diseases and improve gut health.
1. Introduction
Antibiotic-associated diarrhea (AAD) is a common complication developed from systemic antibiotic treatment. Antibiotic therapy is a vital tool to kill pathogenic bacteria but can also potentially lead to the disturbance of indigenous microbiota in the intestine. Specifically, disruption of the normal balance of intestinal flora results in alterations in carbohydrates and bile acids metabolism, reduction in short-chain fatty acids production, and the emergence of enteric pathogens such as Salmonella [].
Bacillus coagulans is an endospore-forming bacteria widely used as a probiotic agent. It can develop spores and possesses good stability against harsh processing conditions such as low pH and high temperature [,]. Following spore germination intragastrically, B. coagulans vegetative cells can proliferate in the intestine, elevate short-chain fatty acid concentrations, and subsequently improve the intestinal milieu []. Previous research has substantiated different therapeutic activities of B coagulans, including attenuated gut dysbiosis, decreased occurrence of abdominal pain and bloating, and alleviation of diarrhea-predominant irritable bowel syndrome through supplementation of B. coagulans spores [,,].
Co-supplementation with a prebiotic could enhance the potential probiotic effects of B. coagulans by elevating its colonization and survival rates []. As previously reported by van der Beek et al. [], orally administrated prebiotics could be fermented in the large intestine to produce short-chain fatty acids (SCFAs) and exert beneficial effects on intestinal health. Another study also indicated that consumption of B. coagulans and prebiotics effectively elevates organic acid production and populations of beneficial bacteria in the large intestine [].
In light of the AAD resulting from disruption of the commensal gut microbiota, it was believed that supplementing the diet with B. coagulans and prebiotics could be a reasonable approach to ameliorating AAD by modulating and restoring gut microbiota []. This present study aimed to compare the prebiotic potential of four different carbohydrate components, including xylo-oligosaccharide, fructo-oligosaccharide, galacto-oligosaccharide, and citrus pectin. The synbiotic effects of B. coagulans BACO-17 and selected prebiotics in a rat diarrhea model were established by challenging the animals with ampicillin and clindamycin together with Salmonella enterica evaluated. More specifically, changes in different fecal parameters (e.g., diarrhea index (DI), moisture, and SCFAs) and histological assessment of colonic tissue after the consumption of B. coagulans BACO-17 and selected prebiotics are discussed.
2. Materials and Methods
2.1. In Vitro Screening of Prebiotics
A pure strain of B. coagulans BACO-17 in spore form was provided by Syngen Biotech Co., Ltd. (Taipei City, Taiwan) and stored at 4 °C until used. In order to select appropriate prebiotics for B. coagulans BACO-17 to create a synbiotic preparation, in vitro growth of B. coagulans BACO-17 in the presence of four different carbohydrate components was analyzed and compared to the probiotic bacteria growth in the presence of glucose as a control group.
B. coagulans BACO-17 cell suspension (about 4.9 Log CFU/mL) was added as 10% (v/v) inoculum into the test tubes containing 9 mL of medium, in which glucose was substituted with each prebiotic (0.5%, w/v) in equal amounts separately. The prebiotics used in the limiting carbon source replacement included citrus pectin, fructo-oligosaccharide (FOS), galacto-oligosaccharide (GOS), and xylo-oligosaccharide (XOS). These prebiotics were obtained from Syngen Biotech Co., Ltd. (Taipei City, Taiwan). GYEA culture medium was used as the substrate. B. coagulans BACO-17 was cultivated at 55 °C for 24 h, and its growth profile in different carbon sources and standard glucose-containing media was observed. The results were used as a reference for subsequent in vivo experiments.
2.2. Diets and In Vivo Experimental Design
The animal study protocol was approved by the Animal Care and Use Committee of National Chung Hsing University, and the laboratory animals were handled in accordance with the institutional ethical guideline.
In this experiment, a total of fifty-four 7-week-old Sprague Dawley (SD) rats weighing 239.3 ± 2.9 g were purchased from BioLASCO Company, Taiwan (IACUC approval number: 109–105). These animals were individually housed in stainless steel cages and placed in a room maintained at 22 ± 1 °C with a 12-h light/dark cycle. After one week of acclimation, SD rats were randomly divided into seven groups, including two control groups and five sample groups. During the whole experimental period, the five sample groups included one probiotic group, administrated with B. coagulans BACO-17 (BC group, 9 log CFU/day), two prebiotic groups, administrated with FOS (FOS group, 1.03 g FOS/kg bw/day) and GOS (GOS group, 0.83 g GOS/kg bw/day), and two synbiotic groups, administrated with B. coagulans BACO-17 combined with FOS (FB group) and GOS (GB group). The FB group was fed with a mixture of B. coagulans BACO-17 (9 log CFU) and FOS (1.03 g/kg bw) each day. The GB group was fed with a mixture of B. coagulans BACO-17 (9 log CFU) and GOS (1.03 g/kg bw) each day. The negative control group was fed a basal chow diet and treated with the same diarrheal disease model as the sample groups. The normal control group was only provided with a basal chow diet (LabDiet 5001).
During the experiment, all rats had free access to LabDiet 5001 and drinking water. Samples were given via oral gavage. Body weight, food consumption, and water intake were recorded every day. The daily health status of the rats was also monitored. The diarrhea model was performed based on the methods described by van Ampting et al. [] with slight modifications in antibiotic dosage and days of Salmonella infection. As indicated in Figure 1, from day 15 to day 17 of the experiment, rats in the negative control and sample groups were given 100 mg of ampicillin (Sigma-Aldrich, St. Louis, MO, USA; CAS69-52-4) and 15 mg of clindamycin (Goldbio, St. Louis, MO, USA; CAS21462-39-5) which were mixed with basal diet. The morphology of rat feces was recorded, and the fecal DI was determined. From day 18 to day 22 of the experiment, 1 × 109 CFU of S. enterica subsp. enterica (strain number: BCRC10747) culture was given to the negative control and sample groups every day. The fecal morphology and diarrhea index were recorded.
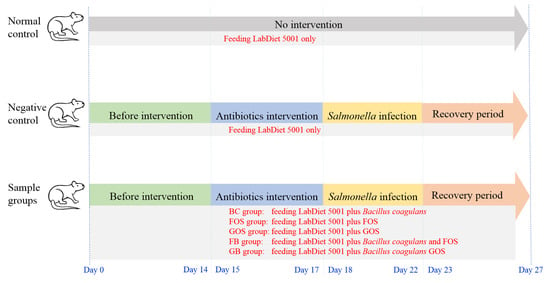
Figure 1.
Schematic diagram of the experimental design in vivo.
2.3. Analysis of Fecal Bacteria
After the second day of the experiment, freshly voided feces were collected in an aseptic tube and analyzed by conventional microbiological methods within 2 h. A series of ten-fold dilutions of the homogenized samples were prepared using sterile and anaerobic dilution solution. B. coagulans, Bifidobacterium spp., E. coli, and Salmonella were enumerated by spread plating onto different selective mediums, which were glucose yeast extract agar (GYEA), Bifidobacteria iodoacetate medium 25 (BIM-25), Levine eosin methylene blue (LEMB) agar (Merck KGaA, Darmstadt, Germany), and mannitol lysine crystal violet brilliant green (MLCB) agar (Cyrusbioscience, New Taipei City, Taiwan), respectively. B. coagulans was aerobically cultivated at 55 °C for 48 h []. E. coli were aerobically cultivated at 37 °C for 72 h and 48 h, respectively. Salmonella was aerobically cultivated at 40 °C for 24 h, and Bifidobacterium spp. was anaerobically cultivated at 37 °C for 48 h.
The colonies of B. coagulans and Salmonella isolated in the fecal samples were confirmed genetically by a molecular probing technique using 16S ribosomal RNA gene primers (Supplementary Table S3 and Figures S1 and S2).
2.4. Determination of Fecal Moisture
Fresh rat feces were collected and accurately weighed. The samples were dried in a hot air oven at 80 °C for 6 h and subsequently dried at 105 °C until a constant weight was reached. The fecal moisture content was calculated by the equation:
Fecal moisture content (g/100 g feces) = (initial weight − final weight)/initial weight
2.5. Diarrhea Index Assessment
Diarrhea assessments were performed according to the methods described by Sakai et al. [] with slight modifications. The severity of diarrhea was scored using the following scale, 1: normal (normal and dark brown stool), 2: slight (slightly wet and yellow black stool), 3: moderate (wet and unformed, brown stool), and 4: severe (watery stool, cannot be picked up with tweezers). The incidence of each diarrhea score (1 to 4) and the average diarrhea score were used to evaluate the severity of diarrhea (Figure 2).

Figure 2.
Morphology of stool in different diarrhea index.
2.6. Determination of SCFAs
Fecal SCFAs were determined according to the method described by Saw et al. [] with slight modifications. Fresh fecal samples were homogenized with deionized water at a ratio of 1:10 (w/v), followed by centrifugation at 1006× g for 10 min. Two milliliters of supernatant were combined with 10 μL of isocaporic acid (internal standard) and 20 μL of 50% (w/v) sulfuric acid. Diethyl ether was used to extract the SCFAs. The SCFAs in the ether layer (1 μL) were analyzed using Agilent J and WHP-INNOWax GC Column (30 m, 0.25 mm, 0.25 μm). As a carrier gas, helium gas was used with a constant flow rate of 7 mL/min. An Agilent Technologies 7890A system equipped with a flame ionization detector (FID) was used for chromatographic analysis. During the analysis, the initial oven temperature at 80 °C was maintained for 1 min before being raised to 140 °C at a rate of 20 °C/min and held for another 1 min. The temperature was again increased to 220 °C at a rate of 20 °C/min, and lastly, held at 220 °C for 2 min. The temperatures of the detector and the injector were 250 °C and 140 °C, respectively. SCFAs were quantified by comparing the spectrum obtained from the fecal samples of each group with those of standard compounds.
2.7. Histological Assessment of Colonic Inflammation
Rats were sacrificed at the end of the experiment. The procedure described by Aleisa et al. [] was used to determine the inflammation of colonic tissue. Colon tissue was removed, cut longitudinally, and washed with saline. Fixed colonic tissue (2 cm) in 10% buffered formalin was used for histological evaluation. Embedded specimens were cut into sections of 5 µm thickness in paraffin wax blocks. Colon segments were stained with Hematoxylin and then counterstained with Eosin. Tissue inflammatory infiltration, severity of edema, cellular necrosis, and production of regenerative cells were observed microscopically. Degree of lesions was graded from 1 to 5 depending on severity: 1 = minimal (<1%); 2 = slight (1–25%); 3 = moderate (26–50%); 4 = moderate/severe (51–75%); 5 = severe/high (76–100%).
2.8. Statistical Analysis
Statistical analysis was carried out using the SPSS statistics program (Version 20.0; SPSS, Armonk, NY, USA). All parameters were analyzed by one-way analysis of variance (ANOVA) followed by Duncan’s multiple range test, with the significance level set at p < 0.05. The data were expressed as mean ± standard deviation (SD).
3. Results and Discussion
3.1. In Vitro Prebiotic Activity
Table 1 shows the prebiotic impact of different carbohydrate components on the growth of B. coagulans BACO-17. The results were compared to the rate of probiotic bacteria growth in the presence of glucose. In this study, among the four carbohydrate samples, the pectin group yielded significantly higher (p < 0.05) B. coagulans count than other groups after the sixth hour of cultivation. This indicated that B. coagulans BACO-17 grew faster using pectin as its carbon energy source. A similar growth profile was observed across all prebiotic samples; specifically, the highest bacterial count in the range of 6.83–7.20 Log CFU/mL was observed at the sixteenth hour of incubation. The data suggested that GOS created the best growth condition, followed by FOS.

Table 1.
Effects of various carbon sources at the level of 0.5% (w/v) on the growth of B. coagulans (Log cfu/g) in vitro.
It was observed that B. coagulans BACO-17 was capable of utilizing all of the tested prebiotics. Noticeably, although B. coagulans BACO-17 initially grew faster using pectin as its energy source, its growth lagged behind other groups after 24 h of cultivation (6.86 Log CFU/mL). This indicated that pectin was not utilized as efficiently as the other prebiotics. The growth rates of B. coagulans BACO-17 in the FOS and GOS groups were similar to that of the glucose control. Our results indicated that GOS might better support the growth of B. coagulans BACO-17. These findings were consistent with the observations made by Cano Roca [], stating that culturing B. coagulans using GOS led to a more significant change in absorbance value and better coagulation efficiency compared with that using FOS. Therefore, in this study, FOS and GOS were selected for further evaluation of their prebiotic potential with B. coagulans BACO-17 in vivo.
3.2. Food Intake and Body Weight Gain
There were no apparent differences in food intake (24.1–25.8 g/day) and body weight gain (2.3–2.7 g/day) among all animal groups before the intervention (Supplementary Table S1). The daily consumption of the B. coagulans BACO-17 and prebiotic samples did not affect healthy rats’ appetite or body weight.
After the antibiotic intervention and Salmonella infection, it was found that food intakes in the D (negative control) and samples groups were significantly (p < 0.05) reduced. Previous findings from Tulstrup et al. [,] have demonstrated that antibiotic therapies cause decreases in body weight and food intake due to antibiotic-perturbed low-diversity microbiota and disturbed appetite. During the diarrhea period, the rate of weight gain in negative control and sample groups was reduced by 16.3–38.7% versus the normal control, with the GB group having the lowest decrease in weight gain (Supplementary Table S1). It has been reported that galacto-oligosaccharide consumption could resist drug-induced body weight loss in a dose-dependent manner []. Therefore, it was inferred that combining B. coagulans BACO-17 and GOS as a synbiotic could help alleviate antibiotics- and salmonella-induced decrease in body weight gain.
3.3. Assessment of Fecal Moisture and Diarrhea Index
Figure 3 illustrates that three days of antibiotic intervention for the animals in the negative control and sample groups starts on day 15. After the antibiotic supplementation, these animals were subsequently infected orally with S. enterica culture for 5 days (between day 18 and day 22), followed by another 5 days of the recovery period.
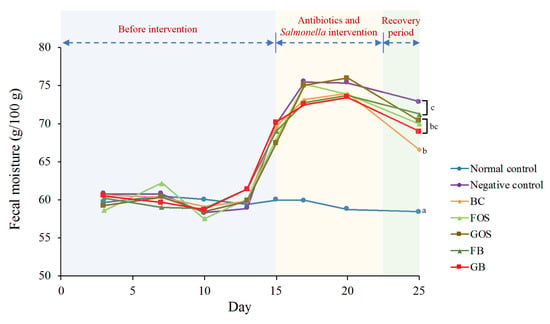
Figure 3.
Change in fecal moisture among different animal groups. Blue zone (days 0 to 14) indicates the period before intervention. Yellow zone (days 15 to 22) indicates the period of antibiotics intervention and Salmonella infection. Green zone (days 23 to 25) indicates the recovery period. Mean values on the curves bearing different letters (a–c) indicate significant differences (p < 0.05) among different groups on day 25.
Before the antibiotic intervention (from day 0 to day 14), Figure 3 shows no apparent difference in fecal moisture content among all seven dietary groups (57.6–62.2 g/100 g feces). After the supplementation of antibiotics, fecal moisture content of the negative control and five sample groups significantly (p < 0.05) increased by about 25% to the highest values (up to 72.5–75.9 g/100 g feces) between day 17 and day 20, followed by a subsequent reduction. During the recovery period (day 25), a significant (p < 0.05) reduction in the fecal moisture content was observed only in the BC, GOS, and GB groups (9.0%, 7.3%, and 6.1%, respectively) against their corresponding highest values, implying a better recovery in the animal groups fed either B. coagulans BACO-17 or GOS, alone or in combination.
Regarding the diarrhea index, rats fed a normal diet had diarrhea indexes ranging between 1.13–1.22. After the antibiotic intervention, rats in all dietary groups (except normal control) discharged diarrheic feces, and the severity of diarrhea gradually increased to their highest values (2.66–2.92) between day 17 and day 20 (Figure 4). It appeared that the approach to introducing an antibiotic intervention successfully induced diarrhea symptoms in this study. At the end of this experiment, all five sample groups had a significant (p < 0.05) decline in diarrhea indexes, which ranged from 14.5 to 26.9%. Notably, the BC group had the most drastic reduction among these dietary groups (26.9%), followed by the GB group (18.7%). B. coagulans consumption could ameliorate diarrhea symptoms and inhibit gastrointestinal motility in rats [] and was reported to be able to improve the intestinal environment, defecation frequency, and fecal characteristics in humans [].
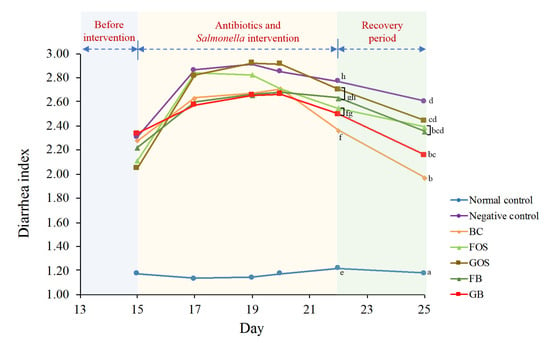
Figure 4.
Changes in diarrhea index among different animal groups. Blue zone (days 13 to 14) indicates the period before intervention. Yellow zone (days 15 to 22) indicates the period of antibiotics intervention and Salmonella infection. Green zone (days 23 to 25) indicates the recovery period. Mean values on the curves bearing different letters (e–h) indicate significant differences (p < 0.05) among different groups on day 22. Mean values on the curves bearing different letters (a–d) indicate significant differences (p < 0.05) among different groups on day 25.
Interestingly, the BC group had a superior performance in reducing incidences and severity of diarrhea compared with other dietary groups. In sum, using B. coagulans BACO-17 alone and in combination with prebiotics, particularly GOS, could effectively relieve diarrhea.
3.4. Changes in Fecal Microflora
In the first two weeks of this feeding experiment (Figure 1), there were no statistical differences in fecal B. coagulans, Bifidobacteria, and E. coli counts among the seven animal groups (Figure 5A–D). These fecal bacterial counts remained stable before the intervention with antibiotics.
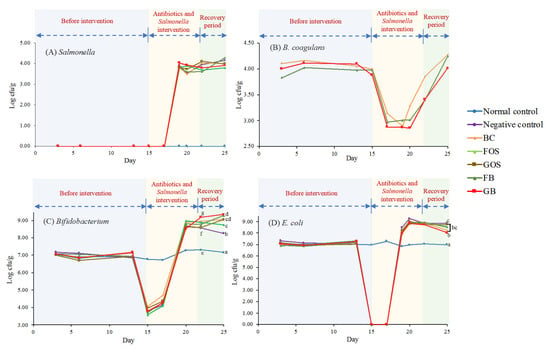
Figure 5.
Change in different fecal bacteria counts (Log cfu/g) among different animal groups. (A) fecal Salmonella; (B) fecal B. coagulans; (C) fecal Bifidobacterium spp.; (D) fecal Escherichia coli. Blue zone (days 0 to 14) indicates the period before intervention. Yellow zone (days 15 to 22) indicates the period of antibiotics intervention and Salmonella infection. Green zone (days 23 to 25) indicates the recovery period. Mean values on the curves bearing different letters (e–g) indicate significant differences (p < 0.05) among different groups on day 22. Mean values on the curves bearing different letters (a–d) indicate significant differences (p < 0.05) among different groups on day 25.
Figure 5A reveals that no Salmonella was detected in the feces among all different dietary groups in the first two weeks. It indicated that the Salmonella count was below the detectable range, although the existence of Salmonella was generally expected in the small intestine. The observation was consistent with a previous study claiming that fecal Salmonella was undetectable without infection []. After the Salmonella infection, fecal Salmonella counts increased from non-detectable to 3.80–4.03 Log CFU/g on day 19. However, during the rest of the experiment (from days 19 to 25), no further variation in the fecal Salmonella counts (3.47–4.28 Log CFU/g) was observed.
After the antibiotic intervention at day 15, a significant (p < 0.05) reduction (ranging from 21.5% to 27.8%) in the fecal B. coagulans count was observed in the subsequent 4 days among the BC, FB, and GB groups (Figure 5B), followed by a rebound of bacterial growth on day 19. Although GOS could support a better growth for B. coagulans BACO-17 in the in vitro experiment, no apparent difference in the fecal B. coagulans count was noted in the in vivo experiment. It was believed that the discrepancy between the in vitro and in vivo experimental results was likely because the in vitro experimental condition was not complex enough to mimic the intestinal microflora in feces and thoroughly examine specific substrates’ prebiotic potential [].
A similar pattern was also observed in the growth of fecal Bifidobacteria in the negative control and sample groups (Figure 5C). The dramatic drop in fecal Bifidobacteria counts indicated that antibiotics might affect the growth of fecal bacteria. On day 20 of the experiment, the fecal Bifidobacteria counts ascended to a higher level (8.54–9.98 Log CFU/g). It was inferred that continuous B. coagulans supplement provided necessary organic acids and other metabolites for the growth of lactic acid bacteria [] and hence aided in restoring and increasing the total Bifidobacteria counts in feces.
From days 15 to 17 of the experiment, it was interesting to note that the intervention with antibiotics resulted in a significant (p < 0.05) reduction in the fecal E. coli counts in both the negative control group and the other five sample groups (Figure 5D). The phenomenon was probably attributed to the fact that antibiotics suppressed the number of E. coli in feces below the detectable range []. After the Salmonella infection on day 18, the apparently (p < 0.05) higher fecal E. coli counts versus with their pre-infection state was in agreement with the observations previously reported by Polishchuk et al. [].
During the recovery period (day 25), Figure 5C,D indicate that the GB group had the highest Bifidobacteria count in feces and the most prominent downward trend in the E. coli counts in feces. It was postulated that the combination of B. coagulans BACO-17 with appropriate prebiotics, particularly GOS, could be explored as a potential synbiotic mixture for improving dysbiosis in feces.
3.5. Changes in Fecal SCFAs
Table 2 presents the concentrations of total SCFAs in rat feces collected on day 14 (before antibiotic intervention), day 19 (after antibiotic intervention and Salmonella infection), and day 25 (end of the experiment). The administration of B. coagulans BACO-17 and the two prebiotics (i.e., FOS and GOS) for 14 days effectively promoted the production of total SCFAs in feces (37.9–64.9%) relative to the normal control. It is worth noting that the supplementation of synbiotic mixtures in GB and FB groups would further result in an approximately two-fold increase (p < 0.05) in the fecal levels of total SCFAs compared with the normal control.

Table 2.
The total concentrations of fecal short-chain fatty acids (μmol/g) at different times.
After the antibiotic intervention and Salmonella infection, the total concentrations of fecal SCFAs in all animal groups (except normal control) were significantly decreased (p < 0.05). Noticeably, the negative control group had the least total SCFAs (52.4 μmol/g). Mekonnen et al. [] reported that antibiotic administration could induce an imbalance of gut microbiota and impact carbohydrate metabolism, resulting in lower levels of acetic acid, propionic acid, butyric acid, and total SCFAs in feces. Our findings, as presented in Supplementary Table S2, also demonstrated a dramatic decline in the fecal levels of acetic acid and butyric acid.
During the recovery period (day 25), a general increase in the fecal SCFA concentrations was noted in Table 2. Among the five sample groups, the GB group showed the most remarkable improvement in the elevation of fecal total SCFA levels (182.1 μmol/g), which was almost 2-fold higher than the negative control. More specifically, the BC and GB groups showed a more promising increase in fecal butyric acid concentrations (Supplementary Table S2). Some previous studies have demonstrated that B. coagulans combined with prebiotics remarkably increased intestinal Lactobacillus and Bifidobacterium levels and boosted the production of lactic acid and SCFAs from prebiotics [,]. These findings might support the idea of developing a synbiotic by combining B. coagulans BACO-17 and GOS through their enhanced synthesis of fecal SCFAs.
3.6. Histopathological Studies
The H&E staining of colon tissue in each group is shown in Figure 6. The arrowheads indicate submucosae infiltrated with inflammatory cells. The results show that the normal control group had no apparent inflammatory cell infiltration in the lamina propria. In contrast, the negative control had significantly greater severity of inflammatory cell infiltration (at least a 2-fold increase) and extent of edema than the normal control (Table 3). It is worth noting that the administration of B. coagulans BACO-17 alone (BC group) or in combination with GOS (GB, synbiotic group) was more effective in reducing intestinal inflammation (27–31%) and mucosal necrosis (82%) over the negative control. The antibiotics treatment was supposed to cause disturbance of intestinal microbiota and facilitate colonization and infectivity of intestinal pathogens [].
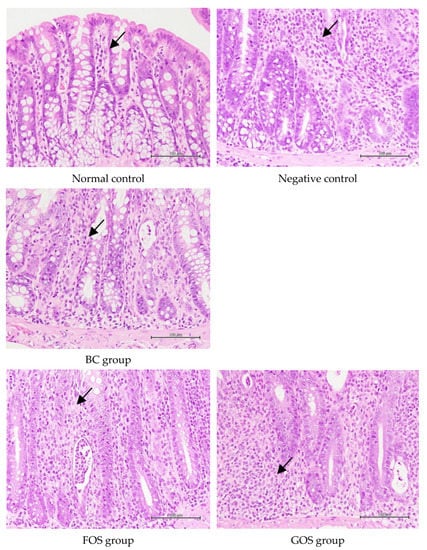
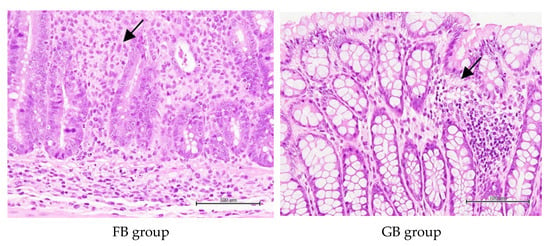
Figure 6.
Representative H&E stained colon tissues among different animal groups. The black arrowheads indicate that submucosae were infiltrated with inflammatory cells.

Table 3.
Histopathological scoring of inflammation grading based on H&E staining in different animal groups.
This indicated that antibiotic administration and Salmonella infection have successfully caused colon injury and cell damage in rats. As presented in Figure 5A,D, it is believed that the inflammatory symptoms caused by Salmonella infection not only promoted the growth of Salmonella in feces but also facilitated the proliferation of fecal commensal bacteria (such as E. coli) []. Compared with the negative control group, GB and BC groups presented less inflammation infiltration in mucosal lamina propria and submucosa and less cell necrosis in colonic tissues (Figure 6). It was postulated that the apparent improvement observed in BC and GB groups might be partly attributed to the remarkable elevation in the fecal butyric acid concentrations. Alonso and Guarner [] suggested that butyrate might serve as a primary energy source for colon cells, achieve anti-inflammatory effects by inhibiting the NF-κB pathway and reducing the expression of pro-inflammatory genes, enhance the integrity of the intestinal mucosal barrier, and regulate apoptosis.
The short-chain fatty acids produced during digestion might help maintain the intestinal mucosal barrier and exert immune regulation. Specifically, high acetic acid and butyric acid concentrations could exert potent anti-inflammatory effects []. It was speculated that the relatively higher fecal concentrations of butyric acid in BC and GB groups might contribute to reducing the extent and severity of colon inflammation in these groups. These results suggested that B. coagulans BACO-17 alone or in combination with GOS could effectively relieve intestinal inflammation.
4. Conclusions
Intestinal dysbiosis triggered by the antibiotic treatment could result in diarrhea. In the BC and GB groups, it was found that the beneficial bacteria counts and fecal SCFA concentrations were increased; meanwhile, the diarrhea symptoms were ameliorated. However, none of the sample groups effectively inhibited Salmonella colonization in the intestine. It was speculated that the antibiotics intervention had disturbed gut microbiota balance and caused inflammation and that Salmonella had competitive advantages over the commensal microbiota under such a condition. Based on the results of this study, the preventive effects of B. coagulans BACO-17 alone or combined with GOS as a synbiotic mixture on intestinal inflammatory diseases could be a promising adjuvant therapy; however, clinical trials are needed to further validate the therapeutic effects. Further studies are also needed for a more complete understanding of the changes in microbial biofilm associated with intestinal tissue and complex interactions between the host and gut microbiota.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pr10102123/s1, Figure S1: Agarose gel electrophoresis of the amplified target of 16S rRNA gene. (A) Bacillus coagulans BACO-17, (B) Salmonella enterica subsp. enterica BCRC 10747; Figure S2: Partial sequence of 16S rRNA gene and top listed BLAST results. (A) Bacillus coagulans BACO-17, (B) Salmonella enterica subsp. enterica BCRC 10747; Table S1: Comparisons of the average food intake, water intake, and body weight gains before and after intervention among different animal groups; Table S2. (A–C) The concentrations of acetic acid (μmol/g) in fecal samples collected at different time points; Table S3: Primers used for 16S rRNA gene analysis [,].
Author Contributions
Conceptualization, C.-F.C., M.-Z.W., Y.-W.H. and W.-J.C.; methodology, M.-Z.W., Y.-W.H., T.-C.S., H.-F.C. and C.-F.C.; formal analysis, M.-Z.W., Y.-W.H., T.-C.S. and C.-Y.L.; data curation, M.-Z.W., Y.-W.H., T.-C.S., H.-F.C., C.-Y.L. and C.-F.C.; writing-original draft preparation, M.-Z.W., Y.-W.H., Y.-C.W., T.-C.S. and C.-F.C.; writing-review and editing, C.-F.C., M.-Z.W., Y.-C.W. and C.-Y.L.; supervision, C.-F.C.; project administration, C.-F.C.; funding acquisition, C.-F.C. and W.-J.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Science and Technology Council of the Republic of China (MOST 111-2320-B-005-004-MY2).
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bergogne-Berezin, E. Treatment and prevention of antibiotic associated diarrhea. Int. J. Antimicrob. Agents 2000, 16, 521–526. [Google Scholar] [CrossRef]
- Elshaghabee, F.F.M.; Rokana, N.; Gulhane, R.D.; Sharma, C.; Panwar, H. Bacillus as potential probiotics: Status, concerns, and future perspectives. Front. Microbiol. 2017, 8, 1490. [Google Scholar] [CrossRef] [PubMed]
- Kapse, N.G.; Engineer, A.S.; Gowdaman, V.; Wagh, S.; Dhakephalkar, P.K. Functional annotation of the genome unravels probiotic potential of Bacillus coagulans HS243. Genomics 2019, 111, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Saw, C.Y.; Chang, T.J.; Chen, P.Y.; Dai, F.J.; Lau, Y.Q.; Chen, T.Y.; Chau, C.F. Presence of Bacillus coagulans spores and vegetative cells in rat intestine and feces and their physiological effects. Biosci. Biotechnol. Biochem. 2019, 83, 2327–2333. [Google Scholar] [CrossRef]
- Hun, L. Bacillus coagulans significantly improved abdominal pain and bloating in patients with IBS. Postgrad. Med. 2009, 121, 119–124. [Google Scholar] [CrossRef]
- Sudha, M.R.; Jayanthi, N.; Aasin, M.; Dhanashri, R.D.; Anirudh, T. Efficacy of Bacillus coagulans Unique IS2 in treatment of irritable bowel syndrome in children: A double blind, randomised placebo controlled study. Benef. Microbes 2018, 9, 563–572. [Google Scholar] [CrossRef]
- Maity, C.; Gupta, A.K. A prospective, interventional, randomized, double-blind, placebo-controlled clinical study to evaluate the efficacy and safety of Bacillus coagulans LBSC in the treatment of acute diarrhea with abdominal discomfort. Eur. J. Clin. Pharmacol. 2019, 75, 21–31. [Google Scholar] [CrossRef]
- Kolida, S.; Gibson, G.R. Synbiotics in health and disease. Annu. Rev. Food Sci. Technol. 2011, 2, 373–393. [Google Scholar] [CrossRef]
- van der Beek, C.M.; Dejong, C.H.; Troost, F.J.; Masclee, A.A.; Lenaerts, K. Role of short-chain fatty acids in colonic inflammation, carcinogenesis, and mucosal protection and healing. Nutr. Rev. 2017, 75, 286–305. [Google Scholar] [CrossRef]
- Nyangale, E.P.; Farmer, S.; Keller, D.; Chernoff, D.; Gibson, G.R. Effect of prebiotics on the fecal microbiota of elderly volunteers after dietary supplementation of Bacillus coagulans GBI-30, 6086. Anaerobe 2014, 30, 75–81. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, B.; Xu, J.; Liu, Y.; Qiu, E.; Li, Z.; Li, Z.; He, Y.; Zhou, H.; Bai, Y.; et al. Bacteroides fragilis protects against antibiotic-associated diarrhea in rats by modulating intestinal defenses. Front. Immunol. 2018, 9, 1040. [Google Scholar] [CrossRef] [PubMed]
- van Ampting, M.T.; Schonewille, A.J.; Vink, C.; Brummer, R.J.M.; van der Meer, R.; Bovee-Oudenhoven, I.M. Damage to the intestinal epithelial barrier by antibiotic pretreatment of Salmonella-infected rats is lessened by dietary calcium or tannic acid. J. Nutr. 2010, 140, 2167–2172. [Google Scholar] [CrossRef] [PubMed]
- Sakai, H.; Sagara, A.; Matsumoto, K.; Hasegawa, S.; Sato, K.; Nishizaki, M.; Shoji, T.; Syunji Horie, S.; Nakagawa, T.; Tokuyama, S.; et al. 5-Fluorouracil induces diarrhea with changes in the expression of inflammatory cytokines and aquaporins in mouse intestines. PLoS ONE 2013, 8, e54788. [Google Scholar] [CrossRef]
- Aleisa, A.M.; Al-Rejaie, S.S.; Abuohashish, H.M.; Ola, M.S.; Parmar, M.Y.; Ahmed, M.M. Pretreatment of Gymnema sylvestre revealed the protection against acetic acid-induced ulcerative colitis in rats. BMC Complement. Altern. Med. 2014, 14, 49. [Google Scholar] [CrossRef]
- Cano Roca, C.L. Characterization of Commercial Probiotics: Antibiotic Resistance, Acid and Bile Resistance, and Prebiotic Utilization. Master’s Thesis, University of Nebraska, Lincoln, NE, USA, 2014. [Google Scholar]
- Tulstrup, M.V.L.; Christensen, E.G.; Carvalho, V.; Linninge, C.; Ahrné, S.; Højberg, O.; Licht, T.R.; Bahl, M.I. Antibiotic treatment affects intestinal permeability and gut microbial composition in Wistar rats dependent on antibiotic class. PLoS ONE 2015, 10, e0144854. [Google Scholar] [CrossRef] [PubMed]
- Tulstrup, M.V.L.; Roager, H.M.; Thaarup, I.C.; Frandsen, H.L.; Frøkiær, H.; Licht, T.R.; Bahl, M.I. Antibiotic treatment of rat dams affects bacterial colonization and causes decreased weight gain in pups. Commun. Biol. 2018, 1, 145. [Google Scholar] [CrossRef] [PubMed]
- Qamar, T.R.; Syed, F.; Nasir, M.; Rehman, H.; Zahid, M.N.; Liu, R.H.; Iqbal, S. Novel combination of prebiotics galacto-oligosaccharides and inulin-inhibited aberrant crypt foci formation and biomarkers of colon cancer in Wistar rats. Nutrients 2016, 8, 465. [Google Scholar] [CrossRef] [PubMed]
- Majeed, M.; Natarajan, S.; Sivakumar, A.; Ali, F.; Pande, A.; Majeed, S.; Karri, S.K. Evaluation of anti-diarrhoeal activity of Bacillus coagulans MTCC 5856 and its effect on gastrointestinal motility in Wistar rats. Int. J. Pharma Bio Sci. 2016, 7, 311. [Google Scholar]
- Ara, K.; Meguro, S.; Hase, T.; Tokimitsu, I.; Otsuji, K.; Kawai, S.; Ito, S.; Iino, H. Effect of spore-bearing lactic acid-forming bacteria (Bacillus coagulans SANK 70258) administration on the intestinal environment, defecation frequency, fecal characteristics and dermal characteristics in humans and rats. Microb. Ecol. Health Dis. 2002, 14, 4–13. [Google Scholar] [CrossRef]
- Bovee-Oudenhoven, I.M.J.; Ten Bruggencate, S.J.M.; Lettink-Wissink, M.L.G.; Van der Meer, R. Dietary fructo-oligosaccharides and lactulose inhibit intestinal colonisation but stimulate translocation of salmonella in rats. Gut 2003, 52, 1572–1578. [Google Scholar] [CrossRef]
- Amorim, C.; Silvério, S.C.; Cardoso, B.B.; Alves, J.I.; Pereira, M.A.; Rodrigues, L.R. In vitro assessment of prebiotic properties of xylooligosaccharides produced by Bacillus subtilis 3610. Carbohydr. Polym. 2020, 229, 115460. [Google Scholar] [CrossRef] [PubMed]
- Haldar, L.; Gandhi, D.N. Effect of oral administration of Bacillus coagulans B37 and Bacillus pumilus B9 strains on fecal coliforms, Lactobacillus and Bacillus spp. in rat animal model. Vet. World 2016, 9, 766. [Google Scholar] [CrossRef] [PubMed]
- Holota, Y.; Dovbynchuk, T.; Kaji, I.; Vareniuk, I.; Dzyubenko, N.; Chervinska, T.; Zakordonets, L.; Stetska, V.; Ostapchenko, L.; Serhiychuk, T.; et al. The long-term consequences of antibiotic therapy: Role of colonic short-chain fatty acids (SCFA) system and intestinal barrier integrity. PLoS ONE 2019, 14, e0220642. [Google Scholar] [CrossRef] [PubMed]
- Polishchuk, N.N.; Bachurin, H.V.; Cherkovska, O.S.; Zinych, O.L.; Lazaryk, O.L.; Bezugly, M.B. Salmonella-induced changes of the rat intestinal microbiota. Russ. J. Infect. Immun. 2021, 11, 865–874. [Google Scholar] [CrossRef]
- Mekonnen, S.A.; Merenstein, D.; Fraser, C.M.; Marco, M.L. Molecular mechanisms of probiotic prevention of antibiotic-associated diarrhea. Curr. Opin. Biotechnol. 2020, 61, 226–234. [Google Scholar] [CrossRef]
- Cao, J.; Yu, Z.; Liu, W.; Zhao, J.; Zhang, H.; Zhai, Q.; Chen, W. Probiotic characteristics of Bacillus coagulans and associated implications for human health and diseases. J. Funct. Foods 2020, 64, 103643. [Google Scholar] [CrossRef]
- Kleessen, B.; Hartmann, L.; Blaut, M. Oligofructose and long-chain inulin: Influence on the gut microbial ecology of rats associated with a human faecal flora. Br. J. Nutr. 2001, 86, 291–300. [Google Scholar] [CrossRef]
- Kempf, F.; Menanteau, P.; Rychlik, I.; Kubasová, T.; Trotereau, J.; Virlogeux-Payant, I.; Schaeffer, S.; Schouler, C.; Drumo, R.; Guitton, E.; et al. Gut microbiota composition before infection determines the Salmonella super- and low-shedder phenotypes in chicken. Microb. Biotechnol. 2020, 13, 1611–1630. [Google Scholar] [CrossRef]
- Stecher, B.; Denzler, R.; Maier, L.; Bernet, F.; Sanders, M.J.; Pickard, D.J.; Barthel, M.; Westendorf, A.M.; Krogfelt, K.A.; Walker, A.W.; et al. Gut inflammation can boost horizontal gene transfer between pathogenic and commensal Enterobacteriaceae. Proc. Natl. Acad. Sci. USA 2012, 109, 1269–1274. [Google Scholar] [CrossRef]
- Alonso, V.R.; Guarner, F. Linking the gut microbiota to human health. Br. J. Nutr. 2013, 109, S21–S26. [Google Scholar] [CrossRef]
- Liu, Y.; Gibson, G.R.; Walton, G.E. An in vitro approach to study effects of prebiotics and probiotics on the faecal microbiota and selected immune parameters relevant to the elderly. PLoS ONE 2016, 11, e0162604. [Google Scholar] [CrossRef] [PubMed]
- Weisburg, W.G.; Barns, S.M.; Pelletier, D.A.; Lane, D.J. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 1991, 173, 697–703. [Google Scholar] [CrossRef]
- Gupta, R.S.; Patel, S.; Saini, N.; Chen, S. Robust demarcation of 17 distinct Bacillus species clades, proposed as novel Bacillaceae genera, by phylogenomics and comparative genomic analyses: Description of Robertmurraya kyonggiensis sp. nov. and proposal for an emended genus Bacillus limiting it only to the members of the Subtilis and Cereus clades of species. Int. J. Syst. Evol. Microbiol. 2020, 70, 5753–5798. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).