Abstract
Metallic coronary stents have been developed to overcome acute complications after percutaneous coronary intervention. However, this durable implant is associated with complications in the long-term, such as stent fracture, re-stenosis and thrombosis. Hence, a coronary implant that will provide temporary support before being resorbed has been proposed as a valuable alternative and is currently under investigation. In this article, we report the one-year clinical outcome after implantation of the first bio-resorbable everolimuseluting vascular scaffold (BVS) performed at our institution.
Introduction
The safety and efficacy of percutaneous coronary interventions (PCI) has continuously improved since its inception more than 30 years ago. The breathtaking growth of PCI was rendered possible by the advent of stents that remarkably improved the safety of PCI by reducing peri-procedural vessel closure due to dissections and the need for emergent coronary artery bypass graft surgery. Since the first coronary stent was developed in Lausanne by Hans Wallsten, Ake Senning and Ulrich Sigwart [1] in the early 1980s, the technology drastically evolved in 2002 with the launch of the drugeluting stent (DES) that consistently lowered neointimal hyperplasia and the risk of re-stenosis [2].
Since then, however, concerns about the potential toxicity associated with late stent thrombosis stimulated the development of safer and more effective next-generation drug-eluting stents. One of the most extreme innovations in this area was the development of drug-eluting coronary implants from a bio-erodible platform [3,4].
Here, we report the one-year follow-up of the first patient with a BVS bio-resorbable everolimuseluting vascular scaffold in Switzerland. This device has a bio-resorbable polymer backbone of poly-L-lactic acid with a polymer coating of poly-D,L-lactide that contains and controls the release of the anti-proliferative drug, everolimus, and is currently under investigation at our institution (ABSORB Cohort B and ABSORB EXTEND trials) [2]. Polylactic acid is a biodegradable, thermoplastic and aliphatic polyester derived from cornstarch (“maize”).
Case report
A 51-year-old male was referred for coronary angiography. His known cardiovascular risk factors were insulin-dependent diabetes mellitus, dyslipidaemia, arterial hypertension, obesity (BMI 33 kg/m2) and a family history of coronary artery disease. Coronary angiography (Figure 1) showed patent left main (LM), left anterior descending artery (LAD) and circumflex artery (LCx) without obstructive disease. A second obtuse marginal branch (OM2) and the distal right coronary artery (RCA) demonstrated high-grade stenoses. After angioplasty of OM2 with subsequent implantation of one 2.25 × 12 mm-everolimus-eluting stent, (XIENCE V, Abbott Vascular, Santa Clara, CA), at 16 atm, the stenosis in the RCA was treated with a bio-resorbable everolimus-eluting coronary scaffold. Briefly, a 6F Judkins right 4 guiding catheter was positioned at the RCA ostium. A 0.014’’ guidewire (BMW, Abbott Vascular, Santa Clara, CA) was advanced into the posterolateral branch and the lesion was treated by single balloon inflation (3.0 × 12 mm-Apex, Boston Scientific, Nantik, MA), at 12 atm, which was followed by the implantation of one 3.0 × 18 mm-everolimus-eluting bioresorbable vascular scaffold, (BVS, Abbott Vascular, Santa Clara, CA), at 12 atm. The control angiography, the intravascular ultrasound and the optical coherence tomography showed satisfactory results. The recovery was uneventful and the patient was discharged on the following day. A lifelong prescription of aspirin (100 mg daily) was prescribed, and clopidogrel (75 mg daily) was prescribed for 12 months. At 6-month follow-up (Figure 2), control angiography was performed and demonstrated a smooth artery without visual angiographic late loss. Both an intravascular ultrasound study and optical coherence tomography revealed neoendothelialisation of the scaffold struts without significant vessel shrinkage or excessive neointimal hyperplasia. Clinical follow-up to 12 months was uncomplicated.
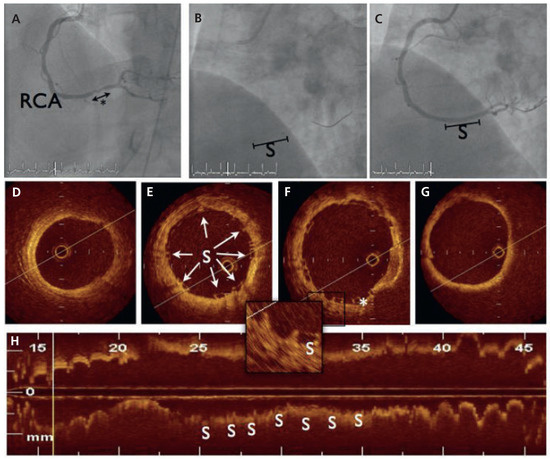
Figure 1.
Angiographic and optical coherence tomography (OCT) findings during index procedure. (A) Dye injection in the right coronary artery (RCA) demonstrated one 10 mm-stenosis of the distal part (*). (B) BVS scaffold platform is radiolucent but two small platinum markers allow accurate placement of the device. (C) Final angiogram after scaffold implantation. (D–G). Cross-sectional OCT imaging from distal to proximal: the BVS struts (S) are discernible with sites showing sharply defined, bright reflection borders, usually described as having a “box-shaped” appearance (arrows). One strut was not apposed to the vessel wall (*). (H) Longitudinal reconstruction.
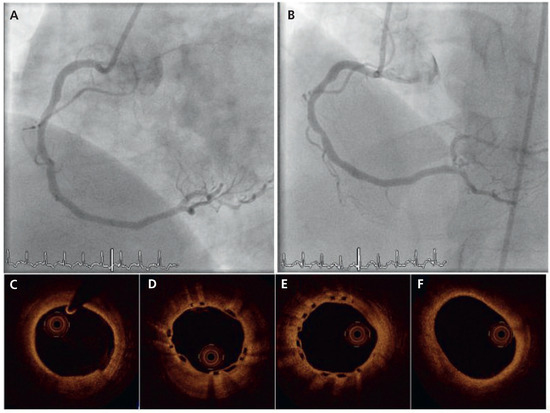
Figure 2.
Angiographic (A,B) and OCT findings (C–F) at 6-month angiographic follow-up.
Discussion
A stent is designed to overcome acute complications after PCI by sealing dissection and decreasing vessel recoil. After this early period, a durable implant is probably useless and increases the stiffness of the vessel, precludes surgical anastomoses and carries a non-trivial risk of late thrombosis. A bio-resorbable vascular scaffold has been conceptually developed in order to temporarily protect against acute PCI-associated complications without exposure to long-term hurdles. This first patient does not prove the rule, but validates this new concept.
Conflicts of Interest
The authors certify that there is no actual or potential conflict of interest in relation to this article.
References
- Sigwart, U.; Puel, J.; Mirkovitch, V.; Joffre, F.; Kappenberger, L. Intravascular stents to prevent occlusion and restenosis after transluminal angioplasty. N Engl J Med. 1987, 316, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.; Windecker, S. Coronary artery stent. In Cardiac Catheterization Coronary & Peripheral Angiography and Interventional Procedures; Informa Healthcare: London, 2009. [Google Scholar]
- Ormiston, J.A.; Serruys, P.W. Bioabsorbable coronary stents. Circ Cardiovasc Interv. 2009, 2, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Tamai, H.; Igaki, K.; Kyo, E.; Kosuga, K.; Kawashima, A.; Matsui, S.; et al. Initial and 6-month results of biodegradable poly-l-lactic acid coronary stents in humans. Circulation 2000, 102, 399–404. [Google Scholar] [CrossRef] [PubMed]
© 2011 by the author. Attribution - Non-Commercial - NoDerivatives 4.0.