Abstract
We are presenting an overview of the retracted clinical trials about the Coronavirus Disease (COVID)-19 published in PubMed using the descriptors ((COVID-19 OR SARS-CoV-2) AND (Clinical Trial)). We collected the information for i) the first author’s country; ii) the journal name where the study was published; iii) the impact factor of the journal; iv) the main objective of the study; v) methods including population, intervention, study design, and outcomes; and vi) results and conclusions. We collected complete information from the retraction notes published by the journals and the number of publications/retractions related to non-COVID-19 clinical trials published simultaneously. We also included the Altmetric index for the clinical trials and the retraction notes about COVID-19 to compare the accessibility to both studies’ indexes. The retraction of clinical trials occurred in four countries (one in Lebanon, one in India, one in Brazil, and five in Egypt) and six journals (one in Viruses, one in Archives of Virology, one in Expert Review of Anti-infective Therapy, one in Frontiers in Medicine, two in Scientific Reports, and two in The American Journal of Tropical Medicine and Hygiene). Eight drugs were tested (Ivermectin, Vitamin D, Proxalutamide, Hydroxychloroquine, Remdesevir, Favipiravir, and Sofosbuvir + Daclatasvir) in the studies. One of the retractions was suggested by the authors due to an error in the statistical analysis, which compromised their results and conclusions. Also, the methods, mainly the allocation, were not well conducted in the two studies, and the studies were retracted. In addition, the studies performed by Dabbous et al. presented several issues, mainly including several raw datasets that did not prove their findings. Moreover, two studies were retracted due to data overlap and copying. Significant concerns were raised about the integrity of the data and reported results in another article. We identified a higher Altmetric index for the original studies, proving that the retracted studies were accessed more than the retraction notes. Interestingly, the impact of the original articles is much higher than their retraction notes. The different Altmetric indexes show that possibly people who read those retracted articles are not reading their retraction notes and are unaware of the erroneous information they share. COVID-19- related clinical trials were ~two-time times more retracted than the other clinical trials performed during the same time.
1. Introduction
Coronavirus Disease (COVID)-19 is the most widespread and severe pandemic in modern times. The world, including the scientific community, turned their efforts to investigate the disease, searching for pathophysiological mechanisms, risk factors, possible treatments, and vaccines. Following the “publish or perish” mantra, the urge for discoveries regarding COVID-19 has taken its toll on evidence-based science, resulting in a significant number of papers published about COVID-19 [1]. Valencise et al. (2022) showed the impact COVID-19 caused on the publication of papers regarding the 25 leading death causes, according to world region, implicating that while everyone was focused on COVID-19-related research, the leading causes of death had their research postponed or canceled [2].
With such a fast income of COVID-19 publications, followed by their retractions, the characteristics of the removed papers can provide important details into the nature of the removals and the review process [3,4]. Articles may be retracted when their findings are not trustworthy. This can occur due to scientific misconduct or error (e.g., data manipulation, fraudulent data, unsupported conclusions, questionable data validity, non-replicability, data errors—even if unintended) or ethical guidelines violations (e.g., duplicate publication, plagiarism, missing credit, no institutional review board, ownership issues, authorship issues) [5,6,7]. In first-quartile journals like The Lancet and The New England Journal of Medicine, retractions of papers from journals like these are in the minority in retraction but raise a more significant concern for evidence-based medicine [3]. Moreover, it is becoming harder to understand the reasons behind the removal of the papers, making the importance of the retraction note significantly higher. In addition, due to the high number of published papers, it is difficult to read all of them, even the retractions notes.
In this matter, several articles were rushed and published under poor evaluation, causing the number of retractions to escalate vigorously [8], including the clinical trials studies about COVID-19, which comprised the utmost importance articles, causing the need for several retraction notes [9,10,11,12,13,14,15,16]. In theory, once a paper is retracted, it should only be cited by other studies in the context of their retraction [7,17]. However, in reality, there are an impressive number of citations on retracted papers without mentioning the retraction of said papers, which raised several concerns in the scientific community [7,17]. Furthermore, not only is rushing papers to publication, sometimes under poor evaluation, dangerous, the retraction notes provided by the journals bring very little information regarding the reasons for retraction and do not get as many visualizations as the original article, which implies that not all people that read the paper got access to the retraction note.
This problem gets aggravated during a pandemic such as COVID-19 once everyone searches for pathogenesis and possible treatments. Rush-publishing papers in this scenario impact the quality of treatment for the disease, making it possible for medications that were not properly evaluated to gain momentum in the great masses, disseminating unverified information [18].
In this context, we aimed to present an overview of the retracted clinical trials during the COVID-19 pandemic published in PubMed and to discuss why this issue was aggravated during the pandemic, calling for attention to this unaddressed problem, its impact on COVID-19 treatment and why it may put science in jeopardy once it stimulates quantity over quality.
2. Materials and Methods
In the present brief report, we present an overview of the retracted clinical trials during the COVID-19 pandemic and for COVID-19 interventions published in PubMed using the descriptors ((COVID-19 OR SARS-CoV-2) AND (Clinical Trial)). PubMed database was selected to standardize the study because PubMed database uses an algorithm that searches the title, abstract, and headings of articles in the National Library of Medicine database—specific to medicine and health [19]. Also, we summarize clinical trials published during the COVID-19 pandemic (from the year 2019 to 12 December 2022) not related to COVID-19 published in PubMed using the descriptors ((Clinical Trial) NOT (COVID-19 OR SARS-CoV-2)). We presented the papers as the indexation type: “clinical trial Phase I”, “clinical trial Phase II”, “clinical trial Phase III”, “clinical trial Phase IV”, “clinical trial protocol”, and “randomized clinical trial”. We presented the proportion of each type of paper by the total number of clinical trials published. In addition, we selected the types of papers classified only as “retracted publication” or “retraction of publication” (COVID-19-related) to present a complete description of their findings. We excluded the papers not associated with COVID-19 interventions.
In this context, considering only the retracted papers for COVID-19-related interventions, we collected the information for (i) the first author’s country; (ii) the journal name where the study was published; (iii) the impact factor of the journal; (iv) the main objective of the study; (v) methods including population, intervention, study design, and outcomes; and (vi) results and conclusions. We also collected complete information from the retraction notes published by the journals, the Altmetric index for the clinical trials and the retraction notes to compare the accessibility to both studies’ indexes (https://www.altmetric.com/ accessed on 16 December 2022). In addition, the number of citations achieved from the PubMed database was described.
The Altmetric is an index that collects and collates all of the disparate information about research to provide the Scientific community with a visually engaging and informative view of the online activity surrounding the scholarly content. In brief, as described by the developers, “Altmetrics are metrics and qualitative data that are complementary to traditional, citation-based metrics. They can include (but are not limited to) peer reviews on Faculty of 1000, citations on Wikipedia and in public policy documents, discussions on research blogs, mainstream media coverage, bookmarks on reference managers like Mendeley, and mentions on social networks such as Twitter. Sourced from the Web, Altmetrics can tell you a lot about how often journal articles and other scholarly outputs like datasets are discussed and used around the world. For that reason, Altmetrics have been incorporated into researchers’ websites, institutional repositories, journal websites, and more.”.
We compared the proportion between the number of retracted clinical trials by the total number of published clinical trials considering the ratio between COVID-19-related papers and other clinical trials.
3. Results
On 12 December 2022, a total of 8445 studies were published in PubMed using the descriptors ((COVID-19 OR SARS-CoV-2) AND (Clinical Trial)). From them, 142 were Phase I studies, 250 were Phase II studies, 156 were Phase III studies, and 17 were Phase IV studies. In addition, 2086 studies were indexed as randomized controlled trials and 549 as clinical trial protocols (Table 1). Also, using the descriptors, nine studies were retracted [9,10,11,12,13,14,15,16,20]; however, one study was not associated with a COVID-19 intervention, and it was excluded, as well as its retraction note [20] (Table 1 and Table 2). During the same period, from January 2019 to December 12, 2022, we observed a total of 202,398 studies published in PubMed using the descriptors ((Clinical Trial) NOT (COVID-19 OR SARS-CoV-2)). From them, 4774 were Phase I studies, 7426 were Phase II studies, 5445 were Phase III studies, and 594 were Phase IV studies (Table 1). In addition, 92,111 studies were indexed as randomized controlled trials and 7942 as clinical trial protocols. Also, 111 clinical trial studies were retracted (Table 1).

Table 1.
Number of clinical trials published in PubMed during the Coronavirus Disease (COVID)-19 pandemic and the proportion between COVID-19-related clinical trials and other clinical trials.

Table 2.
Title of the retracted studies, first author’s country, and journal where the study was published.
Until 12 December 2022, the COVID-19-related articles (clinical trials) were 1.73 times (8/8445 by 111/202,398) more retracted than other clinical trials during the same period (Table 1). Curiously, clinical trial protocols were proportionally more published for COVID-19-related clinical trials than non-COVID-19-related clinical trials; in contrast, all clinical trial phases and the descriptor randomized clinical trial were more evident for non-COVID-19-related clinical trials.
The retraction of clinical trials occurred in four countries (one in Lebanon, one in India, one in Brazil, and five in Egypt) and six Journals (one in Viruses, one in Archives of Virology, one in Expert Review of Anti-infective Therapy, one in Frontiers in Medicine, two in Scientific Reports, and two in The American Journal of Tropical Medicine and Hygiene) (Table 2).
In Table 3, we presented the characterization of the clinical trials about COVID-19 retracted after publication, including their objective, methods (intervention, study design, and outcomes), results, and conclusions. In addition, we presented the retraction notes and the Altmetrics indexes (crude data) for the clinical trials (original study) and their retraction note. In Figure 1, we presented the number of citations of the clinical trials (original study) and their retraction notes. Curiously, all clinical trials were cited, and two received more than 100 citations [13,15]. Besides that, only four retractions notes were cited by eight, one, five, and three studies [9,10,12,13]. Between both studies that received more than 100 citations, only one had citations for the retraction note [13]. Also, the retraction note with the highest number of citations (eight) was associated with one study with 37 citations [9].

Table 3.
Characterization of the clinical trials about Coronavirus Disease (COVID)-19 retracted after publication.
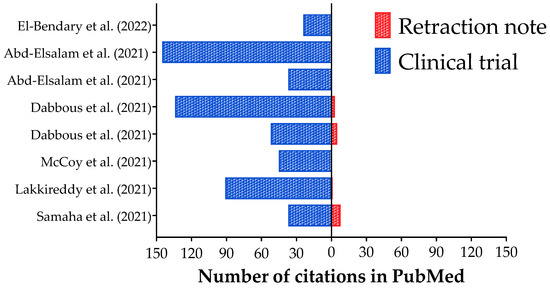
Figure 1.
Number of citations in Pubmed database for the retracted clinical trials about Coronavirus Disease (COVID)-19 interventions and their retractions notes. References: [9,10,11,12,13,14,15,16].
One of the retractions was suggested by the authors due to an error in the statistical analysis, which compromised their results and conclusions [9]. Also, the methods, mainly the allocation, were not well done in the two studies, and the studies were retracted [10,11]. In addition, the studies performed by Dabbous et al. (2021, 2022) [12,13] presented several issues, mainly including several raw datasets that did not prove their findings. Moreover, two studies were retracted together due to data overlap and/or copying; in this case, the authors have not provided a reasonable explanation for this significant problem, and the authors have not provided adequate data error-checking or validation to ensure that the remaining results presented in the paper accurately represent the sourced data [14,15]. Also, significant concerns have been raised about the integrity of the data and reported results in one article; the authors have been unable to address the concerns raised fully and cannot provide sufficient supporting information [16] (Table 3).
Figure 2 presents the Altmetrics indexes for clinical trials (original studies) and their retraction notes. In our data, there is a high amplitude between the Altmetrics indexes for the clinical trials, which ranged between one [16] to 1763 [10] (Figure 2a); however, the amplitude was low among the retractions notes which ranged between zero [13,16] and 344 [9] (Figure 2b). In addition, we calculated the proportion between the Altmetrics indexes for the clinical trials and their retraction notes (Figure 2c). The clinical trial with the highest Altmetric index had a higher ratio (16.17×) [10]. To note, two clinical studies had a higher Altmetric index for the retraction note [12,14], and maybe one of them can be related to the fact that the retraction note [14] was made for two simultaneous clinical trials [14,15].
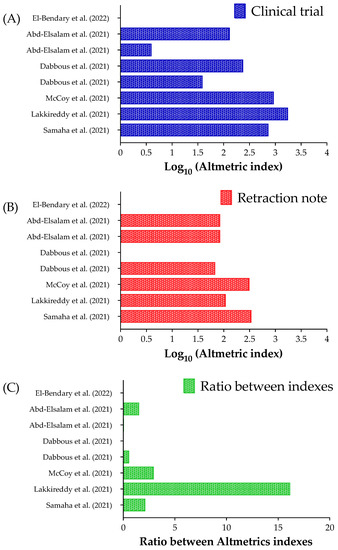
Figure 2.
Altmetric index for the clinical trials and their retraction notes related to Coronavirus Disease (COVID-19) interventions. (A) Altmetric index for the clinical trials. (B) Altmetric index for the retraction notes. (C) The ratio between the Altmetric index for the clinical trials and their retraction notes. We presented the Altmetric index using a Log10 scale in (A,B). It was impossible to calculate the ratio between the Altmetrics indexes for two clinical trials and their retractions notes [13,16]. The Altmetric index was obtained using the “Altmetric it!” when logged in Pubmed for each clinical trial and its retraction note. References: [9,10,11,12,13,14,15,16].
Of those retracted papers, one evaluated Ivermectin (PubChem CID: #6321424; C48H74O14) as a potential agent to control the viral load of SARS-CoV-2 among asymptomatic participants in Lebanon [9]. The paper was later retracted because the authors identified an error in files during the statistical analysis, which comprised the study and its findings [9]. Initially, the authors described that Ivermectin could cause fewer symptoms, lower viral load, and reduce hospital admissions in patients infected with SARS-CoV-2 but asymptomatic on inclusion.
A third clinical trial was retracted, and it evaluated the inclusion of Pulse D therapy to reduce the inflammatory markers of COVID-19 [10]. In this study, the authors described that vitamin D levels increased after Pulse D therapy in the vitamin D group and were associated with a reduction of the measured inflammatory markers. In addition, the authors identified a significant difference in the decrease in inflammatory markers between the study groups [10]. The study was later retracted because significant differences in the baseline parameters were identified, indicating that randomization may not have been performed correctly [10]. In this context, a post-publication peer review identified that the allocation method used was not appropriate. In brief, the study was retracted because the methods were unsuitable for affirming that Pulse D therapy was the only factor associated with the different outcomes between both study groups [10].
An American first author conducted a Brazilian study to describe if Proxalutamide (PubChem CID: #60194102; C24H19F4N5O2S), an androgen receptor antagonist, was effective in treating men with COVID-19 in an outpatient setting [11]. In the study, the authors included 268 men that received Proxalutamide (N = 134) or placebo (N = 134). In brief, the authors noticed that the 30-day hospitalization rate was 2.2% in men taking Proxalutamide when compared to 26% in placebo [11]. After the publication, several concerns were raised about the methods, and the study was retracted mainly due to errors in the allocation process, which was not random [11].
Two studies were performed in Egypt by Dabbous et al. (2021, 2022) [12,13] to evaluate the benefits of Favipiravir (PubChem CID: #492405; C5H4FN3O2) versus Hydroxychloroquine (PubChem CID: #3652; C18H26ClN3O) and oral Oseltamivir (PubChem CID: #65028; C16H28N2O4) in managing patients with COVID-19. The first study concluded that Favipiravir was a safe and effective alternative to Hydroxychloroquine in mild or moderate SARS-CoV-2 infected participants [12]. However, after the publication, several concerns were raised by the scientific community, and the editors requested the raw data. Notably, the authors sent several datasets, and none presented the same data and results as those published. In addition, it was demonstrated that the randomization procedure was not entirely performed at random, e.g., the distribution of male and female patients was equal between the groups. However, it is unlikely because sex was not considered during the allocation as a covariate [12]. The second study presented a similar conclusion to the first one. It concluded that Favipiravir is a promising drug for the treatment of patients with COVID-19 that might decrease the duration of the hospital stay and the need for mechanical ventilation [13]. In addition, the study was retracted due to the divergences in the raw data and the differences between the groups at the baseline for several features [13].
Another two retracted clinical trial studies performed in Egypt were included in our review study [14,15]. The first study aimed to assess the efficacy of Remdesivir (PubChem CID: #121304016; C27H35N6O8P) in hospitalized Egyptian patients with COVID-19. It concluded that Remdesivir positively influenced the length of hospital stay, but it had no mortality benefit in Egyptian patients with COVID-19 [14]. The second study aimed to evaluate the safety and efficacy of Hydroxychloroquine added to standard care in patients with COVID-19 [15]. The authors found that the overall mortality did not differ between the two groups. Univariate logistic regression analysis showed that Hydroxychloroquine treatment was not significantly associated with decreased mortality [15]. In brief, both studies were retracted in the same retraction note, mainly due to data overlap and/or copying between the manuscripts [14,15].
Finally, one more study from Egypt was included in our review [16]. The study aimed to evaluate the efficacy of generic Sofosbuvir/Daclatasvir (PubChem CID: #45375808; C22H29FN3O9P/PubChem CID: #25154714; C40H50N8O6) in treating COVID-19 patients with pneumonia [16]. In the study, the authors observed a lower mortality rate in the group that received Sofosbuvir/Daclatasvir. After one month of therapy, no differences were found in intensive care unit admission rates, oxygen therapy, or ventilation support. Additionally, a statistically significant shorter duration of hospital stays (9 vs. 12%) and a faster achievement of polymerase chain reaction negativity at day 14 (84 vs. 47%) were noticed in the treatment group [16]. The article was retracted due to significant concerns raised regarding the integrity of the data and reported results in the article [16].
In brief, the retraction of clinical trials for COVID-19 occurred in four countries (one in Lebanon, one in India, one in Brazil, and five in Egypt) and in six scientific journals (one in Viruses, one in Archives of Virology, one in Expert Review of Anti-infective Therapy, one in Frontiers in Medicine, two in Scientific Reports, and two The American Journal of Tropical Medicine and Hygiene). In total, eight drugs were tested (Ivermectin, Vitamin D, Proxalutamide, Hydroxychloroquine, Remdesevir, Favipiravir, and lastly, Sofosbuvir combined with Daclatasvir) [9,10,11,12,13,14,15,16].
Moreover, we identified a higher Altmetric index for the original studies, proving that the retracted studies were read more than the retraction notes. Interestingly, the impact of the original articles is much higher than their retraction notes. The Altmetrics indexes show that people who read those retracted articles are not reading their retraction notes and are unaware of the erroneous information they share.
We briefly explained the paper retraction (causes and implications) in Figure 3.
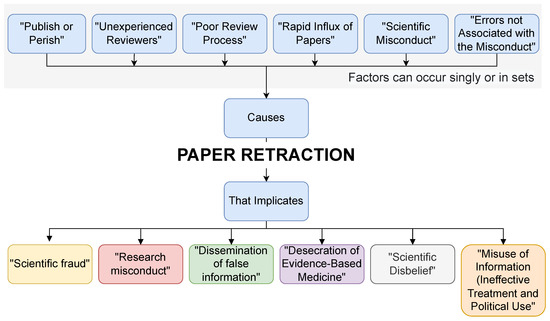
Figure 3.
Paper retraction (causes and implications).
4. Discussion
It is worrisome that the number of retractions in the era of the COVID-19 pandemic is high [3,21,22], even for clinical trials [9,10,11,12,13,14,15,16]. Yeo-Teh & Tang (2021) described that a PubMed search showed 7440 COVID-19-related articles as of 3rd May 2020. This number escalated to 17,559 articles as of 8th June 2020, leading to exponential growth in publication during a short period [3]. The retractions from clinical trials can compromise the politics of treating patients with COVID-19, mainly in countries with intense scientific disbelief, such as Brazil [18,23].
In the literature, Frampton, Woods, & Scott (2021) identified several problems in how COVID-19 papers were retracted, like lack of clarity on the timing of and reasons for retractions, and continued availability of retracted articles, often from multiple sources, which raises attention on how difficult it is to prove the facts behind the publication of clinical trials and their retraction, which included the implementation of a fast review process and the review by inexperienced reviewers compromising the quality of the review process, the implementation of good study design to give the correct randomization reducing the influence of baseline features into the outcomes, error during the data collection and the statistical analysis, or the misconduct of the authors [22]. However, suppose it is difficult to determine the causes of the publication followed by its retraction. In that case, it is even more challenging to decide whether the published papers were correctly performed or how this type of publication affected health measures. For example, among the cited papers, several were used and cited in systematic and meta-analysis studies, even after retraction. Importantly, Kataoka et al. investigated in a meta-epidemiological study if the systematic reviews and clinical practice guidelines, which included retracted randomized controlled trials, performed a correction letter [24]. Curiously, only 5% of the studies published before the retraction corrected or retracted their results. In addition, several studies cited already retracted randomized controlled trials after publication. Sadly, a significant part of the articles cited those studies in the evidence analysis and did not consider the retraction note, even in the discussion section. We need to better prepare health students and health professionals to read and interpret a scientific article, mainly a publication of a clinical trial. In that sense, the student and professional will be able to perform their conclusion based on the study findings, and they will create a critical view of science.
One question remains: why were the retraction rates on COVID-19 papers higher during the pandemic? After consideration, the authors ended up with three possible reasons for this phenomenon:
- (1)
- This was due to pressure to publish, which, despite not being demonstrated empirically for any of these articles, is a valid possibility and further studies are to elucidate this possibility.
- (2)
- That retractions are due to insufficient peer review, which implies that skilled reviewers can detect randomization errors, research misconduct, and similar minor errors resulting in work that is not reproducible. It is unclear whether skilled and experienced reviewers would request raw data from the studies or be able to detect some of the issues identified as the cause of retractions upon review.
- (3)
- That the rapid influx of papers causes retractions. In this sense, due to the urgency for new information and possible treatment for a new deadly disease, all researchers turned to COVID-19. This converged in a high number of articles being submitted simultaneously.
The main limitations of our study include that the authors only analyzed eight retracted articles, which is a low number when compared to the immense number of published articles during the COVID-19 pandemic. But, despite the low number of retracted articles analyzed, this paper calls for attention to a severe problem. Also, proportionally, there is double the number of papers retracted that are related to COVID-19 interventions than non-COVID-19-related clinical trials during the same period. Moreover, very little information was available regarding the retraction note and its justifications. In addition, it is difficult to assess the quality of clinical trials, including randomized controlled trials, after publication, and it demands an intensive collaboration between the authors, editorial staff, and the scientific community. Finally, we did not evaluate the timeframe after retraction between the publication of the clinical trial and its retraction note.
5. Conclusions
It is necessary to constantly check information before taking it as trustworthy and truthful. We live in a time where information is only a few clicks away, which makes it amazingly easy to spread false and unverified information online. Scientific journals are among the safest spaces to seek and read quality and proven information. But, in a rush to publish COVID-19-related articles, in the heat of the pandemic, some low-quality articles with erroneous information were published, reaching thousands of people who trust journals to prove information before publication. Real quality science, evidence-based, can always be checked and proven. No “publish or perish” is worth the sacrifice and jeopardy of quality research. More and more articles are published every day. While only a few of them end up retracted, of those that are, their retraction notes need to obtain as many views as their original paper for people not to take erroneous information as truthful. In brief, as warned by Prof. Ambrosino to younger researchers (and the oldest ones): “Reproducible and transparent procedures should be incorporated into research. Publications should provide sufficient information about materials, protocols, raw data, statistical analysis, and other indicators. Clinical decisions may depend on replicable or refutable results” [25]. Then, we need to improve our efforts to perform a high-standard science because it is the best way to treat those under severe conditions, such as infection with SARS-CoV-2.
Author Contributions
Conceptualization, F.E.V., C.V.C.P. and F.A.L.M.; methodology, F.E.V. and F.A.L.M.; validation, C.V.C.P.; formal analysis, F.E.V. and F.A.L.M.; investigation, F.E.V. and F.A.L.M.; writing—original draft preparation, F.E.V., C.V.C.P. and F.A.L.M.; writing—review and editing, F.E.V., C.V.C.P. and F.A.L.M.; supervision, F.A.L.M.; project administration, F.A.L.M.; funding acquisition, F.A.L.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Fundação de Amparo à Pesquisa do Estado de São Paulo (São Paulo Research Foundation, grant number 2021/08437-5).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Carvalho, T.; Lima, T.; Melani, V.; Mendes, M.; Pereira, L.; Marson, F.A.L. The scientific production during 2009 swine flu pandemic and 2019/2020 COVID-19 pandemic. Pulmonology 2020, 26, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Valencise, F.E.; Boschiero, M.N.; Palamim, C.V.C.; Marson, F.A.L. The COVID-19 impact on the scientific production on the 25 main death causes according to world region. Pulmonology 2022, 28, 1–3. [Google Scholar] [CrossRef]
- Yeo-Teh, N.S.L.; Tang, B.L. An alarming retraction rate for scientific publications on Coronavirus Disease 2019 (COVID-19). Account. Res. 2021, 28, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.; Nugent, K.; Peterson, C. Academic Journal Retractions and the COVID-19 Panemic. J. Prim. Care Community Health 2021, 12, 21501327211015592. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.C.; Casadevall, A. Retracted science and the retraction index. Infect. Immun. 2011, 79, 3855–3859. [Google Scholar] [CrossRef] [PubMed]
- Bar-Ilan, J.; Halevi, G. Temporal characteristics of retracted articles. Scientometrics 2018, 116, 1771–1783. [Google Scholar] [CrossRef]
- Marcus, M.A.; Abritis, A.J.; Oransky, M.I. How to Stop the Unknowing Citation of Retracted Papers. Anesthesiology 2022, 137, 280–282. [Google Scholar] [CrossRef]
- Boschiero, M.N.; Carvalho, T.A.; Marson, F.A.D.L. Retraction in the era of COVID-19 and its influence on evidence-based medicine: Is science in jeopardy? Pulmonology 2021, 27, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Samaha, A.; Mouawia, H.; Fawaz, M.; Hassan, H.; Salami, A.; Bazzal, A.; Saab, H.; Al-Wakeel, M.; Alsaabi, A.; Chouman, M.; et al. Effects of a Single Dose of Ivermectin on Viral and Clinical Outcomes in Asymptomatic SARS-CoV-2 Infected Subjects: A Pilot Clinical Trial in Lebanon. Viruses 2021, 13, 989, Retraction on Viruses 2021, 13, 2154. [Google Scholar] [CrossRef]
- Lakkireddy, M.; Gadiga, S.G.; Malathi, R.D.; Karra, M.L.; Raju, I.S.S.V.P.M.; Ragini; Chinapaka, S.; Baba, K.S.S.S.; Kandakatla, M. Impact of daily high dose oral vitamin D therapy on the inflammatory markers in patients with COVID-19 disease. Sci. Rep. 2021, 11, 10641, Retraction on Sci. Rep. 2022, 12, 6487. [Google Scholar] [CrossRef]
- McCoy, J.; Goren, A.; Cadegiani, F.A.; Vaño-Galván, S.; Kovacevic, M.; Situm, M.; Shapiro, J.; Sinclair, R.; Tosti, A.; Stanimirovic, A.; et al. Proxalutamide Reduces the Rate of Hospitalization for COVID-19 Male Outpatients: A Randomized Double-Blinded Placebo-Controlled Trial. Front. Med. (Lausanne) 2021, 8, 668698, Retraction on Front. Med. (Lausanne) 2022, 9, 964099. [Google Scholar] [CrossRef] [PubMed]
- Dabbous, H.M.; El-Sayed, M.H.; El Assal, G.; Elghazaly, H.; Ebeid, F.F.S.; Sherief, A.F.; Elgaafary, M.; Fawzy, E.; Hassany, S.M.; Riad, A.R.; et al. Safety and efficacy of favipiravir versus Hydroxychloroquine in management of COVID-19: A randomised controlled trial. Sci. Rep. 2021, 11, 7282, Retraction on Sci. Rep. 2021, 11, 18983. [Google Scholar] [CrossRef] [PubMed]
- Dabbous, H.M.; Abd-Elsalam, S.; El-Sayed, M.H.; Sherief, A.F.; Ebeid, F.F.S.; El Ghafar, M.S.A.; Soliman, S.; Elbahnasawy, M.; Badawi, R.; Tageldin, M.A. Efficacy of favipiravir in COVID-19 treatment: A multi-center randomized study. Arch. Virol. 2021, 166, 949–954, Retraction on Arch. Virol. 2022, 167, 277. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elsalam, S.; Ahmed, O.A.; Mansour, N.O.; Abdelaziz, D.H.; Salama, M.; Fouad, M.H.A.; Soliman, S.; Naguib, A.M.; Hantera, M.S.; Ibrahim, I.S.; et al. Remdesivir Efficacy in COVID-19 Treatment: A Randomized Controlled Trial. Am. J. Trop. Med. Hyg. 2021, 106, 886–890, Retraction on Am. J. Trop. Med. Hyg. 2022, 107, 1. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elsalam, S.; Esmail, E.S.; Khalaf, M.; Abdo, E.F.; Medhat, M.A.; El Ghafar, M.S.A.; Ahmed, O.A.; Soliman, S.; Serangawy, G.N.; Alboraie, M. Hydroxychloroquine in the treatment of COVID-19: A multi-center randomized controlled study. Am. J. Trop. Med. Hyg. 2020, 103, 1635–1639, Retraction on Am. J. Trop. Med. Hyg. 2022, 107, 1. Epub ahead of print. [Google Scholar] [CrossRef]
- El-Bendary, M.; Abd-Elsalam, S.; Elbaz, T.; El-Akel, W.; Cordie, A.; Elhadidy, T.; Elalfy, H.; Farid, K.; Elegezy, M.; El-Badrawy, A.; et al. Efficacy of combined Sofosbuvir and Daclatasvir in the treatment of COVID-19 patients with pneumonia: A multi-center Egyptian study. Expert Rev. Anti Infect. Ther. 2022, 20, 291–295, Retraction on Expert Rev. Anti Infect. Ther. 2022, 20, 1243. [Google Scholar] [CrossRef]
- Bolland, M.J.; Grey, A.; Avenell, A. Citation of retracted publications: A challenging problem. Account. Res. 2022, 29, 18–25. [Google Scholar] [CrossRef]
- Boschiero, M.N.; Palamim, C.V.C.; Ortega, M.M.; Mauch, R.M.; Marson, F.A.L. One Year of Coronavirus Disease 2019 (COVID-19) in Brazil: A Political and Social Overview. Ann. Glob. Health 2021, 87, 44. [Google Scholar] [CrossRef]
- Morshed, T.; Hayden, S. Google Versus PubMed: Comparison of Google and PubMed’s Search Tools for Answering Clinical Questions in the Emergency Department. Ann. Emerg. Med. 2020, 75, 408–415. [Google Scholar] [CrossRef]
- Alessi, J.; Becker, A.S.; Amaral, B.; de Oliveira, G.B.; Franco, D.W.; Knijnik, C.P.; Kobe, G.L.; de Brito, A.; de Carvalho, T.R.; Telo, G.H.; et al. Type 1 diabetes and the challenges of emotional support in crisis situations: Results from a randomized clinical trial of a multidisciplinary teleintervention. Sci. Rep. 2022, 12, 3086, Retraction on Sci. Rep. 2022, 12, 4265. [Google Scholar] [CrossRef]
- Guzman-Prado, Y. Retraction of Studies on Potential Drug Therapies for COVID-19: A Call for Reliability and Scientific Integrity. Am. J. Cardiol. 2020, 132, 173. [Google Scholar] [CrossRef] [PubMed]
- Frampton, G.; Woods, L.; Scott, D.A. Inconsistent and incomplete retraction of published research: A cross-sectional study on Covid-19 retractions and recommendations to mitigate risks for research, policy, and practice. PloS ONE 2021, 16, e0258935. [Google Scholar] [CrossRef] [PubMed]
- Marson, F.; Ortega, M. COVID-19 in Brazil. Pulmonology 2020, 26, 241–244. [Google Scholar] [CrossRef]
- Kataoka, Y.; Banno, M.; Tsujimoto, Y.; Ariie, T.; Taito, S.; Suzuki, T.; Oide, S.; Furukawa, T.A. Retracted randomized controlled trials were cited and not corrected in systematic reviews and clinical practice guidelines. J. Clin. Epidemiol. 2022, 150, 90–97. [Google Scholar] [CrossRef]
- Ambrosino, N.; Pacini, F. Publish or perish? Perish to publish? (Unrequested advices to young researchers). Pulmonology 2022, 28, 327–329. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).