Abstract
An unprecedented increase in human life expectancy has taken place over past centuries, thus raising the number of elderly people globally. Despite advances in medicine, cardiovascular disease remains the major cause of mortality and morbidity among elderly people and poses a great burden to healthcare services. As aging is inevitable and represents a risk factor for cardiovascular disease, a better understanding of its underlying mechanism will be crucial to designing tailored therapeutic options. Indeed, the dysregulation of aging and longevity genes has been implicated in lifespan by regulating numerous cellular processes that alter both structural and functional aspects of the cardiovascular system. In this article, several lifespan-determining genes are reviewed, in particular for their involvement in regulating cardiovascular health via inflammation and oxidative stress. Lastly, several promising prevention and treatment approaches such as an antiaging and age-associated cardiovascular disease intervention are also described.
Introduction
Aging is the most important determinant of cardiovascular health and represents the main risk factor for cardiovascular disease [1]. Owing to advances in current medical knowledge, treatment, and technology, average human lifespan is increasing. According to the World Health Organization, the proportion of the worldwide population over 60 years of age will grow exponentially from 12% in 2015 to 22% in 2050. This implies that the number of aged individuals is expected to increase substantially, as well as the number of aging-related diseases, such as diabetes, and neurodegenerative and cardiovascular diseases [1,2].
Aging is a fundamental human biological process that leads to a decline in biological function and increases the risk of many diseases. Over the last decade, various aging studies have demonstrated the role of genes governing the aging process. Indeed, several genes have been recognised to regulate molecular mechanisms in aging, thus determining lifespan [3]. Some of these genes are also critically involved in the development of cardiovascular diseases, since the latter are mediated by similar molecular mechanisms to aging. Those mechanisms often involve increased oxidative stress, chronic systemic inflammation and metabolic dysfunction [3,4].
This review article summarises the implication of genes regulating lifespan (i.e., longevity and aging genes) in age-dependent cardiovascular disease via fundamental molecular mechanisms of aging. The importance of these genes in cardiovascular disease will be drawn from previous animal and clinical studies. Finally, their potential as therapeutic targets for future intervention is also discussed.
Aging in the cardiovascular system
Cardiovascular aging is defined as an age-dependent progressive degeneration of the cardiovascular system resulting in a stress-susceptible heart and blood vessels [5,6]. Several complex modifications occur in the aged heart and vasculature, referred to as cardiovascular aging phenotype, that cause the development of age-associated cardiovascular disease. In the aging heart, aging involves not only cardiomyocytes, but also interstitial fibroblast and vascular cells, which will lead to chronic heart remodeling [5]. Meanwhile, arterial wall thickening and vascular stiffness also take place in the vasculature during the aging process. Vascular aging is characterised by molecular, structural and physiological changes of the blood vessel, in which vascular inflammation, oxidative stress and endothelial dysfunction promote vascular aging [7]. Consequently, the changes observed in the heart and vasculature contribute to the increased risk of promoting cardiovascular disease, including heart failure, cardiac fibrosis, and stroke.
Molecular aspects of cardiovascular aging
Telomere shortening
Telomere shortening occurring with age is one of the hallmarks of molecular aging. Known as the biological clock, telomeres are repetitive regions of nucleotide sequences (TTAGGG) capping the end of chromosomes [8]. They promote stability during cell replication by repairing and protecting the genome from nucleolytic degradation and interchromosomal fusion. Nonetheless, telomeres shorten at each DNA replication up to a critical length that leads to genomic instability and induces chromosomal degradation, senescence and cell death (Figure 1) [9].
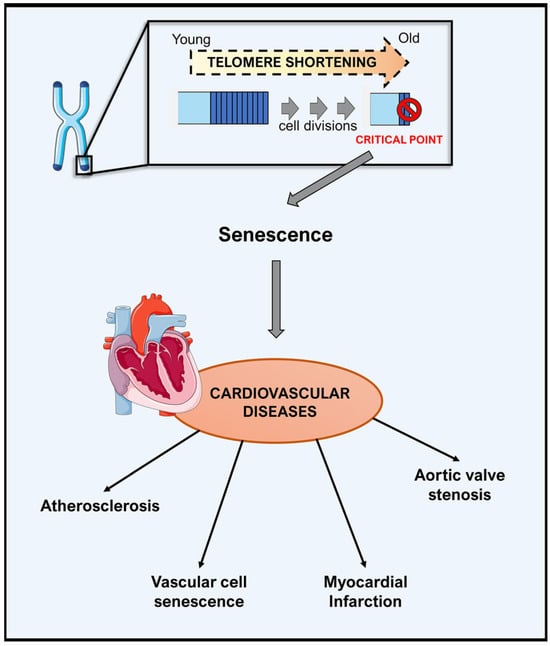
Figure 1.
Telomere shortening in aging and cardiovascular disease. Telomeres shorten with each round of cell division. Critically short telomeres lead to cellular senescence and dysfunction, contributing to reducing regenerative capacity in the cardiovascular system. As a consequence, it promotes cardiovascular aging and the development of cardiovascular diseases.
Telomerase is the enzyme responsible for telomere maintenance and regulation by preserving telomere length. It synthesises new telomeric DNAs to compensate for telomere loss during each cell division. Somatic cells have low or undetectable levels of telomerase activity, resulting in progressive telomere shortening and limited longevity. On the other hand, telomerase is highly expressed in cancers where tumour cells reactivate its activity to avoid replicative senescence and gain immortality [10].
Since telomere shortening is proposed as one of the causes of physiological aging, its relevance in cardiovascular disease has been investigated in detail. In humans, telomere length is measured systemically in circulating lymphocytes, referred to as leucocyte telomere length, which associates with vascular cell senescence, atherosclerosis, aortic valve stenosis and myocardial infarction [6,11]. To date, several hypotheses have been postulated, based on numerous animal and epidemiological studies, to describe the link between telomere attrition and cardiovascular disease. The first hypothesis defines the causal role of telomere length in cardiovascular disease. For instance, inherited shorter telomeres may lead to atherosclerosis, and telomere attrition, either local (atherosclerotic lesions) or systemic, which determines the onset of cardiovascular disease. On the other hand, telomere shortening has also been proposed as the consequence of atherosclerosis and cardiovascular disease, and thus may act as a biomarker [12]. Despite these hypotheses, epidemiological studies show a small effect size and the underlying mechanism remains unclear. Therefore, further investigations are required to address and verify the identified mechanistic insights.
Reactive oxygen species and oxidative stress
The free radical theory of aging was proposed in 1956 by Dr D Harman. Since then, it has become the most widely accepted theory to account for the mechanisms of aging in which reactive oxygen species (ROS) play a crucial role as the driving force of aging and determining lifespan [13]. According to this theory, ROS are produced during fundamental metabolic processes and accumulate with age, thus leading to oxidative damage in cellular lipids, proteins and DNA. In addition, previous aging studies reported an increase in ROS production by mitochondria and increased damage to mitochondrial DNA by ROS. This finding confirms the contribution of a progressive accumulation of oxidative DNA damage in the aging process [14].
Several enzymatic sources of superoxide have been recognised, including NADPH oxidase (Nox), mitochondria, cytochrome p450 and uncoupled endothelial nitric oxide synthase (eNOS) [15]. Meanwhile, in physiological conditions levels of intracellular ROS are kept low by enzymatic and nonenzymatic antioxidants. To date, oxidative stress is considered a key player in the development of cardiovascular pathologies. For instance, Nox has been associated with vascular diseases, shown by Nox-1 overexpression in mice that develop increased blood pressure in response to angiotensin II [16]. Superoxide anions (O2 -) generated by Nox will further promote eNOS uncoupling, thus causing blunted nitric oxide (NO) bioavailability, as well as increasing levels of ROS [16]. This process is observed in human endothelial dysfunction and represents a characteristic feature of vascular aging. Furthermore, the production of total antioxidants (i.e., superoxide dismutase, catalase and glutathione) is decreased in aging, resulting in inadequate defense against the accumulated oxidants. Of note, the accumulated O2 when binding to NO generates an additional toxic species, peroxynitrite (ONOO-), thus causing disruption of mitochondrial activity and lipoprotein oxidation. Consequently, ROS accumulation in the cardiovascular system may lead to age-related arterial oxidative stress (Figure 2) [4,17,18].
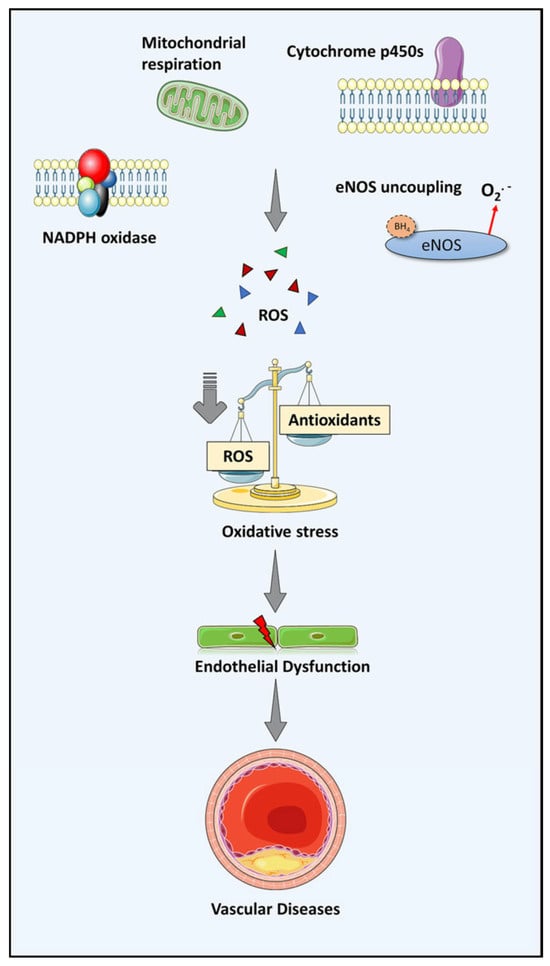
Figure 2.
Oxidative stress as the source of vascular disease via endothelial dysfunction. The level of ROS in the vascular cell is well maintained in physiological conditions. Nevertheless, an imbalance between oxidant and anti-oxidant production occurs in aging due to increased ROS production and less generated anti-oxidants. Consequently, endothelial dysfunction arises due to vascular oxidative stress and further leads to vascular diseases.
Chronic low-grade inflammation: inflammaging
Inflammation is a crucial defense system of all living organisms against harmful agents, such as pathogens, allergens and toxins. Acute responses of the immune system enable the repair of damaged tissue and pathogen elimination to maintain body homeostasis [19]. However, chronic inflammation is usually a deleterious process. During aging, chronic inflammation typically manifests in a low-grade manner for a prolonged period of time, a phenomenon known as “inflammaging”. [20]
Increased levels of circulating markers of inflammation is a characteristic of age-related sterile inflammation. Increased circulating C-reactive protein (CRP) and various inflammatory cytokines (i.e., tumour necrosis factor-α, interleukin (IL)-6 and vascular cell adhesion molecule-1) have been previously reported [17]. At the molecular level, nuclear factor (NF)-κB is a crucial factor in the inflammatory process. This transcription factor upregulates the expression of proinflammatory genes, chemokines and adhesion molecules, which have been linked with diverse pathophysiological conditions such as atherosclerosis [21]. Interestingly, activation of the proinflammatory transcription factor NF-κB can also be triggered by increased ROS production, implying an important link between ROS and inflammation in aging [4]. Additionally, senescent cells exhibit a senescence-associated secretory phenotype (SASP), including the release of a wide range of inflammatory cytokines, chemokines, growth factors and proteases [22,23]. By acting in a paracrine manner, the released secretory molecules facilitate further cellular senescence, thus contributing to the fueling of inflammaging and age-related pathologies (Figure 3) [24,25].
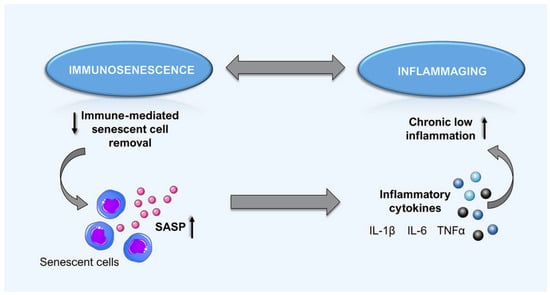
Figure 3.
The vicious circle of immune alteration in aging. Deleterious alteration of immune response occurs in aged individuals. A reduction of functional immunity cells occurs (immunosenescence), which contributes to ineffective senescent cell removal. This counterproductive condition causes an accumulation of senescent cells and eventually promotes systemic manifestation of senescence-associated secretory phenotypes (SASP), including the release of various inflammatory cytokines. Consequently, chronic inflammation due to the SASP accumulation further promotes cellular senescence and age-associated pathology.
The involvement of a chronic inflammatory state was investigated in various age-related diseases, such as atherosclerosis and hypertension. In such settings, the inflammatory process plays a deleterious role in promoting the thrombotic complications of atherosclerosis, which underlie acute stroke and myocardial infarction [4,26]. Indeed, such inflammatory markers, in particular CRP, have become a predictive biomarker of cardiovascular disease. CRP and IL-6 have also been correlated with impairment of endothelial-dependent dilatation and stiffness of large arteries [27,28]. Meanwhile, IL-1β activity has been a crucial factor in the development of atherosclerosis as its signaling pathway determines atheroma formation [29]. Notably, the CANTOS trial showed a significant reduction of recurrent cardiovascular events upon treatment with a human monoclonal anti-IL-1β antibody, canakinumab, in patients with previous myocardial infarction and increased systemic inflammation [30].
Lifespan regulating genes: implications on the cardiovascular aging
Adaptor protein p66shc
The adaptor protein p66shc is a key regulator of aging encoded by the SHC1 gene along with the other two isoforms, p46shc and p52shc [3,8]. Despite their identical molecular structures, these three isoforms share functional domains yet exert different functions. Compared with p46shc and p52shc, p66shc protein has an additional collagen-homology domain (CH2) containing an important phosphorylation site, serine 36 (S36). Once phosphorylated at S36, p66shc protein translocates from the cytosol to the mitochondria, mediating the release of cytochrome C into the cytoplasm, increased ROS production and, eventually, cell death via apoptosis [31]. Previous studies suggested the implication of p66shc in aging, as well as in regulating cellular redox state. p66shc-/- cells display a reduction in intracellular free radicals [32]. At the same time, deletion of p66shc in mice results in a 30% prolonged life span, increased resistance to oxidative stress-induced apoptosis and decreased production of intracellular oxidants [33].
Increased ROS production taking place in aging vessels leads to decreased NO bioavailability and impaired endothelial function [17]. Initially described for its role in regulating the cellular redox state, p66shc mitochondrial protein was considered to partake in age-dependent endothelial dysfunction. In fact, mice lacking p66shc are protected against endothelial dysfunction by means of a preserved endothelial NO bioavailability and decreased ROS production [3]. Higher oxidative stress via p66shc activation is also induced by several risk factors. High levels of blood glucose were shown to promote phosphorylation of p66shc at S36, thus leading to increased ROS formation. The link between this protein and hyperglycaemia in the context of vascular function is also supported by evidence from in vivo studies. The diabetic mouse model revealed a reduction in aortic NO bioavailability mediated by p66shc activation, whereas p66shc gene silencing restores endothelium-dependent relaxation, and suppresses ROS production and apoptosis [32,34].
Notably, p66shc knockout mice are also protected against age-associated endothelial dysfunction of the basilar artery, indicating the relevance of this protein for cerebrovascular disease also [35]. In a recent study, we showed that p66shc-/- mice display blunted cerebral ROS production and a reduction in stroke size following ischaemia-reperfusion brain injury [6,35,36]. Also, in a clinically relevant experimental setup whereby p66shc gene silencing was performed post-ischaemically to mimic a more relevant clinical setup, we showed an improved stroke outcome in mice, mainly due to a preserved blood-brain barrier integrity. In line with the above, we also described increased p66shc gene expression in acute ischaemic stroke patients, where the expression correlated with the patient’s short-term neurological outcome [36].
Besides protection against aging and age-dependent endothelial dysfunction, a role for p66shc in the context of atherosclerotic heart disease was also suggested, specifically in lesion formation and progression. Indeed, a lower rate of early atherogenesis, including decreased accumulation of intimal foam cells, arterial oxidised low-density lipoprotein (LDL) and systemic oxidative stress, was observed in mice lacking p66shc [37,38]. Multiple clinical studies have also shown the involvement of this protein in coronary artery disease. In fact, coronary heart disease patients showed a significant increase of p66shc mRNA levels in peripheral blood mononuclear cells [39]. p66shc transcript levels are also higher in acute coronary syndrome patients as compared with patients with stable coronary artery disease and healthy controls, thus indicating a potential role for p66shc in myocardial infarction [40]. Nevertheless, the detrimental role of this gene in myocardial infarction is still controversial. Indeed, a recent study in which transient ligation of the left anterior coronary artery was performed in p66shc knockout mice reported larger myocardial injuries following a blunted activation of protective pathways, such as Akt and Stat3 [3].
Experimental and clinical data altogether strongly implicate the aging gene p66shc in the development of the age-dependent cardiovascular pathologies, highlighting its potential as a valuable therapeutic target in the setting of age-related cardiovascular disease.
Target of rapamycin/rapamycin
The target of rapamycin (TOR) is an evolutionarily conserved serine/threonine kinase that belongs to the phosphoinositide kinase-related family. It governs cell cycle progression and cell growth by integrating various nutrients and growth factor signals [41]. TOR (mTOR in mammals) is a catalytic subunit of two distinct complexes, namely mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2). These two protein complexes have different protein partners and substrates, and differ functionally in regulation of downstream processes and in their sensitivity to rapamycin. For instance, mTORC1 is involved in cell growth, whereas mTORC2, which is less sensitive to rapamycin, plays a crucial role in cell architecture by regulating the actin cytoskeleton [41,42,43]. Given its multiple functions, previous observations implicated mTOR in various aging-associated processes. Increased mTOR activity correlates with senescence and autophagy deficiency, and mTORC1 inhibition results in a prolonged lifespan. Hence, dysregulation of mTOR signaling may lead to the development of several pathologies, including age-dependent cardiovascular diseases [3,44].
The mTOR complex is an essential factor in the embryonic development of the cardiovascular system as well as postnatal cardiovascular function. Systemic deletion of mTOR in mice results in a high rate of embryonic lethality, with multiple cardiac and vascular integrity disruptions. Similarly, adult mice with a cardiac-specific mTOR deletion suffer from fatal cardiac dilation and heart failure [44]. On the other hand, available evidence suggests cardioprotective effects of selective and partial inhibition of mTORC1 in aging, via activation of autophagy, and reduction in misfolded protein accumulation and energy expenditure. Pharmacological mTORC1 inhibition with rapamycin decreases heart-specific inflammation and fibrosis, leading to a reduction in age-related cardiac abnormalities, including cardiac hypertrophy and systolic dysfunction [44,45].
In aging, vascular inflammation, endothelial dysfunction and oxidative stress are closely connected and accelerate the vascular aging process. In a recent study, Reho et al. assessed the direct involvement of mTORC1 signaling in regulating vascular endothelial function. They showed that an increment of mTORC1 activity in the vasculature contributes to reducing endothelial-mediated vasorelaxation by escalating ROS signaling [46]. Likewise, long-term mTOR inhibition with rapamycin in mice enhances vasodilatory effects via a specific endothelial-dependent mechanism [47]. In addition to its protective properties in the setting of endothelial function, a beneficial effect of rapamycin on arterial stiffness was also suggested, since rapamycin-treated kidney transplant patients develop less central arterial stiffness [48]. Additionally, long-term dietary supplementation with rapamycin in old mice showed attenuation of age-related arterial stiffness by reducing aortic collagen content [17].
In summary, inhibition of mTOR signaling has shown promising effects in improving and maintaining cardiovascular health during the aging process. Nevertheless, additional studies will be necessary to investigate the effect of specific targets of mTOR signaling as a therapeutic strategy to improve cardiovascular health in the elderly.
Sirtuins
Sirtuins (silent information regulator 2 proteins) belong to the class III histone deacetylase family characterised by a nicotinamide adenine dinucleotide (NAD+)-dependent enzymatic activity. In mammals, there are seven different members (SIRT1–7) of the sirtuin family, each with distinct subcellular localisation, cellular function and tissue distribution [3,8,49]. Decreased sirtuin activity has been observed with ascending age whereas sirtuin function has been associated with numerous physiological and pathological processes linked to cardiovascular diseases (i.e., oxidative stress, inflammation, autophagy, cellular senescence and apoptosis) [49]. Thus, coinciding with a decline of vascular NAD+ levels with aging, the implication of sirtuins in age-related cardiovascular disease is noteworthy.
To date, SIRT1 is one of the best characterised isoforms of sirtuins. SIRT1 has been associated with a protective role in atherosclerosis; SIRT1 overexpression shows atheroprotective effects in ApoE-/- mice fed with a high-fat diet by decreasing endothelial ROS formation and blunting NF-κB activity in endothelial cells [50,51,52]. Via the same pathway, SIRT1 also prevents vascular thrombosis by inhibiting tissue factor activation [50]. Besides the observations linked to SIRT1, accelerated arterial thrombus formation was also reported in SIRT3-/- animals, denoting the thrombo-protective effect of SIRT3. SIRT3 significance in arterial thrombosis is also supported by the clinical findings that SIRT3 mRNA expression on human peripheral blood CD14+ monocytes is lower in ST-elevation myocardial infarction patients than a healthy control group [53]. In addition, SIRT3 knockout mice fed a high cholesterol diet exhibit mild endothelial dysfunction paralleled by a higher accumulation of mitochondrial superoxide [54]. Nevertheless, SIRT3 deficiency does not affect atherosclerosis, in terms of neither plaque burden nor plaque vulnerability [55].
In addition to their atheroprotective properties, SIRTs also preserve cardiac health. According to previous evidence, both SIRT1 and SIRT3 mediate protection against ischaemia/reperfusion myocardial damage. Mice lacking cardiac-specific SIRT1 suffer larger myocardial infarction areas following left anterior descending artery (LAD) ligation, whereas overexpression of this protein results in a reduction of cardiac injury, thus denoting a protective role of this enzyme against ischemic myocardial injury [56]. Similarly, more cardiac damage following ischaemia/reperfusion injury is also observed in SIRT3 deficient mice [57]. Located in the mitochondria, SIRT3 also preserves mitochondrial function. As such, it is crucial for the development of cardiac hypertrophy and heart failure as seen in SIRT3 knockout mice, which exhibit a lower ejection fraction than wild typemice following transverse aortic constriction. Cardiac hyperthrophy and heart failure also develope in SIRT6-deficient mice, whereas SIRT6 overexpression protects from hypertrophic stimuli [58,59]
In addition to its cardiac protective effect, SIRT6 has also been implicated in stroke [50,60]. In a recent study reported by our group, mice lacking endothelial-specific SIRT6 developed larger cerebral infarct volumes following transient middle cerebral artery occlusion. The same mice also showed a higher mortality rate compared with wild type animals exposed to the same procedure. The detrimental effect of SIRT6 deficiency is due to SIRT6’s role in preserving blood-brain barrier integrity, thus having a protective function in ischaemic stroke. Indeed, our team demonstrated a reduction in stroke size by 50% and improved neurological outcomes on SIRT6-overexpressing mice as compared with controls. The strong link between SIRT6 and ischaemic stroke was further supported by our findings on SIRT6 expression measured in peripheral blood mononuclear cells of stroke patients. In this recent study, we showed that SIRT6 gene expression correlates with the patient’s short-term neurological outcome [60].
On the contrary, SIRT5, which is mainly located in the mitochondria, plays a detrimental role in ischaemia/reperfusion cerebral injury. We found that peripheral blood mononuclear cells collected from acute ischaemic stroke patients expressed a SIRT5 gene more highly than healthy controls. Indeed, in vivo SIRT5 gene silencing results in reduced stroke size, improved stroke outcome and preserved blood brain barrier permeability [61].
Accumulated evidence undoubtedly reveals the role of sirtuins in longevity and, particularly, in cardiovascular diseases. Although several sirtuins exhibit a deleterious role against cardiovascular disease, the majority of sirtuin members appear to have a protective function in cardiac remodeling, hypertension and stroke. Hence, sirtuin activator compounds, specifically for SIRT1, have emerged as a promising therapeutic approach for cardiovascular disease. However, more profound study is still required to elucidate the specific actions of different sirtuins on cardiac and vascular cells, in order to unveil the potential of other sirtuins as valuable therapeutic targets.
Transcription factor JunD
Activating protein-1 (AP-1) is a heterodimeric transcription factor composed of three DNA-binding proteins: Jun, Fos and ATF/CREB, which determine its activitiy based on their composition [6,62]. Multiple evidence indicates an important role of the AP-1 associated transcription factor JunD in age-related diseases, by regulating cell growth and survival and modulating oxidative stress levels. A previous study by Lorent et al. showed that JunD-/- mice develop signs of premature aging, such as cataract, alopecia and graying, despite a normal appearance at birth. The same mice also have a lifespan reduced by 17% as compared with wild type littermates [63].
Various evidence suggests the relevance of JunD in vascular aging, given that JunD knockout mice display premature features of vascular senescence. In fact, premature endothelial dysfunction is developed in the absence of JunD via increased ROS production, whereas JunD overexpression is able to preserve endothelial function. Regulation of JunD expression was also investigated in the setting of aging where aged animals displayed downregulation of JunD. Similarly, reduced expression of JunD was also detected in monocytes of elderly individuals as compared with young healthy subjects [62]. It is reported that reduced expression of JunD, as observed with aging, alters the equilibrium between oxidants (i.e., NADPH oxidase) and scavenger enzymes (i.e., manganese superoxide dismutase and aldehyde dehydrogenase), and that this imbalance results in ROS accumulation, mitochondrial dysfunction and later endothelial dysfunction [62].
JunD also plays a role in ischaemia/reperfusionbrain injury. Our team previously showed that JunD knockout mice suffer from larger-sized strokes and worsened neurological function following transient middle cerebral artery occlusion. A closer investigation in this setting indicated increased systemic inflammation, including higher IL-1β levels in brain tissue. Interestingly, anti-IL-1β antibody treatment upon reperfusion could rescue the deleterious effect of JunD silencing, suggesting the protective role of AP-1 transcription factor JunD against neuronal damage due to ischaemia/reperfusion-induced cerebral injury via IL-1β suppression [64].
Klotho
In 1997, the KLOTHO gene was first identified by Kuro et al. as an aging suppressor gene. The original study demonstrated that a defect in KLOTHO gene expression in mice generates a series of phenotypes such as a short lifespan, skin atrophy and aorta calcification that resemble human aging [65]. Hence, as it is implicated in human longevity, the Klotho protein is widely studied in multiple research areas including in cardiovascular research.
Accumulating evidence associates cardiovascular disease with soluble Klotho, which is released into the circulation and acts as a humoral factor. Previous clinical studies reported an independent association between low levels of circulating Klotho and the severity of coronary artery disease [66,67]. Low levels of soluble Klotho were related to ectopic calcifications of cardiac valves and the aorta whereas increased levels were shown to alleviate cardiac remodeling [68,69].
Emerging data have indicated the critical role of this longevity gene in vascular health, given the fact that Klotho knockout mice develop different vascular phenotypes. In fact, restoration of Klotho expression reverses vascular aging phenotypes, including vascular calcification, endothelial dysfunction and atherosclerosis [70]. Protective effects of Klotho were also reported with respect to endothelial homeostasis and vascular function. Impairment of vascular vasodilatation was observed in Klotho deficient mice which, interestingly, also showed decreased urinary excretion of NO metabolites (NO - and NO -), indicating lower vascular and systemic NO production [71]. Moreover, Klotho overexpression results in an upregulation of mitochondrial antioxidant enzymes and decreased oxidative stress [70]. This finding suggests a role for Klotho in blunting oxidative stress – a crucial factor in vascular disease development.
In 2016, Corsetti et al. reported for the first time the Klotho expression on the human myocardium. Their study showed that Klotho expression is down-regulated in high cardiovascular risk subjects parallel to the augmentation of oxidative stress, inflammation and fibrosis [72]. An in vitro study demonstrated that recombinant Klotho treatment of human cardiomyocytes results in higher cell viability and improved cell metabolism, which is beneficial in the setting of ischaemia/reperfusion injury [73]. Interestingly, the Klotho protein level is increased in cardiac tissue subjected to ischaemic damage [73]. A similar trend was identified in cardiac tissue of patients with cardiomyopathy in which Klotho mRNA levels are upregulated simultaneously with the upregulation of fibroblast growth factor-23 – a hormone linked to heart failure progression and cardiac remodeling [74]. To date, it is still not fully understood whether this phenomenon represents a response to or a cause of cardiac disease. Nevertheless, Klotho upregulation is postulated to be a compensatory mechanism in acute injury and the chronic progression of the disease [73,74]. Thus, further investigation is required in this regard.
Future perspective: aging and longevity genes as therapeutic targets
Previous studies clearly point at oxidative stress as being crucial in the pathogenesis of age-related diseases. Maintaining the balance between pro- and antioxidants was suggested as a plausible strategy to attain healthy aging. Hence, natural or synthetic exogenous antioxidants were investigated and developed in order to counterbalance oxidative stress. However, clinical studies on antioxidant- based therapy often show contrasting results. This discouraging outcome is frequently due to the nonspecific effects of the antioxidants used. Besides, the effect of antioxidant supplementation may also be influenced by different redox states and the genetic background of each individual [75].
A large amount of experimental evidence indicates that aging and longevity genes are critical mediators of age-dependent cardiovascular disease, mainly via oxidative stress and inflammation (Figure 4). Given the importance of aging and longevity genes in lifespan determination, different approaches to modulating their activities have been assessed as potential strategies to delay senescence process. In principle, these methods include dietary intervention and the use of low molecular weight compounds.
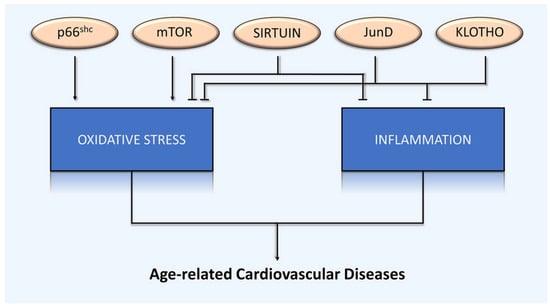
Figure 4.
Lifespan-regulating genes in age-related cardiovascular disease. Lifespan-regulating genes play a critical pathophysiological role in age-related cardiovascular disease, mediated by common underlying mechanisms: oxidative stress and inflammation.
Calorie restriction was shown to slow down aging and prolong lifespan. To date, accumulating evidence demonstrates the protective effect of calorie restriction against age-related cardiovascular diseases. Indeed, long-term calorie restriction blunts age-related endothelial dysfunction by maintaining NO bioavailability and blunting arterial oxidative stress. This dietary intervention also shows an impact on large elastic arteries in prevention of arterial stiffening [76]. Even more, reduced myocardial fibrosis and an improvement of cardiac function are mentioned as other beneficial outcomes of calorie restriction [77]. Previous studies suggested that calorie restriction improves vascular health by modulating nutrient-sensing pathways that are dysregulated with advancing age, namely mTOR, sirtuins and AMP-activated protein kinase. Because of the benefits of calorie restriction, pharmacological approaches targeting the same pathways are being developed, and thus may also be effective in impeding cardiovascular aging.
The potential of the mTOR inhibitor, rapamycin, in treatment of age-related cardiovascular diseases has been studied extensively. Originally used as an immunosuppressant drug, this compound was assessed in clinical trials for its antiatherosclerotic and vasoprotective properties. In kidney transplant patients, rapamycin was reported to attenuate arterial stiffness and blood pressure [78]. Furthermore, rapamycin administration in old mice results in improved myocardial contractile function and decreased age-related inflammation [79]. Nevertheless, rapamycin has serious adverse effects (i.e., hyperlipidaemia, hyperglycaemia and insulin resistance), which limit the broadening of its indications [78]. In this regard, further studies are required in order to develop a safer rapamycin analogue to minimise its adverse effects.
Modulating sirtuin pathways, especially SIRT1, is also an alternative pharmacological strategy to promote healthy aging. Several compounds have been used to modulate SIRT1 activity, for instance, resveratrol and other sirtuinactivating compounds (STACs) [80]. Naturally present in grapeskin and red wine, resveratrol is a potent SIRT1 activator and has shown vasoprotective effects in multiple studies. Via the SIRT1 pathway, resveratrol induces protein deacetylation and autophagy, which are beneficial in regulating oxidative stress, inflammation, cellular senescence and endothelial dysfunction [80,81]. Indeed, in rodents, resveratrol promotes protections against hypertension, atherosclerosis and heart failure [77].
However, resveratrol is a nonspecific compound that targets various enzymes, receptors and kinases [82]. As a consequence, the complex pharmacodynamics of resveratrol may complicate its application in the clinic. Besides, it will be difficult to predict the tolerability of this compound in a varied and large clinical setting. Therefore, nowadays, different small molecule activators of SIRT1 are developed due to the shortcoming. In fact, previous studies show the beneficial effect of SRT1720 and SRT2104 in extending healthspan and lifespan [80]. A SIRT1 activator compound, SRT3025, also has atheroprotective properties on ApoE knockout mice [83]. To date, several STACs have undergone clinical trials for different indications, including cardiovascular disease, diabetes and cancer [84]. Therefore, due to their promising outcome, continued research and development of SIRT1 activators in the future is strongly encouraged [85].
Conclusions
Recent advances in gerontology have contributed to a better understanding of aging and aging-related diseases. Various lifespan regulating genes have been identified and demonstrated to be crucial for the pathophysiology of age- dependent cardiovascular disease via oxidative stress and inflammation. Indeed, several strategies modulating the genes pathways and activities have been developed and are currently under investigation. However, there are still significant knowledge gaps on this topic and many practical challenges in the clinic. Indeed, those strategies have so far not been well translated into therapeutic or preventive strategies against age-dependent cardiovascular disease, despite promising results. In this respect, future and ongoing investigations will be key in providing the missing knowledge. New pharmacological agents and novel interventions to restore healthy aging are currently being tested with the goal of blunting the incidence of age-dependent cardiovascular disease and reducing mortality.
Acknowledgments
Figures were designed using Servier Medical Art by Servier under a Creative Commons Attribution 3.0 Unported License.
Financial disclosure
GGC received support for his work from the Swiss National Science Foundation [310030_197510], the Swiss Heart Foundation, the Alfred and Annemarie von Sick Grants for Translational and Clinical Research Cardiology and Oncology, and the Foundation for Cardiovascular Research – Zurich Heart House. GGC is also a recipient of a Sheikh Khalifa's Foundation Ass. Professorship at the Faculty of Medicine, University of Zurich.
Potential competing interests
GGC is a co-investor on a provisional patent application that was filed in May 2019. The patent relates to the use of antibodies which specifically bind IL-1α to reduce various sequelae of ischaemia-reperfusion injury to the central nervous system. The other authors report no conflict of interest.
References
- North, B.J.; Sinclair, D.A. The intersection between aging and cardiovascular disease. Circ Res. 2012, 110, 1097–1108. [Google Scholar] [CrossRef] [PubMed]
- Camici, G.G.; Liberale, L. Aging: the next cardiovascular disease? Eur Heart J. 2017, 38, 1621–1623. [Google Scholar] [CrossRef] [PubMed]
- Liberale, L.; Kraler, S.; Camici, G.G.; Lüscher, T.F. Ageing and longevity genes in cardiovascular diseases. Basic Clin Pharmacol Toxicol. 2020, 127, 120–131. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, A.J.P.O.; Ribeiro, T.P.; de Medeiros, I.A. Aging: Molecular Pathways and Implications on the Cardiovascular System. Oxid Med Cell Longev. 2017, 2017, 7941563. [Google Scholar] [CrossRef]
- Ming, X.F.; Montani, J.P.; Yang, Z. Perspectives of targeting mTORC1-S6K1 in cardiovascular aging. Front Physiol. 2012, 3, 5. [Google Scholar] [CrossRef]
- Paneni, F.; Diaz Cañestro, C.; Libby, P.; Lüscher, T.F.; Camici, G.G. The Aging Cardiovascular System: Understanding It at the Cellular and Clinical Levels. J Am Coll Cardiol. 2017, 69, 1952–1967. [Google Scholar] [CrossRef]
- Ungvari, Z.; Kaley, G.; de Cabo, R.; Sonntag, W.E.; Csiszar, A. Mechanisms of vascular aging: new perspectives. J Gerontol A Biol Sci Med Sci. 2010, 65, 1028–1041. [Google Scholar] [CrossRef]
- Camici, G.G.; Savarese, G.; Akhmedov, A.; Lüscher, T.F. Molecular mechanism of endothelial and vascular aging: implications for cardiovascular disease. Eur Heart J. 2015, 36, 3392–3403. [Google Scholar] [CrossRef]
- Yeh, J.K.; Lin, M.H.; Wang, C.Y. Telomeres as Therapeutic Targets in Heart Disease. JACC Basic Transl Sci. 2019, 4, 855–865. [Google Scholar] [CrossRef]
- De Meyer, T.; Rietzschel, E.R.; De Buyzere, M.L.; Van Criekinge, W.; Bekaert, S. Telomere length and cardiovascular aging: the means to the ends? Ageing Res Rev. 2011, 10, 297–303. [Google Scholar] [CrossRef]
- Aviv, A. Genetics of leukocyte telomere length and its role in atherosclerosis. Mutat Res. 2012, 730, 68–74. [Google Scholar] [CrossRef]
- De Meyer, T.; Nawrot, T.; Bekaert, S.; De Buyzere, M.L.; Rietzschel, E.R.; Andrés, V. Telomere Length as Cardiovascular Aging Biomarker: JACC Review Topic of the Week. J Am Coll Cardiol. 2018, 72, 805–813. [Google Scholar] [CrossRef]
- Clemson, L.; et al. Free-Radical Theory of Aging. In Encyclopedia of Behavioral Medicine; Gellman, M.D., Turner, J.R., Eds.; Springer: New York, NY, USA, 2013. [Google Scholar] [CrossRef]
- Cui, H.; Kong, Y.; Zhang, H. Oxidative stress, mitochondrial dysfunction, and aging. J Signal Transduct. 2012, 2012, 646354. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, A.J.P.O.; Ribeiro, T.P.; de Medeiros, IA. Aging: Molecular Pathways and Implications on the Cardiovascular System. Oxid Med Cell Longev. 2017, 2017, 7941563. [Google Scholar] [CrossRef] [PubMed]
- Dikalova, A.; Clempus, R.; Lassègue, B.; Cheng, G.; McCoy, J.; Dikalov, S.; et al. Nox1 overexpression potentiates angiotensin II-induced hypertension and vascular smooth muscle hypertrophy in transgenic mice. Circulation. 2005, 112, 2668–2676. [Google Scholar] [CrossRef]
- Donato, A.J.; Machin, D.R.; Lesniewski, L.A. Mechanisms of dysfunction in the aging vasculature and role in age-related disease. Circ Res. 2018, 123, 825–848. [Google Scholar] [CrossRef]
- Münzel, T.; Camici, G.G.; Maack, C.; Bonetti, N.R.; Fuster, V.; Kovacic, J.C. Impact of Oxidative Stress on the Heart and Vasculature: Part 2 of a 3-Part Series. J Am Coll Cardiol. 2017, 70, 212–229. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; et al. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget. 2017, 9, 7204–7218. [Google Scholar] [CrossRef]
- Fulop, T.; Larbi, A.; Dupuis, G.; Le Page, A.; Frost, E.H.; Cohen, A.A.; et al. Immunosenescence and inflamm-aging as two sides of the same coin: Friends or Foes? Front Immunol. 2018, 8, 1960. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct Target Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Sanada, F.; Taniyama, Y.; Muratsu, J.; Otsu, R.; Shimizu, H.; Rakugi, H.; et al. Source of Chronic Inflammation in Aging. Front Cardiovasc Med. 2018, 5, 12. [Google Scholar] [CrossRef]
- Olivieri, F.; Prattichizzo, F.; Grillari, J.; Balistreri, C.R. Cellular senescence and inflammaging in age-Related diseases. Mediators Inflamm. 2018, 2018, 9076485. [Google Scholar] [CrossRef]
- Ferrucci, L.; Fabbri, E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol. 2018, 15, 505–522. [Google Scholar] [CrossRef]
- Heath, J.J.; Grant, M.D. The Immune Response Against Human Cytomegalovirus Links Cellular to Systemic Senescence. Cells. 2020, 9, 766. [Google Scholar] [CrossRef]
- Chung, H.Y.; Kim, D.H.; Lee, E.K.; Chung, K.W.; Chung, S.; Lee, B.; et al. Redefining chronic inflammation in aging and age-related diseases: Proposal of the senoinflammation concept. Aging Dis. 2019, 10, 367–382. [Google Scholar] [CrossRef]
- Vita, J.A.; Keaney, J.F.; Jr Larson, M.G.; Keyes, M.J.; Massaro, J.M.; Lipinska, I.; et al. Brachial artery vasodilator function and systemic inflammation in the Framingham Offspring Study. Circulation. 2004, 110, 3604–3609. [Google Scholar] [CrossRef]
- Schnabel, R.; Larson, M.G.; Dupuis, J.; Lunetta, K.L.; Lipinska, I.; Meigs, J.B.; et al. Relations of inflammatory biomarkers and common genetic variants with arterial stiffness and wave reflection. Hypertension. 2008, 51, 1651–1657. [Google Scholar] [CrossRef]
- Liu, D.; Richardson, G.; Benli, F.M.; Park, C.; de Souza, J.V.; Bronowska, A.K.; et al. Inflammageing in the cardiovascular system: mechanisms, emerging targets, and novel therapeutic strategies. Clin Sci (Lond). 2020, 134, 2243–2262. [Google Scholar] [CrossRef]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; et al. CANTOS Trial Group. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef]
- Galimov, E.R. The Role of p66shc in Oxidative Stress and Apoptosis. Acta Naturae. 2010, 2, 44–51. [Google Scholar] [CrossRef]
- Cosentino, F.; Francia, P.; Camici, G.G.; Pelicci, P.G.; Lüscher, T.F.; Volpe, M. Final common molecular pathways of aging and cardiovascular disease: role of the p66Shc protein. Arterioscler Thromb Vasc Biol. 2008, 28, 622–628. [Google Scholar] [CrossRef]
- Francia, P.; delli Gatti, C.; Bachschmid, M.; Martin-Padura, I.; Savoia, C.; Migliaccio, E.; et al. Deletion of p66shc gene protects against age-related endothelial dysfunction. Circulation. 2004, 110, 2889–2895. [Google Scholar] [CrossRef]
- Boengler, K.; Bornbaum, J.; Schlüter, K.-D.; Schulz, R. P66shc and its role in ischemic cardiovascular diseases. Basic Res Cardiol. 2019, 114, 29. [Google Scholar] [CrossRef]
- Spescha, R.D.; Shi, Y.; Wegener, S.; Keller, S.; Weber, B.; Wyss, M.M.; et al. Deletion of the ageing gene p66(Shc) reduces early stroke size following ischaemia/reperfusion brain injury. Eur Heart J. 2013, 34, 96–103. [Google Scholar] [CrossRef]
- Spescha, R.D.; Klohs, J.; Semerano, A.; Giacalone, G.; Derungs, R.S.; Reiner, M.F.; et al. Post-ischaemic silencing of p66Shc reduces ischaemia/reperfusion brain injury and its expression correlates to clinical outcome in stroke. Eur Heart J. 2015, 36, 1590–1600. [Google Scholar] [CrossRef]
- Napoli, C.; Martin-Padura, I.; de Nigris, F.; Giorgio, M.; Mansueto, G.; Somma, P.; et al. Deletion of the p66Shc longevity gene reduces systemic and tissue oxidative stress, vascular cell apoptosis, and early atherogenesis in mice fed a high-fat diet. Proc Natl Acad Sci USA. 2003, 100, 2112–2116. [Google Scholar] [CrossRef]
- Camici, G.G.; Cosentino, F.; Tanner, F.C.; Lüscher, T.F. The role of p66Shc deletion in age-associated arterial dysfunction and disease states. J Appl Physiol (1985). 2008, 105, 1628–1631. [Google Scholar] [CrossRef]
- Miao, Q.; Wang, Q.; Dong, L.; Wang, Y.; Tan, Y.; Zhang, X. The expression of p66shc in peripheral blood monocytes is increased in patients with coronary heart disease and correlated with endothelium-dependent vasodilatation. Heart Vessels. 2015, 30, 451–457. [Google Scholar] [CrossRef]
- Franzeck, F.C.; Hof, D.; Spescha, R.D.; Hasun, M.; Akhmedov, A.; Steffel, J.; et al. Expression of the aging gene p66Shc is increased in peripheral blood monocytes of patients with acute coronary syndrome but not with stable coronary artery disease. Atherosclerosis. 2012, 220, 282–286. [Google Scholar] [CrossRef]
- Fingar, D.C.; Blenis, J. Target of rapamycin (TOR): an integrator of nutrient and growth factor signals and coordinator of cell growth and cell cycle progression. Oncogene. 2004, 23, 3151–3171. [Google Scholar] [CrossRef]
- Papadopoli, D.; Boulay, K.; Kazak, L.; Pollak, M.; Mallette, F.; Topisirovic, I.; et al. mTOR as a central regulator of lifespan and aging. F1000 Res. 2019, 8, 998. [Google Scholar] [CrossRef]
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell. 2017, 168, 960–976. [Google Scholar] [CrossRef]
- Sciarretta, S.; Volpe, M.; Sadoshima, J. Mammalian target of rapamycin signaling in cardiac physiology and disease. Circ Res. 2014, 114, 549–564. [Google Scholar] [CrossRef]
- Sciarretta, S.; Forte, M.; Frati, G.; Sadoshima, J. New insights into the role of mtor signaling in the cardiovascular system. Circ Res. 2018, 122, 489–505. [Google Scholar] [CrossRef]
- Reho, J.J.; Guo, D.F.; Rahmouni, K. Mechanistic Target of Rapamycin Complex 1 Signaling Modulates Vascular Endothelial Function Through Reactive Oxygen Species. J Am Heart Assoc. 2019, 8, e010662. [Google Scholar] [CrossRef] [PubMed]
- Parlar, A.; Can, C.; Erol, A.; Ülker, S. Posttransplantation therapeutic rapamycin concentration protects nitric oxide-related vascular endothelial function: comparative effects in rat thoracic aorta and coronary endothelial cell culture. Transplant Proc. 2010, 42, 1923–1930. [Google Scholar] [CrossRef] [PubMed]
- Joannidès, R.; Monteil, C.; de Ligny, B.H.; Westeel, P.F.; Iacob, M.; Thervet, E.; et al. Immunosuppressant regimen based on sirolimus decreases aortic stiffness in renal transplant recipients in comparison to cyclosporine. Am J Transplant. 2011, 11, 2414–2422. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Bai, P.; Jin, Z.G. Sirtuins in Cardiovascular Health and Diseases. Trends Endocrinol Metab. 2016, 27, 677–678. [Google Scholar] [CrossRef]
- Winnik, S.; Auwerx, J.; Sinclair, D.A.; Matter, C.M. Protective effects of sirtuins in cardiovascular diseases: from bench to bedside. Eur Heart J. 2015, 36, 3404–3412. [Google Scholar] [CrossRef]
- Winnik, S.; Stein, S.; Matter, C.M. SIRT1 an anti-inflammatory pathway at the crossroads between metabolic disease and atherosclerosis. Curr Vasc Pharmacol. 2012, 10, 693–696. [Google Scholar] [CrossRef]
- Stein, S.; Schäfer, N.; Breitenstein, A.; Besler, C.; Winnik, S.; Lohmann, C.; et al. SIRT1 reduces endothelial activation without affecting vascular function in ApoE-/- mice. Aging (Albany NY). 2010, 2, 353–360. [Google Scholar] [CrossRef]
- Gaul, D.S.; Weber, J.; van Tits, L.J.; Sluka, S.; Pasterk, L.; Reiner, M.F.; et al. Loss of Sirt3 accelerates arterial thrombosis by increasing formation of neutrophil extracellular traps and plasma tissue factor activity. Cardiovasc Res. 2018, 114, 1178–1188. [Google Scholar] [CrossRef]
- Winnik, S.; Gaul, D.S.; Siciliani, G.; Lohmann, C.; Pasterk, L.; Calatayud, N.; et al. Mild endothelial dysfunction in Sirt3 knockout mice fed a high-cholesterol diet: protective role of a novel C/EBP-β-dependent feedback regulation of SOD2. Basic Res Cardiol. 2016, 111, 33. [Google Scholar] [CrossRef] [PubMed]
- Winnik, S.; Gaul, D.S.; Preitner, F.; Lohmann, C.; Weber, J.; Miranda, M.X.; et al. Deletion of Sirt3 does not affect atherosclerosis but accelerates weight gain and impairs rapid metabolic adaptation in LDL receptor knockout mice: implications for cardiovascular risk factor development. Basic Res Cardiol. 2014, 109, 399. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.P.; Zhai, P.; Yamamoto, T.; Maejima, Y.; Matsushima, S.; Hariharan, N.; et al. Silent information regulator 1 protects the heart from ischemia/reperfusion. Circulation. 2010, 122, 2170–2182. [Google Scholar] [CrossRef]
- Porter, K.E.; Turner, N.A. Cardiac fibroblasts: at the heart of myocardial remodeling. Pharmacol Ther. 2009, 123, 255–278. [Google Scholar] [CrossRef]
- Mostoslavsky, R.; Chua, K.F.; Lombard, D.B.; Pang, W.W.; Fischer, M.R.; Gellon, L.; et al. Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell. 2006, 124, 315–329. [Google Scholar] [CrossRef]
- Sundaresan, N.R.; Vasudevan, P.; Zhong, L.; Kim, G.; Samant, S.; Parekh, V.; et al. The sirtuin SIRT6 blocks IGF-Akt signaling and development of cardiac hypertrophy by targeting c-Jun. Nat Med. 2012, 18, 1643–1650. [Google Scholar] [CrossRef]
- Liberale, L.; Gaul, D.S.; Akhmedov, A.; Bonetti, N.R.; Nageswaran, V.; Costantino, S.; et al. Endothelial SIRT6 blunts stroke size and neurological deficit by preserving blood-brain barrier integrity: a translational study. Eur Heart J. 2020, 41, 1575–1587. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Cañestro, C.; Merlini, M.; Bonetti, N.R.; Liberale, L.; Wüst, P.; Briand-Schumacher, S.; et al. Sirtuin 5 as a novel target to blunt blood-brain barrier damage induced by cerebral ischemia/reperfusion injury. Int J Cardiol. 2018, 260, 148–55. [Google Scholar] [CrossRef]
- Paneni, F.; Osto, E.; Costantino, S.; Mateescu, B.; Briand, S.; Coppolino, G.; et al. Deletion of the activated protein-1 transcription factor JunD induces oxidative stress and accelerates age-related endothelial dysfunction. Circulation. 2013, 127, 1229–1240. [Google Scholar] [CrossRef] [PubMed]
- Laurent, G.; Solari, F.; Mateescu, B.; Karaca, M.; Castel, J.; Bourachot, B.; et al. Oxidative stress contributes to aging by enhancing pancreatic angiogenesis and insulin signaling. Cell Metab. 2008, 7, 113–124. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Diaz-Cañestro, C.; Reiner, M.F.; Bonetti, N.R.; Liberale, L.; Merlini, M.; Wüst, P.; et al. AP-1 (Activated Protein-1) Transcription Factor JunD Regulates Ischemia/Reperfusion Brain Damage via IL-1β (Interleukin-1β). Stroke. 2019, 50, 469–477. [Google Scholar] [CrossRef]
- Kuro-o, M.; Matsumura, Y.; Aizawa, H.; Kawaguchi, H.; Suga, T.; Utsugi, T.; et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997, 390, 45–51. [Google Scholar] [CrossRef]
- Martín-Núñez, E.; Donate-Correa, J.; López-Castillo, Á.; Delgado-Molinos, A.; Ferri, C.; Rodríguez-Ramos, S.; et al. Soluble levels and endogenous vascular gene expression of KLOTHO are related to inflammation in human atherosclerotic disease. Clin Sci (Lond). 2017, 131, 2601–2609. [Google Scholar] [CrossRef]
- Navarro-González, J.F.; Donate-Correa, J.; Muros de Fuentes, M.; Pérez-Hernández, H.; Martínez-Sanz, R.; Mora-Fernández, C. Reduced Klotho is associated with the presence and severity of coronary artery disease. Heart. 2014, 100, 34–40. [Google Scholar] [CrossRef]
- Xie, J.; Yoon, J.; An, S.W.; Kuro-o, M.; Huang, C.L. Soluble Klotho protects against uremic cardiomyopathy independently of fibroblast growth factor 23 and phosphate. J Am Soc Nephrol. 2015, 26, 1150–1160. [Google Scholar] [CrossRef]
- Donate-Correa, J.; Martín-Núñez, E.; Mora-Fernández, C.; Muros-de-Fuentes, M.; Pérez-Delgado, N.; Navarro-González, J.F. Klotho in cardiovascular disease: Current and future perspectives. World J Biol Chem. 2015, 6, 351–357. [Google Scholar] [CrossRef]
- Lim, K.; Halim, A.; Lu, T.S.; Ashworth, A.; Chong, I. Klotho: A Major Shareholder in Vascular Aging Enterprises. Int J Mol Sci. 2019, 20, 4637. [Google Scholar] [CrossRef]
- Saito, Y.; Yamagishi, T.; Nakamura, T.; Ohyama, Y.; Aizawa, H.; Suga, T.; et al. Klotho protein protects against endothelial dysfunction. Biochem Biophys Res Commun. 1998, 248, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Corsetti, G.; Pasini, E.; Scarabelli, T.M.; Romano, C.; Agrawal, P.R.; Chen-Scarabelli, C.; et al. Decreased expression of Klotho in cardiac atria biopsy samples from patients at higher risk of atherosclerotic cardiovascular disease. J Geriatr Cardiol. 2016, 13, 701–711. [Google Scholar] [PubMed]
- Olejnik, A.; Krzywonos-Zawadzka, A.; Banaszkiewicz, M.; Bil-Lula, I. Klotho protein contributes to cardioprotection during ischaemia/reperfusion injury. J Cell Mol Med. 2020, 24, 6448–6458. [Google Scholar] [CrossRef]
- Poelzl, G.; Ghadge, S.K.; Messner, M.; Haubner, B.; Wuertinger, P.; Griesmacher, A.; et al. Klotho is upregulated in human cardiomyopathy independently of circulating Klotho levels. Sci Rep. 2018, 8, 8429. [Google Scholar] [CrossRef] [PubMed]
- Conti, V.; Izzo, V.; Corbi, G.; Russomanno, G.; Manzo, V.; De Lise, F.; et al. Antioxidant Supplementation in the Treatment of Aging-Associated Diseases. Front Pharmacol. 2016, 7, 24. [Google Scholar] [CrossRef]
- Donato, A.J.; Walker, A.E.; Magerko, K.A.; Bramwell, R.C.; Black, A.D.; Henson, G.D.; et al. Life-long caloric restriction reduces oxidative stress and preserves nitric oxide bioavailability and function in arteries of old mice. Aging Cell. 2013, 12, 772–783. [Google Scholar] [CrossRef]
- Alfaras, I.; Di Germanio, C.; Bernier, M.; Csiszar, A.; Ungvari, Z.; Lakatta, E.G.; et al. Pharmacological Strategies to Retard Cardiovascular Aging. Circ Res. 2016, 118, 1626–1642. [Google Scholar] [CrossRef] [PubMed]
- Hamczyk, M.R.; Nevado, R.M.; Barettino, A.; Fuster, V.; Andrés, V. Biological Versus Chronological Aging: JACC Focus Seminar. J Am Coll Cardiol. 2020, 75, 919–930. [Google Scholar] [CrossRef]
- Flynn, J.M.; O’Leary, M.N.; Zambataro, C.A.; Academia, E.C.; Presley, M.P.; Garrett, B.J.; et al. Late-life rapamycin treatment reverses age-related heart dysfunction. Aging Cell. 2013, 12, 851–862. [Google Scholar] [CrossRef]
- Madeo, F.; Carmona-Gutierrez, D.; Hofer, S.J.; Kroemer, G. Caloric Restriction Mimetics against Age-Associated Disease: Targets, Mechanisms, and Therapeutic Potential. Cell Metab. 2019, 29, 592–610. [Google Scholar] [CrossRef]
- D’Onofrio, N.; Vitiello, M.; Casale, R.; Servillo, L.; Giovane, A.; Balestrieri, M.L. Sirtuins in vascular diseases: Emerging roles and therapeutic potential. Biochim Biophys Acta. 2015, 1852, 1311–1322. [Google Scholar] [CrossRef]
- Bhullar, K.S.; Hubbard, B.P. Lifespan and healthspan extension by resveratrol. Biochim Biophys Acta. 2015, 1852, 1209–1218. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.X.; van Tits, L.J.; Lohmann, C.; Arsiwala, T.; Winnik, S.; Tailleux, A.; et al. The Sirt1 activator SRT3025 provides atheroprotection in Apoe-/- mice by reducing hepatic Pcsk9 secretion and enhancing Ldlr expression. Eur Heart J. 2015, 36, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Bonkowski, M.S.; Sinclair, D.A. Slowing ageing by design: the rise of NAD+ and sirtuin-activating compounds. Nat Rev Mol Cell Biol. 2016, 17, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Sinclair, D.A.; Ellis, J.L.; Steegborn, C. Sirtuin activators and inhibitors: Promises, achievements, and challenges. Pharmacol Ther. 2018, 188, 140–54. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2021 by the author. Attribution-Non-Commercial-NoDerivatives 4.0.