Summary
Cardiovascular diseases are the main cause of sudden death (SD) in athletes. Preparticipation screening for sports eligibility is able to detect apparently healthy subjects who are carriers of concealed cardiac defects which may cause SD during the practice of sport. Effort is a trigger of cardiac arrest in subjects with diseases of the aorta, coronary arteries, myocardium, valves, conduction system and ion channels. In young athletes the incidence of SD is 3-fold that in non-athletes. Disqualification from sports activities in young subjects with hidden diseases removes the “trigger” and is lifesaving. In the last decade the incidence of SD has declined in the Veneto Region of Italy, chiefly due to identification and sports disqualification of young athletes affected by hypertrophic cardiomyopathy and arrhythmogenic right ventricular cardiomyopathy. Nowadays clinicians are well aware of these morbid entities, and their diagnosis is possible even in apparently healthy people by use of the ECG and, if requested, echo or possibly MRI and CT. Use of non-invasive diagnostic tools for imaging of the coronary artery tree, plus genetic screening for inherited cardiomyopathies, will in the future contribute to improving early diagnosis and enhance the effectiveness of SD prevention.
Introduction
That sport is a symbol of civilisation is unquestionable, the Olympic Games being a prime example. Sports activities are a source of stimulus and pleasure not only for those who practice them but also those who watch them. It promotes a feeling of well-being and is a counterweight to work and mental stress. Although physical activity prevents atherosclerosis by lowering LDL cholesterol and increasing HDL cholesterol, sudden cardiac death (SD) can strike down apparently healthy athletes, with the ensuing major media impact.
A recent example is the soccer player of Sevilla, Puerta, who fainted during a match and died soon after, arrhythmogenic right ventricular cardiomyopathy/dysplasia (ARVC/D) being found at postmortem.
The experience we have accumulated in the last 30 years on SD in the young and in athletes in the Veneto Region, Italy, shows that sport does not represent a risk in normal subjects; physical activity may unmask a concealed, potentially lethal cardiac anomaly by triggering life-threatening arrhythmias. In our recent epidemiological study on 300 subjects who died suddenly under the age of 35 (sudden infant death syndrome excluded), an SD rate of 1/100 000 young subjects/year was estimated [1]. Splitting the data between athletes and non-athletes, the rate was 0.9/100 000/ year in nonathletes and 2.3/100 000/year in athletes. Thus, the risk of cardiac SD in athletes is nearly 3-fold that in non-athletes.
Cardiac structures at risk of sudden death Aorta
The aorta may suddenly undergo spontaneous laceration during effort, caused by fragility of the tunica media due to disruption and loss of elastic fibres, typically in Marfan subjects. Marfan syndrome results from mutations of the fibrillin gene, a protein which plays a crucial role in the interaction between elastic fibres and smooth muscle cells of the media. The phenotypical expression may vary, from the classical stigmata (i.e., arachnodactyly, ectopia lentis, ligamentous laxity) to concealed disease confined to the cardiovascular system, with a dilated ascending aorta and aortic incompetence [2].
Aortic rupture is also present in patients with bicuspid aortic valves, with or without isthmal coarctation (Figure 1). The malformation may be hereditary, in contrast to the majority of congenital heart diseases. For thus far unknown reasons the tunica media of some subjects with a bicuspid aortic valve shows elastic abnormalities, similar to those in Marfan patients, even in the absence of fibrillin mutations [2]. The only reasonable explanation for the combined defects is that both the aortic valve and tunica media derive embryologically from the neural crest.
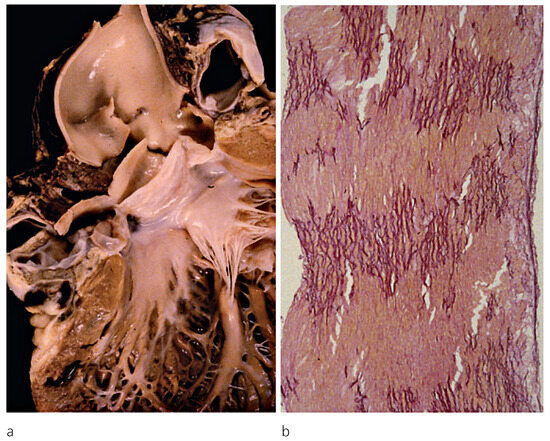
Figure 1.
a. Acute aortic dissection with external rupture and haemopericardiumin a 23-year-old male with normally functioning bicuspid aortic valve; b. Histology of the aortic wall with severe atrophy of elastic lamellae (Weiger Van Giesons stain × 60); from Basso et al. [2], modified.
Echocardiographic monitoring of ascending aorta dilatation in both the sinusal and tubular portions, which usually precedes aortic rupture, and of elastic properties, is useful for identification of subjects at risk [3,4].
Coronary arteries
Atherosclerosis, which usually affects adults and the elderly, may be accelerated in some young subjects, especially smokers, or in the presence of hypertension and/or high serum cholesterol levels. The pathology in the young usually consists of a single obstructive atherosclerotic plaque located in the proximal left anterior descending coronary artery [5]. The mechanism precipitating acute coronary occlusion resulting in ventricular fibrillation is rarely an occlusive coronary thrombosis, complicating plaque rupture or endothelial erosion [5]. More frequently coronary vasospasm is involved and occurs on a plaque rich in smooth muscle cells, and ventricular fibrillation is triggered by reperfusion following prolonged vasospastic occlusion [6].
Congenital coronary anomalies are also a risk factor for SD, particularly if both coronary arteries arise from the same sinus (right or left) [7]. The coronary artery originating from the wrong sinus exhibits an acute take-off and intramural aortic course with a slit-like lumen which has difficulty in coping with increased coronary blood flow demand during effort (Figure 2). Recurrent ischaemic episodes during sports activity may lead to myocardial injury and repair with fibrosis, as well as reperfusion injury with contraction band necrosis, all substrates for ventricular fibrillation. Unlike in adults and elderly people, overt myocardial infarction is quite a rare observation in coronary SD in the young. Ventricular fibrillation occurs early, and hence the availability of an external cardioverter defibrillator on site is crucial for resuscitation.
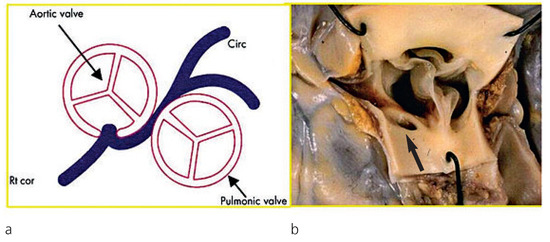
Figure 2.
Anomalous origin of the left coronary artery from the right aortic sinus. Note the proximal course of the left main trunk between the aorta and pulmonary artery (a) with an acute angle take-off (b) (arrow); from Basso et al. [7], modified.
Unfortunately, coronary atherosclerosis and congenital anomalies may be clinically silent both for angina and ECG abnormalities, thus furnishing a false negative result at preparticipation screening. The advent of non-invasive imaging of coronary arteries, such as magnetic resonance and computed tomography, opens up new avenues for more effective screening.
Myocardium
Cardiomyopathies are heart muscle diseases with mechanical or electrical dysfunction, in whose natural history SD is a frequent occurrence.
In hypertrophic cardiomyopathy (HCM), an inherited autosomal dominant disease with gene defects of the sarcomeric proteins (β-myosin heavy chain, myosinbinding protein C, tropomyosin, troponins), the arrhythmic substrate relies on asymmetric left ventricular hypertrophy with left ventricular outflow tract obstruction, histological disarrangement of the myocardium (“myocardial disarray”) (Figure 3) and ischaemic damage with fibrotic scars [8].
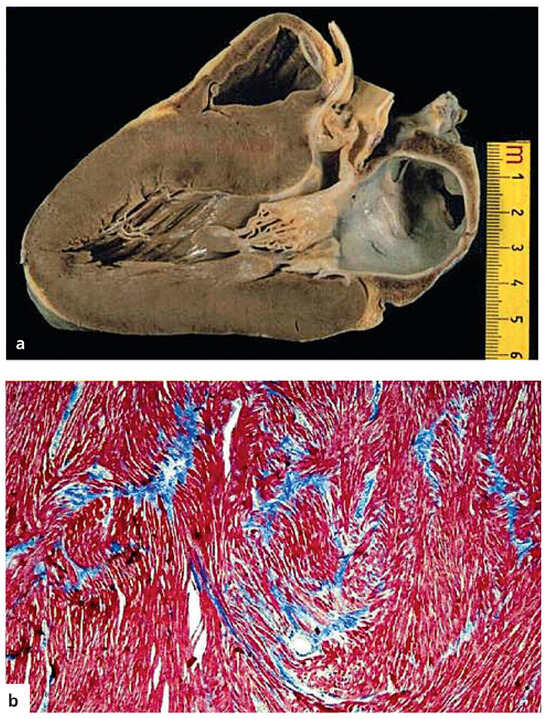
Figure 3.
Hypertrophic cardiomyopathy with sudden death in a 17-year-old boy. a. Long axis view with asymmetric septal hypertrophy and subaortic plaque; b. Myocardial disarray at histology (Azan stain ×120); from Basso et al. [17], modified.
All these substrates account for electrical instability carrying a risk of ventricular tachycardia or fibrillation. The disorder is easily diagnosed by 12-lead ECG and echo. 80% of patients with HCM exhibit ECG abnormalities [9]. In the USA, where neither ECG nor echo is performed in sports preparticipation screening, HCM is the most frequent cause of SD in athletes (up to 36%) [10,11]. In Italy, where ECG and, if this is positive, echo are compulsory at preparticipation screening, affected individuals are identified and disqualified. This may explain why in our experience HCM is a rare cause of SD in athletes [9].
ARVC/D, an inherited autosomal heart muscle disease, is associated with ventricular electrical instability [12,13] due to fibrofatty replacement (Figure 4).
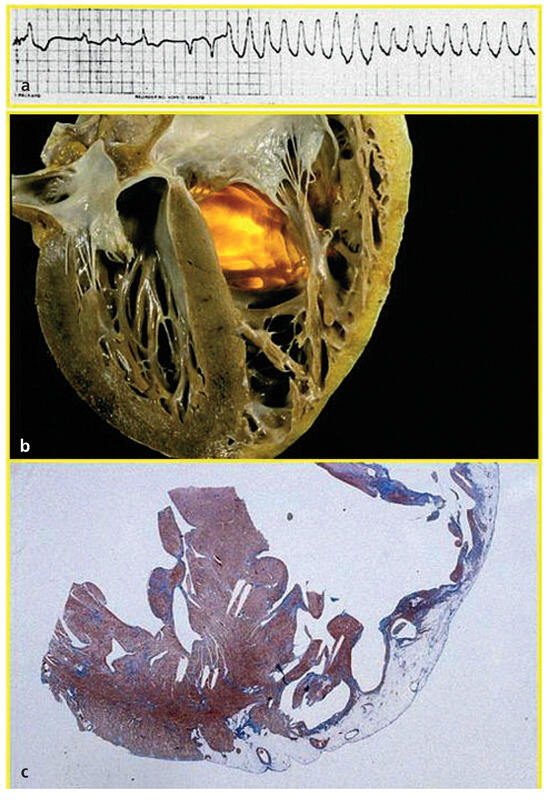
Figure 4.
Sudden death due to ARVC/D in a 26-year-old male. a Ventricular tachycardia in ECG; b Gross four chamber section of the heart with fibro-fatty replacement of the RV free wall; c Histology with fibro-fatty replacement confined to the RV, whereas septum and left ventricle appear spared (Azan stain ×1); from Marcus et al. [14], modified.
Myocardial atrophy of the RV accounts for both depolarisation–repolarisation abnormalities with inverted T waves in right precordial leads, late potentials with epsilon wave and ventricular arrhythmias with left bundle branch block morphology [14]. The RV is usually dilated and the free wall may show abnormalities, with aneurysms and diskinesia, quite pathognomonic of the disease and detectable by echo and magnetic resonance. Molecular studies have revealed that ARVC/D is a desmosomal disease due to gene defects of cell junction proteins (desmoplakin, plakoglobin, plakophillin, desmoglein, desmocollin) [15]. Recessive forms of ARVC/D (Naxos disease, Carvajal syndrome) are associated with palmo-plantar keratosis and woolly hair (cardiocutaneous syndromes) [16].
In Italy ARVC/D is the most frequent cause of SD in athletes [9] and the second among the young in general [17,18]. Widespread knowledge of the disease and its diagnostic criteria, with the use of ECG and echo at preparticipation screening for sport eligibility, has resulted in a substantial fall in SD occurrence among athletes with ARVC/D [19].
Inherited cardiomyopathies without morphological substrates exist and account for so-called “mors sine materia” (sudden unexplained death) observed in nearly 10–20% of SD cases. The defect is often limited to genetic mutations of ion channels (sodium, potassium and calcium), which control depolarisation–repolarisation of the cardiomyocyte and electromechanical coupling. Long and short QT syndromes are characterised by prolongation or shortening of repolarisation, usually due to mutations of potassium channel genes [20]. Brugada syndrome is characterised by non-ischaemic ST-segment elevation due to mutations of sodium channel gene 5A (SCN5A); the abnormalities are detectable in basal 12-lead ECG and thus affected subjects are potentially identifiable [21].
On the other hand, in catecholaminergic polymorphic ventricular tachycardia syndrome the ECG abnormalities are induced only by effort, when the threshold of 120–130 beats per minute is exceeded. The gene defect resides in cardiac ryanodine receptor 2 (RYR2), which controls the release of calcium from smooth sarcoplasmic reticulum for electromechanical coupling [22,23]. A feature of note is that the basal ECG is normal and the stress test may be false-negative. Genetic screening is crucial for identification of asymptomatic carriers [24].
Finally, inflammatory cardiomyopathy accounts for nearly 10% of SD in the young and athletes [17]. Myocarditis usually has an infective aetiology, due to cardiotropic viruses such as enterovirus (RNA virus) or adenovirus (DNA virus), and may be a complication of airway or gastrointestinal infections. When the myocardium is inflamed effort may trigger fatal arrhythmias even if mechanical performance is still preserved. Molecular biology techniques are nowadays available to detect microorganisms, both in vivo through endomyocardial biopsy or at postmortem [25], allowing an aetiological diagnosis (Figure 5).
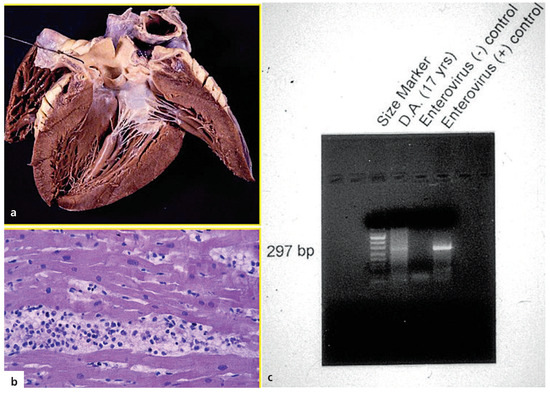
Figure 5.
Sudden death in a 17-year-old male during an influenza episode. A. Normal heart with naked eye; b. Inflammatory infiltrates at histology, in keeping with lymphocytic myocarditis (HE stain ×240); c. Enterovirus in molecular biology investigation; from Basso et al. [17], modified.
Valves
In aortic valve stenosis, which in the young is usually due to congenital valve defects, arrhythmias are related to subendocardial ischaemia, due to left ventricular hypertrophy and systolic overload. Supravalvular aortic stenosis, due to deletion of the elastic gene, is a rare cause of SD [26].
In mitral valve prolapse the electrical instability is most probably due to extracellular matrix abnormalities of the myocardium. Also, mitral valve prolapse may be inherited, and a gene defect has recently been discovered in filamin A (FLNA) [27].
Conduction system
The conduction system, which consists of specialised myocardium controlling the origin (sinus node) and transmission (AV node, His bundle and bundle branches) of electrical impulses, may also show abnormalities which are at risk of SD.
The first condition at risk is the Wolff-ParkinsonWhite (WPW) syndrome, a tiny congenital malformation characterised by abnormal connection of working myocardium between the atria and ventricles, outside the regular track.
The anomalous fascicle, 0.1–0.2 mm thick, does not possess the typical decremental properties of the AV conducting tissues. Thus, the electrical transmission travels quickly and preexcites the ventricle. If an episode of atrial fibrillation occurs, the impulse propagates to the ventricles without the usual delay, and thus atrial fibrillation may degenerate into ventricular fibrillation. Atrial myocarditis has been reported as a possible substrate triggering atrial fibrillation and cardiac arrest in these patients [28].
The disease is diagnosed by identifying in the ECG the short PQ and Δ wave of ventricular preexcitation, togetherwith supraventricular tachycardia due to ventriculo-atrial reentry. The aberrant fascicle is nowadays easily detected and ablated, and thus SD in WPW syndrome is progressively dying out.
Non-ischaemic, familial AV block (Lenègre’s disease) consists structurally of genetically determined fibrosis of the His bundle and bifurcation, due to gene defects of sodium channel (SCN5A) equal to those in Brugada syndrome, with which it may overlap. Complete AV block, with dissociation between atrial and ventricular electrical activities, may occur suddenly to account for asystole and abrupt cardiac arrest. In the majority of cases the complete AV block is preceded by progressive PQ interval prolongation in the ECG, 2–3:1 AV conduction or bundle branch block, with syncope or epileptic episodes due to transient cerebral ischaemia [29].
Main causes of sudden death in the young and athletes
According to the Veneto Region experience of SD in the young (≤35 years), coronary atherosclerosis represents the main cause of SD, followed by ARVC/D, myocarditis, congenital coronary artery anomalies, valve disease, HCM and pathology of the conduction system. This order is overturned in athletes, where ARVC/D comes first, followed by coronary atherosclerosis and congenital coronary artery anomalies.
Effort is an additional risk factor for SD, particularly in congenital coronary artery anomalies (79-fold), in ARVC/D (5.4-fold) and in coronary atherosclerosis (2.6-fold) (Figure 6).
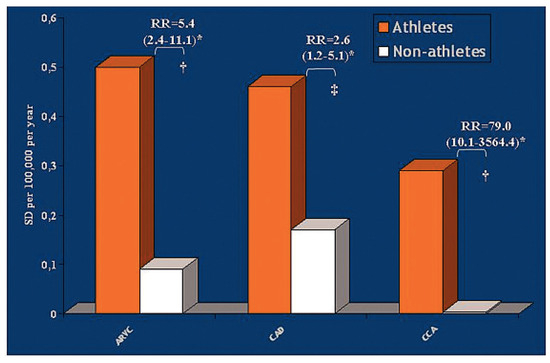
Figure 6.
Comparison of risk ratio (RR) between athletes and non-athletes in ARVC/D, atherosclerotic coronary artery disease (CAD) and congenital coronary artery anomalies (CCA); from Corrado et al. [1], modified. † p = .00001, † p = .009.
HCM, which represents the main cause of SD in athletes in the USA, is a rare cause in Italy since it is easily identified at preparticipation screening, with disqualification of the affected youth.
Is prevention of sudden death in athletes feasible?
There are a multiplicity of ways to reduce SD, by improving diagnosis and treatment and by implementing preventive measures.
One possibility is to intervene in the final step of cardiac arrest by using an external or implantable cardiac defibrillator to convert ventricular fibrillation to sinus rhythm. It is a lifesaving tool: the implantable cardiac defibrillator has proven highly effective both in patients with ARVC/D [30] and HCM [31] by resuscitating patients otherwise destined for certain death. At present its use is indicated for secondary prevention in patients who have experienced previous episodes of syncope, cardiac arrest or ventricular fibrillation.
The presence of an external defibrillator on the spot in the sports arena, with an electric shock delivered to the chest in the event of cardiac arrest, may represent a lifesaving tool if promptly employed (within 2–3 minutes).
For those affected by impending AV block, implantation of a pacemaker is warranted.
For those suffering from monomorphic recurrent ventricular tachycardia, radiofrequency ablation of the arrhythmic focus may be effective, but recurrences are frequently reported due to spontaneous progression of the underlying disease [32].
Antiarrhythmic drug therapy is also available, in particular β-blockers, sotalol and amiodarone [32], but its efficacy in preventing SD is questioned [33].
All the above-mentioned measures are of course palliative. The radical cure will be available when it is possible to intervene on the aetiology or aetiopathogenetic mechanisms of phenotypic disease expression and progression. Gene therapy is still far away and preimplantation diagnosis in the embryo is also under consideration for inherited cardiovascular disease. Genetic counselling, on disease transmission and family planning with birth control, are available options for parents.
Returning to the context of sport, lifestyle may be an effective preventive measure. Taking into account the danger that effort may precipitate life-threatening arrhythmias, the goal is to identify apparently healthy athletes who are carriers of concealed, risky cardiac defects and disqualify them from sports activities. In a study we published in the New England Journal of Medicine in 1998 [34], identification by ECG and echo, at the time of preparticipation screening, of 22 patients affected by HCM resulted in disqualification and longterm survival for a mean of 7 years. In other words, it has been proven that non-eligibility from competitive sport is “lifesaving” simply by avoiding sports activities.
In 1982 a law was introduced in Italy to protect individuals against the risk of SD during sports activities [35]. Under its terms, preparticipation clinical screening is compulsory as a prerequisite for sports eligibility. As far as the cardiovascular system is concerned, the check consists of personal and family history, clinical examination with blood pressure measurement and instrumental investigations, with basal and step test ECG. If the results are doubtful, 2-D echo is mandatory.
This protocol of investigation is quite different from that employed in USA, which involves only history and a clinical investigation, usually conducted by nurses [36].
The efficacy of the Italian programme has recently been proven [19]. Since 1979, an epidemiological and pathological study of all SD in the young (12 to 35 years of age) of the Veneto Region has been in progress. A parallel study was conducted among athletes who underwent preparticipation screening at Padua Sports Medicine Centre in the period 1982–2004. During the same period, 55 SD occurred in athletes and 265 in non-athletes. An 89% decrease of SD in athletes was observed (from 3.6/100 000/year in 1979–80, before implementation of the law, to 0.4/100 000/year in 2003– 2004, p <0.001) whereas the rate of SD in non-athletes did not change (0.9/100 000/year) (Figure 7).
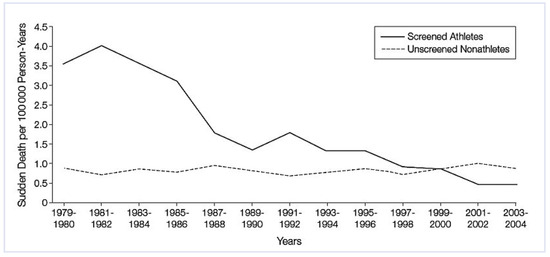
Figure 7.
Sharp decline of SD in athletes in the Veneto Region from 1979 (before the implementation of preparticipation screening) to 2002. Note, in contrast, the stability of the rate of SD in non-athletes; from Corrado et al. [19], modified.
The reduced mortality may be ascribed to the rise in the number of identified patients with cardiomyopathies (including ARVC/D) who were disqualified. The number of disqualified athletes with cardiomyopathies increased from 20 in the period 1982–1992 (4.4% of all disqualifications) to 40 in the period 1993– 2004 (9.4%). Contemporarily, SD due to cardiomyopathies decreased from 1.5/100 000/year, before the preparticipation screening era, to 0.15/100 000/year in 2002–2004 (p <0.002).
Thus, the rate of SD in young competitive athletes has fallen sharply in the Veneto Region since the introduction of the preparticipation screening programme, a system which has revealed itself to be truly lifesaving. The reduced mortality has been chiefly ascribable to a net decrease in the number of deaths from cardiomyopathies, which was parallel to the increase in the identification of cardiomyopathy carriers at the time of eligibility screening.
This indisputable benefit is such that a similar strategy should be implemented elsewhere, i.e., in other European countries and North America, where this type of preparticipation screening is not carried out yet [37]. However, this raises major financial questions. According to our study, to save 3 young athletes/100 000/ year, nearly 100 000 screenings should be performed, including ECG in all and echo in nearly 9000. In a health system based on insurances, the cost/benefit ratio of 100 000 visits vs 3 saved lives is considered to be prohibitive.
Future perspectives
There are still diseases which escape identification at preparticipation screening. Coronary artery diseases, both congenital and acquired, frequently present with a negative ECG even during stress testing. They represent the “bugbear” in the prevention of arrhythmic risk during sport activity. Only the use of non-invasive imaging, such as cardiac MRI and computed tomography, may help to identify patients at risk.
The use of genetic screening is the next goal. There are asymptomatic carriers of inherited cardiovascular diseases not detected with the standard screening for sports eligibility, probably because of low penetrance or late phenotypic expression of the gene defect. Unfortunately, SD may be the first manifestation of the disease. Only systematic genetic screening of families may help to identify gene carriers at the pre-symptomatic stage.
Several disease-causing genes are now known and thus available for mutation molecular analysis, which may become a complement to clinical preparticipation screening in selected cases.
References
- Corrado, D.; Basso, C.; Rizzoli, G.; Schiavon, M.; Thiene, G. Does sports activity enhance the risk of sudden death in adolescents and young adults? J. Am. Coll. Cardiol. 2003, 42, 1959–1963. [Google Scholar] [CrossRef]
- Basso, C.; Frescura, C.; Corrado, D.; Muriago, M.; Angelini, A.; Daliento, L.; et al. Congenital heart disease and sudden death in the young. Hum. Pathos. 1995, 26, 1065–1072. [Google Scholar] [CrossRef]
- Nistri, S.; Sorbo, M.D.; Marin, M.; Palisi, M.; Scognamiglio, R.; Thiene, G. Aortic root dilatation in young men with normally functioning bicuspid aortic valves. Heart 1999, 82, 19–22. [Google Scholar] [CrossRef]
- Nistri, S.; Grande-Allen, J.; Noale, M.; Basso, C.; Siviero, P.; Maggi, S.; et al. Aortic elasticity and size in bicuspid aortic valve syndrome. Eur. Heart J. 2008, 29, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Corrado, D.; Basso, C.; Poletti, A.; Angelini, A.; Valente, M.; Thiene, G. Sudden death in the young: is coronary thrombosis the major precipitating factor? Circulation. 1994, 90, 2315–2323. [Google Scholar] [CrossRef] [PubMed]
- Corrado, D.; Thiene, G.; Buja, G.F.; Pantaleoni, A.; Maiolino, P. The relationship between growth of atherosclerotic plaques, variant angina and sudden death. Int. J. Cardiol. 1990, 26, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Basso, C.; Maron, B.J.; Corrado, D.; Thiene, G. Clinical profile of congenital coronary artery anomalies with origin from the wrong aortic sinus leading to sudden death in young competitive athletes. J. Am. Coll. Cardiol. 2000, 35, 1493–1501. [Google Scholar] [CrossRef]
- Basso, C.; Thiene, G.; Corrado, D.; Buja, G.; Melacini, P.; Nava, A. Hypertrophic cardiomyopathy: pathologic evidence of ischemic damage in young sudden death victims. Hum. Pathol. 2000, 31, 988–998. [Google Scholar] [CrossRef]
- Corrado, D.; Thiene, G.; Nava, A.; Rossi, L. Sudden death in young competitive athletes: clinicopathologic correlations in 22 cases. Am. J. Med. 1990, 89, 588–596. [Google Scholar] [CrossRef]
- Maron, B.J.; Shirani, J.; Poliac, L.C.; Mathenge, R.; Roberts, W.C.; Mueller, F.O. Sudden death in young competitive athletes. Clinical, demographic, and pathological profiles. JAMA. 1996, 276, 199–204. [Google Scholar] [CrossRef]
- Maron, B.J. Sudden death in young athletes. N. Engl. J. Med. 2003, 349, 1064–1075. [Google Scholar] [CrossRef] [PubMed]
- Thiene, G.; Nava, A.; Corrado, D.; Rossi, L.; Pennelli, N. Right ventricular cardiomyopathy and sudden death in young people. N. Engl. J. Med. 1988, 318, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Basso, C.; Thiene, G.; Corrado, D.; Angelini, A.; Nava, A.; Valente, M. Arrhythmogenic right ventricular cardiomyopathy: dysplasia, dystrophy, or myocarditis? Circulation 1996, 94, 983–991. [Google Scholar] [CrossRef] [PubMed]
- Marcus, F.I.; Nava, A.; Thiene, G. Arrhythmogenic RV cardiomyopathy/dysplasia: recent advances; Springer: Milano, Italy, 2007. [Google Scholar]
- Rampazzo, A.; Danieli, G.A. Advances in genetics: dominant forms. In Arrhythmogenic RV cardiomyopathy/dysplasia – Recent advances; Marcus, F.I., Nava, A., Thiene, G., Eds.; Springer: Milano, Italy, 2007; pp. 7–14. [Google Scholar]
- Protonotarios, N.; Tsatsopoulou, A. Advances in genetics: recessive forms. In Arrhythmogenic RV cardiomyopathy/dysplasia: recent advances; Marcus, F.I., Nava, A., Thiene, G., Eds.; Springer: Milano, Italy, 2007; pp. 17–20. [Google Scholar]
- Basso, C.; Calabrese, F.; Corrado, D.; Thiene, G. Postmortem diagnosis in sudden cardiac death victims: macroscopic, microscopic and molecular findings. Cardiovasc. Res. 2001, 50, 290–300. [Google Scholar] [CrossRef]
- Thiene, G.; Basso, C.; Corrado, D. Cardiovascular causes of sudden death. In Cardiovascular pathology, 3rd ed.; Silver, M.D., Gotlieb, A.I., Schoen, F.J., Eds.; Churchill Livingstone: Philadelphia, PA, USA, 2001; pp. 326–74. [Google Scholar]
- Corrado, D.; Basso, C.; Pavei, A.; Michieli, P.; Schiavon, M.; Thiene, G. Trends in sudden cardiovascular death in young competitive athletes after implementation of a preparticipation screening program. JAMA 2006, 296, 1593–1601. [Google Scholar] [CrossRef]
- Crotti, L.; Celano, G.; Dagradi, F.; Schwartz, P.J. Congenital long QT syndrome. Orphanet J. Rare Dis. 2008, 3, 18. [Google Scholar] [CrossRef]
- Napolitano, C.; Priori, S.G. Brugada syndrome. Orphanet J. Rare Dis. 2006, 1, 35. [Google Scholar] [CrossRef]
- Tiso, N.; Stephan, D.A.; Nava, A.; Bagattin, A.; Devaney, J.M.; Stanchi, F.; et al. Identification of mutations in the cardiac ryanodine receptor gene in families affected with arrhythmogenic right ventricular cardiomyopathy type 2 (ARVD2). Hum. Mol. Gen. 2001, 10, 189–194. [Google Scholar] [CrossRef]
- Priori, S.; Napolitano, C.; Tiso, N.; Memmi, M.; Vignati, G.; Bloise, R.; et al. Mutations in the cardiac ryanodine receptor gene (hRyR2) underlie catecholaminergic polymorphic ventricular tachicardia. Circulation 2001, 103, 196–200. [Google Scholar] [CrossRef]
- Bauce, B.; Rampazzo, A.; Basso, C.; Bagattin, A.; Daliento, L.; Tiso, N.; et al. Screening for ryanodine receptor type 2 mutations in families with effort-induced polymorphic ventricular arrhythmias and sudden death: early diagnosis of asymptomatic carriers. J. Am. Coll. Cardiol. 2002, 40, 341–349. [Google Scholar] [CrossRef]
- Calabrese, F.; Thiene, G. Myocarditis and inflammatory cardiomyopathy: microbiological and molecular biological aspects. Cardiovasc. Res. 2003, 60, 11–25. [Google Scholar] [CrossRef]
- Thiene, G.; Ho, S.Y. Aortic root pathology and sudden death in youth: review of anatomical varieties. Appl. Pathol. 1986, 4, 237–245. [Google Scholar]
- Kyndt, F.; Gueffet, J.P.; Probst, V.; Jaafar, P.; Legendre, A.; Le Bouffant, F.; et al. Mutations in the gene encoding filamin A as a cause for familial cardiac valvular dystrophy. Circulation 2007, 115, 40–49. [Google Scholar] [CrossRef]
- Basso, C.; Corrado, D.; Rossi, L.; Thiene, G. Ventricular preexcitation in children and young adults: atrial myocarditis as a possible trigger of sudden death. Circulation 2001, 103, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Probst, V.; Kyndt, F.; Allouis, M.; Schott, J.J.; Le Marec, H. Genetic aspects of cardiac conduction defects. Arch. Mal. Coeur Vaiss. 2003, 96, 1067–1073. [Google Scholar] [PubMed]
- Corrado, D.; Leoni, L.; Link, M.S.; Della Bella, P.; Gaita, F.; Curnis, A.; et al. Implantable cardioverter-defibrillator therapy for prevention of sudden death in patients with arrhythmogenic right ventricular cardiomyopathy. Circulation 2003, 108, 3084–3091. [Google Scholar] [CrossRef] [PubMed]
- Maron, B.J.; Spirito, P.; Shen, W.K.; Haas, T.S.; Formisano, F.; Link, M.S.; et al. Implantable cardioverter-defibrillators and prevention of sudden cardiac death in hypertrophic cardiomyopathy. JAMA 2007, 298, 405–412. [Google Scholar] [CrossRef]
- Wichter, T.; Paul, T.M.; Eckardt, L.; Gerdes, P.; Kirchof, P.; Bocker, D.; et al. Arrhythmogenic right ventricular cardiomyopathy. Antiarrhythmic drugs, catheter ablation, or ICD. Herz 2005, 30, 91–101. [Google Scholar] [CrossRef]
- Melacini, P.; Maron, B.J.; Bobbo, F.; Basso, C.; Tokajuk, B.; Zucchetto, M.; et al. Evidence that pharmacological strategies lack efficacy for the prevention of sudden death in hypertrophic cardiomyopathy. Heart 2007, 93, 708–710. [Google Scholar] [CrossRef]
- Corrado, D.; Basso, C.; Schiavon, M.; Thiene, G. Screening for hypertrophic cardiomyopathy in young athletes. N. Engl. J. Med. 1998, 339, 364–369. [Google Scholar] [CrossRef]
- Decree of the Italian Ministry of Health, February 18, 1982. Norme per la tutela sanitaria dell’attività sportiva agonistica [rules concerning the medical protection of athletic activity]. Gazzetta Ufficiale della Repubblica Italiana, 5 March 1982; 63. [Google Scholar]
- Maron, B.J.; Thompson, P.D.; Puffer, J.C.; McGrew, C.A.; Strong, W.B.; Douglas, P.S.; et al. Cardiovascular preparticipation screening of competitive athletes: a statement for health professionals from the Sudden Death Committee (clinical cardiology) and Congenital Cardiac Defects Committee (cardiovascular disease in the young), American Heart Association. Circulation. 1996, 94, 850–856. [Google Scholar] [PubMed]
- Corrado, D.; Pelliccia, A.; Bjørnstad, H.H.; Vanhees, L.; Biffi, A.; Borjesson, M.; et al. Cardiovascular preparticipation screening of young competitive athletes for prevention of sudden death: proposal for a common European protocol. Consensus Statement of the Study Group of Sport Cardiology of the Working Group of Cardiac Rehabilitation and Exercise Physiology and the Working Group of Myocardial and Pericardial Diseases of the European Society of Cardiology. Eur. Heart J. 2005, 26, 516–524. [Google Scholar]
© 2009 by the author. Attribution - Non-Commercial - NoDerivatives 4.0