Summary
Occasionally, cardiac disease may manifest acutely with extracardiac symptoms. we present a patient case with pulmonary haemorrhage as the first manifestation of rheumatic mitral stenosis, which resolved completely after mitral valve surgery, and discuss the pathophysiology and clinical management of this rare manifestation of rheumatic heart disease.
Case description
A 46-year-old man presented to the emergency department because of recurrent minor haemoptysis and marked shortness of breath when walking short distances or even at rest (equivalent to New York Heart Association Class III to IV). Two years earlier, he had experienced similar symptoms, but a full pulmonary evaluation did not reveal the aetiology. He had unintentionally lost 15 kg of body weight during the previous 5 months, but had no fever or night sweats. His past medical history was remarkable for hepatitis B and alcohol abuse. The patient was living alone, had a lower socioeconomic background, and had migrated from Eastern Europe to Switzerland more than 10 years ago.
On admission, the patient’s blood pressure was 127/77 mm Hg, heart rate was 97/min, and oxygen saturation was 98% while breathing ambient air. On physical examination, discrete wheezing on pulmonary auscultation and slight oedema of the legs were found. Cardiac auscultation was remarkable for a holosystolic murmur and a rumbling diastolic murmur, both best heard in the apex. The ECG showed sinus rhythm with right axis deviation, inferolateral early repolarisation pattern and a bifid p wave (fig. 1). Chest X-ray showed normal cardiac size and a diffuse interstitial abnormality (fig. 2A). A computed tomography (CT) scan revealed a centrilobular ground-glass pattern of the lung parenchyma and thickened bronchial walls (fig. 2B). There were no other signs indicative for lung cancer, pneumonia, pulmonary embolism or bronchiectasis (fig. 2B).
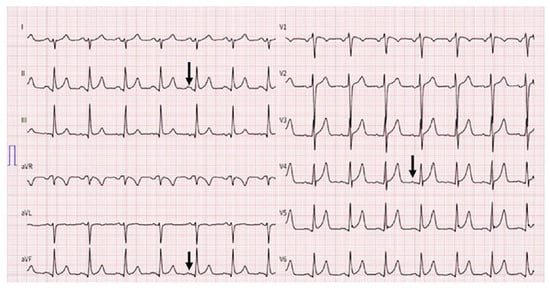
Figure 1.
ECG at admission. Sinus rhythm with bifid (“mitral”) p waves (arrow), right axis deviation and signs of early repolarisation patterns with J waves in leads V4–6, ST segment elevation in leads V4–6 and, less pronounced, in II , aVF.
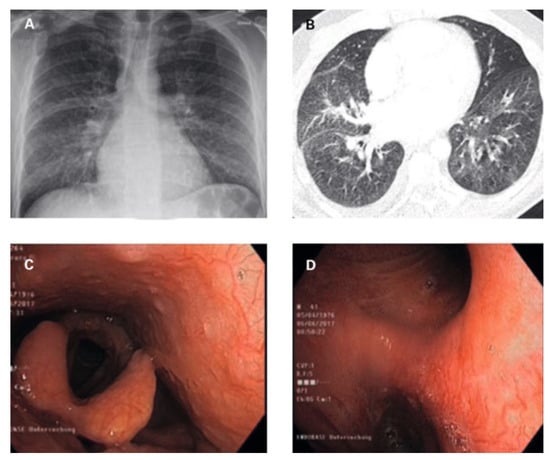
Figure 2.
Imaging and bronchoscopy findings (description in text).
Bronchoscopy revealed hyperaemic bronchial mucosa with ectatic and tortuous vessels, with a high tendency towards bleeding on contact with the bronchoscope, identified as the obvious source of the recurrent haemoptysis (fig. 2C,D). Broncho-alveolar lavage was remarkable macroscopically for recovery of slight haemorrhagic fluid , and microscopically for a high haemosiderin-laden macrophage count. Leucocyte
To exclude left-heart disease as a possible cause of the congested bronchial vessels and the alveolar haemorrhage syndrome, first transthoracic and later transoesophageal echocardiography were performed.
count and haemoglobin levels were normal. C-reactive protein was 32 mg/l (reference <5 mg/l). Further workup with bacterial, virological and rheumatological panels were negative.
To exclude left-heart disease as a possible cause of the congested bronchial vessels and the alveolar haemorrhage syndrome, first transthoracic and later transoesophageal echocardiography were performed. The main finding was a grotesque doming of thickened mitral leaflets with a severe subvalvular mitral stenosis (transthoracic echocardiography figure 3A–F and transoesophageal echocardiography figures 4A–D; mean pressure gradient 16.2 mm Hg, reference <5 mm Hg, at a heart rate of 82/min, calculated mitral valve area by pressure half-time method 1.3 cm2, reference >1.5 cm2; figure 3f; planimetry of mitral valve area 0.74 cm2 in TOE approach, figure 4d+e) and severe mitral regurgitation with pulmonary vein backflow due to restricted leaflet motion (fig. 4a). The right ventricular / right atrial pressure gradient across the tricuspid valve could not be obtained.
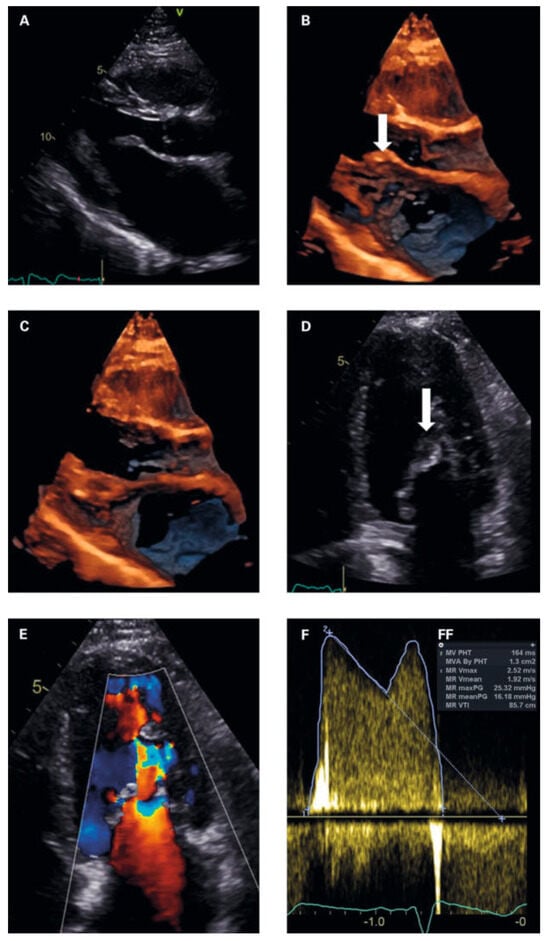
Figure 3.
(a) Parasternal long axis in diastole. Normal sized left ventricle. dilated left atrium. thickened anterior mitral leaflet with typical hockey stick appearance (arrow) and restricted posterior leaflet. (B, C) 4d imaging parasternal long axis in diastole (3B) and systole (3C) showing doming and extensive subvalvular thickening of the mitral apparatus. (D) apical 4 chamber view at diastole. Arrow subvalvular doming and thickening. (e) Colour doppler at diastole showing subvalvular flow increase with PiSa phenomenon. (F) Continous wave doppler measurements of the mitral valve gradients. (FF) Measurements mitral valve area and gradients.
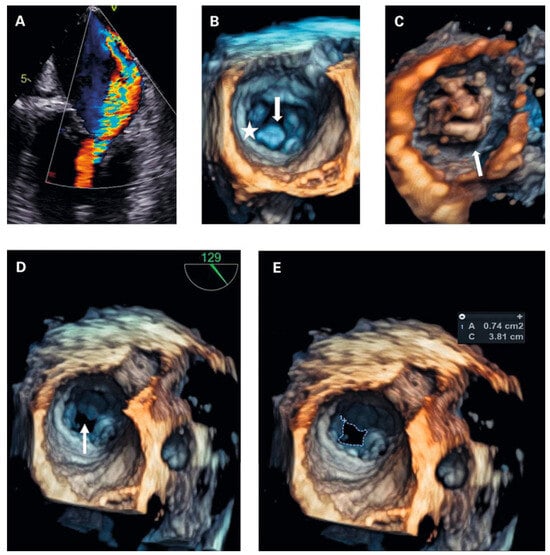
Figure 4.
(a) transoesophageal echocardiography (tOe, 30°): Colour doppler revealed severe mitral regurgitation, which was not apparently visible in transthoracic echocardiography. (B) 4d tOe (surgical view at diastole) showing normal valve orifice at valvular level. Arrow subvalvular thickening. Asterisk posterior mitral valve annulus. (C) 4d tOe (ventricular view at diastole) visualising extensive thickening of the subvalvular apparatus as cause of stenosis. (D) 4d tOe surgical view of stenotic mitral valve area (arrow). (e) 4d tOe planimetry of mitral valve area from surgical view.
Transthoracic and transoesophageal 4D imaging revealed extensive fusion of thickened and shortened chordal structures extending down to the papillary muscles, a shortened posterior leaflet and a prolonged and entirely thickened anterior leaflet with restricted mobility, and mild calcification (fig 3 and fig 4), adding up to a Wilkins Score of 13 points (range 4–16).
The patient received 10 mg of torasemide daily and his dyspnoea improved to class II. No anticoagulation was initiated because of the current haemoptysis.
Because of concerns with regard to oral anticoagulation in a homeless patient, a biological instead of a mechanical prosthesis was implanted.
Further invasive haemodynamic assessment 6 weeks later confirmed the diagnosis of severe mitral stenosis and revealed a pulmonary artery wedge pressure / left ventricular pressure gradient of 25 mm Hg at a heart rate of 100/min, an estimated mitral valve area of 0.9 cm2, and severe postcapillary pulmonary hypertension (mean pulmonary artery pressure 56 mm Hg; fig 5A,B). Coronary angiography was normal.
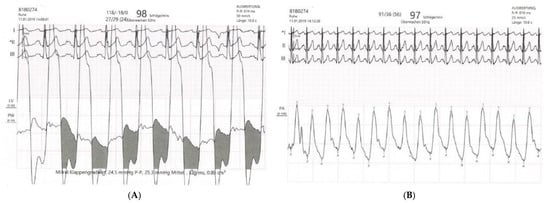
Figure 5.
(A) Invasive haemodynamic assessment of pulmonary artery wedge pressure / left ventricular pressure gradient (mean gradient 24.5 mm Hg, calculated mitral valve area 0.9 cm2). (B) Right heart catheterisation with assessment of mean pulmonary artery pressure (56 mm Hg).
Due to the complex anatomy and combined severe stenosis and severe regurgitation, mitral valve replacement with an Edwards Lifesciences C-E Perimount Magna Mitral Ease 29 mm bioprosthesis was performed. Because of concerns with regard to oral anticoagulation in a homeless patient, a biological instead of a mechanical prosthesis was implanted.
Histology confirmed the fusion of the subvalvular apparatus and was remarkable for extensive fibrosis and mucoid degeneration of the valve leaflets. Rheumatic nodules and calcifications were absent.
Three months after surgery, the haemoptysis and dyspnoea had resolved completely. Echocardiography showed a normally appearing mitral bioprosthesis with an effective valve orifice of 2.3 cm2, and normal pressure gradients. Six months after surgery the patient reported a weight gain of 40 kg, and mild shortness of breath during ordinary activity, which was a ttributed to overweight and physical unfitness.
Discussion
Rheumatic heart disease is still the leading cause of acquired heart disease in children and young adults living in developing countries [1], despite a decrease in the prevalence of rheumatic heart disease worldwide. It still accounts for 15% of all patients with heart failure in endemic countries, often affecting those with inadequate access to health care and unrecognised exposure to group A Streptococcus (GAS). A first episode of acute rheumatic fever due to the crossreactive autoimmune response in GAS pharyngitis can result in carditis and valvulitis. A single severe episode or more often repetitive infections may lead to significant rheumatic heart disease with evolving valvular lesions over the following decades [2], and the mitral valve is involved in most of the cases.
Mitral stenosis typically presents with exertional dyspnoea and reduced exercise tolerance. Haemoptysis occurs in up to 50% of patients with severe mitral stenosis [3].
Bronchial bleeding in left-heart disease – such as mitral stenosis – is due to rupture of engorged submucosal bronchial veins. Although the bronchial arterial blood supply is part of the high pressure systemic circulation, the bronchial veins drain mainly into the (normally low pressure) pulmonary circulation [4] by anastomoses to the pulmonary veins [5]. Under conditions of elevated pulmonary venous and left atrial pressures caused by left heart disease, these veins enlarge and become prone to rupture, for example triggered by mechanical stress induced by coughing [6].
In the treatment of haemoptysis in general, it is most important to secure the airways and maintain oxygenation. Beside treating the cause, specific therapies such as bronchoscopic interventions, bronchial artery embolisation or surgery have to be considered in cases of life-threatening bleeding [7]. However, most bleeding events are self-limiting with conservative measures. If haemoptysis occurs owing to left heart disease, as in this case, reduction of the elevated left atrial pressure is crucial [6].
The overall survival rate of patients with untreated symptomatic mitral stenosis is inversely related to functional NYHA class [8]. The 5-year survival rate is about 60% in NYHA class II, but only 10% in NYHA class IV [8,9]. As in our patient, weight loss may result from chronic advanced heart failure.
Percutaneous mitral balloon commissurotomy (PMBC) is generally preferred to open surgical commissurotomy in symptomatic patients with favourable valve morphology. Several scoring systems such as the Wilkins score can be used to assess the suitability for PMBC [10]. However, in this case the anatomy was not favourable and furthermore, the severe mitral regurgitation was a contraindication for PMBC.
The discrepancy between severe mitral regurgitation at the time of diagnosis and the lack of prominent V waves on pulmonary artery catheterisation is best explained as a result of the diuretic therapy and the time delay to invasive assessment (which was because of insurance issues). This may have decreased the grade of severity.
Multimodality echocardiography with additional 4D imaging did add important anatomical information to the understanding of this valve pathology and guiding therapeutic decisions, and should be included in the evaluation of mitral valve disease.
Conclusion
Today, severe rheumatic heart disease is a rare entity in the Western world, and therefore we hope that this case will increase the awareness of rheumatic heart disease, especially in patients with extracardiac manifestations, which may lead to a challenging diagnostic pathway like in this case.
Disclosure statement
No financial support and no other potential conflict of interest relevant to this article was reported. We thank Dr. Achim Häussler, Cardiac Surgery, Stadtspital Triemli, Zurich, for his support.
References
- Horstkotte, D.; Niehues, R.; Strauer, B.E. Pathomorphological aspects, aetiology and natural history of acquired mitral valve stenosis. Eur Heart J. 1991, 12 (Suppl B), 55–60. [Google Scholar] [CrossRef] [PubMed]
- Carapetis, J.R.; Beaton, A.; Cunningham, M.W.; Guilherme, L.; Karthikeyan, G.; Mayosi, B.M.; et al. Acute rheumatic fever and rheumatic heart disease. Nat Rev Dis Primers. 2016, 2, 15084. [Google Scholar] [CrossRef] [PubMed]
- Abdulmalak, C.; Cottenet, J.; Beltramo, G.; Georges, M.; Camus, P.; Bonniaud, P.; et al. Haemoptysis in adults: a 5-year study using the French nationwide hospital administrative database. Eur Respir J. 2015, 46, 503–11. [Google Scholar] [CrossRef] [PubMed]
- Charan, N.B.; Thompson, W.H.; Carvalho, P. Functional anatomy of bronchial veins. Pulm Pharmacol Ther. 2007, 20, 100–3. [Google Scholar] [CrossRef] [PubMed]
- Woolley, K.; Stark, P. Pulmonary parenchymal manifestations of mitral valve disease. Radiographics 1999, 19, 965–72. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Raju, S.; Das, A.; Mehta, A.C. Vessels of the Central Airways: A Bronchoscopic Perspective. Chest 2016, 149, 869–81. [Google Scholar] [CrossRef] [PubMed]
- Ittrich, H.; Bockhorn, M.; Klose, H.; Simon, M. The diagnosis and treatment of hemoptysis. Dtsch Arztebl Int. 2017, 114, 371–81. [Google Scholar] [CrossRef] [PubMed]
- Chandrashekhar, Y.; Westaby, S.; Narula, J. Mitral stenosis. Lancet 2009, 374, 1271–83. [Google Scholar] [CrossRef] [PubMed]
- Olesen, K.H. The natural history of 271 patients with mitral stenosis under medical treatment. Br Heart J. 1962, 24, 349–57. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, H.; Falk, V.; Bax, J.J.; De Bonis, M.; Hamm, C.; Holm, P.J.; et al. ESCScientific Document Group 2017 ESC/EACTSGuidelines for the management of valvular heart disease. Eur Heart J. 2017, 38, 2739–91. [Google Scholar] [CrossRef]
© 2022 by the author. Attribution - Non-Commercial - NoDerivatives 4.0.