Abstract
Moringa oleifera Lam. (Moringaceae) is one of the most essential medicinal plants primarily found in the rainforest area and forest ecosystem, but is now well-adapted in an organized cultivation system. Moringa oleifera (M. oleifera) is well-known as Drumstick tree, Moringa kai, color, Marengo, Moringe, mulangay, Sahjan, and Sajna, which are its native names commonly used. It has nourishing, beneficial, and preventive effects when taken as food and has an extensive scope of high restorative properties with huge dietary benefits. Different parts of the M. oleifera plants, such as leaves, flowers, fruits, seeds, and roots, contain a significant amount of protein, ß-carotene, amino acids, important minerals, and various phenolic compounds. Because of its multifarious health benefits for its therapeutic value, it is considered an essential plant. The plant is found to be blessed with several medicinal characteristics such as antitumor, anti-inflammatory, antiulcer, antipyretic, antiepileptic, antispasmodic, diuretic, antihypertensive, antidiabetic, cholesterol-level down, cell reinforcement, and hepatoprotective. Moreover, it is used traditionally in the local curative system against cardiac problems, and the antifungal properties are efficiently utilized for the treatment of a wide range of ailments. The present review article was designed to explore the nutritional and economic benefits, medicinal and therapeutic applications, and the future biomedical prospects of Moringa with a view towards human wellbeing.
1. Introduction
The drumstick plant is a nutrient-rich green tree of the Moringaceae family with many applications and is grown around many parts of the world including the United States [1,2]. In English, this plant is known as Moringa oleifera (M. oleifera), horseradish tree, or drumstick tree. It is not only used by humans and animals, but it also has many industrial uses [3]. The leaves, fruit, flowers and youthful branches of this tree are utilized as a profoundly nourishing vegetable in various nations including India, the Philippines, Hawaii, Pakistan, and many African countries. In particular, individuals in India have been utilizing it for their day-to-day nourishment for almost 5000 years [4,5,6]. Starting its journey from the northern parts of India, it quickly spread to the southern portions, where ‘Murungai keerai’ (Moringa leaves) and ‘Murungaikaai’ (drumsticks) are among the most popular sources of vegetables. The moringa tree has essentially been colonized throughout the entire Asia, nearly all of Africa, South America, a tiny section of North America, and a few scars in Europe [7,8]. They essentially have versatile roles as nutritional supplement, soil quality enhancement, and use in the water purification system. Moringa plants are also a good source of oil, which is, therefore, the most popular and significant sources of revenue. The majority of the available bioactive phenolic compounds belong to flavonoid groups such as quercetin and kaempferol. Based on the reported results in several literatures, moringa leaves have a potential source of natural antioxidants due to their discernible qualities of protecting cells against free radicals [9]. Furthermore, water coagulation, proteins, and fatty acid methyl esters (FAME) from the M. oleifera seeds are reviewed, to explore their possible industrial applications, in biodiesel production and in the water purification system [10]. The leaves are abundant in nutrition with vitamins C and A, β-carotene, calcium, iron, potassium, and phosphorus including a protein level of 27 percent [8]. Moringa leaves have the same calcium content as four glasses of milk, the same amount of vitamin C as found in seven oranges, and three times the potassium found in bananas [11,12]. They further contain three times as much iron as spinach, four times as much vitamin A as in carrots, and half the protein of milk, according to research reported [13,14].
2. Methodology: Literature Search
Several electronic databases such as Pubmed, Medline, Embase, and Google Scholar were accessed for retrieving the available relevant literatures. We thoroughly searched to identify articles published till 2021, on different aspects of antidiabetic, anticancerous, phytochemical properties and their mode of action against several cancer cell lines. The keywords and terms used for the search included single and various combinations of the following: “Moringa oleifera”, “medicinal” “pharmaceuticals”, “nutritional”, “economic importance” “therapeutics” and “horticultural crops”. Under each of the searches, the abstracts and titles were screened and the full text versions of articles that met the criteria were downloaded.
3. Traditional and Other Uses of Moringa oleifera
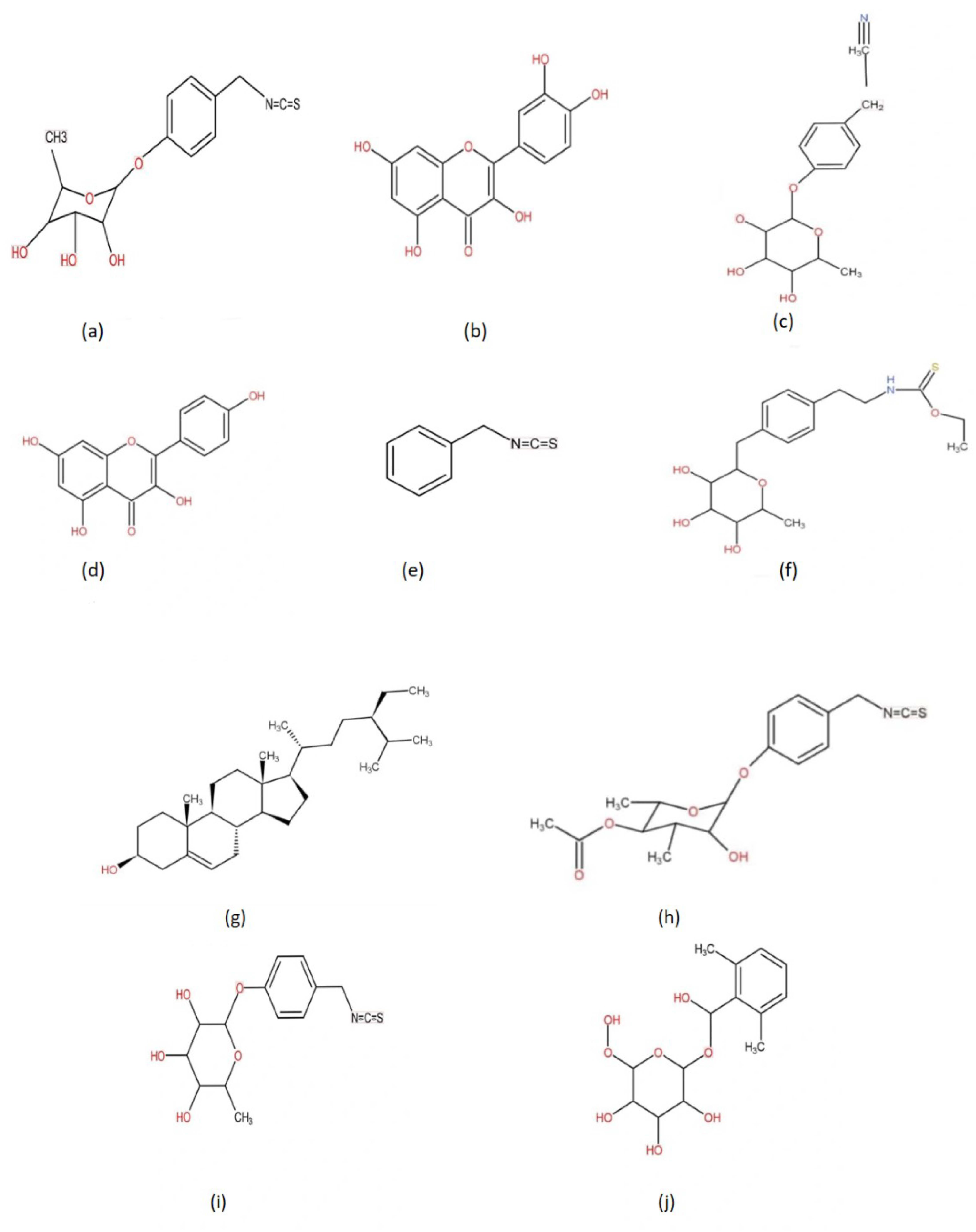
The M. oleifera tree has a wide range of therapeutic applications, including both prevention and therapy. Its bark, seeds, oil, sap, leaves, roots, and flowers are used in conventional medicine. It provides an immediate remedy for stomach, catarrh, malignancy, cancer, ulcer, blood sugar, nerve, cramps, hemorrhoids, cerebral aches, sore gums, stomach-related diseases, respiratory, gastric, and resistant frameworks [15,16]. It also boosts bone density by increasing calcium levels. Flowers act as cholagogue, stimulant, tonic, and diuretic that can help to enhance bile flow. The plant is antibacterial as well and aids in the preparation of heart circulatory tonic [17]. It strengthens the eyes, skin, cerebrum, liver, and also acts as a blood erythropoietin-stimulating agent. The leaves are used as a poultice for mid-region wounds to eradicate intestinal heat in a traditional therapy. The Indian Ayurvedic Pharmacopoeia recommended the uses of dried root bark for goitre, glycosuria, and lipid problems (together with dried seeds), and leaf, seed, root bark, and stem bark for internal abscess and piles [1]. To cure conjunctivitis, the leaves are used as eyewash while a drink made from the leaves of a drumstick tree is good for people with asthma and bronchitis when consumed regularly. A dish of soup is made from the decoction of drumstick leaves. Decoction produced with young drumstick flowers and cow milk is a fantastic tonic for male and female sexual infertility and practical failure [12,18]. Humans have long history of consuming this highly valued tree for a variety of domestic uses [19]. The powdered form of the bark works on the activity of sperm and corrects abnormalities like early discharge in males. An effective home-grown cure for cholera is the integration of new leaf extract of moringa, one spoon of honey, and one glass drink of exquisite coconut water [20]. It is beneficial against diarrhea, jaundice, and also colitis. A typical remedy for dysuria and a high acidic rate in urine is a new leaf extract of moringa combined with carrot or cucumber juice to cure pimples and clogged pores. Aging spots can efficiently be treated with drumstick leaf extract treated with lime juice which enhances the normal brilliance of the complexion [21]. Many medicinal properties such as antitumor, anti-inflammatory, antiulcer, antipyretic, antiepileptic, antispasmodic, diuretic, antihypertensive, and antidiabetic are mentioned in Table 1. Various M. oleifera plant-derived bioactive compounds are shown in Figure 1.

Table 1.
Various beneficial phytochemicals of M. oleifera and their medicinal effect.
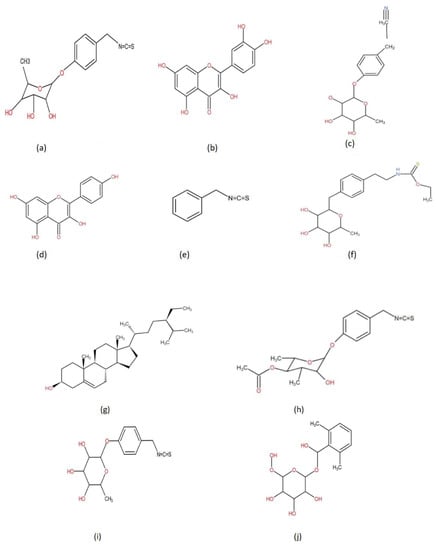
Figure 1.
Selected bioactive compounds from Moringa oleifera: (a) 4-(-L-rhamnopyranosyloxy) benzlisothiocyanate, (b) Quercetin, (c) Niazirin, (d) Kaempferol, (e) Benzyl isothiocyanate, (f) Niazimisin, (g) β-sitosterol, (h) 4-(4-O-acetyl-a-L-rhamnosyloxy) benzyl isothiocyanate, (i) 4-(α-l-rhamnopyranosyloxy) benzyl isothiocyanate, (j) Moringine.
Several parts of the M. oleifera tree served as great reservoir of extraordinary glucosinolates, flavonoids and phenolic compounds [26,27], carotenoids, tocopherols, polyunsaturated fats (PUFAs), profoundly bioavailable minerals, and folate [28]. Among glucosinolates, 4-O-(a-L-rhamnopyranosyloxy)-benzyl glucosinolate (glucomoringin) is the most transcendent in the stem, leaves, flowers, fruits, and seeds of M. oleifera [26]. In the roots, benzyl glucosinolate (glucotropaeolin) is the most prominent bioactive compound present. The most elevated substance of glucosinolate belonging to a group of sulphur-containing glycosides is found in the leaves and seeds. Among the flavonoids group, flavonol glycosides (glucosides, rutinosides, and malonyl glucosides) of quercetin > kaempferol > isorhamnetin are mostly found in different parts of the tree, along with the roots and seeds. In the roots, benzyl glucosinolate (glucotropaeolin) is the most prominent. The highest level of glucosinolate is found in the leaves and seeds. Almost all the parts of M. oleifera are beneficial for human wellness as well as having economic importance as shown in Table 2 and Figure 2.

Table 2.
Various extracts of Moringa oleifera and their biological activities.

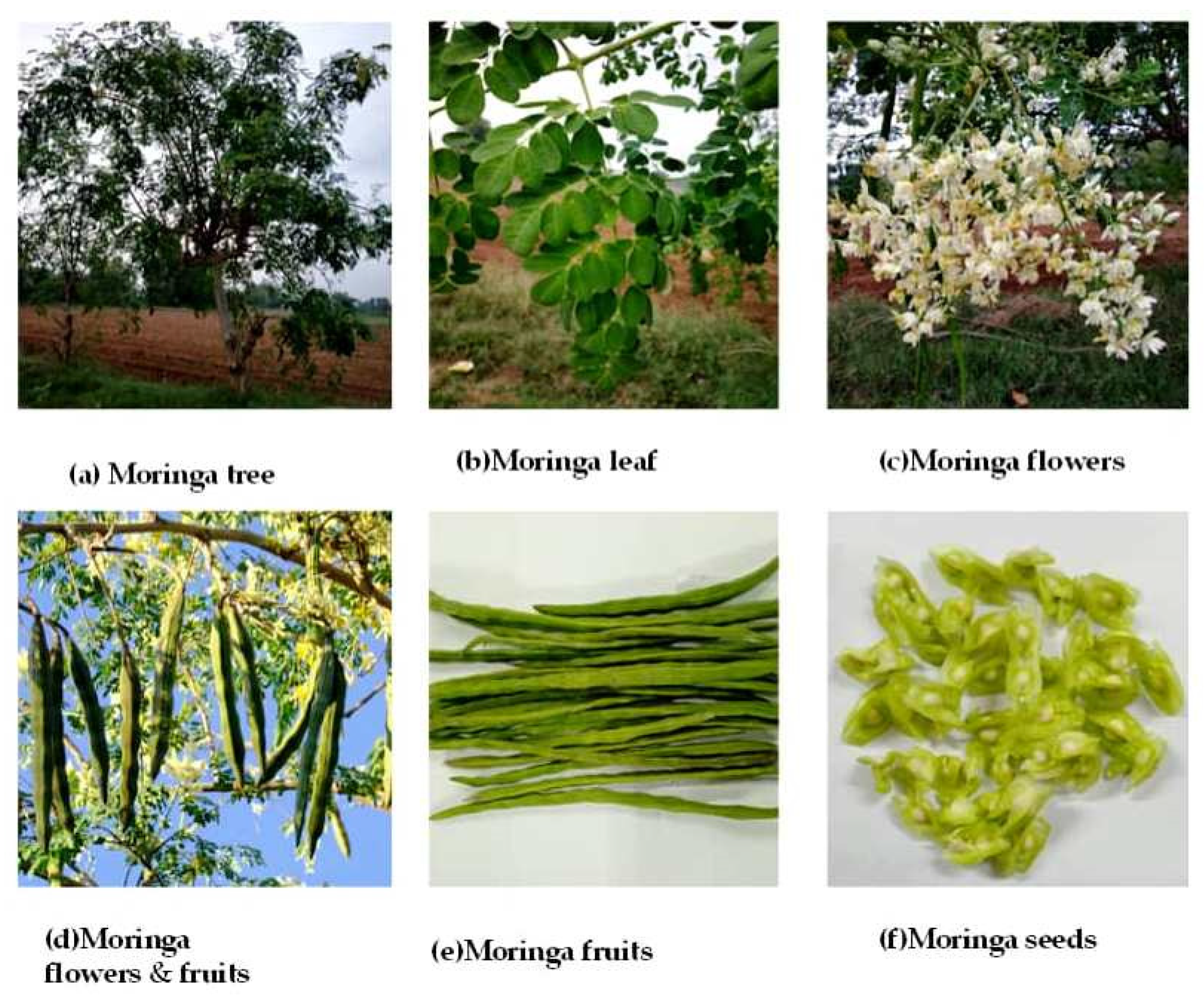
Figure 2.
(a–f). Different economic plant parts of Moringa.
3.1. Leaves
M. oleifera leaves (Figure 2b) are a good source of beta-carotene, iron, protein, vitamin C, and potassium. Cooked leaves are used in a similar way as spinach is used. Its leaves are dried and converted into powder form; sulfur-bearing methionine and cysteine are two major amino acids present in moringa leaves [39]. They are also found in lesser amounts in several green leafy vegetables.
3.2. Flowers
When cooked, the flowers are edible and have a mushroom flavor. Cough medicine is prepared from flowers soaked with honey. The picture of moringa flower is shown in Figure 2c,d.
3.3. Ben Oil
M. oleifera seeds provide around 38–40% oil which is transparent, odorless, and poses the concerns of rancidity similar to other plant oils. The seed cake that remains as residue after the oil has been further extracted can be used as manure or as medium to purify the contaminated water. Oil derived from the moringa seed is beneficial to treat ear infections and skin ailments in salves. Mosquitoes are supposed to be deterred by applying oil to the skin and in this way it can be used as repellant.
4. Morphology of Moringa oleifera
M. oleifera is a long-lived, evergreen tree with a straight trunk and corky, whitish bark that grows up to 6 m in height. The tree has a tuberous tap root and a weak stem. The leaves are bright green, compound, tripinnate and 30–60 cm long. The horizontal pamphlets are elliptic fit, while the terminal one is obovate. The natural product units are pendulous, green, turning to greenish-brown, three-sided, and split longwise into three sections when dry. The units are 30–120 cm long 1.8 cm wide and tightening at the two closures. The cases contain around 10 to 20 seeds inserted in the plump essence (Figure 2a–f).
5. Cultivation
Trees developed from seeds have longer roots (which helps with stability and availability to water) than those grown from cuttings, which have much shorter roots [40,41]. It grows nicely in semi-bone-dry, hot, and moist or sandy loam soils. The range of seeds is 3000 to 9000. Seeds germinate in two weeks at a maximum depth of two centimeters. Seedlings can be transferred when they reach 30 cm (3–6 weeks after germination) when planting is scheduled in a nursery [22]. When seedlings reach 30 cm (3–6 weeks following germination), they can be transported to a nursery for planting [42]. When seeds are few and/or labor is not a constraint, cutting is the recommended method [43] by ensuring that moringa production and growing practices are significantly improved. Plants grown from seeds generate lower-quality fruits, according to research [24]. When hard wood cuttings (1–2 m long, 4–16 cm diameter) [24,25,28,29] from mature trees are planted during the rainy season, burying one-third of them in the soil, they lead to the establishment of roots quickly and attain a significant size in a few months [26]. From June to August, it is propagated by planting 1–2 m long limb cuttings and after 6–8 months of planting, it begins to yield pods, but does not bear after the second year. Cultivation is primarily dependent on creating the correct environment for the plant. In well-draining soil, a seed is sown an inch below the surface which germinates with specified ambient parameters as mentioned in Table 3. Plant production is between 1.1 and 1.3 million tonnes per year, with a total area of 380 km2 [27]. Hot temperatures are significant for germination. Seeds after sowing should be protected from mice and wooded reptiles, as the seed is nutty and considered a delicious piece by those small animals. Stem cuttings, 10–60cm long, can likewise be planted in summer and spring.

Table 3.
Different parameters for cultivation of M. oleifera.
6. Industrial Applications
6.1. Treatment of Water
Seeds of the M. oleifera are subjected to a fine powder for treating the muddy, unclean water [1,17]. A series of electrical charges is used to purify the water. In between the sloppy particles suspended in the water and the slick particles hanging in the smashed seeds, the small particles are continuously pushed to the pond’s bottom by gravity after approximately 60 min. Examination revealed that the seed settles in the dirt and carries more than 90% of tiny organisms and illnesses with it. Moringa seeds can also be applied to a cleaning agent in the water treatment process [1]. The sloppy particles are continually drawn by gravity as the water sinks to the bottom, after around 60 min. It also carries more than 90% of germs and diseases with it and moringa seeds can also be utilized further as a source of disinfectant. This kind of drinking water treatment has also been reported by other groups of researchers [19,50,51].
6.2. Great Fodder for Cattle
Moringa served as a great feed for cows which may lead to a significantly increase in the weight of domesticated animals by 32 percent and increased the milk output of cows by 43 percent. In addition to hay, one farmer fed his cows only 2 kg of moringa dry matter per day to their regular feed with a 58 percent increase in milk production. It can further be expanded up to 3 kg each day, and milk production by 65% [21,46].
6.3. Bio-Gas
Methane could also be produced from the leaves of Moringa. Various reports showed that each hectare may create 4400 cubic meters of biogas per year. When bacteria decompose organic matter (biomass) in the absence of oxygen, biogas is produced [39]. Moringa plants were mashed together with water when they were around 30 days old. Filtration of the fiber via a mesh with 5 mm holes separated the liquid fraction, which was then put into a biogas reactor with an average volatile solids feed of 5.7 g and 580 L of gas was produced per kg of volatile solids. The gas had an average methane concentration of 81% [50,51].
6.4. Standard Plate Count (SPC) Method
Because of the high microbial load, drinking water can be dangerous to consume. The SPC method involves a complete bacterial count of this water determined quantitatively. It was also reported that the M. oleifera seed powder works as an antibacterial agent against microorganisms [52] through the process of coagulation or flocculation of produced water [53]. The M. oleifera seed powder treatment had the added benefit of lowering the microbial load. After treatment, the quantities of bacterial colonies significantly reduced and the SPC was found in the medium range within the permissible limit (102–105) in case of groundwater. The addition of 100 mg/L and 150 mg/L of M. oleifera seeds significantly reduces the colonies in the plaque. It was seen that the M. oleifera seed moves as an antimicrobial specialist against microorganisms [54]. The presence of active antimicrobial properties in the M. oleifera such as O-ethyl-4-(α-l-rhamnosylox) benzyl carbamate) oxy benzyl isothiocyanate, proved to disengage any strong matter with the elimination of a large portion of the suspended microorganisms in water. In underground and surface water samples, a pure distilled water and extract of M. oleifera seed powder resulted in 90 to 95 percent sedimentation of suspended particles. Duckweed-based waste water stabilization ponds for waste water treatment are a low-cost technique for small urban areas in Zimbabwe [9]. In underground and surface water samples, a pure distilled water and extract of M. oleifera seed powder resulted in 90 to 95 percent sedimentation of suspended particles.
6.5. Most Probable Number (MPN)
MPN method is used for counting total coliforms, which are quantifiable. The presence of coliforms indicates that the water has been gravely contaminated and is unfit for human consumption. Hence the MPN for drinking water should be zero. The value represented the coliform MPN per 100 mL of water sample [55]. MPN was found to be over the WHO groundwater standards and MPN/100 mL coliform was reduced after treatment. After treatment, MPN levels in all samples ranged from 500 to 1200 coliforms/mL, indicating that they exceeded the limit as set by WHO standards. The presence of coliforms/mL by the MPN method confirms the presence of hazardous pollutants in water, demonstrating that treated samples are bacteriologically unfit for drinking. Using chlorine with seed powder can result in a negative MPN test. The fraction of M. oleifera seed powder was 50 and 100 mg/L for reducing pH, TS, TDS, hardness, chloride, turbidity, causticity, and alkalinity, respectively 150 mg/L for SPC and MPN [56].
6.6. Other Industrial Uses
The oil is used for the modification of perfumes and hair dressings. Due to its little tendency to deteriorate and become rancid and sticky, it is also utilized as lubricating materials for watches as well as in other delicate hardwares [17]. It is a polyuronide made up of arabinose, galactose and glucoronic acid in a 10:7:2 molar ratios; rhamnose is present in trace amounts [57]. Dehulled seed (kernel) contains about 42% oil which is responsible for the yellowish color of the seed. It is utilized as a lubricant for fine machinery such as watches. After extraction of oil, it is further pressed to make a cake which can be utilized as manure. Moringa seed oil has 13 percent saturated fatty acid content and 82 percent unsaturated fatty acid content [58]. The wood of the drumstick tree is used to make paper and materials, the bark is used to make tanning, and the seeds are employed in the purification of water. Gum of the tree M. oleifera has been reported to have gel-forming potential for topical application [59].
7. Economic Potential
India is the world’s greatest producer of Moringa, with a 380 km2 region producing 1.1 to 1.3 million tonnes of delicate natural products each year. Andhra Pradesh (156.65 km2) is the largest state in terms of both area and population, followed by Karnataka (102.8 km2) and Tamil Nadu (103.8 km2) which come in second and third, respectively. Its production and management are made simple by the relative simplicity with which it propagates both sexually and asexually, along with its low demand for soil nutrients and water after planting. The introduction of this plant to a farm with a biodiverse setting may benefit both the farm owner and the surrounding ecosystem [60]. Drumstick trees could be used to extract oil without hindering their water purification capabilities. Drumstick oil is a premium product with a high market worth which could be of use for cooking oil and as a fundamental ingredient in creating a cleaner one [61].
8. Toxicity Levels
Two alkaloids found in root/bark extract are related to lethal hypotensive M. oleifera. In the animal trial of alkaloids obtained from M. oleifera, nazanin A and niaziminin B isolated from the ethanol extract caused hypotension, and bradycardia. Consistent consumption of large amounts of alcohol may result in liver and kidney damage; whereas, excess and/or repeated consumption of the alkaloid spirochin leads to toxic nerve loss and morbidity.
9. Medicinal Properties and Biomedical Applications
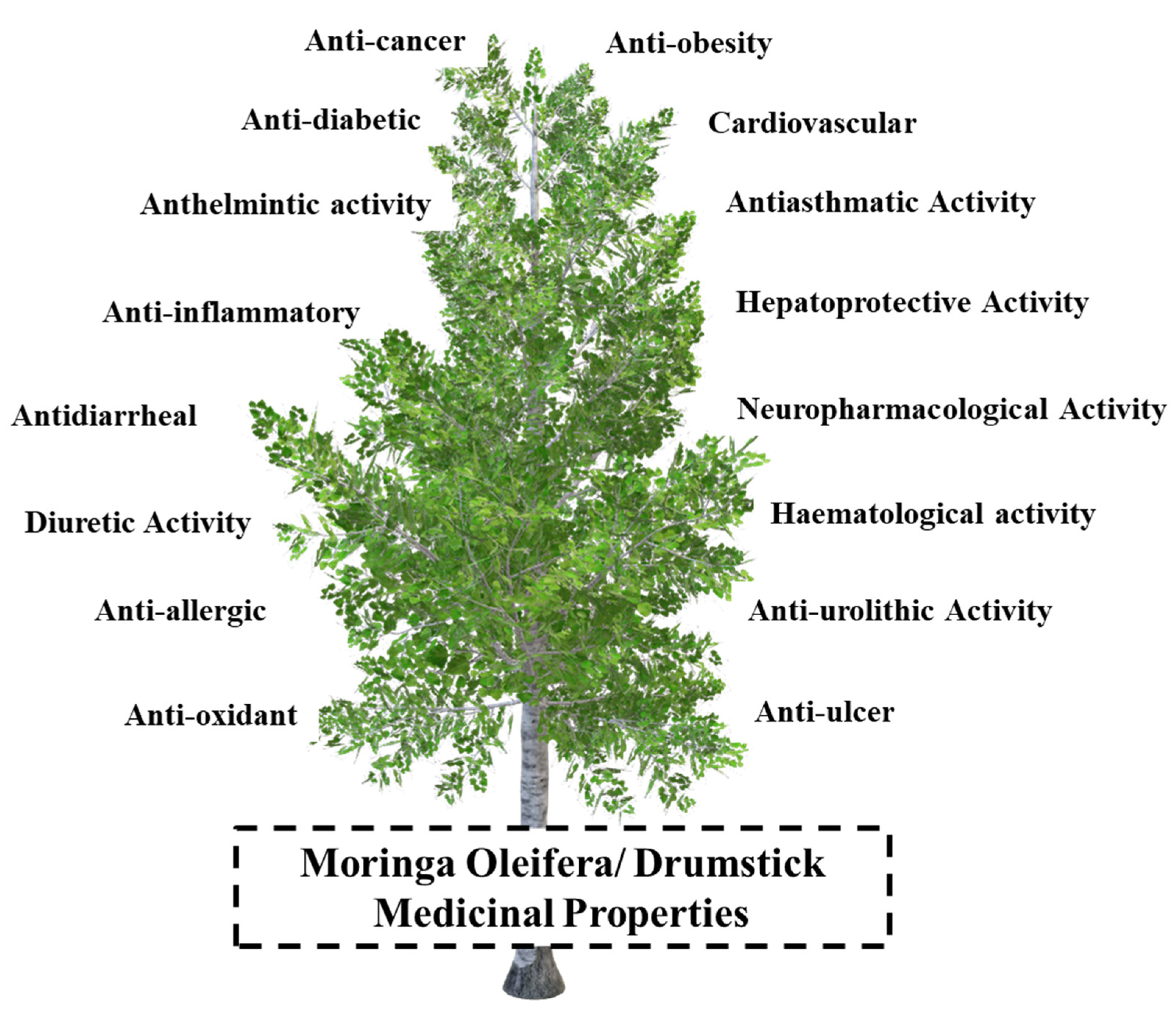
M. oleifera has a variety of activities, including the ones to be used as a galactagogue, rubefacient, antiscorbutic, diuretic, stimulant, purgative, antimicrobial, antibacterial [62], anti-inflammatory, antitumor, antioxidant, anti-aging agent, hypoglycaemic, antipoetryroidism, anti-cellular [63], hypocholesterolemic, antispasmodic. Moreover, it lowers, circulatory strain, reduces cerebral pains, and lessens headaches. Various therapeutic properties have been credited to different portions of this profoundly regarded tree as shown in Figure 3. Practically each and every part of this plant such as bark, gum, root, natural product (cases), flowers, leaf, seed, and seed oil has their own significance. They have been used to control a broad range of illnesses in the form of traditional folk medicine ailments [64]. Several phytochemicals available in M. oleifera and their medicinal uses are shown in Table 4.
Figure 3.
Various medicinal properties of M. oleifera.

Table 4.
M. oleifera phytochemicals and their medical uses.
9.1. Analgesic, Anti-Inflammatory, and Antipyretic Activities
All aspects of this marvel tree have been found to show pain-relieving mechanisms like that of indomethacin in various animal models. Extracts from leaves, seeds, and bark showed significant pain-relieving action in both focal (hot plate technique) and fringe models (acid-induced squirming strategy), in a dose-dependent manner [84,85]. The practical application demonstrated viability against neuropathic pain caused by multiple sclerosis [17]. In a carrageenan-induced paw edema model, reducing leaf removal movement was observed [86]. Extracts of bark showed calming action comparable to diclofenac in a similar model. Root has also been found to have calming qualities [86,87]. The neutrophil guideline and the c-Jun N-terminal kinase pathway may be responsible for the moderating effect [66]. Tannins and other active fixes are involved in the mitigating effect. Some other chemical compounds including alkaloids, flavonoids, phenols, carotenoids, sitosterol, hydroxymellein, vanillin, moringine, sitostenone, moringinine, and 9-octadecenoic acid, to name a few, are reported in this regard [88]. Leaf extract showed critical antipyretic action in brewer’s yeast-induced pyrexia model [89]. Ethanol and the source of ethyl acetic acid of seeds likewise showed huge antipyretic activity [90].
9.2. Neuropharmacological Activity
Aqueous extract of M. oleifera leaves has been seen as assurance against Alzheimer’s disease in a colchicine-instigated Alzheimer’s disease model utilizing social testing [91]. Protected Alzheimer’s disease can be fought by controlling electrical activity and monoamine levels in the brain [92]. Another study looked at the toluene-ethyl acetic acid derivation component of the methanolic concentrate of leaf and found that it had intense nootropic action [90]. The vitamins C and E found in the extracted leaf of M. oleifera play a crucial part in memory formation in Alzheimer’s disease patients [70,92].
Anticonvulsant action of leaf was demonstrated in male albino mice utilizing pentylenetetrazole and maximal electric shock paradigms [93]. Penicillin-induced epileptic convulsions were reduced in adult albino rats by aqueous extract of the root [94,95]. In actophotometer and rotarod apparatuses, ethanolic extract of leaves revealed both central nervous system depressant and muscle relaxant effects respectively [96,97,98]. In the staircase test and elevated plus maze test, it also showed considerable anxiolytic action that was in accordance with a dose-dependent manner [99,100].
9.3. Anticancerous Activity
In mouse melanoma tumor model tests, alcoholic and hydromethanolic extracts of leaves and fruits exhibited a considerable growth delay in tumor kinetics [101,102]. M. oleifera leaf extract has antiproliferative action against A549 lung cancer cell line, [70,103]. The exposure of leaf extract into chick chorioallantoic membrane leads to antiangiogenic action that was in accordance with dose-dependent manner, demonstrating their strong anticancer potential [102,104,105,106]. Another study found that pod extract protected male Institute of Cancer Research (ICR) mice from azoxymethane and dextran sodium sulfate-induced colon damage [29]. Studies reported that Breast cancer, hepatocarcinoma, and colorectal cancer cells in vitro, as well as cisplatin-resistant ovarian cancer cells, were all killed by a root and leaf extract of M. oleifera [34,107,108,109]. These findings imply that M. oleifera has regeneration potential in addition to its anti-cancerous potential, since flower extract promoted cell proliferation in normal cells but not in cancer cells, while leaf extract demonstrated substantial antitumor and hepatoprotective effects [110]. The anticancerous potential of this plant is attributed to phytoconstituents such as niazimicin, carbamates, thiocarbamates, nitrile glycosides, and others such as quercetin and kaempferol [111,112]. The effect of M. oleifera extract on various cancer cell lines is listed in Table 5.

Table 5.
Anticancerous activity of M. oleifera plant extract on different cancer cell lines.
9.4. Antioxidant Activity
M. oleifera foods offer significant antioxidant properties against a variety of free radicals [28]. Prepared leaf extract showed a considerable decrease in malondialdehyde levels and a significant increase in glutathione levels In vivo studies. Several extracts prepared from natural sources showed useful in scavenging of free radicals activity of roots altogether decreased iron and FeSO4-activated microsomal lipid peroxidation in a part subordinate way [31,114,118]. Antioxidant activity of pods through the 2-diphenyl-2-picryl hydroxyl (DPPH) method has been reported by researchers [121,122]. In a male BALB/c rat model of acetaminophen-induced nephrotoxicity, M. oleifera leaf extract demonstrated a nephroprotective effect in addition to antiproliferative effect [123,124]. Several bioactive compounds found in the M. oleifera such as triterpenoids, monopalmitic moringine, di-oleic fatty acids, campesterol, avenasterol, stigmasterol, sterol, β-sitosterol, vitamin A, and its precursor beta-carotene are only a few of the compounds that have been reported which may serve as cancer prevention agent [29,125,126,127,128,129,130,131].
9.5. Hepatoprotective Activity
An extract of the moringa leaves had hepatoprotective effects in Sprague Dawley rats [132,133]. They had been made aware of carbon tetrachloride or acetaminophen-induced liver toxicity [134,135,136,137,138,139]. Furthermore, hepatoprotection against antitubercular medicines and liver damage caused due to alloxan treatment [140]. The M. oleifera plant-based daily therapy for the period of 21 days was demonstrated to have huge potential in constricting hepatic injury [65,66]. Ascorbic acid, quercetin, kaempferol, and benzyl glucosinolate have all been discovered with hepatoprotective properties [141,142].
9.6. Anti-Ulcer and Gastroprotective Properties
The extract of leaves significantly reduced ulcer biomass in a gastric ulcer model caused by ibuprofen and a pyloric ligation test [143] and in addition to a considerable decrease in duodenal ulcers and stress ulcers caused by cysteamine [144]. This property could be enhanced by flavonoids and biphenyls [145].
9.7. Cardiovascular Activity
In male Wistar rats, an extract of M. oleifera leaf reduced cholesterol levels and acted as a defense against hyperlipidemia caused by iron deficiency [146]. In lower chronotropic and inotropic effects in damaged frog hearts, leaf extract had an antihypertensive effect on diseased hypertensive rodents [146,147]. Nazanin B, niazinin A, and miasmic are active ingredients for hypotensive activity [148]. In Male Wistar rats model, Isoproterenol-induced myocardial infarction was also inhibited by a leaf extract. The component responsible for this cardioprotective action was cell proliferation, lipid peroxidation prevention, and protection against isoproterenol-induced ultrastructure and histopathology unsettling effects [149]. M. oleifera lam function in irritation and lipid build-up in several tissue frameworks [150].
9.8. Antiobesity Activity
There was a considerable weight loss as compared to the fat control grouped by using oral therapy with leaf powder extract of M. oleifera [151]. Treatment of hypercholesterolemia animals with methanolic M. oleifera leaf extract for 49 days resulted in a major reduction in cholesterol level, body weight, fatty acids, as well as blood glucose level, liver indicators, and organ weight levels [152,153]. In heavy rats, downregulation of leptin and resistant mRNA articulation and overexpression of adiponectin quality articulation are among the mechanisms [154].
9.9. Antiasthmatic Activity
Extract of seeds showed assurance significant efficacy against asthma as researched in different models; an immediate bronchodilator effect was hypothesized for this effect, together with moderating and antibacterial actions [155] and prudence of prompt, easily affected reaction [156]. In bronchoalveolar lavage, an ethanolic extract of seeds showed potent efficacy against ovalbumin-induced bronchoalveolar lavage, guinea pigs showed a significant expansion of respiratory boundaries and a decrease in interleukin release. [157].
9.10. Hematological Activity
A randomized, double-blind, placebo-controlled trial was conducted on ladies who were pallid with hemoglobin levels somewhere in the mean hemoglobin, and mean corpuscular hemoglobin concentrations increased after being treated with an aqueous extract of moringa leaf in the 8–12 g/dL range [158]. Another review uncovered the potential of moringa for healthy human volunteers for 14 days aiding in a significant increase in platelet count [159,160].
9.11. Antidiabetic Activity
In normal and abnormal circumstances alloxan-induced or cysteamine-induced duodenal and peptic ulcers, the leaf extract had a significant antihyperglycemic and hypoglycemic effect [31,161,162,163]. With type 1 diabetic mouse models, an extensive review was conducted to determine the impact of the elimination of lipid profile, glucose, oral glucose resilience, body weight, and plasma insulin. The homeostatic model evaluation by different experiments on mice showing antidiabetic activity is described in Table 6.

Table 6.
Antidiabetic activity of M. oleifera plant extracts.
9.12. Anti-Urolithic Activity
In a hyperoxaluria-induced mouse model [171,172] and ethylene glycol-induced urolithiasis model, aqueous and ethanolic extract of this plant showed anti-urolithiatic activity [173].
9.13. Diuretic Activity
Seeds, roots, leaves, flowers, and bark extract expanded urine yield in rodents; extract of leaf showed a portion subordinate diuretic activity more prominent than control yet not as much as hydrochlorothiazide. This activity was attributed due to the presence of campesterol, stigmasterol, β-sitosterol, and avenasterol [174].
9.14. Anti-Allergic Activity
Ethanolic extract of seeds hindered latent cutaneous hypersensitivity incited by hostile to Immunoglobulin G (IgG) and histamine release from pole cells; the mechanism is hidden, yet its activity could be harmful in layer settling action [175] and more decreased scratching recurrence in an ovalbumin refinement model [176].
9.15. Anthelmintic Activity
It took a very less effort to incapacitate Indians because the plant had great anthelmintic activity [177]. Ethanolic extract and aqueous extract, separately and in larvicidal measure, showed 95.89 percent and 81.72 percent egg incubates hindrance, respectively, in ovicidal examination. They were deemed adequate for 56.94 percent of the time and 92.50 percent of the time [178].
9.16. Antidiarrheal Activity
In male Wister rats, extract of moringa seeds demonstrated a considerable decrease in gastrointestinal motility and were considered viable in castor oil mediated loose bowels [179,180,181]. Tannins, saponins, and flavonoids are phytochemical compounds that have antidiarrheal properties [168].
9.17. Diabetes and Diverse Effects
Leaf extract shows a decrease in undesirable sebum secretion from sebaceous organs during winter in humans [158]. This herb has unambiguously been identified as a source of “galactagogue” derived from the Greek word “galacta” which means milk—is a kind of herb and drug or food, which enhances the production of breast milk [159]. Diabetes is defined by metabolic dysregulation, especially of carbohydrate metabolism, as seen by hyperglycemia due to insulin secretion and action due to abnormal Insulin levels were not analyzed [166,167,168]. There was no change in the number of lactic acid bacteria counted as described in Table 5. In frog models, methanolic root concentrate demonstrated local sedative action; whereas, in guinea pig model [160], M. oleifera leaf extract has a significant inhibitory effect on CYP3A4 [161]. Thus, M. oleifera has an extraordinary potential for herb-drug formulations.
10. Summary and Future Research
Drumstick plant is a tropical tree with a diverse range of applications and is attracting increasing international attention for exploring more therapeutic interventions. It should be broadly developed in the great majority of places where climatic conditions are difficult to predict for its ideal development. Various studies on M. oleifera have been conducted so far. The primary goal of this research was to uncover and analyze the pharmacological and healing benefits of M. oleifera. This plant has been shown to be effective in preclinical studies and found to have pain-relieving, calming, anthelmintic, anticancer, local sedative, nootropic, hepatoprotective, gastroprotective, anti-hypersensitivity, anti-ulcer, cancer preventive, asthmatic, diuretic, cardiovascular, anti-stoutness, antidiabetic, antiepileptic, anti-urolithiasis, injury-repairing potentialities.
Moringa plant being a rich source of phytoconstituents, have the prospects to develop functional food and nutraceuticals. However, detailed in vitro and in vivo evaluations of bioavailability and biological activities are compulsory to permit reasonable and appropriate recommendations of phytoconstituents for future drug development. Proper attention is required to be devoted to developing cultivars with higher foliage yield by means of specific breeding works, as the foliage is the richest source of carotenoids, ascorbic acids, glucosinolate, and other bioactives, compared to other edible parts. Elicitors (abiotic and biotic) and signaling molecules, such as, methyl jasmonate and salicylic acid were studied for the augmentation of carotenoids and tocopherols in the foliage of M. oleifera. Overall, M. oleifera is emerging as one of the prospective industrial crops in tropical and subtropical countries.
Author Contributions
Conceptualization, C.P. and T.K.U.; methodology, M.A. and A.B.S.; software, N.M.A. and K.M.; validation, F.A.A.-S., M.S., K.M. and N.M.A.; formal analysis, T.K.U. and A.B.S.; investigation, T.K.U. and M.S.; resources, M.S.; data curation, N.M.A., K.M., F.A.A.-S. and M.S.; writing—original draft preparation, C.P., T.K.U. and A.B.S.; writing—review and editing, M.S., A.B.S., T.K.U., K.M. and N.M.A.; visualization, K.M., N.M.A. and F.A.A.-S.; supervision, T.K.U.; project administration, T.K.U., A.B.S. and M.S.; funding acquisition, M.S., K.M., N.M.A. and F.A.A.-S. All authors have read and agreed to the published version of the manuscript.
Funding
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University, KSA, for funding this work through a large research group program under grant number RGP. 2/233/43.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University, KSA, for funding this work through a large research group program under grant number RGP. 2/233/43. Also, the authors are very grateful to Geetika Madan Patel, Medical Director and Chairperson, Centre of Research for Development (CR4D), Parul University, Vadodara, Gujarat, India for providing the facility during the compilation of ideas in the form of the manuscript.
Conflicts of Interest
No affiliations or financial involvements by the authors are related to any organization or entity with a financial interest in or financial conflict with the subject matter or materials in the manuscript, other than those disclosed. The authors declare no conflict of interest.
References
- Ramachandran, C.; Peter, K.V.; Gopalakrishnan, P.K. Drumstick (Moringa oleifera): A multipurpose Indian vegetable. Econ. Bot. 1980, 34, 276–283. [Google Scholar] [CrossRef]
- Nadkarni, K.; Nadkarni, A. Indian Materia Medica; Popular Prakashan Pvt. Ltd.: Mumbai, India, 1976; Volume 1, p. 1799. [Google Scholar]
- Kardam, A.; Raj, K.R.; Arora, J.K.; Srivastava, M.M.; Srivastava, S. Artificial Neural Network Modeling for Sorption of Cadmium from Aqueous System by Shelled Moringa Oleifera Seed Powder as an Agricultural Waste. J. Water Resour. Prot. 2010, 2, 339–344. [Google Scholar] [CrossRef]
- Anwar, F.; Bhanger, M.I. Analytical Characterization of Moringa oleifera Seed Oil Grown in Temperate Regions of Pakistan. J. Agric. Food Chem. 2003, 51, 6558–6563. [Google Scholar] [CrossRef]
- D’souza, J.; Kulkarni, A. Comparative Studies on Nutritive Values of Tender Foliage of Seedlings, and Mature Plants of Moringa oleifera (Lamk). Indian J. Nutr. Dietetics 1990, 27, 205–212. [Google Scholar]
- Somali, M.A.; Bajneid, M.A.; Al-Fhaimani, S.S. Chemical composition and characteristics of Moringa peregrina seeds and seeds oil. J. Am. Oil Chem. Soc. 1984, 61, 85–86. [Google Scholar] [CrossRef]
- Oinam, N.; Urooj, A.; Phillips, P.P.; Niranjan, N.P. Effect of dietary lipids and drumstick leaves (Moringa oleifera) on lipid profile & antioxidant parameters in rats. Food Nutr. Sci. 2012, 3, 141–145. [Google Scholar] [CrossRef]
- Dillard, C.J.; German, J.B. Phytochemicals: Nutraceuticals and human health. J. Sci. Food Agric. 2000, 80, 1744–1756. [Google Scholar] [CrossRef]
- Siddhuraju, P.; Becker, K. Antioxidant Properties of Various Solvent Extracts of Total Phenolic Constituents from Three Different Agroclimatic Origins of Drumstick Tree (Moringa oleifera Lam.) Leaves. J. Agric. Food Chem. 2003, 51, 2144–2155. [Google Scholar] [CrossRef]
- Saini, R.K.; Sivanesan, I.; Keum, Y.-S. Phytochemicals of Moringa oleifera: A review of their nutritional, therapeutic and industrial significance. 3 Biotech 2016, 6, 203. [Google Scholar] [CrossRef]
- Patel, S.; Thakur, A.S.; Chandy, A.; Manigauha, A. Moringa oleifera: A review of there medicinal and economical importance to the health and nation. Drug Invent. Today 2010, 2, 339–342. [Google Scholar]
- Kirtikar, K.; Basu, B. New Cannaught Place; M/s Bishen Singh Mahendrapal Singh: Dehradun, India, 1975. [Google Scholar]
- Lea, M. Bioremediation of Turbid Surface Water Using Seed Extract from the Moringa oleifera Lam. (Drumstick) Tree. Curr. Protoc. Microbiol. 2014, 33, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Tahiliani, P.; Kar, A. Role of Moringa oleifera leaf extract in the regulation of thyroid hormone status in adult male and female rats. Pharmacol. Res. 2000, 41, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Ojiako, F.; Adikuru, N.; Emenyonu, C. Critical issues in Investment, Production and Marketing of Moringa oleifera as an Industrial Agricultural raw material in Nigeria. J. Agric. Res. Dev. 2011, 10, 1039–1056. [Google Scholar]
- Bartha, D. Enzyklopädie der Holzgewächse: Handbuch und Atlas der Dendrologie; Wiley Online Library: Hoboken, NJ, USA, 2001. [Google Scholar]
- Morton, J.F. The horseradish tree, Moringa pterygosperma (Moringaceae)—A boon to arid lands? Econ. Bot. 1991, 45, 318–333. [Google Scholar] [CrossRef]
- Anwar, F.; Latif, S.; Ashraf, M.; Gilani, A.H. Moringa oleifera: A food plant with multiple medicinal uses. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2007, 21, 17–25. [Google Scholar] [CrossRef]
- Jahn, S.; Musnad, H.A.; Burgstaller, H. The tree that purifies water: Cultivating multipurpose Moringaceae in the Sudan. Unasylva 1986, 38, 23–28. [Google Scholar]
- Nasir, S.; Aguilar, D. Congestive Heart Failure and Diabetes Mellitus: Balancing Glycemic Control with Heart Failure Improvement. Am. J. Cardiol. 2012, 110, 50B–57B. [Google Scholar] [CrossRef]
- Rajangam, J.; Azahakia Manavalan, R.S.; Thangaraj, T.; Vijayakumar, A.; Muthukrishan, N. Status of Production and Utilization of Moringa in Southern India. Development Potential for Moringa Product. Dar Es Salaam, Tanzania. 2001. Available online: http://www.moringanews.org/actes/rajangam_en.doc (accessed on 10 January 2022).
- Amaglo, N.K.; Bennett, R.N.; Lo Curto, R.B.; Rosa, E.A.S.; Lo Turco, V.; Giuffrida, A.; Curto, A.L.; Crea, F.; Timpo, G.M. Profiling selected phytochemicals and nutrients in different tissues of the multipurpose tree Moringa oleifera L., grown in Ghana. Food Chem. 2010, 122, 1047–1054. [Google Scholar] [CrossRef]
- Coppin, J.P.; Xu, Y.; Chen, H.; Pan, M.-H.; Ho, C.-T.; Juliani, R.; Simon, J.E.; Wu, Q. Determination of flavonoids by LC/MS and anti-inflammatory activity in Moringa oleifera. J. Funct. Foods 2013, 5, 1892–1899. [Google Scholar] [CrossRef]
- Odebiyi, O.O.; Sofowora, E.A. Phytochemical screening of Nigerian medicinal plants II. Lloydia 1978, 41, 234–246. [Google Scholar]
- Gassenschmidt, U.; Jany, K.D.; Tauscher, B.; Niebergall, H. Isolation and characterization of a flocculating protein from Moringa oleifera Lam. Biochim. Biophys. Acta 1995, 1243, 477–481. [Google Scholar] [CrossRef]
- Nadkarni, K. Indian Materia Medica; Popular Prakashan: Mumbai, India, 1954; Volume 6, p. 629. [Google Scholar]
- Alam, M.I. Inhibition of Toxic Effects of Viper and Cobra Venom by Indian Medicinal Plants. Pharmacol. Pharm. 2014, 5, 828–837. [Google Scholar] [CrossRef]
- Luqman, S.; Srivastava, S.; Kumar, R.; Maurya, A.K.; Chanda, D. Experimental Assessment of Moringa oleifera Leaf and Fruit for Its Antistress, Antioxidant, and Scavenging Potential Using In Vitro and In Vivo Assays. Evid. Based Complement. Altern. Med. 2012, 2012, 519084. [Google Scholar] [CrossRef]
- Kumar, S.; Bhattacharya, A.; Tiwari, P.; Sahu, P. A review of the phytochemical and pharmacological characteristics of Moringa oleifera. J. Pharm. Bioallied Sci. 2018, 10, 181–191. [Google Scholar] [CrossRef]
- Berkovich, L.; Earon, G.; Ron, I.; Rimmon, A.; Vexler, A.; Lev-Ari, S. Moringa Oleifera aqueous leaf extract down-regulates nuclear factor-kappaB and increases cytotoxic effect of chemotherapy in pancreatic cancer cells. BMC Complement. Altern. Med. 2013, 13, 212. [Google Scholar] [CrossRef]
- Caceres, A.; Saravia, A.; Rizzo, S.; Zabala, L.; De Leon, E.; Nave, F. Pharmacologic properties of Moringa oleifera. 2: Screening for antispasmodic, antiinflammatory and diuretic activity. J. Ethnopharmacol. 1992, 36, 233–237. [Google Scholar] [CrossRef]
- Das, A.J. Moringa oleifera (Lamm.): A plant with immense importance. J. Biol. Act. Prod. Nat. 2012, 2, 307–315. [Google Scholar] [CrossRef]
- Shanmugavel, G.; Prabakaran, K.; George, B. Evaluation of phytochemical constituents of Moringa oleifera (Lam.) leaves collected from Puducherry region, South India. Int. J. Zool. Appl. Biosci. 2018, 3, 1–8. [Google Scholar]
- Sreelatha, S.; Jeyachitra, A.; Padma, P. Antiproliferation and induction of apoptosis by Moringa oleifera leaf extract on human cancer cells. Food Chem. Toxicol. 2011, 49, 1270–1275. [Google Scholar] [CrossRef]
- Ndiaye, M.; Dieye, A.M.; Mariko, F.; Tall, A.; Diallo, A.S.; Faye, B. Contribution to the study of the anti-inflammatory activity of Moringa oleifera (moringaceae). Dakar Med. 2002, 47, 210–212. [Google Scholar]
- Minaiyan, M.; Asghari, G.; Taheri, D.; Saeidi, M.; Nasr-Esfahani, S. Anti-inflammatory effect of Moringa oleifera Lam. seeds on acetic acid-induced acute colitis in rats. Avicenna J. Phytomed. 2014, 4, 127–136. [Google Scholar] [PubMed]
- Moyo, B.; Masika, P.J.; Muchenje, V. Antimicrobial activities of Moringa oleifera Lam leaf extracts. Afr. J. Biotechnol. 2012, 11, 2797–2802. [Google Scholar] [CrossRef]
- Al-Malki, A.L.; El Rabey, H.A. The Antidiabetic Effect of Low Doses of Moringa oleifera Lam. Seeds on Streptozotocin Induced Diabetes and Diabetic Nephropathy in Male Rats. BioMed Res. Int. 2015, 2015, 381040. [Google Scholar] [CrossRef]
- James, A.; Zikankuba, V. Moringa oleifera a potential tree for nutrition security in sub-Sahara Africa. Am. J. Res. Commun. 2017, 5, 1–14. [Google Scholar]
- Isitua, C.C.; Lozano, M.J.S.-M.; Jaramillo, C.J.; Dutan, F. Phytochemical and nutritional properties of dried leaf powder of Moringa oleifera Lam. from machala el oro province of ecuador. Asian J. Plant Sci. Res. 2015, 5, 8–16. [Google Scholar]
- Ain, Q.-U.; Khan, H.; Mubarak, M.S.; Pervaiz, A. Plant Alkaloids as Antiplatelet Agent: Drugs of the Future in the Light of Recent Developments. Front. Pharmacol. 2016, 7, 292. [Google Scholar] [CrossRef]
- Pal, S.K.; Mukherjee, P.K.; Saha, B.P. Studies on the antiulcer activity of Moringa oleifera leaf extract on gastric ulcer models in rats. Phytother. Res. 1995, 9, 463–465. [Google Scholar] [CrossRef]
- Babushkina, E.A.; Belokopytova, L.V.; Grachev, A.M.; Meko, D.; Vaganov, E.A. Variation of the hydrological regime of Bele-Shira closed basin in Southern Siberia and its reflection in the radial growth of Larix sibirica. Reg. Environ. Chang. 2017, 17, 1725–1737. [Google Scholar] [CrossRef]
- Tsaknis, J.; Lalas, S.; Gergis, V.; Dourtoglou, V.; Spiliotis, V. Characterization of Moringa oleifera Variety Mbololo Seed Oil of Kenya. J. Agric. Food Chem. 1999, 47, 4495–4499. [Google Scholar] [CrossRef]
- Berger, M.R.; Habs, M.; Jahn, S.A.; Schmahl, D. Toxicological assessment of seeds from Moringa oleifera and Moringa stenopetala, two highly efficient primary coagulants for domestic water treatment of tropical raw waters. East Afr. Med. J. 1984, 61, 712–716. [Google Scholar]
- Baptista, A.T.A.; Silva, M.O.; Gomes, R.G.; Bergamasco, R.; Vieira, M.F.; Vieira, A.M.S. Protein fractionation of seeds of Moringa oleifera lam and its application in superficial water treatment. Sep. Purif. Technol. 2017, 180, 114–124. [Google Scholar] [CrossRef]
- Olsen, A. Low technology water purification by bentonite clay and Moringa oleifera seed flocculation as performed in sudanese villages: Effects on Schistosoma mansoni cercariae. Water Res. 1987, 21, 517–522. [Google Scholar] [CrossRef]
- Barakat, H.; Ghazal, G.A. Physicochemical Properties of Moringa oleifera Seeds and Their Edible Oil Cultivated at Different Regions in Egypt. Food Nutr. Sci. 2016, 7, 472–484. [Google Scholar] [CrossRef]
- Wai, K.T.; Idris, A.; Johari, M.M.N.M.; Mohammad, T.A.; Ghazali, A.H.; Muyibi, S.A. Evaluation on different forms of Moringa oleifera seeds dosing on sewage sludge conditioning. Desalin. Water Treat. 2009, 10, 87–94. [Google Scholar] [CrossRef]
- Radovich, T. Farm and Forestry Production and Marketing Profile for Moringa (Moringa oleifera); Permanent Agriculture Resources (PAR): Holualoa, HI, USA, 2011. [Google Scholar]
- Trigo, C.; Castelló, M.L.; Ortolá, M.D.; García-Mares, F.J.; Soriano, M.D. Moringa oleifera: An Unknown Crop in Developed Countries with Great Potential for Industry and Adapted to Climate Change. Foods 2020, 10, 31. [Google Scholar] [CrossRef]
- Gautam, R.K.; Sankaran, M.; Zamir Ahmed, S.K.; AI Sunder, J.; Ram, N.; Dam Roy, S. Custodian Farmers and Communities of Biodiversity Conservation and Utilization in Andaman & Nicobar Islands, India. ICAR-CIARI, Port Blair; 2014. Available online: http://krishi.icar.gov.in/jspui/handle/123456789/20006 (accessed on 11 April 2022).
- Palada, M.C. Moringa (Moringa oleifera Lam.): A Versatile Tree Crop with Horticultural Potential in the Subtropical United States. HortScience 1996, 31, 794–797. [Google Scholar] [CrossRef]
- Godino, M.; Arias, C.; Izquierdo, M. Interés forestal de la Moringa Oleifera y Posibles Zonas de Implantación en España. In 6° Congreso Forestal Español: “Montes: Servicios y Desarrollo Rural”; Sociedad Española de Ciencias Forestales: Barcelona, Spain, 2013. [Google Scholar]
- Liu, Y.; Wang, X.-Y.; Wei, X.-M.; Gao, Z.-T.; Han, J.-P. Values, properties and utility of different parts of Moringa oleifera: An overview. Chin. Herb. Med. 2018, 10, 371–378. [Google Scholar] [CrossRef]
- Senthilkumar, A.; Karuvantevida, N.; Rastrelli, L.; Kurup, S.S.; Cheruth, A.J. Traditional Uses, Pharmacological Efficacy, and Phytochemistry of Moringa peregrina (Forssk.) Fiori. —A Review. Front. Pharmacol. 2018, 9, 465. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Agrawal, D.; Sahu, P.K.; Kumar, S.; Mishra, S.S.; Patnaik, S. Analgesic effect of ethanolic leaf extract of Moringa oleifera on albino mice. Indian J. Pain 2014, 28, 89. [Google Scholar] [CrossRef]
- Bosch, C. Moringa stenopetala (Baker f.) Cufod. PROTA 2004, 2, 395–397. [Google Scholar]
- George, T.T.; Oyenihi, A.B.; Rautenbach, F.; Obilana, A.O. Characterization of Moringa oleifera Leaf Powder Extract Encapsulated in Maltodextrin and/or Gum Arabic Coatings. Foods 2021, 10, 3044. [Google Scholar] [CrossRef] [PubMed]
- Chawla, S.; Saxena, A.; Seshadri, S. In-vitro availability of iron in various green leafy vegetables. J. Sci. Food Agric. 1988, 46, 125–127. [Google Scholar] [CrossRef]
- Dogra, P.; Singh, B.; Tandon, S. Vitamin C content in moringa pod vegetable. Curr. Sci. 1975, 44, 31. [Google Scholar]
- Azam, M.M.; Waris, A.; Nahar, N. Prospects and potential of fatty acid methyl esters of some non-traditional seed oils for use as biodiesel in India. Biomass Bioenergy 2005, 29, 293–302. [Google Scholar] [CrossRef]
- Nadkarni, K.M. Indian materia medica: With Ayurvedic, Unani-Tibbi, Siddha, allopathic, homeopathic, naturopathic & home remedies, appendices & indexes. 1. In Indian Materia Medica; Popular Prakashan: Mumbai, India, 1996; Volume 1. [Google Scholar]
- Gilani, A.; Janbaz, K.H.; Shah, B.H. 85 Quercetin exhibits hepatoprotective activity in rats. Biochem. Soc. Trans. 1997, 25, S619. [Google Scholar] [CrossRef]
- Ruckmani, K.; Kavimani, S.; Anandan, R.; Jaykar, B. Effect of Moringa oleifera Lam on paracetamol-induced hepatotoxicity. Indian J. Pharm. Sci. 1998, 60, 33. [Google Scholar]
- Jarald, E.E.; Sumati, S.; Edwin, S.; Ahmad, S.; Patni, S.; Daud, A. Characterization of Moringa oleifera Lam. gum to establish it as a pharmaceutical excipient. Indian J. Pharm. Educ. Res. 2012, 46, 211–216. [Google Scholar]
- Wang, Y.; Gao, Y.; Ding, H.; Liu, S.; Han, X.; Gui, J.; Liu, D. Subcritical ethanol extraction of flavonoids from Moringa oleifera leaf and evaluation of antioxidant activity. Food Chem. 2017, 218, 152–158. [Google Scholar] [CrossRef]
- Gothai, S.; Arulselvan, P.; Tan, W.; Fakurazi, S. Wound healing properties of ethyl acetate fraction of Moringa oleifera in normal human dermal fibroblasts. J. Intercult. Ethnopharmacol. 2016, 5, 1–6. [Google Scholar] [CrossRef]
- Álvarez, R.; Vaz, B.; Gronemeyer, H.; de Lera, Á.R. Functions, Therapeutic Applications, and Synthesis of Retinoids and Carotenoids. Chem. Rev. 2014, 114, 1–125. [Google Scholar] [CrossRef]
- Ndabigengesere, A.; Narasiah, K.S. Quality of water treated by coagulation using Moringa oleifera seeds. Water Res. 1998, 32, 781–791. [Google Scholar] [CrossRef]
- Padmarao, P.; Acharya, B.M.; Dennis, T.J. Pharmacognostic study on stembark of Moringa oleifera Lam. Bull. Med.-Ethno-Bot. Res. 1996, 17, 151. [Google Scholar]
- Dahot, M. Vitamin contents of flowers and seeds of Moringa oleifera. Pak. J. Biochem. 1988, 21, 21–24. [Google Scholar]
- Bhattacharya, S.B.; Das, A.K.; Banerji, N. Chemical investigations on the gum exudates from Sonja (Moringa oleifera). Carbohydr. Res. 1982, 102, 253–262. [Google Scholar] [CrossRef]
- Mehta, K.; Balaraman, R.; Amin, A.; Bafna, P.; Gulati, O. Effect of fruits of Moringa oleifera on the lipid profile of normal and hypercholesterolaemic rabbits. J. Ethnopharmacol. 2003, 86, 191–195. [Google Scholar] [CrossRef]
- Daba, M. Miracle Tree: A Review on Multi-purposes of Moringa oleifera and Its Implication for Climate Change Mitigation. J. Earth Sci. Clim. Chang. 2016, 7, 1000366. [Google Scholar] [CrossRef]
- Makonnen, E.; Hunde, A.; Damecha, G. Hypoglycaemic effect of Moringa stenopetala aqueous extract in rabbits. Phytother. Res. 1997, 17, 147–148. [Google Scholar] [CrossRef]
- Faizi, S.; Siddiqui, B.; Saleem, R.; Aftab, K.; Shaheen, F.; Gilani, A.-U. Hypotensive Constituents from the Pods of Moringa oleifera. Planta Medica 1998, 64, 225–228. [Google Scholar] [CrossRef]
- Lalas, S.; Tsaknis, J. Extraction and identification of natural antioxidant from the seeds of the Moringa oleifera tree variety of Malawi. J. Am. Oil Chem. Soc. 2002, 79, 677–683. [Google Scholar] [CrossRef]
- Santos, D.; Segtovich, I.; Teixeira, F.; Alvarez, V.H.; Mattedi, S. Vapor liquid equilibrium calculations for alcohol and hydrocarbon mixtures using COSMO SAC, NRTL, and UNIQUAC Models. Braz. J. Pet. Gas 2015, 8, 4. [Google Scholar]
- Standnes, D.C.; Skjevrak, I. Literature review of implemented polymer field projects. J. Pet. Sci. Eng. 2014, 122, 761–775. [Google Scholar] [CrossRef]
- Mizielinska, S.M.; Greenwood, S.M.; Tummala, H.; Connolly, C.N. Rapid dendritic and axonal responses to neuronal insults. Biochem. Soc. Trans. 2009, 37, 1389–1393. [Google Scholar] [CrossRef] [PubMed]
- Samanta, A.; Bera, A.; Ojha, K.; Mandal, A. Effects of Alkali, Salts, and Surfactant on Rheological Behavior of Partially Hydrolyzed Polyacrylamide Solutions. J. Chem. Eng. Data 2010, 55, 4315–4322. [Google Scholar] [CrossRef]
- Sandiford, B. Laboratory and Field Studies of Water Floods Using Polymer Solutions to Increase Oil Recoveries. J. Pet. Technol. 1964, 16, 917–922. [Google Scholar] [CrossRef]
- Woomer, P.L. Most Probable Number Counts. In Methods of Soil Analysis: Part 2 Microbiological and Biochemical Properties; Wiley Online Library: Hoboken, NJ, USA, 2018; pp. 59–79. [Google Scholar] [CrossRef]
- Adedapo, A.; Falayi, F.; Oyagbemi, A. Evaluation of the analgesic, anti-inflammatory, anti-oxidant, phytochemical and toxicological properties of the methanolic leaf extract of commercially processed Moringa oleifera in some laboratory animals. J. Basic Clin. Physiol. Pharmacol. 2015, 26, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Ferrao, A.M.B.C.; Ferrao, M.J.E. Ácidos gordos em óleo de Moringueiro (Moringa oleifera Lam.). Agron. Angolana 1970, 8, 3–16. [Google Scholar]
- Fuglie, L.J. The Miracle Tree: Moringa Oleifera. Natural Nutrition for the Tropics; Food and Agriculture Organization of the United Nations: Rome, Italy, 1999. [Google Scholar]
- Verma, S.C.; Banerji, R.; Misra, G.; Nigam, S.K. Nutritional value of Moringa. Curr. Sci. 1976, 45, 769–770. [Google Scholar] [CrossRef]
- Sutar, N.G.; Bonde, C.G.; Patil, V.V.; Narkhede, S.B.; Patil, A.P.; Kakade, R.T. Analgesic activity of seeds of Moringa oleifera Lam. Int. J. Green Pharm. 2008, 2. [Google Scholar] [CrossRef]
- Manaheji, H. Analgesic effects of methanolic extracts of the leaf or root of Moringa oleifera on complete Freund’s adjuvant-induced arthritis in rats. J. Chin. Integr. Med. 2011, 9, 216–222. [Google Scholar] [CrossRef]
- Upadhye, K.; Rangari, V.; Mathur, V. Antimigraine activity study of Moringa oleifera leaf juice. Int. J. Green Pharm. 2012, 6, 204. [Google Scholar] [CrossRef]
- Velaga, V.S.A.R.; Suryadevara, N.; Chee, L.; Ismail, N.E. Phytochemical analysis and immuno-modulatory effect of Moringa oleifera flowers. Int. J. Pharm. Pharmaceut. 2017, 9, 24–28. [Google Scholar] [CrossRef][Green Version]
- Ezeamuzie, I.C.; Ambakederemo, A.W.; Shode, F.O.; Ekwebelem, S.C. Antiinflammatory Effects of Moringa oleifera Root Extract. Int. J. Pharmacogn. 1996, 34, 207–212. [Google Scholar] [CrossRef]
- Kinase, J. Moringa tea blocks acute lung inflammation induced by swine confinement dust through a mechanism involving TNF-α expression, c-Jun N-terminal kinase activation and neutrophil regulation. Am. J. Immunol. 2014, 10, 73–87. [Google Scholar]
- Rani, N.Z.A.; Husain, K.; Kumolosasi, E. Moringa Genus: A Review of Phytochemistry and Pharmacology. Front. Pharmacol. 2018, 9, 108. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, L.; Doriya, K.; Kumar, D.S. Moringa oleifera: A review on nutritive importance and its medicinal application. Food Sci. Hum. Wellness 2016, 5, 49–56. [Google Scholar] [CrossRef]
- Ganguly, R.; Hazra, R.; Ray, K.; Guha, D. Effect of Moringa oleifera in experimental model of Alzheimer’s disease: Role of antioxidants. Ann. Neurosci. 2010, 12, 33–36. [Google Scholar] [CrossRef]
- Kou, X.; Li, B.; Olayanju, J.B.; Drake, J.M.; Chen, N. Nutraceutical or Pharmacological Potential of Moringa oleifera Lam. Nutrients 2018, 10, 343. [Google Scholar] [CrossRef]
- Mohan, M.; Kaul, N.; Punekar, A.; Girnar, R.; Junnare, P.; Patil, L. Nootropic activity of Moringa oleifera leaves. J. Nat. Remedies 2005, 5, 59–62. [Google Scholar]
- Akram, M.; Nawaz, A. Effects of medicinal plants on Alzheimer’s disease and memory deficits. Neural Regen. Res. 2017, 12, 660–670. [Google Scholar] [CrossRef]
- More, S.V.; Kumar, H.; Cho, D.-Y.; Yun, Y.-S.; Choi, D.-K. Toxin-Induced Experimental Models of Learning and Memory Impairment. Int. J. Mol. Sci. 2016, 17, 1447. [Google Scholar] [CrossRef]
- Ray, K.; Guha, D. Effect of Moringa oleifera root extract on penicillin-induced epileptic rats. Biog. Amines 2005, 19, 223–231. [Google Scholar] [CrossRef]
- Fathima, S.N.; Vasudevamurthy, S.; Rajkumar, N. A review on phytoextracts with antiepileptic property. J. Pharm. Sci. Res. 2015, 7, 994. [Google Scholar]
- Kaur, G.; Invally, M.; Sanzagiri, R.; Buttar, H.S. Evaluation of the antidepressant activity of Moringa oleifera alone and in combination with fluoxetine. J. Ayurveda Integr. Med. 2015, 6, 273–279. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Santra, S.; Mahapatra, S.; Sahu, P.K.; Agrawal, D.; Kumar, S. Study of anxiolytic effect of ethanolic extract of drumstick tree leaves on albino mice in a basic neuropharmacology laboratory of a postgraduate teaching institute. J. Health Res. Rev. 2016, 3, 41. [Google Scholar] [CrossRef]
- Islam, M.T.; Martins, N.; Imran, M.; Hameed, A.; Ali, S.W.; Salehi, B.; Ahmad, I.; Hussain, A.; Sharifi-Rad, J. Anxiolytic-like effects of Moringa oleifera in Swiss mice. Cell. Mol. Biol. 2020, 66, 73–77. [Google Scholar] [CrossRef]
- Jung, I.L.; Lee, J.H.; Kang, S.C. A potential oral anticancer drug candidate, Moringa oleifera leaf extract, induces the apoptosis of human hepatocellular carcinoma cells. Oncol. Lett. 2015, 10, 1597–1604. [Google Scholar] [CrossRef]
- Dulay, M.T.; Zaman, N.; Jaramillo, D.; Mody, A.C.; Zare, R.N. Pathogen-Imprinted Organosiloxane Polymers as Selective Biosensors for the Detection of Targeted E. coli. C 2018, 4, 29. [Google Scholar] [CrossRef]
- Tiloke, C.; Phulukdaree, A.; Chuturgoon, A.A. The antiproliferative effect of Moringa oleifera crude aqueous leaf extract on cancerous human alveolar epithelial cells. BMC Complement. Altern. Med. 2013, 13, 226. [Google Scholar] [CrossRef]
- Al-Asmari, A.K.; AlBalawi, S.M.; Athar, T.; Khan, A.Q.; Al-Shahrani, H.; Islam, M. Moringa oleifera as an Anti-Cancer Agent against Breast and Colorectal Cancer Cell Lines. PLoS ONE 2015, 10, e0135814. [Google Scholar] [CrossRef]
- Mojzis, J.; Varinska, L.; Mojzisova, G.; Kostova, I.; Mirossay, L. Antiangiogenic effects of flavonoids and chalcones. Pharmacol. Res. 2008, 57, 259–265. [Google Scholar] [CrossRef]
- Tragulpakseerojn, J.; Yamaguchi, N.; Pamonsinlapatham, P.; Wetwitayaklung, P.; Yoneyama, T.; Ishikawa, N.; Ishibashi, M.; Apirakaramwong, A. Anti-proliferative effect of Moringa oleifera Lam (Moringaceae) leaf extract on human colon cancer HCT116 cell line. Trop. J. Pharm. Res. 2017, 16, 371–378. [Google Scholar] [CrossRef]
- Budda, S.; Butryee, C.; Tuntipopipat, S.; Rungsipipat, A.; Wangnaithum, S.; Lee, J.-S.; Kupradinun, P. Suppressive effects of Moringa oleifera Lam pod against mouse colon carcinogenesis induced by azoxymethane and dextran sodium sulfate. Asian Pac. J. Cancer Prev. 2011, 12, 3221–3228. [Google Scholar] [PubMed]
- Abd-Rabou, A.A.; Abdalla, A.M.; Ali, N.A.; Zoheir, K.M.A. Moringa oleifera Root Induces Cancer Apoptosis more Effectively than Leave Nanocomposites and Its Free Counterpart. Asian Pac. J. Cancer Prev. 2017, 18, 2141–2149. [Google Scholar] [PubMed]
- Zayas-Viera, M.D.M. Anticancer effect of Moringa oleifera leaf extract in human cancer cell lines. J. Health Disparities Res. Pract. 2016, 9, 102. [Google Scholar]
- Moghe, A.S.; Fernandes, E.E.; Pulwale, A.V.; Patil, G.A. Probing regenerative potential of Moringa oleifera aqueous extracts using In vitro cellular assays. Pharmacogn. Res. 2016, 8, 231–237. [Google Scholar] [CrossRef]
- Fisall, U.F.M.; Ismail, N.Z.; Adebayo, I.A.; Arsad, H. Dichloromethane fraction of Moringa oleifera leaf methanolic extract selectively inhibits breast cancer cells (MCF7) by induction of apoptosis via upregulation of Bax, p53 and caspase 8 expressions. Mol. Biol. Rep. 2021, 48, 4465–4475. [Google Scholar] [CrossRef]
- Purwal, L.; Pathak, A.; Jain, U. In vivo anticancer activity of the leaves and fruits of Moringa oleifera on mouse melanoma. Pharmacologyonline 2010, 1, 655–665. [Google Scholar]
- Charoensin, S. Antioxidant and anticancer activities of Moringa oleifera leaves. J. Med. Plants Res. 2014, 8, 318–325. [Google Scholar]
- Karin, M.; Lin, A. NF-kappaB at the crossroads of life and death. Nat. Immunol. 2002, 3, 221–227. [Google Scholar] [CrossRef]
- Greenhalf, W.; Thomas, A. Combination therapy for the treatment of pancreatic cancer. Anti-Cancer Agents Med. Chem. 2011, 11, 418–426. [Google Scholar] [CrossRef]
- Sreelatha, S.; Padma, P.R. Antioxidant Activity and Total Phenolic Content of Moringa oleifera Leaves in Two Stages of Maturity. Mater. Veg. 2009, 64, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Shameer, P.; Mohamed, K.; Sukhen, S. Effect of Moringa oleifera on stress induced brain lipid peroxidation in rats. Res. J. Pharm. Biol. Chem. Sci. 2010, 1, 336–342. [Google Scholar]
- Kumar, V.; Pandey, N.; Mohan, N.; Singh, R.P. Antibacterial & antioxidant activity of different extract of Moringa oleifera Leaves—An in vitro study. Int. J. Pharm. Sci. Rev. Res. 2012, 12, 89–94. [Google Scholar]
- Singh, B.N.; Singh, B.R.; Singh, R.L.; Prakash, D.; Dhakarey, R.; Upadhyay, G.; Singh, H.B. Oxidative DNA damage protective activity, antioxidant and anti-quorum sensing potentials of Moringa oleifera. Food Chem. Toxicol. 2009, 47, 1109–1116. [Google Scholar] [CrossRef]
- Satish, A.; Reddy, P.V.; Sairam, S.; Ahmed, F.; Urooj, A. Antioxidative Effect and DNA Protecting Property of Moringa oleifera Root Extracts. J. Herbs Spices Med. Plants 2014, 20, 209–220. [Google Scholar] [CrossRef]
- Laoung-On, J.; Jaikang, C.; Saenphet, K.; Sudwan, P. Phytochemical Screening, Antioxidant and Sperm Viability of Nelumbo nucifera Petal Extracts. Plants 2021, 10, 1375. [Google Scholar] [CrossRef]
- Sinha, M.; Das, D.K.; Bhattacharjee, S.; Majumdar, S.; Dey, S. Leaf Extract of Moringa oleifera Prevents Ionizing Radiation-Induced Oxidative Stress in Mice. J. Med. Food 2011, 14, 1167–1172. [Google Scholar] [CrossRef]
- Paliwal, R.; Sharma, V.; Sharma, S. Elucidation of free radical scavenging and antioxidant activity of aqueous and hydro-ethanolic extracts of Moringa oleifera pods. Res. J. Pharm. Technol. 2011, 4, 566–571. [Google Scholar]
- Atawodi, S.E.; Atawodi, J.C.; Idakwo, G.A.; Pfundstein, B.; Haubner, R.; Wurtele, G.; Bartsch, H.; Owen, R.W. Evaluation of the Polyphenol Content and Antioxidant Properties of Methanol Extracts of the Leaves, Stem, and Root Barks of Moringa oleifera Lam. J. Med. Food 2010, 13, 710–716. [Google Scholar] [CrossRef]
- Patel, R.K.; Patel, M.M.; Kanzariya, N.R.; Vaghela, K.R.; Patel, N.J. In-vitro hepatoprotective activity of Moringa oleifera Lam. leave on isolated rat hepatocytes. Int. J. Pharm. Sci. 2010, 2, 457–463. [Google Scholar]
- Hamza, A.A. Ameliorative effects of Moringa oleifera Lam seed extract on liver fibrosis in rats. Food Chem. Toxicol. 2010, 48, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Fakurazi, S.; Hairuszah, I.; Nanthini, U. Moringa oleifera Lam prevents acetaminophen induced liver injury through restoration of glutathione level. Food Chem. Toxicol. 2008, 46, 2611–2615. [Google Scholar] [CrossRef] [PubMed]
- Das, N.; Sikder, K.; Ghosh, S.; Fromenty, B.; Dey, S. Moringa oleifera Lam. leaf extract prevents early liver injury and restores antioxidant status in mice fed with high-fat diet. Indian J. Exp. Biol. 2012, 50, 404–412. [Google Scholar] [PubMed]
- Suganthi, U.R.; Parvatham, R. Efficacy of Moringa oleifera and Aloe vera on aflatoxin Blinduced hepatotoxicityin rats. Res. J. Biotechnol. 2010, 4, 2024. [Google Scholar]
- Pari, L.; Kumar, N.A. Hepatoprotective activity of Moringa oleifera on antitubercular drug-induced liver damage in rats. J. Med. Food 2002, 5, 171–177. [Google Scholar] [CrossRef]
- Omodanisi, E.; Aboua, Y.G.; Chegou, N.N.; Oguntibeju, O.O. Hepatoprotective, Antihyperlipidemic, and Anti-inflammatory Activity of Moringa oleifera in Diabetic-induced Damage in Male Wistar Rats. Pharmacogn. Res. 2017, 9, 182–187. [Google Scholar] [CrossRef]
- Abd Eldaim, M.A.; Elrasoul, A.S.A.; Elaziz, S.A.A. An aqueous extract from Moringa oleifera leaves ameliorates hepatotoxicity in alloxan-induced diabetic rats. Biochem. Cell Biol. 2017, 95, 524–530. [Google Scholar] [CrossRef]
- Adeyemi, O.S.; Aroge, C.S.; Akanji, M.A. Moringa oleifera-based diet protects against nickel-induced hepatotoxicity in rats. J. Biomed. Res. 2017, 31, 350–357. [Google Scholar] [CrossRef]
- Toppo, R.; Roy, B.K.; Gora, R.H.; Baxla, S.L.; Kumar, P. Hepatoprotective activity of Moringa oleifera against cadmium toxicity in rats. Veter-World 2015, 8, 537–540. [Google Scholar] [CrossRef]
- Debnath, S.; Guha, D. Role of Moringa oleifera on enterochromaffin cell count and serotonin content of experimental ulcer model. Indian J. Exp. Biol. 2007, 45, 726–731. [Google Scholar]
- Ndong, M.; Uehara, M.; Katsumata, S.; Sato, S.; Suzuki, K. Preventive Effects of Moringa oleifera (Lam) on Hyperlipidemia and Hepatocyte Ultrastructural Changes in Iron Deficient Rats. Biosci. Biotechnol. Biochem. 2007, 71, 1826–1833. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dangi, S.; Jolly, C.; Narayanan, S. Antihypertensive Activity of the Total Alkaloids from the Leaves of Moringa oleifera. Pharm. Biol. 2002, 40, 144–148. [Google Scholar] [CrossRef]
- Randriamboavonjy, J.I.; Loirand, G.; Vaillant, N.; Lauzier, B.; Derbré, S.; Michalet, S.; Pacaud, P.; Tesse, A. Cardiac Protective Effects of Moringa oleifera Seeds in Spontaneous Hypertensive Rats. Am. J. Hypertens. 2016, 29, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Gilani, A.H.; Aftab, K.; Suria, A.; Siddiqui, S.; Salem, R.; Siddiqui, B.S.; Faizi, S. Pharmacological studies on hypotensive and spasmolytic activities of pure compounds from Moringa oleifera. Phytother. Res. 1994, 8, 87–91. [Google Scholar] [CrossRef]
- Nandave, M.; Ojha, S.K.; Joshi, S.; Kumari, S.; Arya, D.S. Moringa oleifera leaf extract prevents isoproterenol-induced myocardial damage in rats: Evidence for an antioxidant, antiperoxidative, and cardioprotective intervention. J. Med. Food 2009, 12, 47–55. [Google Scholar] [CrossRef]
- Barbagallo, I.; Vanella, L.; Distefano, A.; Nicolosi, D.; Maravigna, A.; Lazzarino, G.; Di Rosa, M.; Tibullo, D.; Acquaviva, R.; Volti, G.L. Moringa oleifera Lam. improves lipid metabolism during adipogenic differentiation of human stem cells. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 5223–5232. [Google Scholar]
- Nahar, S.; Faisal, F.; Iqbal, J.; Rahman, M.M.; Yusuf, A. Antiobesity activity of Moringa oleifera leaves against high fat diet-induced obesity in rats. Int. J. Basic Clin. Pharmacol. 2016, 5, 1263–1268. [Google Scholar] [CrossRef]
- Bais, S.; Singh, G.S.; Sharma, R. Antiobesity and Hypolipidemic Activity of Moringa oleifera Leaves against High Fat Diet-Induced Obesity in Rats. Adv. Biol. 2014, 2014, 162914. [Google Scholar] [CrossRef]
- Pare, D.; Hilou, A.; Ouedraogo, N.; Guenne, S. Ethnobotanical Study of Medicinal Plants Used as Anti-Obesity Remedies in the Nomad and Hunter Communities of Burkina Faso. Medicines 2016, 3, 9. [Google Scholar] [CrossRef]
- Metwally, F.; Rashad, H.; Ahmed, H.H.; Mahmoud, A.; Raouf, E.A.; Abdalla, A.M. Molecular mechanisms of the anti-obesity potential effect of Moringa oleifera in the experimental model. Asian Pac. J. Trop. Biomed. 2017, 7, 214–221. [Google Scholar] [CrossRef]
- Mehta, A.; Agrawal, B. Investigation into the mechanism of action of Moringa oleifera for its anti-asthmatic activity. Orient. Pharm. Exp. Med. 2008, 8, 24–31. [Google Scholar] [CrossRef]
- Goyal, B.R.; Goyal, R.K.; Mehta, A.A. Investigation Into the Mechanism of Anti-Asthmatic Action of Moringa oleifera. J. Diet. Suppl. 2009, 6, 313–327. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, S.G.; Mehta, A.A. Effect of Moringa oleifera Lam. Seed Extract on Ovalbumin-Induced Airway Inflammation in Guinea Pigs. Inhal. Toxicol. 2008, 20, 897–909. [Google Scholar] [CrossRef] [PubMed]
- Suzana, D.; Suyatna, F.D.; Azizahwati; Andrajati, R.; Santi, P.S.; Mun’im, A. Effect of Moringa oleifera Leaves Extract Against Hematology and Blood Biochemical Value of Patients with Iron Deficiency Anemia. J. Young Pharm. 2017, 9, s79–s84. [Google Scholar] [CrossRef]
- Adegbite, O.A.; Omolaso, B.; Seriki, S.A.; Shatima, C. Effects of Moringa oleifera leaves on hematological indices in humans. Ann. Hematol. Oncol. 2016, 3, 1107. [Google Scholar]
- Archibong, A.N.; Nku, C.O.; Ofem, O.E. Extract of Moringa oleifera attenuates hematological parameters following salt loading. MicroMedicine 2017, 5, 24–30. [Google Scholar]
- Manohar, V.S.; Jayasree, T.; Kiran Kishore, K.; Mohana Rupa, L.; Dixit, R.; Chandrasekhar, N. Evaluation of hypoglycemic and antihyperglycemic effect of freshly prepared aqueous extract of Moringa oleifera leaves in normal and diabetic rabbits. J. Chem. Pharm. Res. 2012, 4, 249–253. [Google Scholar]
- Jaiswal, D.; Rai, P.K.; Kumar, A.; Mehta, S.; Watal, G. Effect of Moringa oleifera Lam. leaves aqueous extract therapy on hyperglycemic rats. J. Ethnopharmacol. 2009, 123, 392–396. [Google Scholar] [CrossRef]
- Yassa, H.D.; Tohamy, A.F. Extract of Moringa oleifera leaves ameliorates streptozotocin-induced Diabetes mellitus in adult rats. Acta Histochem. 2014, 116, 844–854. [Google Scholar] [CrossRef]
- Karadi, R.V.; Gadge, N.B.; Alagawadi, K.; Savadi, R.V. Effect of Moringa oleifera Lam. root-wood on ethylene glycol induced urolithiasis in rats. J. Ethnopharmacol. 2006, 105, 306–311. [Google Scholar] [CrossRef]
- Dhongade, H.K.J.; Paikra, B.K.; Gidwani, B. Phytochemistry and Pharmacology of Moringa oleifera Lam. J. Pharmacopunct. 2017, 20, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Villarruel-López, A.; López-de la Mora, D.A.; Vázquez-Paulino, O.D.; Puebla-Mora, A.G.; Torres-Vitela, M.R.; Guerrero-Quiroz, L.A.; Nuño, K. Effect of Moringa oleifera consumption on diabetic rats. BMC Complement. Altern. Med. 2018, 18, 127. [Google Scholar] [CrossRef] [PubMed]
- Paula, P.C.; Sousa, D.O.B.; Oliveira, J.T.A.; Carvalho, A.F.U.; Alves, B.G.T.; Pereira, M.L.; Farias, D.F.; Viana, M.P.; Santos, F.A.; Morais, T.C.; et al. A Protein Isolate from Moringa oleifera Leaves Has Hypoglycemic and Antioxidant Effects in Alloxan-Induced Diabetic Mice. Molecules 2017, 22, 271. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Muñoz, M.A.; Valdez-Solana, M.A.; Campos-Almazán, M.I.; Flores-Herrera, Ó.; Esparza-Perusquía, M.; Olvera-Sánchez, S.; García-Arenas, G.; Avitia-Domínguez, C.; Téllez-Valencia, A.; Sierra-Campos, E. Streptozotocin-Induced Adaptive Modification of Mitochondrial Supercomplexes in Liver of Wistar Rats and the Protective Effect of Moringa oleifera Lam. Biochem. Res. Int. 2018, 2018, 5681081. [Google Scholar] [CrossRef]
- Hagiwara, A.; Hidaka, M.; Takeda, S.; Yoshida, H.; Kai, H.; Sugita, C.; Watanabe, W.; Kurokawa, M. Anti-Allergic Action of Aqueous Extract of Moringa oleifera Lam. Leaves in Mice. Eur. J. Med. Plants 2016, 16, 1–10. [Google Scholar] [CrossRef]
- Rastogi, T.; Buhtda, V.; Moon, K.; Aswar, P.B.; Khadabadi, S.S. Comparative studies on anthelmintic activity of Moringa oleifera and Vitex negundo. Asian J. Res. Chem. 2009, 2, 181–182. [Google Scholar]
- Cabardo, D.E., Jr.; Portugaliza, H.P. Anthelmintic activity of Moringa oleifera seed aqueous and ethanolic extracts against Haemonchus contortus eggs and third stage larvae. Int. J. Vet. Sci. Med. 2017, 5, 30–34. [Google Scholar] [CrossRef]
- Saralaya, M.G.; Patel, P.; Patel, M.; Roy, S.P.; Patel, A.N. Research article antidiarrheal activity of methanolic extract of Moringa oleifera lam roots in experimental animal models. Int. J. Pharm. Res. 2010, 2, 25–29. [Google Scholar]
- Lakshminarayana, M.; Shivkumar, H.; Rimaben, P.; Bhargava, V.K. Antidiarrhoeal activity of leaf extract of Moringa oleifera in experimentally induced diarrhoea in rats. Int. J. Phytomedicine 2011, 3, 68–74. [Google Scholar]
- Choudhury, S.; Sharan, L.; Sinha, M. Antidiarrhoeal potentiality of leaf extracts of Moringa oleifera. Br. J. Appl. Sci. Technol. 2013, 10, 1086–1096. [Google Scholar] [CrossRef]
- Raguindin, P.F.N.; Dans, L.F.; King, J.F. Moringa oleifera as a Galactagogue. Breastfeed. Med. 2014, 9, 323–324. [Google Scholar] [CrossRef] [PubMed]
- Medhi, B.; Khanikor, H.; Lahon, L.; Mohan, P.; Barua, C. Analgesic, Anti-inflammatory and Local Anaesthetic Activity of Moringa pterygosperma in Laboratory Animals. Pharm. Biol. 2003, 41, 248–252. [Google Scholar] [CrossRef][Green Version]
- Monera, T.G.; Wolfe, A.R.; Maponga, C.C.; Benet, L.Z.; Guglielmo, J. Moringa oleifera leaf extracts inhibit 6beta-hydroxylation of testosterone by CYP3A4. J. Infect. Dev. Ctries. 2008, 2, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Roosdiana, A.; Prasetyawan, S.; Mahdi, C.; Sutrisno, S. Production and Characterization of Bacillus firmus pectinase. J. Pure Appl. Chem. Res. 2013, 2, 35–41. [Google Scholar] [CrossRef]
- Cabeza, M.S.; Baca, F.L.; Puntes, E.M.; Loto, F.; Baigori, M.; Morata, V.I. Selection of psychrotolerant microorganisms producing cold-active pectinases for biotechnological processes at low temperature. Food Technol. Biotechnol. 2011, 49, 187–195. [Google Scholar]
- Das, B.; Chakraborty, A.; Ghosh, S.; Chakrabarti, K. Studies on the effect of pH and carbon sources on enzyme activities of some pectinolytic bacteria isolated from jute retting water. Turk. J. Biol. 2011, 35, 671–678. [Google Scholar]
- Namasivayam, E.; Ravindar, J.D.; Mariappan, K.; Jiji, A.; Kumar, M.; Jayaraj, R.L. Production of Extracellular Pectinase by Bacillus Cereus Isolated from Market Solid Waste. J. Bioanal. Biomed. 2011, 3, 70–75. [Google Scholar] [CrossRef]
- Tripathi, G.D.; Zoya, J.; Singh, A.K. Pectinase production and purification from Bacillus subtilis isolated from soil. Adv. Appl. Sci. Res. 2014, 5, 103–105. [Google Scholar]
- Chandra, D. Analgesic effect of aqueous and alcoholic extracts of Madhuka Longifolia (Koeing). Indian J. Pharmacol. 2001, 33, 108–111. [Google Scholar]
- Makinde, A.I. Effects of inorganic fertilizer on the growth and nutrient composition of Moringa (Moringa oleifera). J. Emerg. Trends Eng. Appl. Sci. 2013, 4, 341–343. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).