Abstract
Cardiovascular diseases (CVD) and complications occur prematurely and approximately 1.5 times more frequently in patients with rheumatoid arthritis (RA) compared to the general population. The higher level of CV complications is insufficiently explained by the traditional cardiovascular (CV) risk factors. There is substantial evidence that chronic systemic inflammation contributes to excess CVD in RA and that effective suppression of RA-associated inflammation reduces CV morbidity. CV risk assessment and management tools were developed many years ago for the general population. A Swiss expert group consisting of cardiologists and rheumatologists has elaborated the following pragmatic guidelines for the management of CV risk in patients with RA. In addition to systematic screening for and reduction of traditional individual CV risk factors based on the national risk score model, the direct link between inflammation and the development of atherosclerosis demands early and effective control of joint and systemic inflammation. Antirheumatic therapy preferably includes methotrexate, a disease modifying antirheumatic drug (DMARD) and, if necessary, tumour necrosis factor-α blockers or other biologics, because they are not only effective in the treatment of RA but might also reduce excess CV events in patients with RA. Additional treatment should be initiated according to the strategies for lowering circulating lipids and blood pressure as published by the European Society of Cardiology and European Atherosclerosis Society. Pharmacological interventions should be complemented by lifestyle changes.
Introduction
1 This is also applicable for other chronic inflammatory diseases such as SLE, ankylosing spondylitis, and psoriatic arthritis.
It is the strong impression of Swiss experts in rheumatology and cardiology that current guidelines, in particular the recent EULAR guidelines [1], are not sufficiently adhered to by specialists and primary care physicians in Switzerland, most likely due to their complexity. Therefore, a Swiss expert group consisting of cardiologists and rheumatologists elaborated user-optimised consensus recommendations on cardiovascular (CV) risk management in patients with rheumatoid arthritis (RA) to address the underappreciated problem of elevated CV risk in RA patients. The ad-hoc expert group convened to discuss and formulate evidence-based consensus opinions without systematic processing of the literature. The aim was to present current “best practice” to primary care physicians and rheumatologists.
Rheumatoid arthritis and risk for cardiovascular disease
Patients with rheumatoid arthritis (RA) have a 50% increased risk for cardiovascular diseases and their complications as compared to the general population.
RA is a chronic inflammatory joint disease of unknown aetiology affecting approximately 1% of the general population [2,3]. As compared to the general population, it is associated with an approximately 50% increase in CV morbidity and mortality, predominantly resulting from accelerated coronary and cerebrovascular atherosclerosis [4–7].1 It has been demonstrated that the prevalence of cardiovascular disease (CVD) in RA patients is increased to an extent comparable to type 2 diabetes mellitus (Figure 1) [8].
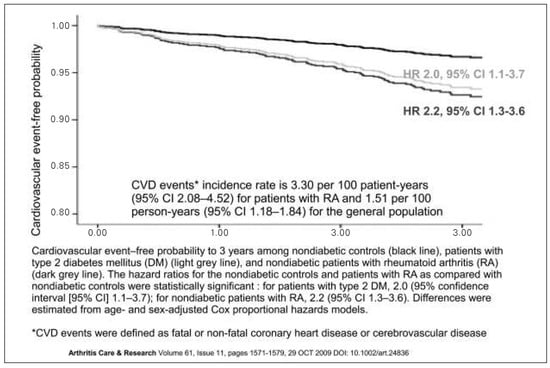
Figure 1.
Prevalence of cardiovascular disease. The prevalence of cardiovascular disease in RA patients is comparable to patients with type 2 diabetes mellitus. From: Peters MJ, et al. Does rheumatoid arthritis equal diabetes mellitus as an independent risk factor for cardiovascular disease? A prospective study. Arthritis Rheum. 2009;61(11):1571–9. Reprinted with permission.
The increased incidence of CVD cannot be explained by traditional CV risk factors alone [9,10]. Various disease-related mechanisms may contribute to premature vascular damage, including an increased synthesis of pro-inflammatory mediators (cytokines, chemokines and adhesion molecules), autoantibodies against endothelial cell components or anti-apoA1 lipoprotein, perturbations in T-cell subsets, genetic polymorphisms, hyperhomocysteinaemia, oxidative stress, abnormal vascular repair, and iatrogenic factors [11,12]. Particularly, the systemic inflammation seems to account for the excess CV risk [13]. There is strong evidence that chronic inflammatory markers are independently associated with CV mortality and morbidity in RA patients [14,15,16]. Immune dysregulation may also play a role [17]. Moreover, the prevalence of CV risk factors such as dyslipidaemia, diabetes, hypertension, higher Body Mass Index, or impaired physical fitness may be increased in RA patients [18,19]. Tobacco smoking is associated with both CVD and the development of RA, in particular in genetically susceptible individuals [20].
Although newer disease modifying antirheumatic drugs (DMARDs) and biologic agents have substantially advanced the management of RA and other forms of inflammatory arthritis in the past few years, CVD morbidity and mortality remain significantly increased in patients with RA [21,22,23]. But the lack of CV risk assessment tools and evidencebased recommendations developed specifically for RA patients has impeded the implementation of this knowledge in the delivery of clinical care.
Treatment goals
Complete clinical remission is the primary treatment goal in the management of RA using early and effective antirheumatic treatment.
There is broad consensus among experts that an adequate control of disease activity is a prerequisite to lower the CV risk in RA patients. Long-term clinical remission has become a realistic treatment goal in many patients due to new drug treatment strategies that improve clinical and radiographic outcomes [1,24]. The current standard for measuring disease activity is the EULAR Disease Activity Score (DAS) and its modified version, the DAS28, including 28 joints [25]. The score is calculated using a formula that incorporates counts of tender and swollen joints (28 joints maximum), an evaluation of the general health by the patient (Visual Analogue Scale 0 to 100), and the erythrocyte sedimentation rate or the C-reactive protein as systemic inflammation markers. Although various definitions of remission have been proposed during the last decade, the most widely used definition in randomised controlled trials has been a DAS28 index with a cut point of <2.6 [26].
Treatments currently used in the management of chronic inflammatory disorders may affect CV risk. Table 1 gives an overview of the current pharmacotherapy with information regarding CV risks associated with each drug class.

Table 1.
Current pharmacotherapy of RA. Effect of different treatment options on cardiovascular risk in RA patients and source of clinical evidence.
Cardiovascular risk assessment and estimation of risk in the general population and RA patients
A systematic CV risk-factor screening must be performed in RA patients using the risk score model presented by the Swiss Society of Cardiology (SGK/SSC) and the working group on lipids and atherosclerosis (AGLA) in 2012 (www. agla.ch).
In RA patients, the derived CV risk estimate should subsequently be multiplied by a factor of 1.5, without any restrictions. For this purpose the risk score chart used in Switzerland was modified accordingly (Figure 2). It allows simple and direct assessment of risk score for RA patients.
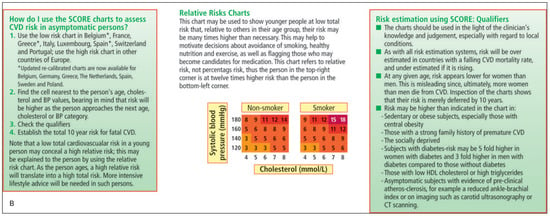
Figure 2.
SCORE – European Low Risk Chart for Rheumatoid Arthritis Patients. From (Figure 2A modified by the authors of this manuscript): Perk J, et al. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). Eur Heart J. 2012;33(13):1635–701. Reprinted with permission.
Assessment of cardiovascular risk should be performed annually in every RA patient.
The major traditional risk factors for CVD are tobacco smoking, elevated blood pressure, elevated serum total cholesterol (TC) and low-density lipoprotein cholesterol (LDLC), low serum high-density lipoprotein cholesterol (HDL-C), diabetes mellitus, and advancing age. The quantitative relationship between these risk factors and coronary heart disease (CHD) risk has been elucidated by the Framingham Heart Study and other studies [27,28]. These show that the major risk factors are additive in predictive power. Accordingly, the total risk of a person can be estimated by a summation of the risks imparted by each individual major risk factor. The risk for CHD is assessed with risk charts, and usually the predicted 10-year risk of fatal
Figure 2.SCORE – European Low Risk Chart for Rheumatoid Arthritis Patients. From (Figure 2A modified by the authors of this manuscript): Perk J, et al. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). Eur Heart J. 2012;33(13):1635–701. Reprinted with permission.
CHD is calculated. The European risk prediction system SCORE (Systemic COronary Risk Evaluation) has been developed to define the lifestyle, risk factor, and therapeutic targets for CVD prevention. SCORE is representative of typical European populations, and the risk score system has been optimised for coronary prevention in European clinical practice.
All risk score charts are presented for TC. However, new data show that HDL-C can substantially contribute to risk estimation if entered as a separate variable as opposed to the TC/HDL-C ratio [29,30]. For example, HDL-C modifies risk at all levels of risk as estimated from the SCORE cholesterol charts. Therefore, a new risk score chart was developed that includes HDL-C.
The Swiss national guidelines for the prevention of CV events were published in 2005 by the Swiss Society of Cardiology (SGK/SSC) and the working group on lipids and atherosclerosis (AGLA) [31]. In these guidelines, CV risk is stratified into low (<10%), intermediate (10–20%) and high (>20%). Primary prevention with statins and/or antihypertensive agents is only indicated in the general population when this 10-year risk is above 10% (Figure 2).
It is very likely that the use of available CV risk score charts underestimates the CV risk in RA patients, since only traditional risk factors are considered. Nontraditional risk factors such as disease severity and duration significantly increase the CV risk in patients with RA [32,33,34]. However, other factors such as rheumatoid factor (RF), anti-cyclic citrullinated peptide antibodies (anti-CCP) positivity are also relevant [35,36]. Therefore, EULAR evidence-based recommendations introduce a multiplication factor 1.5 to adapt the risk score models for RA patients, provided that 2 of the following 3 criteria are fulfilled: Disease duration of over 10 years, RF or anti-CCP positivity, presence of certain extra-articular manifestations [1]. Our Swiss expert group proposes to extend this recommendation to all RA patients in order to simplify management strategy and to acknowledge the fact that most of the epidemiological studies on the risk of CVD in RA did not stratify the patients according to these criteria. In addition to RA, this approach should be implemented in patients with other forms of inflammatory arthritis such as psoriatic arthritis and ankylosing spondylitis, and autoimmune diseases such as systemic lupus erythematosus.
Intervention strategies on the basis of the cardiovascular risk profile
The same intervention strategies for lowering of LDL-C in the normal population with elevated CV risk should be used in RA patients.
The LDL-C goals are derived from the total CV risk score, as proposed by ESC/EAS in their guidelines for the management of dyslipidaemia in 2011 (see Table 2).

Table 2.
Intervention strategies for lowering LDL-C levels in patients with RA. For lowering LDL-C levels in RA patients the same strategies as in the normal population with elevated cardiovascular risk should be used. (Reprinted from: Perk J et al. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). Eur Heart J 2012;33:1635–1701, with permission ).
The management of arterial hypertension in RA patients is based on the levels of systolic and diastolic blood pressure and the CV risk as described in the ESH/ESC guidelines in 2013. The antihypertensive treatment should be initiated according to the recommendations in
Table 3, which was modified by the expert group from the corresponding table in the ESH/ESC guidelines for specific use in RA patients.

Table 3.
Antihypertensive treatment strategies in RA patients. (Modified from Mancia G, et al., Eur Heart J. 2007;28:1462–536, with permission.).
It is well-established that low levels of HDL-C and high levels of TC, LDL-C and TG are associated with an increased risk for CV events in the general population. In particular, the TC/HDL-C ratio is an important prognostic factor for future CV events [37,38]. Intervention trials have demonstrated that cholesterol modification, especially reduction in LDL-C levels is associated with favourable effects on the CV event rate, including CV mortality, especially in patients at high risk [39,40,41,42]. Unfortunately, so far there are no data from intervention trials on the effect of LDL-C lowering pharmacotherapy CV event rate in patients with RA. On the other hand, there is no indication that lipid lowering pharmacotherapy is less efficacious in RA patients.
RA patients with active disease often have low HDL-C levels, high triglyceride levels and unfavourable TC/ HDL-C ratio [43,44]. It appears that DMARDs have beneficial effects on the lipid profile in patients with early active RA [45,46,47,48]. However, the data are conflicting.
Experts from the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS) have developed new guidelines for the management of dyslipidaemia in 2011 [49]. In these guidelines, the SCORE system has been used to categorise patients as very high, high, moderate or low CV risk, as a basis for treatment decisions.
LDL-C remains the primary priority in lipid management. In patients at very high risk (as derived from the SCORE tables), the LDL-C goal is set at <1.8 mmol/l (<70 mg/dl) and/or at least a 50% reduction in LDL-C if the target cannot be reached. In patients at high risk (e.g., marked elevation of only one risk factor), the LDL-C goal is <2.5 mmol/l (<100 mg/dl); in patients with moderate risk, LDL-C should be lowered to <3 mmol/l (<115 mg/dl) (Table 2).
Hypertension should be considered a major risk factor for an array of CV and related diseases [50,51]. Randomised placebo-controlled trials on blood pressure lowering have demonstrated that antihypertensive treatment translates into significant reductions of CV morbidity and mortality [52].
In 2013, ESH/ESC issued new guidelines on the management of hypertension [53]. Blood pressure is categorised as optimal (systolic blood pressure [SBP] less than 120 mm Hg and diastolic blood pressure [DBP] less than 80 mm Hg), normal (SBP 120–129 mm Hg and/or DBP 80–84 mm Hg), and high-normal (SBP 130–139 mm Hg and/or DBP 85–89 mm Hg), followed by 3 grades of hypertension, and a separate category for isolated systolic hypertension, The presence of RA should be considered as a distinct CV risk factor as presented in the modified table of the expert group (Table 3).
According to the new ESH/ESC guidelines, initiation of blood pressure lowering therapy should be based on two criteria, i.e., the level of systolic and diastolic pressure (SBP/DBP) and the CV risk. The goal of treatment is to reduce blood pressure to at least below 140/90 mm Hg (SBP/DBP).
Non-pharmacological interventions
Lifestyle changes should be recommended individually according to the presumed effectiveness and feasibility. Smoking cessation is of paramount interest.
The new ESC/EAS guidelines for the management of dyslipidaemia issued in 2011 stress that lifestyle interventions, including smoking cessation, healthy diet, moderate exercise and moderate alcohol consumption, should be the first step for managing lipids in all patients [49]. Moderate-intensity physical activity is significantly lower in RA patients compared with matched controls [54], and potentially amenable to change. To decrease LDL-C and TC, reduction in dietary saturated fat and trans-fat, as well as cholesterol itself is necessary. Dietary fibre consumption should be increased. It is also recommended to use food enriched with phytosterols. Finally, the guidelines focus on the considerable importance of body weight and central obesity, as they are one of the main problems in western countries.
Pharmacological interventions
Control of the joint disease remains the principal aim, but not all drugs have this far demonstrated additional CV benefits in this population, an element to take into consideration whenever possible, in particular in the high CV risk patient.
Current RA therapy strategies aim at remission with full control of joint and systemic inflammation, a control of the inflammation which is an integral part of the strategy to lower the cardiovascular risk, as suggested in the guidelines of ESC/EAS (2012), EULAR (2010) and ESH/ESC (2013). Classical nonsteroidal antiinflammatory drugs (NSAIDs) and cyclooxygenase-2 inhibitors (COXIBs) are very commonly used in the treatment of RA. There is still controversy about the effects of NSAIDs on CV disease and mortality. According to the findings of a meta-analysis, high dose regimens of ibuprofen and diclofenac are associated with an increased risk of CV events, but high doses of naproxen are not associated with such an excess [55]. In another cohort study, this risk appeared to be mediated mainly by the effect of NSAIDs on blood pressure [56] stressing the importance of BP control. Of note, the anti-inflammatory effect of NSAIDs could potentially counteract this risk, as in a cohort study in RA patients, the use of NSAIDs was not associated with excess CVD or mortality from all causes [57].
Corticosteroids are commonly used in rheumatic patients and may influence the CV risk in two competing ways. On one hand their anti-inflammatory and antiproliferative action on vessel walls may be cardioprotective, and on the other they affect blood pressure, insulin resistance, lipid profiles, body weight, platelet activity and fat distribution, especially when used long-term at high doses [58,59]. High-dose corticosteroid therapy appears to be associated with a dose-dependent CV risk increase in RA patients, while exposure to low doses of corticosteroids for 1–3 years does not [57,60].
There are several indications that DMARDs can alter CV risk by influencing atherosclerotic processes directly through suppression of inflammation or indirectly by affecting CV risk factors. However, few studies have investigated their effects on the occurrence of CVD in RA patients.
Methotrexate (MTX) is a cornerstone of the treatment of RA. It improves the clinical signs of disease activity and slows radiographic progression. Patients with RA who take MTX have lower mortality rates and decreased CV morbidity [4,61,62]. In a large cohort study of RA patients MTX was associated with a significant decrease in the rate of acute myocardial infarction [63]. A recent meta-analysis came to the conclusion that MTX use is associated with a reduced risk of CVD events in patients with RA [64].
The sparse available data on leflunomide suggest that, despite the small percentage of RA patients developing hypertension under leflunomide, it may reduce the risk of myocardial infarction, but the results are controversial [4,65,66,67].
Tumour necrosis factor-α blocking agents (TNF-α blockers) are potent inhibitors of inflammation and radiographic progression in RA patients. According to the current Swiss and EULAR RA treatment recommendations, TNF-α blockers should be favoured as first-line biologics [68,69]. Their role in the prevention of CV events in RA patients is of great interest, a further argument currently favouring their use. Several studies indicate that TNF inhibition might improve endothelial function and be associated with decreased CV morbidity, especially in patients with clinical response to therapy [4,70,71,72]. Two recent meta-analyses support the hypothesis that treatment with anti-TNF agents is associated with a reduced risk of all CV events, myocardial infarction, and cerebrovascular accidents in RA patients [73,74]. The effects of other biologic agents, including abatacept, rituximab and tocilizumab, on CV morbidity in RA remain to be determined [75].
Cardiovascular therapy
Drug treatment of dyslipidaemia and arterial hypertension is identical to that of patients without RA. Preferred first-line treatments are statins, ACE-inhibitors and angiotensin receptor blockers.
Anti-platelet therapy is only recommended in RA patients with established CV disease.
The ESC/EAS guidelines (2011) and the EULAR evidence-based recommendations (2010) propose that if lipid targets are not met with lifestyle changes alone, statins are the treatment of choice for lowering LDL cholesterol levels [1,49]. The effects of statins in patients with RA might be of additional benefit, since they possess potential anti-inflammatory properties. The selection of a specific statin should be based on consideration of the extent of LDL cholesterol lowering required and the individual’s CV risk. The Trial of Atorvastatin in Rheumatoid Arthritis (TARA) randomised 116 patients with RA in a double-blind placebo-controlled trial to receive 40 mg of atorvastatin or placebo as an adjunct to existing DMARDs [76]. The authors found that statins mediated modest but clinically apparent anti-inflammatory effects with lowering of CRP after six months. This study provides some evidence to use statins to selected RA patients. However, it needs confirmation in a larger population of RA patients with assessment of systemic inflammation, morbidity, longer follow-up, CV risk factors outcome (major cardiovascular events). Cost effectiveness and quality of life issues also need to be taken into account. Combination therapy with a cholesterol absorption inhibitor (ezetimibe) or nicotinic acid (niacin) may be considered if the LDL-C target is not met.
Five classes of drugs are considered appropriate as first-line agents for the treatment of hypertension: diuretics; angiotensin-converting enzyme (ACE) inhibitors; calcium channel blockers (CCBs); angiotensin receptor blockers (ARBs), and beta-blockers. However, the use of beta-blockers in hypertension remains controversial.
The EULAR consensus guidelines recommend the preferential use of ACE-inhibitors and ARBs because of their favourable effect on inflammatory markers and endothelial function in patients with RA [77,78]. Anti-platelet therapy is recommended for patients with established CV disease [79]. For patients with increased CV risk the benefit is lower [80]. The benefit of anti-platelet therapy in RA patients is therefore doubtful, and has to be weighted with the increased risk of gastro-intestinal events in patients with concomitant NSAIDs.
Funding/potential competing interests:
No financial support and no other potential conflict of interest relevant to this article were reported.
References
The full list of references is attached to the online version on www.cardiovascmed.ch.
© 2015 by the author. Attribution - Non-Commercial - NoDerivatives 4.0.