Abstract
(1) Background: Local therapy is highly promising in a multimodal approach strategy for patients with low-volume metastatic prostate cancer (mPCa). We aimed to systematically assess and summarize the safety, oncologic, and functional outcomes of cytoreductive prostatectomy (cRP) in mPCa. (2) Methods: Three databases were queried in September 2022 for publications that analyzed mPCa patients treated with cytoreductive prostatectomy without restrictions. The outcomes of interest were progression-free survival (PFS), cancer-specific survival (CSS), overall survival (OS), perioperative complication rates, and functional outcomes following cRP. (3) Results: Overall, 26 studies were included in this systematic review. Among eight population-based studies, cRP was associated with a reduced risk of CSS and OS compared with no local therapy (NLT) after adjusting for the effects of possible confounders. Furthermore, one population-based study showed that cRP reduced the risk of CSS even when compared with radiotherapy (RT) of the prostate after adjusting for the effects of possible confounders. In addition, one randomized controlled trial (RCT) demonstrated that local therapy (comprising 85% of cRP) significantly improved the prostate-specific antigen (PSA)-PFS and OS. Overall, cRP had acceptable perioperative complication rates and functional outcomes. (4) Conclusions: Mounting evidence suggests that cRP offers promising oncological and functional outcomes and technical feasibility and that it is associated with limited complications. Well-designed RCTs that limit selection bias in patients treated with cRP are warranted.
1. Introduction
The management of metastatic hormone-sensitive prostate cancer (mHSPC) has transformed during the past decade owing to the emergence of combination systemic therapies, such as androgen receptor signaling inhibitor and/or docetaxel plus androgen deprivation therapy (ADT) [1,2,3,4,5,6]. Further, mHSPC is a heterogeneous disease entity with varied prognoses. Tumor burden stratified as low- vs. high-volume disease (defined as the presence of visceral metastases, or four or more bone metastases, of which at least one must be located outside the vertebral column or pelvic bone) on the basis of the definition of the CHAARTED trial has been shown to stratify mHSPC into different risk categories [4,6,7,8,9,10,11]. For mHSPC patients with low-volume disease, local destructive therapy for primary prostate cancer and metastasis-directed therapy have gained widespread use [12]. For example, the STAMPEDE trial showed an OS benefit by delivering radiation therapy (RT) to the prostate in mHSPC patients treated with a standard of care for low-volume disease [13]. Since then, there has been increasing interest in local therapy (LT) as a part of the treatment strategy for mHSPC to ensure durability in efficacy and quality of life (i.e., local progression prevention). In addition to RT, cytoreductive prostatectomy (cRP) has been used in this setting. A previous meta-analysis based on population-based studies demonstrated the OS benefit of cRP even in mHSPC patients, including both low- and high-volume diseases [14]. Despite this, there are several limitations in the methodology of the published literature, primarily owing to heterogeneity in the included studies and patient selection [14,15,16]. One such factor is the even-increasing heterogeneity in the low-volume mHSPC group. On the basis of the increasing implementation of prostate-specific membrane antigen (PSMA)–positron emission tomography (PET) scans in clinical practice, an increasing number of oligometastatic patients with mHSPC are being identified [17]. Therefore, we conducted this systematic and comprehensive review to update and assess the safety, oncologic, and functional outcomes in mHSPC patients who underwent cRP.
2. Materials and Methods
The protocol has been registered in the International Prospective Register of Systematic Reviews database (PROSPERO: CRD42022368246).
2.1. Search Strategy
This systematic review was carried out according to the guidelines of the Preferred Reporting Items for Meta-Analyses of Observational Studies in Epidemiology Statement (Supplementary Figure S1) [18]. A literature search on the PubMed, Web of Science, and Scopus databases was performed in September 2022 to identify studies investigating the perioperative, oncologic, or functional outcomes of cRP for mPCa. The detailed search strategy was as follows: (metastatic) AND (prostate cancer) AND (prostatectomy) OR (cytoreductive). The primary outcomes of interest were overall survival (OS) and cancer-specific survival (CSS), and the secondary outcomes of interest were progression-free survival (PFS), perioperative outcomes, and urinary and erectile functional outcomes. The initial screening based on the titles and abstracts aimed to identify eligible studies and was performed by two investigators. Potentially relevant studies were subjected to a full-text review. Disagreements were resolved by consensus with the coauthors.
2.2. Inclusion and Exclusion Criteria
Studies were included if they investigated metastatic PCa (mPCa) patients (patients), who underwent cRP (interventions) compared with those treated with RT or without LT (comparisons), to assess the differential oncologic, perioperative, and functional outcomes (outcome) in randomized controlled studies (RCTs) and in nonrandomized, observational, population-based, or cohort studies (Study design). We excluded studies that compared the differential outcomes of LT vs. non-LT (NLT), not separately reporting the outcomes of cRP or RT unless more than 80% of patients treated with LT were cRP. Studies lacking original patient data, reviews, letters, editorial comments, replies from authors, case reports, and articles not written in English were excluded. References of all papers included were scanned for additional studies of interest.
2.3. Data Extraction
Two authors independently extracted the following data: the first author’s name, publication year, country, inclusion criteria, number of patients, follow-up duration, age, performance status or comorbidity, clinical stage, biopsy Gleason score (GS), pretreatment of prostate-specific antigen (PSA), metastatic site, surgical approach of cRP, lymph node dissection (LND) and number of removed lymph nodes (LNs), estimated blood loss, operation time, catheterization periods, length of hospital stay, all and severe (≧Clavien-Dindo classification Ⅲ) postoperative complication rates, positive surgical margin (PSM), LN involvement, pathologic stage, GS in the resected specimen, continence rates, erectile function, patient-reported quality of life (QOL), OS, CSS, PFS, time to castration-resistant prostate cancer (CRPC), and CRPC-free survival. Subsequently, the hazard ratios (HRs) and 95% confidence intervals (CIs) from Cox regression models for OS and CSS were retrieved. All discrepancies were resolved by consensus with the coauthors.
2.4. Risk-of-Bias Assessment
The study quality and risk of bias were assessed according to the Risk of Bias in Nonrandomized Studies of Interventions (ROBINS-I) tool and the risk-of-bias (RoB version2), referring to the Cochrane Handbook for Systematic Reviews of Interventions [18]. Each bias domain and the overall risk of bias were judged as ‘low’, ‘moderate’, ‘serious’ or ‘critical’. The presence of possible confounders was determined by consensus and a literature review. The ROBINS-I and risk-of-bias assessment of each study were independently conducted by two authors (Supplementary Figure S2 and Table S1).
3. Results
3.1. Study Selection and Characteristics
Our initial search identified 6980 records. After removing duplicates, 4110 records remained for screening titles and abstracts (Figure 1). After screening, a full-text review of 142 articles was performed. According to our inclusion criteria, we finally identified 27 studies eligible for systematic review [19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45]. The demographics of each included study are shown in Table 1 and Table 2. Of the 27 studies, 9 were population-based designs, 11 were comparative (including case-control cohorts and RCTs), and seven included only cRP patients. This section may be divided by subheadings. It should provide a concise and precise description of the experimental results, their interpretation, and the experimental conclusions that can be drawn.
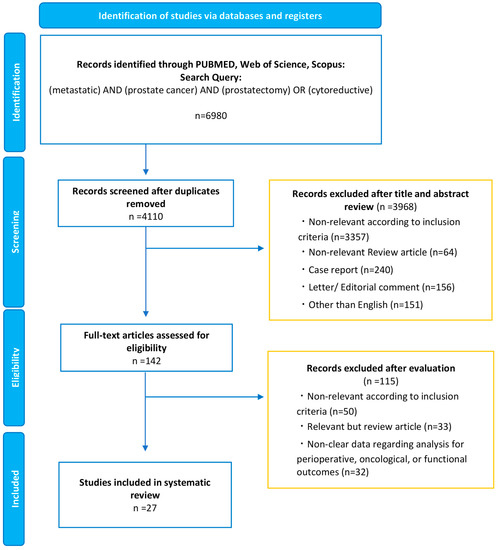
Figure 1.
The Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) flow chart, detailing the article-selection process.

Table 1.
Demographics and oncologic outcomes of population-based study.

Table 2.
Study demographics and oncologic outcomes of cohort studies.
3.2. Oncologic Outcomes
3.2.1. Population-Based Studies
We identified seven studies by using the Surveillance, Epidemiology, and End Results (SEER) database and one study each by using the Munich Cancer Registry database and the National Cancer Database (NCDB). SEER and NCDB reflect the real-world survival data of patients diagnosed with mPCa in the US. However, variables unavailable from SEER, such as patient performance status, comorbidity, and metastatic burden (i.e., including both high- and low-volume disease), undoubtedly limited the granularity and generalizability of the analyses and precluded controlling for the often-existent selection bias [20]. Patient demographics of included population-based studies are summarized in Supplementary Table S2.
cRP vs. NLT
In 2014, Culp et al. for the first time showed the OS and CSS benefit of LT for mPCa among 8185 mPCa patients (NLT: n = 7811, cRP: n = 245, brachytherapy [BT]: n = 129) by using the SEER database from 2004 to 2010 [20]. The authors reported that the 5-year OS and CSS were significantly higher in patients undergoing cRP (67.4% and 75.8%, respectively) or BT (52.6% and 61.3%, respectively) compared with those without LT (22.5% and 48.7%, respectively) [20]. After adjusting for the effects of confounders, such as TNM stage and PSA, using multivariable competing risks regression analysis, statistical significance remained (HR for cRP: 0.38 [95% CI: 0.27–0.53], HR for BT: 0.68 [95% CI: 0.49–0.93]) [20]. Thereafter, Antwi et al. and Satkunasivam et al. performed the additional analyses using propensity scores (PS) in 2014 and 2015 [19,26]. Antwi et al. showed that PS-adjusted HR for OS in patients who underwent cRP was 0.22 (95% CI: 0.17–0.28) compared with those without LT [19]. Satkunasivam et al. assessed the same oncologic outcomes in patients 66 years or older (n = 4069) [26]. Owing to the low number of patients who underwent cRP (n = 47), PS-adjusted HR for OS did not reach statistical significance (HR: 0.55 [95% CI: 0.30–1.02]) [26]. In 2017, Parikh et al. published results from the NCDB comprising 6051 patients (NLT: n = 5224, cRP: n = 622, radiotherapy [RT]: n = 205) by adjusting for the effects of confounders such as age, TN stage, and the Charlson comorbidity index (CCI) [25]. The authors showed that the adjusted HR by using the Cox proportional hazard model for OS in patients who underwent cRP was 0.51 (95% CI: 0.45–0.59) and confirmed the OS benefit of cRP even after PS adjustment (HR: 0.27 [95% CI: 0.22–0.33]) [25].
Since 2020, two studies using the SEER database have been published. Jin et al. updated the study period (2010–2014) from the study conducted by Culp et al., comprising 5849 patients (NLT: n = 5628, cRP: n = 159, BT: n = 62) [24]. The authors corroborated previous findings suggesting an OS and CSS benefit by using the Cox proportional hazard models (HR: 0.60 [95% CI: 0.42–0.87], HR: 0.56 [95% CI: 0.37–0.86], respectively) [24]. In addition, a subgroup analysis revealed that patients with bone metastasis or distant LN metastasis were significantly more likely to benefit from definitive local therapy [24]. Despite the limitation of selection bias derived from a population-based study, the detailed analyses adjusting for the effects of confounders revealed an OS and CSS benefit for cRP over NLT.
cRP vs. RT
Jin et al. compared oncologic outcomes by using the SEER database (2004–2015) comprising 19,612 patients (NLT: n = 18,857, cRP: n = 435, RT: n = 320) [23]. The authors confirmed the OS and CSS benefit of LT over NLT even after adjusting for the effects of unmeasured confounders (HR for OS: 0.57 [95% CI 0.50–0.65], HR for CSS: 0.59 [95% CI 0.51–0.68], respectively.) [23]. Furthermore, the authors showed that cRP was associated with significantly better OS and CSS compared with RT after adjusting for the effects of race, age, marital status, TNM stage, GS, and PSA as well as performance status [23]. However, after adjusting for the effects of unmeasured confounders, this statistical significance diminished (HR for OS: 0.63 [95% CI 0.26–1.54] and HR for CSS: 0.47 [95% CI 0.16–1.35], respectively) [23]. Guo et al. created 1:1 PS-matched cohorts (cRP: n = 148, RT: n = 148) based on data from the SEER database (2004–2016) [22]. The authors failed to show the superiority of cRP over RT in terms of OS (HR: 0.73 [95% CI: 0.48–11]) and CSS (HR: 0.77 [95% CI: 0.46–1.30]) [22]. A recently published study using the SEER database (2004–2016) conducted by Stolzenbach et al. comprised 954 patients who underwent cRP and 3326 patients who underwent RT [27]. Despite short follow-up periods (median follow-up was within 2 years), they showed that cRP is associated with significantly better CSS compared with RT after adjusting for age, initial PSA, biopsy GS, and clinical TNM stages using PS and the competing risk regression (HR: 0.82 [95% CI: 0.71–0.94]) [27]. However, the results from the SEER database differ according to the recruitment periods, statistical methods, and follow-up duration, leaving the potential benefits of cRP over RT controversial.
3.2.2. Case-Control Studies
Patient demographics and oncologic outcomes of included studies are shown in Table 2 and Supplementary Table S3.
cRP vs. NLT
Six case-control studies assessing the differential oncologic outcomes were identified. In 2015, Heidenreich et al. assessed the differential oncologic outcomes of cRP (n = 23) vs. NLT (n = 38) in patients with oligometastatic mPCa (less than three bone metastases) with comparable patient demographics except for baseline PSA [33]. The authors reported significantly better PFS, time to CRPC, and CSS in patients who underwent cRP compared with those who did not undergo LT [33]. In 2017, Poelart et al. reported the preliminary results of the LoMP trial with extremely better oncologic outcomes in terms of 100% of 2-year CSS and OS in patients who underwent cRP (n = 17) compared with patients without LT (n = 29). On the other hand, Moschini et al. and Steuber et al. showed no differences in CSS or OS between patients who underwent cRP and those who did not [39,42]. In addition, updated results from the LoMP trial comprising 40 patients in each arm (NLT vs. cRP) showed no differences in CRPC-free survival on multivariable analysis [29]. However, most recently, Mistretta et al. demonstrated that NLT was associated with higher rates of progression to mCRPC (HR: 0.40; CI 0.19–0.84 adjusted for the effects of the site of metastasis, HR:0.39; CI 0.19–0.84 adjusted for the effect of PSA) while adjusting only for one confounder [38]. Taken together, there is conflicting evidence on the oncologic benefit of cRP from case-control studies. Notably, these studies included only 17 to 43 patients in the cRP group; therefore, these studies suffered from low statistical power. We initially attempted to perform a meta-analysis to integrate them; however, these studies also suffered different inclusion criteria and unmatched comparators. This suggests the need for well-controlled future trials and for international collaborative multicenter studies with more patients.
cRP vs. RT
Lumen et al. reported comparable oncological outcomes between cRP and RT from the LoMP trial [36]. Comparing cRP (n = 48) vs. RT (n = 26), the 2-year CSS were 93% vs. 100%, and the 2-year OS were 93% vs. 100%, respectively [36]. Knipper et al. compared the oncologic outcomes of cRP in mHSPC patients with low-volume disease and the results from the STAMPEDE arm H [13,35]. The authors showed comparable 3-year OS and CSS rates for cRP and RT (OS: 91% vs. 81%, CSS: 92% vs. 86%, respectively) [35]. To date, high-quality evidence regarding the differential oncological outcomes between cRP and RT is lacking; nonetheless, the oncological effectiveness of cRP with PLND may be comparable to pelvic RT.
3.2.3. RCT
Up to now, only one RCT, conducted by Dai et al., has been published [31]. The authors conducted an open-label phase-2 RCT to compare the oncologic outcomes between LT (n = 100) and NLT (n = 100). The LT group comprised 85 (85%) patients who underwent cRP and 11 (11%) patients who underwent RT, whereas 17 patients (17%) eventually received LT in the NLT group. This study showed significantly better OS (HR: 0.44 [95% CI: 0.24–0.81]), radiographic PFS (HR: 0.43 [95% CI: 0.27–0.70]), and PSA-PFS (HR: 0.44 [95% CI: 0.29–0.67]) in patients who underwent LT compared with those who did not during 48 months of median follow-up [31].
3.3. Perioperative Outcomes
3.3.1. Complications
Assessing perioperative outcomes on the basis of different surgical approaches is imperative. Robot-assisted radical prostatectomy (RARP) has recently replaced open and laparoscopic approaches as the standard technique [46,47]. Traditionally, cRP has been performed by using an open approach [33,39,45], while recently, RARP has become the standard procedure even for cRP (Table 3) [29,38,40,41,43].

Table 3.
Perioperative outcomes following cRP.
In total, after combining patients from all studies, there were 155 overall complications reported in 473 patients (33%) and 47 severe complications (CTCAE grade≥ 3) reported in 448 patients (10%) who underwent cRP. Only one rectal injury was reported in 347 patients (0.29%). However, most eligible studies did not report outcome data on cRP for either open or robot-assisted approaches, making meaningful comparisons challenging. The largest multicenter cohort reported by Heidenreich et al. (open: n = 104, RARP: n = 5) showed that the rates of overall and severe complications were 34% and 9.7%, respectively [32].
For open cRP alone, the overall and severe complication rates ranged from 29% to 54% and from 6.5% to 21%, respectively [33,35,39,45]. In comparison, Sooriakumaran et al. reported a 12.5% overall complication rate in patients who underwent cytoreductive RARP (cRARP) [41]; furthermore, Takagi et al., assessing the feasibility of cRARP in 12 patients, reported excellent perioperative outcomes without any complications [43]. Taken together, cRARP seems safer than open cRP, in agreement with the previously demonstrated safety of RARP for localized PCa [48].
3.3.2. Pathologic Outcomes
Of the studies included, 15 provided data on the rates of PSM, ranging from 8.3% to 82% [28,29,30,31,32,33,34,37,39,40,41,42,43,44,45]. Most studies performed concomitant PLND during cRP; 15 studies provided data on the rates of LN involvement, ranging from 31% to 91% [28,29,30,31,32,33,34,37,39,40,41,42,44,45]. The wide range of PSM rates suggests the importance of optimal patient selection and the need for adjuvant RT in some patients. Extended PLND should be performed during cRP given the high likelihood of LN involvement.
3.4. Functional Outcomes
3.4.1. Urinary Function
Obstructive Voiding Dysfunction in NLT Patients
Obstructive voiding dysfunction and relevant lower urinary tract symptoms (LUTSs) due to the local progression of PCa are critical clinical issues in the late stages of mPCa [49]. Evaluating the intervention rates for obstructive urinary dysfunction in NLT patients, a retrospective case-control study by Heidenreich et al. showed that 11 of 38 (29%) patients required surgical or percutaneous intervention [33]. The LoMP trial conducted by Poelaert et al. revealed that 11 of 29 (38%) NLT patients required intervention [29]. In addition, Steuber et al. reported that 14 of 40 (35%) NLT patients experienced severe local complications [42]. Notably, Lumen et al. demonstrated that cRP was associated with higher local event-free rates than NLT on multivariable analysis (HR: 0.36 [95% CI: 0.14–0.94]) [36]. Preventing obstructive voiding dysfunction seems to be an essential rationale for undergoing cRP for mPCa patients in the earlier disease stages before disease progression.
Incontinence after cRP
The timing and tools for assessing urinary incontinence after cRP varied across studies (Table 4). Continence rates, defined as pad 0–1/day 1 year after cRP, ranged from 74% to 88% [28,29,35]. Of note, 0 pad achievement rates at 1-year follow-up after cRP ranged from 53% to 92% [28,29,31,32,35]. For example, Knipper et al. showed that 53% who underwent open cRP (n = 78) did not use any pad/day [35]. Furthermore, another large multicenter study conducted by Heidenreich et al. comprising 113 patients (92% of patients underwent open cRP) found a 68% 0 pad rate at 1-year follow-up [32]. In the recent RCT conducted by Dai et al., excellent continence rates, of 92%, at 1 year and 95% at 2 years after cRP were reported, although only 20% of patients underwent cRARP [31]. There are no robust data regarding urinary function following cRARP.

Table 4.
Functional outcomes following cRP.
Interestingly, Chaloupka et al. compared the functional outcomes between cRP (open cRP: n = 69, cRARP: n = 13) and RP for localized PCa (open RP: n = 116, RARP: n = 216) [30]. This study revealed comparable continence recovery rates (66% vs. 72%, p = 0.4) as well as International Consultation on Incontinence Questionnaire scores (ICIQ-SF; 6.4 ± 5.7 vs. 6.4 ± 5.2 [mean ± SD], p = 1) at 25 months after surgery [30].
3.4.2. Erectile Function
Two studies assessed erectile function before and after cRP using the International Index of Erectile Function (IIEF)-5 score [30,41]. The TroMbone trial revealed comparable IIEF-5 scores between the cRP and NLT groups (Median [IQR]: 5.0 [5.0–6.0] vs. 5.0 [5.0–12.0]) [41]. On the contrary, a study conducted by Chaloupka et al. comparing the functional outcomes between cRP and RP for localized PCa (Open cRP: n = 116, cRARP: n = 216) showed that the IIEF-5 score was significantly lower in the patients who underwent cRP compared with those who underwent RP for localized PCa (mean ± SD: 1.3 ± 4.2 vs. 3.5 ± 6.2, p < 0.001) [30]. The low rates of nerve sparing cRP (cRP: 17% vs. RP for localized PCa: 55%) indeed affect these outcomes [30].
3.4.3. Quality of Life
The TroMbone trial also assessed the patient-reported QOL using the EuroQoL Five Dimensions Five Levels (EQ-5D-5L) questionnaires at baseline and 3 months postrandomization [41]. This study showed a comparative EQ-5D-5L descriptive score at 3 months after randomization between the cRP and NLT groups (median [IQR]: both 1.0 [0.8–1.0]) [41]. Chaloupka et al. compared the general health-related QOL (HRQOL) by global health status (GHS) by using the European Organization for Research and Treatment of Cancer (EORTC) quality-of-life questionnaire (QLQ)-C30 between cRP and RP for the localized PCa group. This study demonstrated no difference in the general HRQOL rates between the two groups at the end of follow-up (44% vs. 56%, p = 0.8) [30]. Interestingly, GHS significantly worsened in localized PCa patients compared with the baseline (–5, p = 0.001), whereas GHS did not significantly change in patients who underwent cRP (+3.2, p = 0.4) [30]. Taken together, cRP seems not to reduce the patient-reported QOL compared with patients with NLT.
4. Conclusions
Population-based studies showed an oncologic benefit to cRP compared with NLT or RT for mPCa, after careful analyses that adjusted for the effects of possible confounders. Nevertheless, these studies suffered from selection bias and lacked relevant data often used for clinical decision-making, such as comorbidity and metastatic burden. Small case-control studies, including only patients with oligometastatic disease, failed to report a clear survival benefit for cRP. Recently, only one RCT, including 85% of cRP patients in the LT group, demonstrated an oncologic benefit of LT in terms of PSA-PFS as well as OS. Perioperative and functional outcomes following cRP seem to be comparable to those of NLT or RP for localized PCa. Taken together, cRP offers promising oncological outcomes, technical feasibility, and acceptable functional outcomes. However, well-designed, adequately powered RCTs with long-term follow-ups are needed to allow a robust and fair comparison of cRP with NLT and RT. Until then, cRP should be considered experimental and assessed only in clinical trials.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/curroncol30020170/s1, Supplementary Figure S1. PRISMA checklist 2009, Supplementary Figure S2. Risk-of-bias assessment of the included RCTs, Supplementary Table S1. Risk-of-bias assessment for NRCTs (ROBINS-I), Supplementary Table S2. Patient characteristics of included population-based studies, Supplementary Table S3. Patient characteristics of included case-control studies.
Author Contributions
T.Y. contributed to the protocol/project development, data collection and management, and manuscript writing/editing. P.R. and T.K. (Tatsushi Kawada) contributed to the data extraction and manuscript writing/editing. K.B., E.L., M.v.D., M.M. and M.C. contributed to the manuscript writing/editing. T.K. (Takahiro Kimura), P.I.K. and A.H. contributed to the manuscript editing. S.F.S. contributed to the supervision, protocol/project development/management, and manuscript editing. All authors have read and agreed to the published version of the manuscript.
Funding
NA (no external funding provided), EUSP Scholarship of the European Association of Urology (PR).
Conflicts of Interest
Takahiro Kimura is a paid consultant/advisor of Astellas, Bayer, Janssen, and Sanofi. Shahrokh F. Shariat has received honoraria from Astellas, AstraZeneca, BMS, Ferring, Ipsen, Janssen, MSD, Olympus, Pfizer, Roche, and Takeda; played a consulting or advisory role with Astellas, AstraZeneca, BMS, Ferring, Ipsen, Janssen, MSD, Olympus, Pfizer, Pierre Fabre, Roche, and Takeda; and was associated with the speakers bureau of Astellas, Astra Zeneca, Bayer, BMS, Ferring, Ipsen, Janssen, MSD, Olympus, Pfizer, Richard Wolf, Roche, and Takeda. The other authors declare no conflict of interest associated with this manuscript.
References
- Cornford, P.; van den Bergh, R.C.N.; Briers, E.; Van den Broeck, T.; Cumberbatch, M.G.; De Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer. Part II-2020 Update: Treatment of Relapsing and Metastatic Prostate Cancer. Eur. Urol. 2021, 79, 263–282. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.J.; Azad, A.A.; Iguchi, T.; Szmulewitz, R.Z.; Petrylak, D.P.; Holzbeierlein, J.; Villers, A.; Alcaraz, A.; Alekseev, B.; Shore, N.D.; et al. Improved Survival with Enzalutamide in Patients with Metastatic Hormone-Sensitive Prostate Cancer. J. Clin. Oncol. 2022, 40, 1616–1622. [Google Scholar] [CrossRef] [PubMed]
- Chi, K.N.; Chowdhury, S.; Bjartell, A.; Chung, B.H.; Pereira de Santana Gomes, A.J.; Given, R.; Juárez, A.; Merseburger, A.S.; Özgüroğlu, M.; Uemura, H.; et al. Apalutamide in Patients with Metastatic Castration-Sensitive Prostate Cancer: Final Survival Analysis of the Randomized, Double-Blind, Phase III TITAN Study. J. Clin. Oncol. 2021, 39, 2294–2303. [Google Scholar] [CrossRef]
- Fizazi, K.; Foulon, S.; Carles, J.; Roubaud, G.; McDermott, R.; Fléchon, A.; Tombal, B.; Supiot, S.; Berthold, D.; Ronchin, P.; et al. Abiraterone plus prednisone added to androgen deprivation therapy and docetaxel in de novo metastatic castration-sensitive prostate cancer (PEACE-1): A multicentre, open-label, randomised, phase 3 study with a 2 × 2 factorial design. Lancet 2022, 399, 1695–1707. [Google Scholar] [CrossRef]
- Smith, M.R.; Hussain, M.; Saad, F.; Fizazi, K.; Sternberg, C.N.; Crawford, E.D.; Kopyltsov, E.; Park, C.H.; Alekseev, B.; Montesa-Pino, Á.; et al. Darolutamide and Survival in Metastatic, Hormone-Sensitive Prostate Cancer. N. Engl. J. Med. 2022, 386, 1132–1142. [Google Scholar] [CrossRef]
- Fizazi, K.; Tran, N.; Fein, L.; Matsubara, N.; Rodriguez-Antolin, A.; Alekseev, B.Y.; Özgüroğlu, M.; Ye, D.; Feyerabend, S.; Protheroe, A.; et al. Abiraterone acetate plus prednisone in patients with newly diagnosed high-risk metastatic castration-sensitive prostate cancer (LATITUDE): Final overall survival analysis of a randomised, double-blind, phase 3 trial. Lancet Oncol. 2019, 20, 686–700. [Google Scholar] [CrossRef]
- Clarke, N.W.; Ali, A.; Ingleby, F.C.; Hoyle, A.; Amos, C.L.; Attard, G.; Brawley, C.D.; Calvert, J.; Chowdhury, S.; Cook, A.; et al. Addition of docetaxel to hormonal therapy in low-and high-burden metastatic hormone sensitive prostate cancer: Long-term survival results from the STAMPEDE trial. Ann. Oncol. 2019, 30, 1992–2003. [Google Scholar] [CrossRef] [PubMed]
- Davis, I.D.; Martin, A.J.; Zielinski, R.R.; Thomson, A.; Tan, T.H.; Sandhu, S.; Reaume, M.N.; Pook, D.W.; Parnis, F.; North, S.A.; et al. Updated overall survival outcomes in ENZAMET (ANZUP 1304), an international, cooperative group trial of enzalutamide in metastatic hormone-sensitive prostate cancer (mHSPC). J. Clin. Oncol. 2022, 40 (Suppl. S17), LBA5004. [Google Scholar] [CrossRef]
- Gravis, G.; Fizazi, K.; Joly, F.; Oudard, S.; Priou, F.; Esterni, B.; Latorzeff, I.; Delva, R.; Krakowski, I.; Laguerre, B.; et al. Androgen-deprivation therapy alone or with docetaxel in non-castrate metastatic prostate cancer (GETUG-AFU 15): A randomised, open-label, phase 3 trial. Lancet. Oncol. 2013, 14, 149–158. [Google Scholar] [CrossRef]
- Kyriakopoulos, C.E.; Chen, Y.H.; Carducci, M.A.; Liu, G.; Jarrard, D.F.; Hahn, N.M.; Shevrin, D.H.; Dreicer, R.; Hussain, M.; Eisenberger, M.; et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer: Long-Term Survival Analysis of the Randomized Phase III E3805 CHAARTED Trial. J. Clin. Oncol. 2018, 36, 1080–1087. [Google Scholar] [CrossRef]
- Yanagisawa, T.; Rajwa, P.; Thibault, C.; Gandaglia, G.; Mori, K.; Kawada, T.; Fukuokaya, W.; Shim, S.R.; Mostafaei, H.; Motlagh, R.S.; et al. Androgen Receptor Signaling Inhibitors in Addition to Docetaxel with Androgen Deprivation Therapy for Metastatic Hormone-sensitive Prostate Cancer: A Systematic Review and Meta-analysis. Eur. Urol. 2022, 82, 584–598. [Google Scholar] [CrossRef] [PubMed]
- von Deimling, M.; Rajwa, P.; Tilki, D.; Heidenreich, A.; Pallauf, M.; Bianchi, A.; Yanagisawa, T.; Kawada, T.; Karakiewicz, P.I.; Gontero, P.; et al. The current role of precision surgery in oligometastatic prostate cancer. ESMO Open. 2022, 7, 100597. [Google Scholar] [CrossRef] [PubMed]
- Parker, C.C.; James, N.D.; Brawley, C.D.; Clarke, N.W.; Hoyle, A.P.; Ali, A.; Ritchie, A.W.S.; Attard, G.; Chowdhury, S.; Cross, W.; et al. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): A randomised controlled phase 3 trial. Lancet 2018, 392, 2353–2366. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qin, Z.; Wang, Y.; Chen, C.; Wang, Y.; Meng, X.; Song, N. The role of radical prostatectomy for the treatment of metastatic prostate cancer: A systematic review and meta-analysis. Biosci. Rep. 2018, 38, BSR20171379. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Hu, M.; Yang, G.; Gao, E.; Xu, W. Cytoreductive prostatectomy improves survival outcomes in patients with oligometastases: A systematic meta-analysis. World J. Surg. Oncol. 2022, 20, 255. [Google Scholar] [CrossRef]
- Shemshaki, H.; Al-Mamari, S.A.; Geelani, I.A.; Kumar, S. Cytoreductive radical prostatectomy versus systemic therapy and radiation therapy in metastatic prostate cancer: A systematic review and meta-analysis. Urologia 2022, 89, 16–30. [Google Scholar] [CrossRef]
- Christ, S.M.; Pohl, K.; Muehlematter, U.J.; Heesen, P.; Kühnis, A.; Willmann, J.; Ahmadsei, M.; Badra, E.V.; Kroeze, S.G.C.; Mayinger, M.; et al. Imaging-Based Prevalence of Oligometastatic Disease: A Single-Center Cross-Sectional Study. Int. J. Radiat. Oncol. Biol. Phys. 2022, 114, 596–602. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gotzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef]
- Antwi, S.; Everson, T.M. Prognostic impact of definitive local therapy of the primary tumor in men with metastatic prostate cancer at diagnosis: A population-based, propensity score analysis. Cancer Epidemiol. 2014, 38, 435–441. [Google Scholar] [CrossRef]
- Culp, S.H.; Schellhammer, P.F.; Williams, M.B. Might men diagnosed with metastatic prostate cancer benefit from definitive treatment of the primary tumor? A SEER-based study. Eur. Urol. 2014, 65, 1058–1066. [Google Scholar] [CrossRef]
- Gratzke, C.; Engel, J.; Stief, C.G. Role of radical prostatectomy in metastatic prostate cancer: Data from the Munich Cancer Registry. Eur. Urol. 2014, 66, 602–603. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Xia, H.; Su, X.; Hou, H.; Zhong, Q.; Wang, J. Comparing the Survival Outcomes of Radical Prostatectomy Versus Radiotherapy for Patients with De Novo Metastasis Prostate Cancer: A Population-Based Study. Front. Oncol. 2021, 11, 797462. [Google Scholar] [CrossRef] [PubMed]
- Jin, K.; Qiu, S.; Jin, H.; Zheng, X.; Zhou, X.; Jin, D.; Li, J.; Yang, L.; Wei, Q. Survival Outcomes for Metastatic Prostate Cancer Patients Treated with Radical Prostatectomy or Radiation Therapy: A SEER-based Study. Clin. Genitourin. Cancer 2020, 18, e705–e722. [Google Scholar] [CrossRef]
- Jin, S.; Wei, J.; Wang, J.; Wang, B.; Wu, J.; Gan, H.; Dai, B.; Qin, X.; Lin, G.; Wei, Y.; et al. Prognostic Value of Local Treatment in Prostate Cancer Patients with Different Metastatic Sites: A Population Based Retrospective Study. Front. Oncol. 2020, 10, 527952. [Google Scholar] [CrossRef] [PubMed]
- Parikh, R.R.; Byun, J.; Goyal, S.; Kim, I.Y. Local Therapy Improves Overall Survival in Patients with Newly Diagnosed Metastatic Prostate Cancer. Prostate 2017, 77, 559–572. [Google Scholar] [CrossRef]
- Satkunasivam, R.; Kim, A.E.; Desai, M.; Nguyen, M.M.; Quinn, D.I.; Ballas, L.; Lewinger, J.P.; Stern, M.C.; Hamilton, A.S.; Aron, M.; et al. Radical Prostatectomy or External Beam Radiation Therapy vs No Local Therapy for Survival Benefit in Metastatic Prostate Cancer: A SEER-Medicare Analysis. J. Urol. 2015, 194, 378–385. [Google Scholar] [CrossRef]
- Stolzenbach, L.F.; Deuker, M.; Collà-Ruvolo, C.; Nocera, L.; Tian, Z.; Maurer, T.; Steuber, T.; Tilki, D.; Briganti, A.; Saad, F.; et al. Radical prostatectomy improves survival in selected metastatic prostate cancer patients: A North American population-based study. Int. J. Urol. 2021, 28, 834–839. [Google Scholar] [CrossRef]
- Babst, C.; Amiel, T.; Maurer, T.; Knipper, S.; Lunger, L.; Tauber, R.; Retz, M.; Herkommer, K.; Eiber, M.; von Amsberg, G.; et al. Cytoreductive radical prostatectomy after chemohormonal therapy in patients with primary metastatic prostate cancer. Asian J. Urol. 2022, 9, 69–74. [Google Scholar] [CrossRef]
- Buelens, S.; Poelaert, F.; Claeys, T.; De Bleser, E.; Dhondt, B.; Verla, W.; Ost, P.; Rappe, B.; De Troyer, B.; Verbaeys, C.; et al. Multicentre, prospective study on local treatment of metastatic prostate cancer (LoMP study). BJU Int. 2022, 129, 699–707. [Google Scholar] [CrossRef]
- Chaloupka, M.; Stoermer, L.; Apfelbeck, M.; Buchner, A.; Wenter, V.; Stief, C.G.; Westhofen, T.; Kretschmer, A. Health-Related Quality of Life following Cytoreductive Radical Prostatectomy in Patients with De-Novo Oligometastatic Prostate Cancer. Cancers 2021, 13, 5636. [Google Scholar] [CrossRef]
- Dai, B.; Zhang, S.; Wan, F.N.; Wang, H.K.; Zhang, J.Y.; Wang, Q.F.; Kong, Y.Y.; Ma, X.J.; Mo, M.; Zhu, Y.; et al. Combination of Androgen Deprivation Therapy with Radical Local Therapy Versus Androgen Deprivation Therapy Alone for Newly Diagnosed Oligometastatic Prostate Cancer: A Phase II Randomized Controlled Trial. Eur. Urol. Oncol. 2022, 5, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, A.; Fossati, N.; Pfister, D.; Suardi, N.; Montorsi, F.; Shariat, S.; Grubmüller, B.; Gandaglia, G.; Briganti, A.; Karnes, R.J. Cytoreductive Radical Prostatectomy in Men with Prostate Cancer and Skeletal Metastases. Eur. Urol. Oncol. 2018, 1, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, A.; Pfister, D.; Porres, D. Cytoreductive radical prostatectomy in patients with prostate cancer and low volume skeletal metastases: Results of a feasibility and case-control study. J. Urol. 2015, 193, 832–838. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.Y.; Mitrofanova, A.; Panja, S.; Sterling, J.; Srivastava, A.; Kim, J.; Kim, S.; Singer, E.A.; Jang, T.L.; Ghodoussipour, S.; et al. Genomic analysis and long-term outcomes of a phase 1 clinical trial on cytoreductive radical prostatectomy. Prostate Int. 2022, 10, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Knipper, S.; Beyer, B.; Mandel, P.; Tennstedt, P.; Tilki, D.; Steuber, T.; Graefen, M. Outcome of patients with newly diagnosed prostate cancer with low metastatic burden treated with radical prostatectomy: A comparison to STAMPEDE arm H. World J. Urol. 2020, 38, 1459–1464. [Google Scholar] [CrossRef]
- Lumen, N.; De Bleser, E.; Buelens, S.; Verla, W.; Poelaert, F.; Claeys, W.; Fonteyne, V.; Verbeke, S.; Villeirs, G.; De Man, K.; et al. The Role of Cytoreductive Radical Prostatectomy in the Treatment of Newly Diagnosed Low-volume Metastatic Prostate Cancer. Results from the Local Treatment of Metastatic Prostate Cancer (LoMP) Registry. Eur. Urol. Open Sci. 2021, 29, 68–76. [Google Scholar] [CrossRef]
- Mandel, P.C.; Huland, H.; Tiebel, A.; Haese, A.; Salomon, G.; Budäus, L.; Tilki, D.; Chun, F.; Heinzer, H.; Graefen, M.; et al. Enumeration and Changes in Circulating Tumor Cells and Their Prognostic Value in Patients Undergoing Cytoreductive Radical Prostatectomy for Oligometastatic Prostate Cancer-Translational Research Results from the Prospective ProMPT trial. Eur. Urol. Focus. 2021, 7, 55–62. [Google Scholar] [CrossRef]
- Mistretta, F.A.; Luzzago, S.; Conti, A.; Verri, E.; Marvaso, G.; Collà Ruvolo, C.; Catellani, M.; Di Trapani, E.; Cozzi, G.; Bianchi, R.; et al. Oligometastatic Prostate Cancer: A Comparison between Multimodality Treatment vs. Androgen Deprivation Therapy Alone. Cancers 2022, 14, 2313. [Google Scholar] [CrossRef]
- Moschini, M.; Morlacco, A.; Kwon, E.; Rangel, L.J.; Karnes, R.J. Treatment of M1a/M1b prostate cancer with or with out radical prostatectomy at diagnosis. Prostate Cancer Prostatic Dis. 2017, 20, 117–121. [Google Scholar] [CrossRef]
- Poelaert, F.; Verbaeys, C.; Rappe, B.; Kimpe, B.; Billiet, I.; Plancke, H.; Decaestecker, K.; Fonteyne, V.; Buelens, S.; Lumen, N. Cytoreductive Prostatectomy for Metastatic Prostate Cancer: First Lessons Learned from the Multicentric Prospective Local Treatment of Metastatic Prostate Cancer (LoMP) Trial. Urology 2017, 106, 146–152. [Google Scholar] [CrossRef]
- Sooriakumaran, P.; Wilson, C.; Rombach, I.; Hassanali, N.; Aning, J.; D Lamb, A.; Cathcart, P.; Eden, C.; Ahmad, I.; Rajan, P.; et al. Feasibility and safety of radical prostatectomy for oligo-metastatic prostate cancer: The Testing Radical prostatectomy in men with prostate cancer and oligo-Metastases to the bone (TRoMbone) trial. BJU Int. 2022, 130, 43–53. [Google Scholar] [CrossRef]
- Steuber, T.; Berg, K.D.; Røder, M.A.; Brasso, K.; Iversen, P.; Huland, H.; Tiebel, A.; Schlomm, T.; Haese, A.; Salomon, G.; et al. Does Cytoreductive Prostatectomy Really Have an Impact on Prognosis in Prostate Cancer Patients with Low-volume Bone Metastasis? Results from a Prospective Case-Control Study. Eur. Urol. Focus. 2017, 3, 646–649. [Google Scholar] [CrossRef] [PubMed]
- Takagi, K.; Kawase, M.; Kato, D.; Kawase, K.; Takai, M.; Iinuma, K.; Nakane, K.; Hagiwara, N.; Yamada, T.; Tomioka, M.; et al. Robot-Assisted Radical Prostatectomy for Potential Cancer Control in Patients with Metastatic Prostate Cancer. Curr. Oncol. 2022, 29, 2864–2870. [Google Scholar] [CrossRef] [PubMed]
- Xue, P.; Wu, Z.; Wang, K.; Gao, G.; Zhuang, M.; Yan, M. Oncological Outcome of Combining Cytoreductive Prostatectomy and Metastasis-Directed Radiotherapy in Patients with Prostate Cancer and Bone Oligometastases: A Retrospective Cohort Study. Cancer Manag. Res. 2020, 12, 8867–8873. [Google Scholar] [CrossRef] [PubMed]
- Gandaglia, G.; Fossati, N.; Stabile, A.; Bandini, M.; Rigatti, P.; Montorsi, F.; Briganti, A. Radical Prostatectomy in Men with Oligometastatic Prostate Cancer: Results of a Single-institution Series with Long-term Follow-up. Eur. Urol. 2017, 72, 289–292. [Google Scholar] [CrossRef]
- Mazzone, E.; Mistretta, F.A.; Knipper, S.; Tian, Z.; Larcher, A.; Widmer, H.; Zorn, K.; Capitanio, U.; Graefen, M.; Montorsi, F.; et al. Contemporary National Assessment of Robot-Assisted Surgery Rates and Total Hospital Charges for Major Surgical Uro-Oncological Procedures in the United States. J. Endourol. 2019, 33, 438–447. [Google Scholar] [CrossRef]
- Ploussard, G.; Grabia, A.; Beauval, J.B.; Barret, E.; Brureau, L.; Dariane, C.; Fiard, G.; Fromont, G.; Gauthé, M.; Mathieu, R.; et al. A 5-Year Contemporary Nationwide Evolution of the Radical Prostatectomy Landscape. Eur. Urol. Open Sci. 2021, 34, 1–4. [Google Scholar] [CrossRef]
- Novara, G.; Ficarra, V.; Rosen, R.C.; Artibani, W.; Costello, A.; Eastham, J.A.; Graefen, M.; Guazzoni, G.; Shariat, S.F.; Stolzenburg, J.U.; et al. Systematic review and meta-analysis of perioperative outcomes and complications after robot-assisted radical prostatectomy. Eur. Urol. 2012, 62, 431–452. [Google Scholar] [CrossRef]
- Won, A.C.; Gurney, H.; Marx, G.; De Souza, P.; Patel, M.I. Primary treatment of the prostate improves local palliation in men who ultimately develop castrate-resistant prostate cancer. BJU Int. 2013, 112, E250–E255. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).