Abstract
Zoonotic parasitic diseases in dogs are particularly concerning in regions with low human development indices due to inadequate sanitary services and insufficient environmental and health education. This study aimed to assess the parasitological status of dogs living in households and evaluate their owners’ knowledge about zoonoses. A total of 183 dogs from Rolim de Moura, Rondônia State, were screened for the presence of ectoparasites, and 163 fecal samples were collected for analysis. The results showed that 74.23% (112/163) of the animals had at least one species of endoparasite. The most identified pathogens were Ancylostoma spp. (68.71%, 112/163), Trichuris vulpis (11.66%, 19/163), Toxocara canis (6.75%, 11/163), Cystoisospora canis (4.91%, 8/163), Dipylidium caninum (1.23%, 2/163), and Hammondia/Neospora (0.61%, 1/163). Ectoparasites were observed in 43.17% (79/183) of the evaluated animals, with Rhipicephalus sanguineus found in 31.15% (57/183) and Ctenocephalides felis felis in 20.77% (38/183). Only 11.48% (7/61) of the owners were familiar with the term “Zoonoses.” However, a significant majority (83.61%, 51/61) believed that dogs can transmit diseases to humans. Our findings highlight the prevalence of parasites in the studied area and associated risk factors, underscoring the urgent need for educational interventions to raise awareness about these diseases and their risks to human health.
1. Introduction
Parasitism in pet dogs is relatively common, and the proximity between these animals and humans may facilitate the transmission of zoonotic parasites. Various biotic and abiotic factors, such as animal behavior, management practices, housing conditions, and parasite control strategies, were shown to influence the dynamics of parasitic infections in dogs [1,2]. However, parasite control is often neglected by owners due to a lack of knowledge about parasitic diseases in dogs and their risks to human health [3]. Therefore, it is crucial to raise awareness and provide education on these topics to promote effective preventive measures and safeguard the health of both animals and humans. Considering the social and economic impacts of these diseases, the adoption of preventive measures becomes necessary [4,5].
Zoonotic parasitic infections have been described in dogs worldwide [1], but they are more frequent in regions with low human development [6]. These regions often face challenges related to a lack of basic sanitation, limited access to healthcare, and inadequate public health infrastructure. In Brazil, the northern, central-western, and northeastern regions of the country, which have low human development indices, record the highest rates of infectious and parasitic diseases in humans [5].
The northern region of Brazil, predominantly located within the Amazon Biome, has experienced relatively recent human occupation, with significant expansion starting in the 1970s. This region is renowned for its biodiversity but faces substantial anthropogenic pressure due to extractive activities and agricultural potential [7]. Gastrointestinal parasitosis in dogs is highly prevalent in this region, exceeding 80% in certain areas [2,8,9,10]. However, there is a dearth of data regarding pet dog owners’ perceptions of parasitic diseases in dogs and their significance as zoonoses in this region, despite the existence of perception studies conducted in other areas of Brazil and globally [3,11,12,13]. Consequently, further studies in unexplored areas are warranted, as risk perception is known to vary across countries and regions due to diverse factors, including cultural, environmental, and governmental influences [1,11].
The objective of this research was to identify the occurrence of parasitosis in pet dogs in a municipality in the state of Rondônia and investigate the population’s perception of parasites and zoonoses.
2. Materials and Methods
2.1. Study Area
The study was conducted in the Rolim de Moura municipality, situated at coordinates 11°48′13″ S and 61°48′12″ W, within the state of Rondônia in the northern region of Brazil. This municipality is located in the Zona da Mata region, which is part of the Amazon Biome, at an elevation of 290 m above sea level. Rolim de Moura covers a total area of 1457.888 km2 (Figure 1). The Anta Atirada River hydrographic basin is found within Rolim de Moura. The region features a tropical climate with a distinct dry season (Aw) that is characterized by an annual temperature range of 19 °C to 36 °C and annual precipitation ranging between 1700 and 1900 mm. Rolim de Moura has a Human Development Index of 0.700, ranking seventh in Rondônia and 1904th among the 5568 Brazilian municipalities. As of 2021, the population of the municipality stood at 55,748 residents, occupying an area of 1457.81 km2. This translates to a population density of 34.74 inhabitants per km2 [14,15].
Figure 1.
Study area. South America, highlighting Brazil (A). State of Rondônia (B). Municipality of Rolim de Moura (C), located in the Zona da Mata region.
The study focused on 12 distinct sectors within the municipality: Beira Rio, Boa Esperança, Centenário, Centro, Cidade Alta, Industrial, Jardim Eldorado, Jardim Tropical, Nova Morada, Olímpico, Planalto, and São Cristóvão (Figure 2).
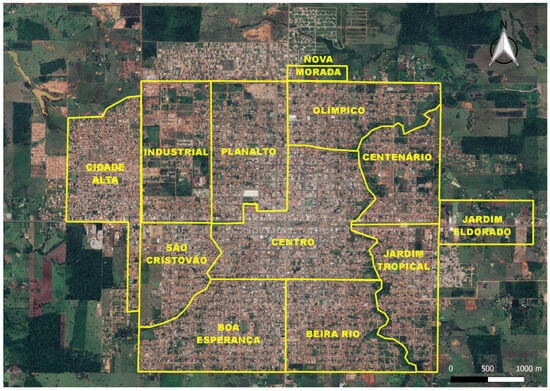
Figure 2.
Municipality of Rolim de Moura, Rondônia State, Brazil, highlighting the 12 sectors where the study was conducted: Beira Rio, Boa Esperança, Centenário, Centro, Cidade Alta, Industrial, Jardim Eldorado, Jardim Tropical, Nova Morada, Olímpico, Planalto, and São Cristóvão.
The inclusion criteria for this study was the consent of dog owners, given the absence of official data on the actual canine population size in the municipality. A total of 183 dogs from 61 households within the study area participated (see Figure 3 and Appendix A).
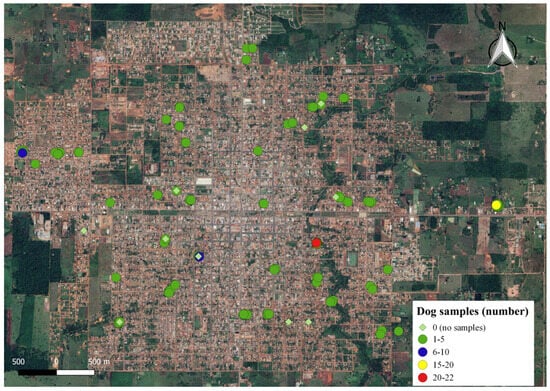
Figure 3.
Number of fecal samples collected per household in the Municipality of Rolim de Moura, Rondônia State, Brazil.
Initially, each animal underwent a comprehensive physical examination, coupled with a meticulous inspection for ectoparasite presence. Given the observed infestation in all animals, ectoparasite samples (28 in total) were collected on a per-household basis and subsequently preserved using 70% ethanol in appropriately labeled containers. The taxonomic identification of ectoparasites was accomplished using specific keys for each arthropod group [16,17,18,19,20,21].
Fecal samples were obtained through enema or immediate defecation. The 163 samples collected were stored in temperature-controlled containers during transportation and processed within 24 h of collection. To diagnose endoparasitic presence, the techniques outlined by Gordon and Whitlock, Willis, Watanabe et al., and Baermann [22] were employed. Descriptor calculations for endoparasitic infection were conducted using the methodology described by Bush et al. [23].
2.2. Evaluation of Owners’ Knowledge and Animal/Environmental Characteristics
A semi-structured interview based on the provided script (Appendix B) was conducted to gather information on the owners’ understanding of canine diseases and zoonoses, as well as details about the animals, methods for parasitic control, and sanitation practices. This questionnaire was administered upon completion of the sample collection.
2.3. Statistical Analyses
The associations between parasite infection/infestation and host and environmental characteristics were examined through univariate and, when applicable, multivariate regression analyses. The explanatory variables encompassed sex, size, breed, age, outdoor access, the use of anthelmintics, employment of ectoparasiticides, residence location in relation to the river, street paving, sewage system, water source, frequency of veterinary consultations, and geographic location. The outcome variables comprised overall gastrointestinal parasite infection, overall ectoparasite infestation, infection by Ancylostoma spp., infection by Toxocara canis, infection by Trichuris vulpis, infection by Cystoisospora canis, infection by Dipylidium caninum, infestation by fleas, and infestation by ticks, as well as coinfections involving Ancylostoma and Toxocara, Ancylostoma and Trichuris, and Ancylostoma and Cystoisospora.
With respect to the dog owners, we assessed their educational level as an explanatory variable and knowledge regarding zoonotic diseases, as well as the potential for dogs to transmit diseases to humans, as outcome variables. Additionally, we evaluated environmental factors (such as water source and sewage system) as explanatory variables and the presence or absence of animals infected/infested by any parasite as outcome variables.
The chi-square test or Fisher’s exact test was utilized to assess associations, considering p-values below 0.2 as indicating significance as screening. Factors demonstrating significant associations underwent further scrutiny through simple logistic regression analysis, with a p-value threshold set at less than 0.05. The statistical analyses were conducted using Epi Info software (version 7.2.3.1, 2019). Variables displaying a significant association (p < 0.05) were then subjected to the Akaike information criterion to identify the optimal model by employing R software (version 4.3.2). Subsequently, a mixed model was developed, considering the significant associations, using the lme4 package in R software. The confidence interval for prevalence was calculated using the exact method implemented in the “binom” package of R software.
3. Results
The analysis of 163 fecal samples revealed that 74.23% (121/163) tested positive for at least one species of endoparasite (Table 1 and Figure 4). The quantification of eggs per gram of feces ranged from 50 to 46,100 eggs. Among the identified parasites, Ancylostoma spp. displayed the highest abundance, with egg counts spanning from 50 to 46,100 eggs per gram. Following Ancylostoma spp., Toxocara canis exhibited egg counts ranging from 200 to 4200 eggs per gram, while Trichuris vulpis demonstrated counts ranging between 50 and 750 eggs per gram. Notably, no lungworm larvae or Strongyloides stercoralis were detected using the Baermann test. Ectoparasites were observed in 43.17% (79/183) of the examined animals. Among these, immature and/or adult stages of Rhipicephalus sanguineus were found in 31.15% (57/183) of the animals, while Ctenocephalides felis felis were identified in 20.77% (38/183). Some animals exhibited co-infestation by both ectoparasite species.

Table 1.
Gastrointestinal parasites in pet dogs from Rolim de Moura, Rondônia state, Brazil, 2019.
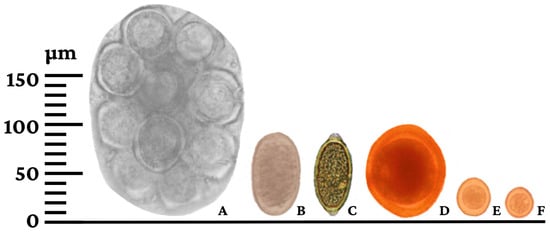
Figure 4.
Helminth eggs and protozoan oocysts diagnosed in pet dogs from Rolim de Moura, Rondônia state, Brazil. (A) Dipylidium caninum; (B) Ancylostoma spp.; (C) Trichuris vulpis; (D) Toxocara canis; (E) Cystoisospora canis; (F) Hammondia/Neospora.
Table 2 provides a detailed breakdown of the descriptors of endoparasitic infection, which were classified according to the municipality sectors. The analysis of these households revealed that 60.66% (37/61) were located on unpaved streets. Given the absence of a sewage system, a significant majority of 90.16% (55/61) relied on septic tanks. Concerning the water supply, 72.13% (44 out of 61) of households accessed water from the public supply, while 37.70% (23/61) relied on artesian wells. Additionally, 9.84% (6/61) had access to water from both sources (further details are available in Table 3 and Table 4).

Table 2.
Descriptors of endoparasitic infection in pet dogs from Rolim de Moura, Rondônia State, Brazil, 2019, grouped in city sectors.

Table 3.
Univariate and multivariate analysis of the association between dog characteristics, environmental characteristics, and frequency of parasitic infection in pet dogs from Rolim de Moura, Rondônia State, Brazil, 2019.

Table 4.
Univariate analysis of the association between in pet dogs characteristics and frequency of infection by Ancylostoma spp., Rolim de Moura, Rondônia State, Brazil, 2019.
Observations indicated that co-infection by multiple parasites occurred more frequently than infection by a single parasite. Notably, co-infection involving Ancylostoma spp. and Toxocara canis was particularly prevalent. The occurrence of polyparasitism was directly associated with contact with other dogs (p = 0.04). Furthermore, the large breed dogs exhibited a higher risk of Ancylostoma spp. and Trichuris vulpis infection (p = 0.047, odds ratio (OR) = 2.8352, confidence interval (CI) = 1.0619–8.8003) compared with the small breed animals.
When considered individually, animals positive for Toxocara canis, Trichuris vulpis (Appendix C and Appendix D), Cystoisospora canis, Dipylidium caninum, and coinfection with Ancylostoma and Cystoisospora did not show significant associations with the studied variables.
The analysis revealed a direct association between the extent of contact with other dogs and the frequency of parasitism, particularly with Ancylostoma spp. Additionally, the infected animals demonstrated a lower prevalence of parasitic infections when subjected to regular health examinations (p = 0.024, OR = 0.80, CI = 0.60–0.95) and had limited or no access to outdoor environments (p = 0.020, OR = 0.23, CI = 0.06–0.84) (Table 3). Furthermore, routine veterinary examinations were found to reduce the risk of Ancylostoma spp. infection (p = 0.02) (refer to Table 4).
The purebred dogs demonstrated significantly lower ectoparasite prevalence in comparison with the mixed breeds (p = 0.03, OR = 0.0105, CI = 2.02 × 10−4–0.1344), particularly in relation to fleas (p = 0.02, OR = 1.91 × 10−6, CI = 0.00–6.05 × 10−3).
As reported by the dog owners, 76.50% (140/183) of them utilized anthelmintic medications for their dogs. However, among the 61 owners interviewed, 62.84% did not adhere to any specific criteria for usage. Of those who did, 24.04% administered them quarterly, 11.48% semiannually, and 1.64% monthly. The most commonly used products were anthelmintic compounds based on 4H-pyrimidine and pro-benzimidazole, either combined or without a pyrazinoisoquinoline derivative. Among the frequently used drug combinations were pyrantel pamoate + praziquantel + febantel (54.10%), pyrantel pamoate + praziquantel (25.68%), and pyrantel pamoate (8.20%). Approximately 3.83% of the owners were uncertain about the specific anthelmintic used.
Concerning ectoparasiticides, 73.22% (134/183) of the owners employed these products. Among the 61 interviewed owners, 56.83% did not follow any specific criteria for ectoparasiticide use. Among those who did, 22.95% applied them semiannually, 9.84% quarterly, 9.84% monthly, and 0.55% annually. Within the group using ectoparasiticides, a majority (39.89%) could not identify the medication used. Amitraz, afoxolaner, and fipronil were mentioned by 38.80%, 6.01%, and 4.92% of the owners, respectively.
Out of the 183 evaluated animals, 91.80% (168/183) had never been taken to veterinary consultations, 3.83% (7/183) were seen annually, 2.73% (5/183) semiannually, and 1.64% (3/183) monthly.
The owners’ educational levels were assessed during interviews. The majority (36.07%, 22/61) had completed high school, 32.79% (20/61) had a college degree or technical education, and 31.15% (19/61) were either illiterate or had only completed elementary school. Although 88.52% (54/61) were unfamiliar with the term “zoonoses,” most of the dog owners (83.61%, 51/61) were aware of the cross-transmission of diseases between dogs and humans. Rabies (32.79%, 20/61), scabies (9.84%, 6/62), leptospirosis, and leishmaniosis (both 4.92%, 3/61) were the diseases mentioned by those aware of the risk of disease transmission. Regarding parasitic diseases, most of the owners mentioned ticks (98.36%), cutaneous larva migrans (96.72%), toxoplasmosis (70.49%), and giardiosis (68.85%). The least known parasites were Cryptosporidium (8.20%), Cystoisospora (6.56%), and Toxocara canis (4.92%). Awareness of zoonoses was associated with the level of education (refer to Table 5); however, the recognition of cross-transmission of diseases between dogs and humans remained consistent across all educational categories (p = 1).

Table 5.
Analysis of the association between the level of education and knowledge of the dog owner about the concept of zoonoses, Rolim de Moura, Rondônia State, Brazil, 2019.
4. Discussion
This study encountered limitations, including the relatively basic laboratory facilities in Rondônia, limited personnel availability, and budgetary constraints. Despite these limitations, it is important to highlight that this study contributes to the understanding of parasitic infections in pet dogs in the state of Rondônia, which remains limited due to the scarcity of research on canine diseases in the northern region of Brazil. Our findings revealed a significant proportion of infected pet dogs in the municipality of Rolim de Moura, underscoring the necessity for further investigation given the substantial sample size. Previous studies showed a high prevalence of gastrointestinal parasite infections in pet dogs in two cities within the state, where infection rates reached 84.2% (72/95) and 87.5% (35/40) [9,10]. Correspondingly, similar research conducted in other states of the northern region also reported elevated infection rates. For instance, a study that evaluated 80 stray dogs in Manaus, Amazonas, revealed positive parasite findings in all animals [11]. Furthermore, another study conducted in Gurupi, Tocantins, exhibited a gastrointestinal parasite infection rate of 39.20% among 126 stray dogs [2].
Ensuring the overall well-being of pet dogs, including their health, requires responsible ownership and proper care. Regular veterinary attention plays a crucial role in this context. However, it is concerning that only 8.20% of the animals in this study received regular veterinary attention. Furthermore, most dog owners incorrectly administered both anthelmintics and ectoparasiticides. According to the guidelines provided by the European Scientific Counsel Companion Animal Parasites and the Tropical Council for Companion Animal Parasites [24], anthelmintics should be administered at least quarterly, while ectoparasiticides should be administered monthly. The misuse of these medications can be attributed to a lack of knowledge and guidance among dog owners regarding responsible pet ownership. Pet owners bear the responsibility of meeting the physical, psychological, and environmental needs of their pets, while also taking measures to prevent risks, such as aggression, disease transmission, and harm to others [25].
The dynamics of certain tropical parasitic diseases have witnessed global changes, which have been influenced by socio-environmental factors [26]. In the Amazon region, the conversion of native forests to other land uses carries significant consequences. Newly populated areas often lack proper basic sanitation infrastructure, leading to the spread of zoonotic helminth infections transmitted through the soil [27]. Despite boasting a relatively high human development index (0.700) in comparison with other cities in Rondônia, only 14.6% of households in Rolim de Moura have access to a suitable water supply and sewage collection [15]. Most homes rely on septic tanks and wells for their water needs. In municipalities lacking adequate sewerage systems, groundwater can become contaminated due to improper septic tank waste disposal or irregular sewage, consequently resulting in environmental contamination [28]. Given the prevalence of zoonotic parasites in this area, this has additional negative impacts on the health of the population, further exacerbating diseases associated with inadequate sanitation. Engaging in activities focused on human and animal health could yield substantial benefits for the local population, including effectively controlling diagnosed parasites and enhancing sanitary conditions for both dogs and humans.
Two important zoonotic parasites, namely, Ancylostoma spp. and Toxocara canis, were found to be prevalent in Rolim de Moura. However, no available data pertains to human infections caused by these parasites in the city. The risk of exposure to these diseases extended beyond the dog owners at home, as 41.53% of dogs had outdoor access. Consequently, other individuals can potentially be exposed to these parasites when the dogs roam the streets during walks in public areas and parks [2]. This inadequate interaction between humans, animals, and the environment heightens the risk of zoonoses, particularly among children who engage in outdoor activities and potential geophagy, rendering them more susceptible to these pathogens [29]. The larval form of Ancylostoma spp., which was identified in 68.71% of the dog samples in this study, is the causative agent of cutaneous larva migrans (CLM) disease. CLM is endemic in tropical and subtropical regions, particularly in Latin America and the Caribbean, Southeast Asia, and Africa. Accurate assessments of the global occurrence of CLM remain lacking. Publication bias might be present due to underreporting and potential underdiagnosis in numerous countries where this condition is often overlooked [30], which also happens in northern Brazil.
Infection by T. canis was observed in 6.75% of the tested dogs. This apparent low frequency may be related to the parasite’s biology, as in dogs older than four to six months, the parasite’s larvae tend to enter a hypobiotic state in the host’s tissues instead of developing into the adult stage [31]. Most sampled dogs were over one year old, suggesting that the observed prevalence might not accurately reflect the true distribution of this nematode. Toxocariosis, which is caused by the nematode T. canis, involves humans as paratenic hosts, harboring larvae of the parasite in their tissues [32]. Presently, toxocariosis ranks among the six most significant neglected parasitic infections in the United States [33]. Apart from ocular damage, human toxocariosis is associated with neurological lesions, leading to cognitive deficits in exposed children [34]. There is inadequate data on the occurrence of T. canis infection in dogs across various Brazilian regions, yet available information suggests that its prevalence could be higher in northern Brazil compared with other areas. Importantly, the burden of human toxocariosis in this country remains largely underestimated [35].
Concerning ectoparasites, Rhipicephalus sanguineus ticks were detected in the evaluated animals, particularly in dogs residing in urban areas [36]. Notably, purebred animals exhibited significantly fewer ectoparasites, possibly due to better care and the use of costly ectoparasiticides, which might be less accessible for mixed-breed dog owners.
It is noteworthy to say that the present study is based on propagule morphology, and the employed methodology could underestimate the presence of parasites with small propagules, such as Giardia spp. and Cryptosporidium spp. The absence of these parasites in our results could be related to the low sensitivity of the adopted techniques. Considering Giardia spp., the combination of rapid tests and fecal flotation could reduce the chances of false negatives [37,38,39,40]. On the other hand, the diagnosis of Cryptosporidium spp. infection depends on specific techniques, such as acid-fast staining or molecular tests [41,42]. Also, as the study area is inserted in one of the most megadiverse regions of the planet [43], the occurrence of rare or scarcely known parasite species in the studied animals should not be ruled out, as recent studies developed in Australia, which is another megadiverse country, revealed the presence of Linguatula serrata affecting dogs and wild carnivores. As these parasites’ eggs resemble the propagules of other parasites, their presence was ignored [44,45]. Therefore, further studies should be encouraged in this region, adopting other techniques and sampling the wild animals associated with anthropized areas.
Campaigns that endorse responsible pet ownership and the prevention of zoonotic diseases can play a pivotal role in bridging this gap, as their scope extends beyond the financial aspect of pet ownership. Additionally, it is essential to underscore that alongside responsible ownership campaigns, health education assumes a pivotal role in mitigating concerns related to animal and public health. Employing questionnaires to gauge the population’s existing knowledge of zoonoses allows for the formulation of tailored health education policies, which can be developed based on the unique requirements of each community. These initiatives can foster collaborative efforts in health education and epidemiological surveillance [46].
5. Conclusions
The prevalence of parasite infection, particularly those with zoonotic potential, among pet dogs was high in Rolim de Moura. The close interaction between humans and infected animals highlights the significance of addressing these potential health risks. Implementing effective educational initiatives can play a crucial role in reducing the incidences of these zoonotic diseases and promoting a safer environment within the municipality.
Author Contributions
Conceptualization, E.G.L.-H. and T.O.M.; methodology, E.G.L.-H. and L.A.M.; software, T.O.M. and L.A.M.; validation, E.G.L.-H., T.O.M. and L.A.M.; formal analysis, E.G.L.-H., T.O.M., L.A.M. and K.P.B., investigation, T.O.M., P.P.P., D.d.S.Z., H.L.d.S., P.H.K.P., I.M.M., J.H.T., L.A.M., K.P.B. and E.G.L.-H.; resources, T.O.M., D.d.S.Z., H.L.d.S., P.H.K.P., I.M.M., J.H.T., L.A.M., K.P.B. and E.G.L.-H.; data curation, T.O.M., L.A.M. and E.G.L.-H.; writing—original draft preparation, T.O.M., L.A.M., K.P.B. and E.G.L.-H.; writing—review and editing, T.O.M., P.P.P., L.A.M., K.P.B. and E.G.L.-H.; visualization, E.G.L.-H.; supervision, E.G.L.-H.; project administration, E.G.L.-H. and T.O.M.; funding acquisition, E.G.L.-H. and K.P.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), grant number 001, and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), grant number 311063/2022-5.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Human Ethics Committee (Plataforma Brasil protocol code 3.560.313/19, 06 Sep 2019). The animal study protocol was approved by the Ethics Committee of FCAV/Unesp (protocol code 006737/19, 3 June 2019).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
General characteristics of pet dogs from Rolim de Moura, Rondônia, Brazil, 2019.
| Variables | Number of Dogs | % | |
| Sex | Male | 115 | 62.84 |
| Female | 68 | 37.16 | |
| Breed | Purebred | 45 | 24.59 |
| Mixed breed | 138 | 75.41 | |
| Size | Small | 97 | 53.00 |
| Medium | 49 | 26.78 | |
| Large | 37 | 20.22 | |
| Age | Up to 12 months | 40 | 21.86 |
| Over 12 months | 143 | 78.14 | |
| Means of acquisition | Gifted | 80 | 43.72 |
| Bred | 51 | 27.87 | |
| Purchased | 36 | 19.67 | |
| Place of origin | Rolim de Moura | 16 | 8.74 |
| Other municipalities | 173 | 94.54 | |
| Close contact with other animals | Dogs | 10 | 5.46 |
| Cats | 170 | 92.90 | |
| Number of contacting dogs | <10 | 86 | 46.99 |
| >10 | 128 | 69.94 | |
| Number of contacting cats | <10 | 42 | 22.95 |
| >10 | 66 | 24.05 | |
| House access | Indoor access | 20 | 10.93 |
| Outdoor only | 2 | 1.09 | |
| Both | 162 | 88.52 | |
| Outdoor access (walks) | No | 19 | 10.38 |
| Yes | 107 | 58.47 | |
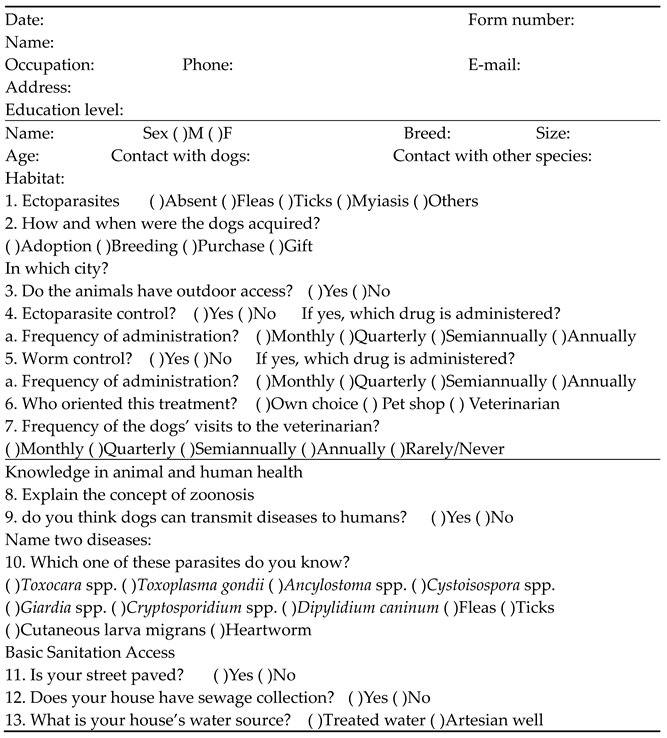
Appendix B
Script of the semi-structured interviews performed in this study.
 |
Appendix C
Univariate analysis of the association between dog characteristics and frequency of infection by Toxocara canis, Rolim de Moura, Rondônia state, Brazil, 2019.
| Variables | EX. | POS. | % | (95% CI) | p | OR (95% CI) |
| Sex | ||||||
| Male | 58 | 6 | 10.34 | 3.89–21.17 | 0.20 | 2.30 (0.67–7.92) |
| Female | 105 | 5 | 4.76 | 1.56–10.76 | ||
| Size | ||||||
| Small | 81 | 4 | 4.94 | 1.36–12.16 | 0.55 | Undefined |
| Medium | 48 | 4 | 8.33 | 2.32–19.98 | ||
| Large | 34 | 3 | 8.82 | 1.86–23.68 | ||
| Breed | ||||||
| Purebred | 41 | 1 | 2.44 | 0.06–12.86 | 0.29 | 0.28 (0.03–2.25) |
| Mixed breed | 122 | 10 | 8.20 | 4.00–12.56 | ||
| Age | ||||||
| Up to 12 months | 37 | 4 | 10.81 | 3.03–25.42 | 0.27 | 0.48 (0.13–1.75) |
| Over 12 months | 126 | 7 | 5.56 | 2.26–11.11 | ||
| Outdoor access (walks) | ||||||
| Yes | 60 | 6 | 10.00 | 3.76–20.51 | 0.21 | 2.17 (0.63–7.46) |
| No | 103 | 5 | 4.85 | 1.59–10.97 | ||
| Use of antihelminthics | ||||||
| Yes | 123 | 9 | 7.32 | 3.40–13.44 | 1.0 | 1.50 (0.31–7.25) |
| No | 40 | 2 | 5.00 | 0.61–16.92 | ||
EX.—number of dogs examined; POS.—number of positive dogs; %—frequency; 95% CI—95% frequency confidence interval; p—p-value; OR (95% CI)—odds ratio and 95% confidence interval.
Appendix D
Univariate analysis of the association between dog characteristics and frequency of infection by Trichuris vulpis, Rolim de Moura, Rondônia state, Brazil, 2019.
| Variables | EX. | POS. | % | (95% CI) | p | OR (95% CI) |
| Sex | ||||||
| Male | 58 | 5 | 8.62 | 2.86–18.98 | 0.45 | 0.61 (0.20–1.79) |
| Female | 105 | 14 | 13.33 | 7.49–21.36 | ||
| Size | ||||||
| Small | 81 | 7 | 8.64 | 3.55–17.00 | 0.20 | Undefined |
| Medium | 48 | 5 | 10.42 | 3.47–22.66 | ||
| Large | 34 | 7 | 20.59 | 8.70–37.90 | ||
| Breed | ||||||
| Purebred | 41 | 1 | 2.44 | 0.06–12.86 | 0.06 | 0.14 (0.01–1.11) |
| Mixed breed | 122 | 18 | 14.75 | 8.98–22.31 | ||
| Age | ||||||
| Up to 12 months | 37 | 3 | 8.11 | 1.70–21.92 | 0.56 | 1.64 (0.45–5.99) |
| Over 12 months | 126 | 16 | 12.70 | 7.44–19.80 | ||
| Outdoor access (walks) | ||||||
| Yes | 60 | 6 | 10.00 | 3.76–20.51 | 0.80 | 0.76 (0.27–2.14) |
| No | 103 | 13 | 12.62 | 6.89–20.62 | ||
| Use of antihelminthics | ||||||
| Yes | 123 | 16 | 13.01 | 7.62–20.26 | 0.41 | 1.84 (0.50–6.69) |
| No | 40 | 3 | 7.50 | 1.57–20.39 | ||
EX.—number of dogs examined; POS.—number of positive dogs; %—frequency; 95% CI—95% frequency confidence interval; p—p-value; OR (95% CI)—odds ratio and 95% confidence interval.
References
- Otranto, D.; Dantas-Torres, F.; Mihalca, A.D.; Traub, R.J.; Lappin, M.; Baneth, G. Zoonotic parasites of sheltered and stray dogs in the era of the global economic and political crisis. Trends Parasitol. 2017, 33, 813–825. [Google Scholar] [CrossRef]
- Nunes, H.C.; Moura, A.S.; Gontijo, E.E.L.; Silva, M.G. Prevalência de parasitas intestinais em cães triados no centro de controle de zoonoses de Gurupi, Tocantins. Rev. Cereus 2018, 10, 27–37. Available online: http://ojs.unirg.edu.br/index.php/1/article/view/2322/695 (accessed on 19 January 2020). (In Portuguese).
- Katagiri, S.; Oliveira-Sequeira, T.C.G. Prevalence of dog intestinal parasites and risk perception of zoonotic infection by dog owners in Sao Paulo State, Brazil. Zoonoses Public Health 2008, 55, 406–413. [Google Scholar] [CrossRef]
- Ferraz, A.; Evaristo, T.A.; dos Santos Pires, B.; de Castro, T.A.; Pinto, D.M.; Nizoli, L.Q. Ocorrência de parasitos gastrointestinais, em fezes de cães, encontradas na orla das praias de Pelotas, RS, Brasil. Atas Saúde Ambient. 2018, 6, 226–234. (In Portuguese) [Google Scholar] [CrossRef]
- De Souza, C.P.; Da Silva, P.F.; Moreno, M.C.; D’Andrea, L.A.Z. Serviços de zoonoses e o seu papel na vigilância em saúde para leishmaniose visceral. Colloq. Vitae 2019, 11, 24–32. (In Portuguese) [Google Scholar] [CrossRef]
- Mascarini-Serra, L.M.; Telles, C.A.; Prado, M.S.; Mattos, S.A.; Strina, A.; Alcantara-Neves, N.M.; Barreto, M.L. Reductions in the prevalence and incidence of geohelminth infections following a city-wide sanitation program in a Brazilian Urban Centre. PLoS Negl. Trop. Dis. 2010, 4, e558. [Google Scholar] [CrossRef]
- Nascimento, C.P. O Processo de Ocupação e Urbanização de Rondônia: Uma Análise das Transformações Sociais e Espaciais. Rev. Geogr. 2010, 27, 2. (In Portuguese) [Google Scholar]
- Labruna, M.B.; Pena, H.F.D.J.; Souza, S.L.P.; Pinter, A.; Silva, J.C.R.; Ragozo, A.M.A.; Camargo, L.M.A.; Gennari, S.M. Prevalência de endoparasitas em cães da área urbana do município de Monte Negro, Rondônia. Arq. Inst. Biol. 2006, 73, 183–193. (In Portuguese) [Google Scholar] [CrossRef]
- Lopes, T.V.; de Souza Morais, W.E.; de Almeida, G.B.M.; Rosas, F.M.P.; de Almeida Souza, T.; Muniz, I.M.; Souza, F.A. Estudo da prevalência de endoparasitos em fezes de cães domiciliados na zona norte de Porto Velho, Rondônia, Brasil. Res. Soc. Dev. 2015, 10, e90101018217. (In Portuguese) [Google Scholar] [CrossRef]
- Pereira Junior, G.; Barbosa, P.S. Prevalência de endoparasitas em cães errantes na cidade de Manaus-AM. Acta Biomed. Bras. 2013, 4, 52–57. (In Portuguese) [Google Scholar]
- Cediel, N.; Conte, V.; Tomassone, L.; TibertiI, D.; Guiso, P.; Romero, J.; Villami, L.C.; De Meneghi, D. Risk perception about zoonoses in immigrants and Italian workers in Northwestern Italy. Rev. Saúde Pública 2012, 46, 850–857. [Google Scholar] [CrossRef]
- Pereira, A.; Martins, A.; Brancal, H.; Vilhena, H.; Silva, P.; Pimenta, P.; Diz-Lopes, D.; Neves, N.; Coimbra, M.; Alves, A.C.; et al. Parasitic zoonoses associated with dogs and cats: A survey of portuguese pet owners’ awareness and deworming practices. Parasit. Vectors 2016, 9, 245. [Google Scholar] [CrossRef]
- Mangesho, P.E.; Neselle, M.O.; Karimuribo, E.D.; Mlangwa, J.E.; Queenan, K.; Mboera, L.E.G. Exploring local knowledge and perceptions on zoonoses among pastoralists in northern and eastern Tanzania. PLoS Negl. Trop. Dis. 2017, 11, e0005345. [Google Scholar] [CrossRef]
- Brazilian Institute of Geography and Statistics. 2010. Available online: https://cidades.ibge.gov.br/brasil/ro/rolim-de-moura/panorama (accessed on 11 July 2023). (In Portuguese)
- Brazilian Institute of Geography and Statistics. 2021. Available online: https://cidades.ibge.gov.br/brasil/ro/rolim-de-moura/panorama (accessed on 11 July 2023). (In Portuguese)
- Linardi, P.M.; Guimarães, L.R. Sifonápteros do Brasil; Editora USP: São Paulo, Brazil, 2000; 291p. (In Portuguese) [Google Scholar]
- Guimarães, J.H.; Tucci, E.C.; Barros-Battesti, D.M. Ectoparasitos de Importância Veterinária; Editora Fapesp: São Paulo, Brazil, 2001; 218p. (In Portuguese) [Google Scholar]
- Martins, T.F.; Onofrio, V.C.; Barros-Battesti, D.M.; Labruna, M.B. Nymphs of the genus Amblyomma (Acari: Ixodidae) of Brazil: Descriptions, redescriptions, and identification key. Ticks Tick Borne Dis. 2010, 1, 75–99. [Google Scholar] [CrossRef]
- Barros-Battesti, D.M.; Ramirez, D.G.; Landulfo, G.A.; Faccini, J.L.H.; Dantas-Torres, F.; Labruna, M.B.; Venzal, J.M.; Onofrio, V.C. Immature argasid ticks: Diagnosis and keys for Neotropical region. Rev. Bras. Parasitol. Vet. 2013, 22, 443–456. [Google Scholar] [CrossRef]
- Martins, T.F.; Barbieri, A.R.; Costa, F.B.; Terassini, F.A.; Camargo, L.M.; Peterka, C.R.; Pacheco, R.C.; Dias, R.A.; Nunes, P.H.; Marcili, A.; et al. Geographical distribution of Amblyomma cajennense (sensu lato) ticks (Parasitiformes: Ixodidae) in Brazil, with description of the nymph of A. cajennense (sensu stricto). Parasit. Vectors 2016, 9, 186. [Google Scholar] [CrossRef]
- Dantas-Torres, F.; Martins, T.F.; Muñoz-Leal, S.; Onofrio, V.C.; Barros-Battesti, D.M. Ticks (Ixodida: Argasidae, Ixodidae) of Brazil: Updated species checklist and taxonomic keys. Ticks Tick Borne Dis. 2019, 10, 101252. [Google Scholar] [CrossRef]
- Zajac, A.M.; Conboy, G.A.; Little, S.E.; Reichard, M.V. Veterinary Clinical Parasitology, 9th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2021. [Google Scholar]
- Bush, A.O.; Lafferty, K.D.; Lotz, J.M.; Shostak, A.W. Parasitology meets ecology on its own terms: Margolis et al. revisited. J. Parasitol. 1997, 83, 575–583. [Google Scholar] [CrossRef]
- Guidelines for the Diagnosis, Treatment and Control of Canine Endoparasites in the Tropics—Tropical Council for Companion Animal Parasites. Available online: https://www.troccap.com/2017press/wp-content/uploads/2019/05/TroCCAP_Canine_Endo_Guidelines_English_Ver2.pdf (accessed on 14 July 2023).
- Santana, L.R.; Oliveira, T.P. Guarda responsável e dignidade dos animais. Rev. Bras. Dir. Anim. 2006, 1, 1. (In Portuguese) [Google Scholar]
- Mandal, F.B. The Changing Ecology of Infectious Diseases in Humans. World Environ. 2011, 1, 14–19. [Google Scholar] [CrossRef]
- Confalonieri, U.E.; Margonari, C.; Quintão, A.F. Environmental change and the dynamics of parasitic diseases in the Amazon. Acta Trop. 2014, 129, 33–41. [Google Scholar] [CrossRef]
- Helbel, A.F.; Nunes, M.L.A.; Marchetto, M. Água subterrânea: Estudo de caso em Ji-Paraná. Águas Subterrâneas. 2008. Available online: https://aguassubterraneas.abas.org/asubterraneas/article/view/23310 (accessed on 19 January 2020). (In Portuguese).
- Cassenote, A.J.; Pinto Neto, J.M.; Lima-Catelani, A.R.; Ferreira, A.W. Contaminação do solo por ovos de geohelmintos com potencial zoonótico na municipalidade de Fernandópolis, Estado de São Paulo, entre 2007 e 2008. Rev. Soc. Bras. Med. Trop. 2011, 44, 371–374. (In Portuguese) [Google Scholar] [CrossRef][Green Version]
- Rodriguez-Morales, A.J.; González-Leal, N.; Montes-Montoya, M.C.; Fernández-Espíndola, L.; Bonilla-Aldana, D.K.; Azeñas-Burgoa, J.M.; Medina, J.C.D.; Rotela-Fisch, V.; Bermudez-Calderon, M.; Arteaga-Livias, K.; et al. Cutaneous larva migrans. Curr. Trop. Med. Rep. 2021, 8, 190–203. [Google Scholar] [CrossRef]
- Taylor, M.A.; Coop, R.L.; Wall, R.L. Parasitologia Veterinária; Guanabara Koogan: Rio de Janeiro, Brazil, 2017. (In Portuguese) [Google Scholar]
- Anderson, R.C. Nematode Parasites of Vertebrates: Their Development and Transmission, 2nd ed.; Cabi: Wallingford, UK, 2000. [Google Scholar]
- Centers for Disease Control and Prevention. Available online: https://www.cdc.gov/parasites/toxocariasis/ (accessed on 11 July 2023).
- Hotez, P. Neglected infections of poverty in the United States and their effects on the brain. JAMA Psychiatry 2014, 71, 1099–1100. [Google Scholar] [CrossRef]
- Dantas-Torres, F. Toxocara prevalence in dogs and cats in Brazil. Adv. Parasitol. 2020, 109, 715–741. [Google Scholar] [CrossRef]
- Labruna, M.B.; Pereira, M.D.C. Carrapato em cães no Brasil. Clín. Vet. 2001, 6, 24–32. (In Portuguese) [Google Scholar]
- Rishniw, M.; Liotta, J.; Bellosa, M.; Bowman, D.; Simpson, K.W. Comparison of 4 Giardia diagnostic tests in diagnosis of naturally acquired canine chronic subclinical giardiasis. J. Vet. Intern. Med. 2010, 24, 293–297. [Google Scholar] [CrossRef]
- Pepe, P.; Ianniello, D.; Alves, L.C.; Morgoglione, M.E.; Maurelli, M.P.; Bosco, A.; Cringoli, G.; Rinaldi, L. Comparative cost-effectiveness of immunoassays and FLOTAC for diagnosing Giardia spp. infection in dogs. Parasites Vectors 2019, 12, 158. [Google Scholar] [CrossRef]
- Uchôa, F.F.M.; Sudré, A.P.; Campos, S.D.E.; Almosny, N.R.P. Assessment of the diagnostic performance of four methods for the detection of Giardia duodenalis in fecal samples from human, canine and feline carriers. J. Microbiol. Methods 2018, 145, 73–78. [Google Scholar] [CrossRef]
- Mekaru, S.R.; Marks, S.L.; Felley, A.J.; Chouicha, N.; Kass, P.H. Comparison of direct immunofluorescence, immunoassays, and fecal flotation for detection of Cryptosporidium spp. and Giardia spp. in naturally exposed cats in 4 Northern California animal shelters. J. Vet. Intern. Med. 2007, 21, 959–965. [Google Scholar] [CrossRef]
- Taghipour, A.; Olfatifar, M.; Bahadory, S.; Godfrey, S.S.; Abdoli, A.; Khatami, A.; Javanmardh, E.; Shahrivar, F. The global prevalence of Cryptosporidium infection in dogs: A systematic review and meta-analysis. Vet. Parasitol. 2020, 281, 109093. [Google Scholar] [CrossRef] [PubMed]
- Mufa, R.M.D.; Lastuti, N.D.R.; Legowo, D. Detection of Cryptosporidiosis in dogs of veterinary clinics in surabaya city using acid-fast staining and PCR. J. Veterinário Mund. 2021, 11, 602–607. [Google Scholar] [CrossRef]
- Outlook, G.B. Global Biodiversity Outlook 3; Secretariat of the Convention on Biological Diversity: Montréal, QC, Canada, 2010. Available online: https://www.cbd.int/gbo1/gbo-pdf.shtml (accessed on 12 December 2023).
- Shamsi, S.; Mc Spadden, K.; Baker, S.; Jenkins, D.J. Occurrence of tongue worm, Linguatula cf. serrata (Pentastomida: Linguatulidae) in wild canids and livestock in south-eastern Australia. Int. J. Parasitol. Parasites Wildl. 2017, 6, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Shamsi, S.; Barton, D.P.; Zhu, X.; Jenkins, D.J. Characterisation of the tongue worm, Linguatula serrata (Pentastomida: Linguatulidae), in Australia. Int. J. Parasitol. Parasites Wildl. 2020, 11, 149–157. [Google Scholar] [CrossRef]
- Oliveira-Neto, R.R.; Souza, V.F.; Carvalho, P.F.G.; Frias, D.F.R. Nível de conhecimento de tutores de cães e gatos sobre zoonoses. Rev. Salud Publica 2018, 20, 198–203. (In Portuguese) [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).