Abstract
Taking into consideration the essential contribution of Mytilus galloprovincialis farming, it is of rising importance to add knowledge regarding bacterial species occurrence in water samples from aquaculture zones from the point of view of both the organism and public health. In the present study, we investigated the bacterial community existing in water samples from six Mytilus galloprovincialis aquaculture areas in the Thermaikos gulf, northern Greece, that may provoke toxicity in aquatic organisms and humans and may indicate environmental pollution in mussel production as well as algal blooms. Bacterial species were identified molecularly by sequencing of a partial 16s rRNA segment and were analyzed phylogenetically for the confirmation of the bacterial taxonomy. The results obtained revealed the presence of four bacterial genera (Halomonas sp., Planococcus sp., Sulfitobacter sp., and Synechocystis sp.). Members of the Halomonas and Sulfitobacter genera have been isolated from highly polluted sites, Planococcus bacteria have been identified in samples derived directly from plastic debris, and Synechocystis bacteria are in line with microcystin detection. In this context, the monitoring of the bacteria community in mussel aquaculture water samples from the Thermaikos gulf, the largest mussel cultivation area in Greece, represents an indicator of water pollution, microplastics presence, algal blooms, and toxin presence.
1. Introduction
Bacterial community alterations caused by marine pollution on account of various sources, some of which are plastics, microplastics, and nanoplastics, exhibit adverse effects by deteriorating the welfare of aquatic organisms and environment services [1]. Microplastics are water-insoluble solid polymer particles that are ≤5 mm in size, while nanoplastics are particles ≤1 μm in size [2]. Micro- and nanoplastics can be easily ingested by aquatic organisms and can thus be responsible for severe effects on organisms’ growth, survival, development, and reproduction [3]. It is estimated that a minimum of 5.25 trillion particles with a total weight of 268,940 tons of plastic items are floating in the world’s marine ecosystems across all five subtropical gyres, including the Mediterranean Sea [4].
Because of their characteristics, such as their small length and distribution, microplastics are easily colonized by bacteria, creating biofilms [5] However, in some cases, the microbial communities inhabiting plastics have been found to be different from those in the water samples around them [6,7,8]. In studies isolating bacteria from polluted sites, such as landfill sites, it has been shown that these bacteria belonged to Proteobacteria, Firmicutes, and Actinobacteria [1]. Studies conducted in the North Sea, the coastal Baltic Sea, the North Atlantic, and several freshwater systems revealed that the main bacterial communities on plastics belong to the families of Flavobacteriaceae, Erythrobacteraceae, Hyphomonadaceae, and Rhodobacteraceae [6,9,10]. In addition, a growing body of evidence suggests that microbial dysbiosis in animals is correlated with the toxic effects caused by environmental contaminants [11,12,13,14]. Although there are many concerns about micro- and nanoplastics’ effects on microorganisms, scarce data exist so far, especially for aquaculture surrounding seawater.
Furthermore, today, harmful algal blooms (HABs) represent a frequent phenomenon associated with water eutrophication. HABs can result in damaged aquatic ecosystems, adverse health effects on humans, and important economic damage. Many abiotic factors are implicated in bloom development, e.g., nutrients, temperature, light, and radiation. Apart from the abiotic factors, a significant influence of the bacterial community on algal bloom dynamics has been observed. Moreover, alterations during the blooms led to changes in bacterial population dynamics, and an increase in bacterial abundance associated with several bacterial groups (e.g., Rhodobacterales) was observed [15]. Many bacterial strains have been identified in samples derived from diatoms and dinoflagellates, which have thus been characterized as the main phycosphere genus [16]. More specifically, HABs occasionally occur in marine mussel and fish farming areas—probably because of intense eutrophication, with fish farming being more detrimental in terms of causing this phenomenon—and are particularly responsible for economic losses in mussel farming because of toxin accumulation in mussel tissues [17].
The Thermaikos gulf (North Greece) is the most important Mytilus galloprovincialis farming area in Greece since 85% to 90% of Greek mussel production is located here, promoting local business activities. A total of 80% of the production, about 30,000 tons per year, is exported to European Union countries, boosting the local economy. Moreover, the Thermaikos gulf attracts tourism, and many recreational activities are observed. Additionally, the deltas of three large rivers, the Loudias, Axios, and Aliakmon, which flow into the gulf, contain a large wetland protected by the Convention of Ramsar that provides great ecological importance to the Thermaikos gulf [18]. However, a plethora of potentially polluting activities take place in the surrounding areas, including agricultural and animal farming enterprises, which may be reflected in the shaping of bacterial communities. Correspondingly, the flow of the aforementioned rivers may occasionally bring urban waste, mainly attributed to Thessaloniki, located in the northern part of the gulf, which is the second-largest city in Greece. Thus, there is an increasing interest in generating new knowledge on bacterial abundance and its diversity in aquaculture water and the surrounding seawater in the coastal area. For instance, Pseudo-nitzschia sp. and enterococci have been well described in this area [19,20]. The Thermaikos gulf constitutes a mesotrophic basin, and because of its location close to the urban area of Thessaloniki Bay, it is facing strong anthropogenic pressure both from the city’s harbor and industrial human activity. In addition, the delta formed by four rivers flowing toward the gulf passes through a hydroelectric power dam located in Western Macedonia correlated with high pollution levels [21] The levels of petroleum hydrocarbons and n-alkanes have been found to be increased in the upper layers of the gulf [22]. Furthermore, little is known concerning the microcystin (MC) presence in the Mediterranean mussel Mytilus galloprovincialis farming area, and in a recent study, MCs were detected for the first time in the mussel production area in the Thermaikos gulf [18]. In addition, limited data refer to the microbial composition of water samples from aquaculture in the Thermaikos gulf. Most of these studies have reported the bacterial communities detected within the tissues of marine bivalves [17,20,23] as well as in sediments [22,24,25].
Taking into consideration the significant contribution of Mytilus galloprovincialis exports to the Greek economy, it is of rising importance to add knowledge concerning the bacterial species presence in water samples from Greek mussel’s aquaculture zones from a public health point of view. Thus, the scope of the current study was the investigation of the bacterial community existing in water samples from Mytilus galloprovincialis aquaculture areas in the Thermaikos gulf, northern Greece, that may provoke toxicity in aquatic organisms and humans, environmental pollution in the mussel production area, and algal blooms. Water samples from the mussel aquaculture zones of the Thermaikos gulf were examined to (a) detect and investigate the taxonomic description of local bacteria populations, (b) investigate their phylogenetic relationships with closely related bacteria, and (c) explore possible assumptions about their origin and their effects on organisms and humans.
2. Materials and Methods
2.1. Sampling Area
The sampling sites included five marine areas, which were within the Mytilus galloprovincialis farming area, whereas two sites originated from brackish waters near the estuaries of the deltas of the Axios and Gallikos rivers located in the Thermaikos gulf; in total six sampling sites (Figure 1). The Thermaikos gulf is a semi-closed estuary with a 90 m maximum depth and a surface of 5100 km2 that is in the northwest Aegean Sea in Central Macedonia, Greece. The sampling sites included Kavoura Chalastra (40°32′20.12′′ N, 22°44′56.63′′ E), Klidi Imathia (40°28′37.03′′ N, 22°39′58.94′′ E), Makrigialos Pieria (40°24′57.98′′ N, 22°37′14.93′′ E), Aggelochori Thessaloniki (40°29′30.05″ N 22°49′11.79″ E), the delta of the Axios river (40°37′50.85″ N, 22°50′46.13″ E), and estuary of the Gallikos river (40°37′50.85′′ N, 22°50′46.13′′ E) (Figure 1). These sites are all considered burdened owing to agricultural, industrial, veterinary, and urban wastes. However, they remain of high economic and environmental importance.
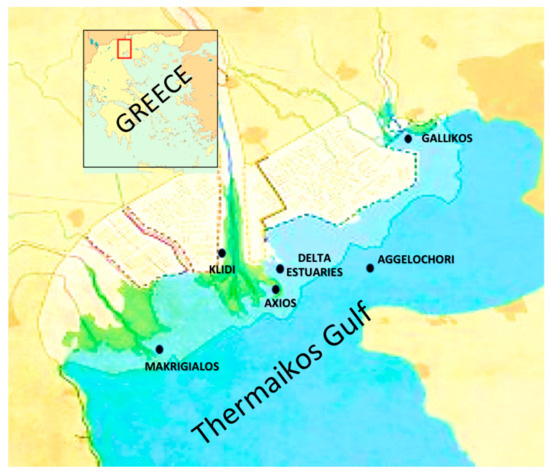
Figure 1.
Sampling sites in Thermaikos gulf [18].
In total, 755 water sample batches were collected twice or three times per month. Most of the samples were collected during the spring, summer, and autumn (645 out of 755) because of the seasonality of the mussel production period and the potential presence of toxic species during the warm periods of the year. The sampling of marine waters was performed by the water column method using a portable hosepipe sampler. Surface samples of brackish waters were collected using a telescopic water sampler.
In addition, measurements of abiotic parameters of the water (temperature, salinity, pH, and dissolved oxygen) were conducted during samplings using a handheld multiparameter instrument (YSI 556, Xylem Inc., Yellow Springs, OH, USA). For sample transportation, a portable refrigerator (at 4 ± 1 °C) was used, and the samples were transferred promptly to the Laboratory of Microbiology and Infectious Diseases, Faculty of Veterinary Medicine, Aristotle University of Thessaloniki, for further analyses.
2.2. Bacteria Isolation and Molecular Identification
A total of 150 mL of each sample was filtered through 0.45 μm pore diameter filters (PALL CORPORATION, 600 South Wagner Road Michigan). Two filters were utilized, with one proceeding to the culture and the other stored at −70 °C. The method for bacterial culture from water samples was applied as described in [26].
Total DNA was isolated from cultures using the QIAamp DNA Mini Kit (Qiagen, Valencia, CA, USA) following the manufacturer’s protocol. The concentration and purity of the isolated DNA were estimated using a NanoDrop spectrophotometer (Shimadzu, Kyoto, Japan). A part of the 16S rRNA in the bacterial genome was amplified using the primers 27f-CM and 1492r [27], which amplify approximately 1400 base pairs, using the MyTaqTM Red Mix (Bioline, London, UK). PCR conditions included an initial desaturation step of 3 min at 95 °C, 38 cycles of 30 s at 95 °C, 40 s at 51 °C, and 50 s at 72 °C, followed by a final extension step of 7 min at 72 °C. The PCR products were checked by electrophoresis in 1.5% agarose gel stained with ethidium bromide, and successfully amplified ones were sequenced to identify the hosting bacteria [28]. The successfully amplified PCR products were purified with the NucleoSpin PCR Clean-up Kit (Macherey-Nagel, Düren, Germany) following the manufacturer’s instructions and sequenced on both strands, applying the Big Dye Terminator v3.1 sequencing method using the forward and reverse primers in an ABI Prism 3730XL automatic capillary sequencer (CeMIA, Larissa, Greece) with both PCR primers. The editing and alignment of individual sequences were performed using the software MEGA version X, applying the MUSCLE algorithm [29]. Since the scope of the phylogenetic analysis was not to evaluate evolution rates but to discriminate the various bacterial species using the pairwise gap deletion and on the basis of p-distances, a neighbor-joining (NJ) tree was constructed using the program MEGA version X, incorporating confidence intervals obtained by 1000-replicate bootstrapping.
3. Results
3.1. Bacterial Detection
Bacterial cultures indicated the presence of cyanobacteria in 58 out of the 755 examined water samples, whereas axenic cultures were obtained in 22 samples. Moreover, other genera of marine bacteria were observed that were not classified as cyanobacteria according to Anagnostidis and Komárek [30]. Cyanobacteria classified morphologically as Synechocystis sp. were chosen for sequencing since they are prone to producing microcystins [31]. Alignment of the sequenced fragments resulted in the read of 976 base pairs, which were further phylogenetically analyzed in comparison with closely related species obtained from GenBank.
3.2. Phylogenetic Analysis
Four bacterial genera were identified after Basic Local Alignment Search Tool (BLAST) searches of the sequenced amplified fragments on the NCBI website (https://www.ncbi.nlm.nih.gov/ (accessed on 10 November 2021)), i.e., Halomonas sp., Planococcus sp., Sulfitobacter sp., and Synechocystis sp., exhibiting sequence similarities greater than 90% in comparison with congeneric haplotypes in the GenBank database. In particular, two haplotypes (THESS1 and THESS4, Figure 2) were categorized as Halomonas sp., very closely related to Halomonas stevensii and Halomonas johnsoniae (Figure 2), both of which were detected in four out of the six sampling sites (Table 1). On the basis of BLAST results as well as the phylogenetic tree in Figure 2, the Planococcus strain (THESS3) sequence was very closely related to Planococcus maritimus species and was only detected at the Makrigialos sampling site. On the other hand, neither Sulfitobacter sp. nor Synechocystis sp. could be further identified at the species level, as depicted in the dendrogram of Figure 2. The Sulfitobacter sp. strain was detected in three out of the six sampling sites, i.e., Gallikos, Chalastra, and Axios (Table 1), all of which are located in the inner part of the Thermaikos gulf. Finally, Synechocystis sp. (THESS, Figure 2) was detected in the Chalastra, Makrigialos, and Aggelochori sampling sites, where the main mussel farming marine area is located.
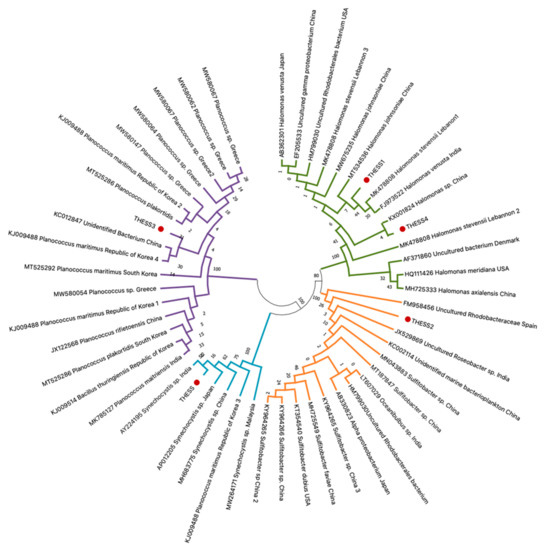
Figure 2.
Neighbor-joining (NJ) dendrogram depicting the phylogenetic relationships of the 16S rRNA haplotypes originating from seawater samples from mussel aquaculture in the Thermaikos gulf, with the most closely related congeneric haplotypes available in the GenBank database. Accession number, taxonomic classification, and geographic origin for each haplotype obtained from GenBank are indicated on each branch. Novel sequences derived in the present study are indicated with red dots. Confidence intervals based on 1000 iterations are demonstrated on each clade.

Table 1.
Geographical distribution of the detected bacterial genera.
4. Discussion
To the best of our knowledge, the present study constitutes the first report of the halophilic bacteria Halomonas sp., Sulfitobacter sp., and Planococcus sp. in water samples from the Thermaikos gulf, the major Mytilus galloprovincialis aquaculture area in Greece. In addition, in line with a recent study by our lab detecting microcystins in the same marine area [28], bacteria belonging to the Synechocystis genus were identified in seawater from the Thermaikos gulf. An investigation conducted from [22] that studied the variability of the sediment bacterial community composition and diversity from sediments in different regions of the Eastern Mediterranean Sea, including the Thermaikos gulf, revealed high richness in the sediment bacterial communities and noteworthy variability in bacterial composition in the different areas. The results revealed that Alpha- and Gammaproteobacteria were observed at high frequencies in most sediments. Another study revealed that in sediments derived from Thermaikos Gulf, the bacterial community included 31.7% Beta- and Gammaproteobacteria, 3.2% Alphaproteobacteria, and 1.6% Firmicutes [25]. In these bacterial groups, some halophilic and moderate halophils were included. It is reported that they can thrive with moderate salt concentrations (3–15% NaCl); however, if the salinity increases to extreme levels, they can occasionally grow as well [32].
The genus Halomonas, detected in most of the sampling sites in the Thermaikos gulf in the current study (Table 1), belongs to the Halomonadaceae family in the class Gammaproteobacteria. Various Gammaproteobacteria members have been reported to grow in the presence of toxins and/or pollutants, such as polyunsaturated aldehydes (PUAs), compounds produced and released from diatoms in natural communities [33,34,35]. It should be noted that the members of this genus are characterized by their preference to grow in saline or hypersaline environments [36], and hence this genus is characterized as halophilic or halotolerant [37]. The bacteria included in the Halomonas genus are distributed in a wide range of environments in terms of pH, temperature, and salinity [38]. Furthermore, microorganisms in this genus are useful for bioremediation uses on account of the degradation activity of hydrocarbons under hypersaline conditions as well as for wastewater treatment [37,39]. In addition, their extracellular exopolysaccharide production promotes the production of biofilm in marine environments. Biofilm production has been recorded in these species, and this could contribute to their persistence in polluted aquatic environments [40,41,42,43]. There are some cases of infections reported, attributed to Halomonas bacteria causing bacteremia [37,44,45,46,47,48], and it was proposed by Kim et al. (2013) [49] that the medical community should be more aware of the pathogenic potential of Halomonas bacterial species.
Furthermore, Halomonas bacteria have been isolated from coastal marine communities of the chronically polluted Priolo Bay on the eastern coast of Sicily, Italy [50]. In addition, Halomonas species were isolated among other bacteria from the Adriatic Sea during a diatom bloom [51] as well as from a shallow pond in Hungary during an algal bloom [52]
Similarly, regarding Halomonas bacteria, Sulfitobacter sp. was detected in the majority of the sampling sites examined, especially the ones in the inner part of the Thermaikos gulf (Table 1, Figure 1). The inner part of the Thermaikos gulf is characterized by a cyclonic flow that prohibits the renewal of the seawater influencing all aquatic organisms [53]. These results are in line with [54], which detected bacteria belonging to the Sulfitobacter genus in mussels, floating particles (FP), biofilm, and water samples from two different sites across Tunisian coastal areas. In various marine regions, the dominance of Proteobacteria in the clone libraries of seawater has been observed, as well in seawater contaminated with the water-soluble fraction (WSF) of crude oil [54,55,56,57,58,59,60]. Most of the Proteobacteria detected in sub-Antarctic seawater samples collected in Ushuaia, Argentina, were members of the family Rhodobacteraceae, closely related to the Sulfitobacter genus [61]. There are studies indicating that members of the Sulfitobacter genus are mainly sulfite oxidizers and hence have been identified in a plethora of habitats, including the Mediterranean Sea [62] and hypersaline environments [63], starfish, and seagrass [64]. Harmful algal blooms usually caused by water eutrophication and bacterial communities seem to shift during a bloom with Rhodobacterales including dominant bacterial groups [15]. In addition, Sulfitobacter members have been isolated during a phytoplankton bloom in the Southern North Sea [65], from a North Atlantic algal bloom [66], from the microbiome of a copepod during an algal bloom in the North Atlantic [67], from surface seawater in the East China Sea during an algal bloom [15], and from Antarctic polynyas, and they are reported to be one of the most abundant clades in eutrophic seas. Consequently, they might be correlated with bacterial bloom as well [68]. Furthermore, many strains of Sulfitobacter have been identified from samples of diatoms and more specifically one strain (Sulfitobacter pseudonitzschiae H46) has been isolated from dinoflagellates. As a result, these observations lead to the conclusion that the genus Sulfitobacter is a phycosphere genus [16]. In addition, Sulfitobacter populations were found to be associated with phytoplankton blooms in the spring and summer [69]. Consequently, it can be estimated that Halomonas sp. and Sulfitobacter sp. detected in the present study might be indicators of some form of pollution in Thermaikos gulf, which is particularly polluted in its inner part [70].
On the other hand, Planococcus sp. was only detected in Makrigialos, located in the outer part of the gulf and characterized by greater depths. Bacteria classified in the Planococcus genus are Gram-positive and characterized as halophilic, and they are mainly known for their ability of hydrocarbon degradation and biosurfactant/bio emulsifier secretion [71] Planococcus species have been isolated from oil-contaminated areas in Iran [72] and from oil-contaminated soil in the Qinghai-Tibetan Plateau [73]. Planococcus were additionally detected in high abundance on plastic debris derived from Haihe Estuary, located in Bohai Bay, China [74]. In general, plastic pollution of marine environments is a problem that is gaining more and more attention. Plastics are widely present in marine environments and may exhibit toxic effects on organisms and humans. Apart from aesthetic degradation, plastics, microplastics, and nanoplastics are correlated with bacterial dysbiosis. There is evidence that microplastics from rivers may accumulate in aquaculture waters [3] and that they can provide the microbiota with a place for further development [75,76,77]. In the case of the Thermaikos gulf, the presence of microplastics can be directly correlated with mussel farming. Particularly at the Makrigialos sampling site, M. galloprovincialis farms are based on the long line farming system, in which mussels grow within tubular nets made of extruded polyethylene. Each plastic tubular net remains in the seawater for half of the growing period of the mussels, approximately 3–6 months, when the tubular nets are replaced with new ones for the other half of the growing period. It should be also noted that, occasionally, used nets are thrown into the sea instead of being collected to be recycled or destroyed, further burdening the already affected benthos [78].
Synechocystis sp. constitutes a potentially toxic cyanobacterium genus that was detected in the main mussel farming area in the Thermaikos gulf (Table 1). Adverse health effects are known to be caused both in human and aquatic organisms by toxic cyanobacteria inhabiting aquatic ecosystems. Toxic compounds with hazardous effects are attributed to a group of secondary metabolites of cyanobacteria known as cyanotoxins [79,80,81]. According to their biological impacts, they can be classified as hepatotoxins (microcystins (MCs) and nodularins), cytotoxins (cylindrospermopsins), or neurotoxins (anatoxins and saxitoxins) [82,83,84]. Among them, microcystins are the most abundant category of cyanotoxins exhibiting high toxicity [5]. Exposure to high doses of cyanotoxins is correlated with respiratory failure and nerve dysfunction, which can lead to death [83,85]. More specifically, severe effects caused by microcystins have been related not only to humans but also to domestic, wild, and aquatic organisms [86]. Their toxicity is reported in many organs, including the kidneys [5], intestines [87], brain [88], heart [89], and lungs [74,90]. The genera of Synechocystis sp. isolated from the Thermaikos gulf in the current study produced microcystins during the experimental exposure of Mediterranean mussels Mytilus galloprovincialis at levels up to 6.85 ± 0.220 μg/kg after 72 h of exposure at a density of 100,000 cells/mL [31]. More than 150 genera of cyanobacteria have been described so far. Among them, at least 40 are characterized as cyanotoxic or toxin-producing cyanobacteria [91,92]. Among the most studied genera that produce MCs are Microcystis, Anabaena (Dolichospermum), Nostoc, Planktothrix, and Chroococcus [93], and MCs are also produced by marine picoplanktonic species, such as Synechococcus and Synechocystis [18,94]. Consequently, there is an emerging need for the detection, identification, and monitoring of local cyanobacteria exhibiting toxicity, especially those that are not usually investigated.
5. Conclusions
In conclusion, bacterial isolation from water samples originating in the Thermaikos gulf, the major mussel aquaculture marine area in Greece, revealed the presence of the Sulfitobacter, Halomonas, Planococcus, and Synechocystis bacterial genera. Members of Halomonas and Sulfitobacter have been isolated from highly polluted sites and may be correlated with water pollution in the Thermaikos gulf as well. On the other hand, Planococcus bacteria have been identified in samples derived directly from plastic debris and may be related to microplastics that are formed on account of plastic net residues utilized in the long line mussel farming system that is applied in the Thermaikos gulf. The detection of Synechocystis bacteria is in line with a recent study detecting microcystins in seawater from the Thermaikos gulf [28], suggesting the need for systematic monitoring in mussel farming areas. Overall, our study indicates that investigating bacterial populations dominating in aquaculture water samples, and not only directly investigating the organisms cultivated, is of high importance. In this manner, associations between dominant bacterial genera and harmful algal blooms, pollution indices, and the presence of toxins may be performed.
Author Contributions
Conceptualization, M.P.K., S.K.K. and E.P.; methodology, M.P.K., M.V.A. and I.A.G.; software, M.P.K., M.V.A. and I.A.G.; validation, S.K.K. and E.P.; formal analysis, K.V.P., M.S. and A.L.; investigation, M.P.K., M.S. and A.L.; resources, M.P.K. and A.L.; data curation, K.V.P.; writing—original draft preparation, M.P.K.; writing—review and editing, I.A.G., S.K.K. and E.P.; visualization, E.P.; supervision, E.P.; project administration, K.V.P.; funding acquisition, S.K.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the research committee Aristotle University of Thessaloniki.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are included within the manuscript or public databases.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Matjašič, T.; Simčič, T.; Medvešček, N.; Bajt, O.; Dreo, T.; Mori, N. Critical evaluation of biodegradation studies on synthetic plastics through a systematic literature review. Sci. Total Environ. 2021, 752, 141959. [Google Scholar] [CrossRef]
- Koelmans, A.A.; Mohamed Nor, N.H.; Hermsen, E.; Kooi, M.; Mintenig, S.M.; De France, J. Microplastics in freshwaters and drinking water: Critical review and assessment of data quality. Water Res. 2019, 155, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Ye, G.; Zhang, X.; Yan, C.; Lin, Y.; Huang, Q. Polystyrene microplastics induce microbial dysbiosis and dysfunction in surrounding seawater. Environ. Int. 2021, 156, 106724. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, M.; Lebreton, L.C.; Carson, H.S.; Thiel, M.; Moore, C.J.; Borerro, J.C.; Galgani, F.; Ryan, P.G.; Reisser, J. Plastic pollution in the world’s oceans: More than 5 trillion plastic pieces weighing over 250,000 tons afloat at sea. PLoS ONE 2014, 9, e111913. [Google Scholar] [CrossRef] [Green Version]
- Xu, S.; Yi, X.; Liu, W.; Zhang, C.; Massey, I.Y.; Yang, F.; Tian, L. A Review of Nephrotoxicity of Microcystins. Toxins 2020, 12, 693. [Google Scholar] [CrossRef] [PubMed]
- Zettler, E.R.; Mincer, T.J.; Amaral-Zettler, L.A. Life in the “Plastisphere”: Microbial communities on plastic marine debris. Environ. Sci. Technol. 2013, 47, 7137–7146. [Google Scholar] [CrossRef]
- Amaral-Zettler, L.A.; Zettler, E.R.; Slikas, B.; Boyd, G.D.; Melvin, D.W.; Morrall, C.E.; Proskurowski, G.; Mincer, T.J. The biogeography of the plastisphere: Implications for policy. Front. Ecol. Environ. 2015, 13, 541–546. [Google Scholar] [CrossRef]
- Oberbeckmann, S.; Löder, M.G.J.; Labrenz, M. Marine microplastic-associated biofilms–a review. Environ. Chem. 2015, 12, 551–562. [Google Scholar] [CrossRef]
- De Tender, C.; Devriese, L.I.; Haegeman, A.; Maes, S.; Vangeyte, J.; Cattrijsse, A.; Dawyndt, P.; Ruttink, T. Temporal dynamics of bacterial and fungal colonization on plastic debris in the North Sea. Environ. Sci. Technol. 2017, 51, 7350–7360. [Google Scholar] [CrossRef]
- Oberbeckmann, S.; Kreikemeyer, B.; Labrenz, M. Environmental factors support the formation of specific bacterial assemblages on microplastics. Front. Microbiol. 2018, 8, 2709. [Google Scholar] [CrossRef] [Green Version]
- Feng, D.; Zhang, H.; Jiang, X.; Zou, J.; Li, Q.; Mai, H.; Su, D.; Ling, W.; Feng, X. Bisphenol A exposure induces gut microbiota dysbiosis and consequent activation of gut-liver axis leading to hepatic steatosis in CD-1 mice. Environ. Pollut. 2020, 265, 114880. [Google Scholar] [CrossRef]
- Huang, J.N.; Wen, B.; Zhu, J.G.; Zhang, Y.S.; Gao, J.Z.; Chen, Z.Z. Exposure to microplastics impairs digestive performance, stimulates immune response and induces microbiota dysbiosis in the gut of juvenile guppy (Poecilia reticulata). Sci. Total Environ. 2020, 733, 138929. [Google Scholar] [CrossRef]
- Kakade, A.; Salama, E.S.; Pengya, F.; Liu, P.u.; Li, X. Long-term exposure of high concentration heavy metals induced toxicity, fatality, and gut microbial dysbiosis in common carp, Cyprinus carpio. Environ. Pollut. 2020, 266, 115293. [Google Scholar] [CrossRef]
- Xia, Y.; Zhu, J.; Xu, Y.; Zhang, H.; Zou, F.; Meng, X. Effects of ecologically relevant concentrations of cadmium on locomotor activity and microbiota in zebrafish. Chemosphere 2020, 257, 127220. [Google Scholar] [CrossRef]
- Zhang, F.; Fan, Y.; Zhang, D.; Chen, S.; Bai, X.; Ma, X.; Xie, Z.; Xu, H. Effect and mechanism of the algicidal bacterium Sulfitobacter porphyrae ZFX1 on the mitigation of harmful algal blooms caused by Prorocentrum donghaiense. Environ. Pollut. 2020, 263, 114475. [Google Scholar] [CrossRef]
- Hu, T.; Wang, S.; Shan, Y.; Zhang, Y.; Zhu, Y.; Zheng, L. Complete Genome of Marine Microalgae Associated Algicidal Bacterium Sulfitobacter pseudonitzschiae H46 with Quorum Sensing System. Curr. Microbiol. 2021, 78, 3741–3750. [Google Scholar] [CrossRef]
- Zgouridou, A.; Tripidaki, E.; Giantsis, I.A.; Theodorou, J.A.; Kalaitzidou, M.; Raitsos, D.E.; Lattos, A.; Mavropoulou, A.M.; Sofianos, S.; Karagiannis, D.; et al. The current situation and potential effects of climate change on the microbial load of marine bivalves of the Greek coastlines: An integrative review. Environ. Microbiol. 2021, in press. [Google Scholar] [CrossRef]
- Kalaitzidou, M.P. Detection of Toxic Cyanobacteria and of the Marine Biotoxin Microcystin-LR in Waters and in Farmed Mussels of Thermaikos Gulf. Ph.D. Thesis, Laboratory of Microbiology and Infectious Diseases, Faculty of Veterinary Medicine, Aristotle University of Thessaloniki, Thessaloniki, Greece, 2021. [Google Scholar]
- Zdragas, A.; Partheniou, P.; Kotzamanidis, C.; Psoni, L.; Koutita, O.; Moraitou, E.; Tzanetakis, N.; Yiangou, M. Molecular characterization of low-level vancomycin-resistant enterococci found in coastal water of Thermaikos Gulf, Northern Greece. Water Res. 2008, 42, 1274–1280. [Google Scholar] [CrossRef]
- Moschandreou, K.K.; Papaefthimiou, D.; Katikou, P.; Kalopesa, E.; Panou, A.; Nikolaidis, G. Morphology, phylogeny and toxin analysis of Pseudonitzschia pseudodelicatissima (Bacillariophyceae) isolated from the Thermaikos Gulf, Greece. Phycologia 2010, 49, 260–273. [Google Scholar] [CrossRef]
- Karageorgis, A.P.; Anagnostou, C.L. Particulate matter spatial-temporal distribution and associated surface sediment properties: Thermaikos Gulf and Sporades Basin, NW Aegean Sea. Cont. Shelf. Res. 2001, 21, 2141–2153. [Google Scholar] [CrossRef]
- Polymenakou, P.; Bertilsson, S.; Tselepides, A.; Stephanou, E.G. Links between Geographic Location, Environmental Factors, and Microbial Community Composition in Sediments of the Eastern Mediterranean Sea. Microb. Ecol. 2005, 49, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Lattos, A.; Giantsis, I.A.; Karagiannis, D.; Michaelidis, B. First detection of the invasive Haplosporidian and Mycobacteria parasites hosting the endangered bivalve Pinna nobilis in Thermaikos Gulf, North Greece. Mar. Environ. Res. 2020, 155, 104889. [Google Scholar] [CrossRef] [PubMed]
- Cotou, E.; Gremare, A.; Charles, F.; Hatzianestis, I.; Sklivagou, E. Potential toxicity of resuspended particulate matter and sediments: Environmental samples from the Bay of Banyuls-sur-Mer and Thermaikos Gulf. Cont. Shelf Res. 2005, 25, 2521–2532. [Google Scholar] [CrossRef]
- Polymenakou, P.N.; Bertilsson, S.; Tselepides, A.; Stephanou, E.G. Bacterial Community Composition in Different Sediments from the Eastern Mediterranean Sea: A Comparison of Four 16S Ribosomal DNA Clone Libraries. Microb. Ecol. 2005, 50, 447–462. [Google Scholar] [CrossRef] [PubMed]
- Kalaitzidou, M.; Filliousis, G.; Petridou, E.; Economou, V.; Theodoridis, A.; Aggelidis, P. Isolation of toxic marine cyanobacteria and detection of microcystins in Thermaikos Gulf in Central Macedonia in Greece. In Proceedings of the 7th International Conference on Information and Communication Technologies in Agriculture, Food and Environment, Kavala, Greece, 17–20 September 2015. [Google Scholar]
- Frank, J.A.; Reich, C.I.; Sharma, S.; Weisbaum, J.S.; Wilson, B.A.; Olsen, G.J. Critical evaluation of two primers commonly used for amplification of bacterial 16S rRNA genes. Appl. Environ. Microbiol. 2008, 74, 2461–2470. [Google Scholar] [CrossRef] [Green Version]
- Kalaitzidou, M.P.; Nannou, C.I.; Lambropoulou, D.A.; Papageorgiou, K.V.; Theodoridis, A.M.; Economou, V.K.; Giantsis, I.A.; Angelidis, P.G.; Kritas, S.K.; Petridou, E.J. First report of detection of microcystins in farmed mediterranean mussels Mytilus galloprovincialis in Thermaikos gulf in Greece. J. Biol. Res. 2021, 28, 8. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547. [Google Scholar] [CrossRef]
- Anagnostidis, K.; Komárek, J. Modern approach to the classification system of cyanophytes. 1-Indroduction. Arch. Hydrobiol. 1985, 71, 291–302. [Google Scholar]
- Kalaitzidou, M.; Papageorgiou, K.; Theodoridis, A.; Economou, V.; Filiousis, G.; Kritas, S.; Angelidis, P.; Petridou, E. Experimental Exposure of the Mediterranean Mussels Mytilus galloprovincialis to Potentially Toxic Cyanobacteria (Synechocystis sp.) and Detection of Microcystins. Acta Vet. Eurasia 2021, 47, 10–18. [Google Scholar] [CrossRef]
- Arahal, D.R.; Ventosa, A. Moderately halophilic and halotolerant species of Bacillus and related genera. In Applications and Systematics of Bacillus and Relatives, 1st ed.; Berkeley, R., Heyndrickx, M., Logan, N., de Vos, P., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2002; Volume 7, pp. 83–99. [Google Scholar] [CrossRef]
- Garrity, G.M.; Bell, J.A.; Lilburn, T. Class III. Gammaproteobacteria class. nov. In Bergey’s Manual of Systematic Bacteriology, 2nd ed.; Brenner, D.J., Krieg, N.R., Staley, J.T., Garrity, G.M., Eds.; Springer: New York, NY, USA, 2005; Volume 2, pp. 159–206. [Google Scholar]
- Balestra, C.; Alonso-Sáez, L.; Gasol, J.M.; Casotti, R. Group-specific effects on coastal bacterioplankton of polyunsaturated aldehydes produced by diatoms. Aquat. Microb. Ecol. 2011, 63, 123–131. [Google Scholar] [CrossRef] [Green Version]
- Bacterionet, 2020. List of Prokaryotic Names with Standing in Nomenclature. 2020. Available online: https://www.bacterio.net/ (accessed on 15 January 2022).
- Arahal, D.R.; Ventosa, A. The family Halomonadaceae. In The Prokaryotes, 3rd ed.; Dworkin, M., Falkow, S., Rosenberg, E., Schleifer, K.H., Stackebrandt, E., Eds.; Springer: New York, NY, USA, 2006; Volume 6, pp. 811–835. [Google Scholar]
- Stevens, D.A.; Kim, K.K.; Johnson, N.; Lee, J.S.; Hamilton, J.R. Halomonas johnsoniae: Review of a medically underappreciated genus of growing human importance. Am. J. Med. Sci. 2013, 345, 335–338. [Google Scholar] [CrossRef]
- Ventosa, A.; Nieto, J.J.; Oren, A. Biology of moderately halophilic aerobic bacteria. Microbiol. Mol. Biol. Rev. 1998, 62, 504–544. [Google Scholar] [CrossRef] [Green Version]
- Mnif, S.; Chamkha, M.; Sayadi, S. Isolation and characterization of Halomonas sp. strain C2SS100, a hydrocarbon-degrading bacterium under hypersaline conditions. J. Appl. Microbiol. 2009, 107, 785–794. [Google Scholar] [CrossRef]
- Poli, A.; Moriello, V.S.; Esposito, E.; Lama, L.; Gambacorta, A.; Nicolaus, B. Exopolysaccharide production by a new Halomonas strain CRSS isolated from saline lake Cape Russell in Antarctica growing on complex and defined media. Biotechnol. Lett. 2004, 26, 1635–1638. [Google Scholar] [CrossRef]
- Heyrman, J.; Balcaen, A.; De Vos, P.; Swings, J. Halomonas muralis sp. nov., isolated from microbial biofilms colonizing the walls and murals of the Saint-Catherine chapel (Castle Herberstein, Austria). Int. J. Syst. Evol. Microbiol. 2002, 52, 2049–2054. [Google Scholar]
- Mata, J.A.; Bejar, V.; Llamas, I.; Arias, S.; Bressollier, P.; Tallon, R.; Urdaci, M.C.; Quesada, E. Exopolysaccharides produced by the recently described halophilic bacteria Halomonas ventosae and Halomonas anticariensis. Res. Microbiol. 2006, 157, 827–835. [Google Scholar] [CrossRef]
- Poli, A.; Esposito, E.; Orlando, P.; Lama, L.; Giordano, A.; de Appolonia, F.; Nicolaus, B.; Gambacorta, A. Halomonas alkaliantartica sp. nov., isolated from saline lake Cape Russell in Antarctica, an alkaliphilic moderately halophilic, exopolysaccharide-producing bacterium. System. Appl. Microbiol. 2007, 30, 31–38. [Google Scholar] [CrossRef]
- Kim, K.K.; Lee, K.C.; Oh, H.M.; Lee, J.S. Halomonas stevensii sp. nov., Halomonas hamiltonii sp. nov. and Halomonas johnsoniae sp. nov., isolated from a renal care centre. Int. J. Syst. Evol. Microbiol. 2010, 60, 369–377. [Google Scholar] [CrossRef] [Green Version]
- Stevens, D.A.; Hamilton, J.R.; Johnson, N.; Kim, K.K.; Lee, J.S. Halomonas, a newly recognized human pathogen, causing infections and contamination in a dialysis center: 3 new species. Medicine 2009, 88, 244–249. [Google Scholar] [CrossRef] [Green Version]
- Von Graevenitz, A.; Bowman, J.; Del Notaro, C.; Ritzler, M. Human infection with Halomonas venusta following fish bite. J. Clin. Microbiol. 2000, 38, 3123–3124. [Google Scholar] [CrossRef] [Green Version]
- Berger, P.; Barguellil, F.; Raoult, D.; Drancourt, M. An outbreak of Halomonas phocaeensis sp. nov. bacteraemia in a neonatal intensive care unit. J. Hosp. Infect. 2007, 67, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Nikkari, S.; Lopez, F.A.; Lepp, P.W.; Cieslak, P.R.; Ladd-Wilson, S.; Passaro, D.; Danila, R.; Relman, D.A. Broad- range bacterial detection and the analysis of unexplained death and critical illness. Emerg. Infect. Dis. 2002, 8, 188–194. [Google Scholar] [CrossRef]
- Kim, K.K.; Lee, K.C.; Stevens, D.A. Microbiology and epidemiology of Halomonas species. Future Microbiol. 2013, 8, 1559–1573. [Google Scholar] [CrossRef]
- Catania, V.; Cappello, S.; Di Giorgi, V.; Santisi, S.; Di Maria, R.; Mazzola, A.; Vizzini, S.; Quatrini, P. Microbial communities of polluted sub-surface marine sediments. Mar. Pollut. Bull. 2018, 131, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Pepi, M.; Heipieper, H.J.; Balestra, C.; Borra, M.; Biffali, E.; Casotti, R. Toxicity of diatom polyunsaturated aldehydes to marine bacterial isolates reveals their mode of action. Chemosphere 2017, 177, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Borsodi, A.; Miseta, R.; Palatinszky, M.; Makk, J.; Márialigeti, K. Spatial and temporal changes of bacterial communities inhabiting the well waters of Harkány spa. Acta microbiol. Immunol. Hung. 2013, 60, 329–343. [Google Scholar] [CrossRef]
- Giantsis, I.A.; Exadactylos, A.; Feidantsis, K.; Michaelidis, B. First insights towards the population genetic structure and the phylogeographic status of the horse mussel (Modiolus barbatus) from the eastern Mediterranean. J. Mar. Biol. Assoc. UK 2019, 99, 1111–1118. [Google Scholar] [CrossRef]
- Bandini, F.; Hchaichi, I.; Zitouni, N.; Missawi, O.; Cocconcelli, P.S.; Puglisi, E.; Banni, M. Bacterial community profiling of floating plastics from South Mediterranean sites: First evidence of effects on mussels as possible vehicles of transmission. J. Hazard. Mater. 2021, 411, 125079. [Google Scholar] [CrossRef]
- Cottrell, M.T.; Kirchman, D.L. Community composition of marine bacterioplankton determined by 16S rRNA gene clone libraries and fluorescence in situ hybridization. Appl. Environ. Microbiol. 2000, 66, 5116–5122. [Google Scholar] [CrossRef] [Green Version]
- McCaig, A.E.; Grayston, S.J.; Prosser, J.I.; Glover, L.A. Impact of cultivation on characterization of species composition of soil bacterial communities. FEMS Microb. Ecol. 2001, 35, 37–48. [Google Scholar] [CrossRef]
- Hagstrom, A.; Pommier, T.; Rohwer, F.; Simu, K.; Stolte, W.; Svensson, D.; Zweifel, U.L. Use of 16S ribosomal DNA for delineation of marine bacterioplankton species. Appl. Environ. Microbiol. 2002, 68, 3628–3633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bowman, J.P.; McCuaig, R.D. Biodiversity, community structural shifts and biogeography of prokaryotes within Antarctic continental shelf sediment. Appl. Environ. Microbiol. 2003, 69, 2463–2483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellis, R.J.; Morgan, P.; Weightman, A.J.; Fry, J.C. Cultivation-dependent and independent approaches for determining bacterial diversity in heavy metal contaminated soil. Appl. Environ. Microbiol. 2003, 69, 3223–3230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shivaji, S.; Reddy, G.S.N.; Aduri, R.P.; Kutty, R.; Ravenschlag, K. Bacterial diversity of a soil sample from Schirmacher Oasis, Antarctica. Cell Mol. Biol. 2004, 50, 525–536. [Google Scholar]
- Prabagaran, S.R.; Manorama, R.; Delille, D.; Shivaji, S. Predominance of Roseobacter, Sulfitobacter, Glaciecola and Psychrobacter in seawater collected off Ushuaia, Argentina, SubAntarctica. FEMS Microbiol. Ecol. 2007, 59, 342–355. [Google Scholar] [CrossRef] [Green Version]
- Pukall, R.; Buntefu, D.; Fruhling, A.; Rohde, M.; Kroppenstedt, R.M.; Burghardt, J.; Lebaron, P.; Bernard, L.; Stackebrandt, E. Sulfitobacter mediterraneus sp. nov., a new sulfite-oxidizing member of the alpha-Proteobacteria. Int. J. Syst. Bacteriol. 1999, 49, 513–519. [Google Scholar] [CrossRef] [Green Version]
- Labrenz, M.; Tindall, B.J.; Lawson, P.A.; Collins, M.D.; Schumann, P.; Hirsch, P. Staleya guttiformis gen. nov., sp. nov. and Sulfitobacter brevis sp. nov., alpha-3-Proteobacteria from hypersaline, heliothermal and meromictic antarctic Ekho Lake. Int. J. Syst. Evol. Microbiol. 2000, 50, 303–331. [Google Scholar] [CrossRef]
- Ivanova, E.P.; Gorshkova, N.M.; Sawabe, T.; Zhukova, N.V.; Hayashi, K.; Kurilenko, V.V.; Alexeeva, Y.; Buljan, V.; Nicolau, D.V.; Mikhailov, V.V.; et al. Sulfitobacter delicates sp. nov. and Sulfitobacter dubius sp. nov. respectively from a starfish (Stellaster equestris) and sea grass (Zostera marina). Int. J. Syst. Evol. Microbiol. 2004, 54, 475–480. [Google Scholar] [CrossRef] [Green Version]
- Hanke, G.; Galgani, F.; Werner, S.; Oosterbaan, L.; Nilsson, P.; Fleet, D.; Kinsey, S.; Thompson, R.; Palatinus, A.; Van Franeker, J.; et al. Guidance on Monitoring of Marine Litter in European Seas: A Guidance Document within the Common Implementation Strategy for the Marine Strategy Framework Directive; European Commission, Joint Research Centre: Luxembourg, 2013; 128p. [Google Scholar]
- Barak-Gavish, N.; Frada, M.J.; Ku, C.; Lee, P.A.; Ditullio, G.R.; Malitsky, S.; Aharoni, A.; Green, S.J.; Rotkopf, R.; Kartvelishvily, E.; et al. Bacterial virulence against an oceanic bloom-forming phytoplankter is mediated by algal DMSP. Sci. Adv. 2018, 4, eaau5716. [Google Scholar] [CrossRef] [Green Version]
- Ku, C.; Barak-Gavish, N.; Maienschein- Cline, M.; Green, S.J.; Vardi, A. Complete genome sequence of Sulfitobacter sp. strain D7, a virulent bacterium isolated from an Emiliania huxleyi algal bloom in the North Atlantic. Microbiol. Resour. Announc. 2018, 7, e01379-18. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.B.; Kim, J.G.; Jung, M.Y.; Kim, S.J.; Min, U.G.; Si, O.J.; Park, S.J.; Yeon Hwang, C.; Park, J.; Lee, S.H.; et al. Cultivation and biochemical characterization of heterotrophic bacteria associated with phytoplankton bloom in the Amundsen sea polynya, Antarctica. Deep Sea Res. Part II Top. Stud. Oceanogr. 2016, 123, 126–134. [Google Scholar] [CrossRef]
- Isaac, A.; Francis, B.; Amann, R.I.; Amin, S.A. Tight Adherence (Tad) Pilus Genes Indicate Putative Niche Differentiation in Phytoplankton Bloom Associated Rhodobacterales. Front. Microbiol. 2021, 2, 2268. [Google Scholar] [CrossRef] [PubMed]
- Giantsis, I.A.; Kravva, N.; Apostolidis, A.P. Genetic characterization and evaluation of anthropogenic impacts on genetic patterns in cultured and wild populations of mussels (Mytilus galloprovincialis) from Greece. Genet. Molec. Res. 2012, 11, 3814–3823. [Google Scholar] [CrossRef] [PubMed]
- Waghmode, S.; Suryavanshi, M.; Sharma, D.; Satpute, S.K. Planococcus Species—An Imminent Resource to Explore Biosurfactant and Bioactive Metabolites for Industrial Applications. Front. Bioeng. Biotechnol. 2020, 8, 996. [Google Scholar] [CrossRef]
- Ebrahimipour, G.; Gilavand, F.; Karkhane, M.; Kavyanifard, A.; Teymouri, M.; Marzban, A. Bioemulsification activity assessment of an indigenous strain of halotolerant Planococcus and partial characterization of produced biosurfactants. Int. J. Environ. Sci. Technol. 2014, 11, 1379–1386. [Google Scholar] [CrossRef] [Green Version]
- Yang, R.; Liu, G.; Chen, T.; Zhang, W.; Zhang, G.; Chang, S. The complete genomic sequence of a novel cold-adapted bacterium, Planococcus maritimus Y42, isolated from crude oil-contaminated soil. Stand. Genom. Sci. 2018, 13, 23. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Y.; Wu, N.; Zhao, Z.; Xu, W.; Ma, Y.; Niu, Z. Colonization Characteristics of Bacterial Communities on Plastic Debris Influenced by Environmental Factors and Polymer Types in the Haihe Estuary of Bohai Bay, China. Environ. Sci. Technol. 2019, 53, 10763–10773. [Google Scholar] [CrossRef]
- Wen, B.; Liu, J.-H.; Zhang, Y.; Zhang, H.-R.; Gao, J.-Z.; Chen, Z.-Z. Community structure and functional diversity of the plastisphere in aquaculture waters: Does plastic color matter? Sci. Total Environ. 2020, 740, 140082. [Google Scholar] [CrossRef]
- Xue, N.; Wang, L.; Li, W.; Wang, S.; Pan, X.; Zhang, D. Increased inheritance of structure and function of bacterial communities and pathogen propagation in plastisphere along a river with increasing antibiotics pollution gradient. Environ. Pollut. 2020, 265, 114641. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, W.; Zhang, Z.; Grossart, H.-P.; Gadd, G.M. Microplastics provide new microbial niches in aquatic environments. Appl. Microbiol. Biotechnol. 2020, 104, 6501–6511. [Google Scholar] [CrossRef]
- Giantsis, I.A.; University of Western Macedonia, Florina, Greece. Personal communication, 2022.
- Carmichael, W.W.; Azevedo, S.M.F.O.; An, J.S.; Molica, R.J.R.; Jochimsen, E.M.; Lau, S.; Rinehart, K.L.; Shaw, G.R.; Eaglesham, G.K. Human fatalities form cyanobacteria: Chemical and biological evidence for cyanotoxins. Environ. Health Perspect. 2001, 109, 663–668. [Google Scholar] [CrossRef]
- Svirčev, Z.; Drobac, D.; Tokodi, N.; Lužanin, Z. Epidemiology of Cancers in Serbia and Possible Connection with Cyanobacterial Blooms. J. Environ. Sci. Health Part C 2014, 32, 319–337. [Google Scholar] [CrossRef]
- Drobac, D.; Tokodi, N.; Kiprovski, B.; Malenčič, D.; Važić, T.; Nybom, S.; Meriluoto, J.; Svirčev, Z. Microcystin accumulation and potential effects on antioxidant capacity of leaves and fruits of Capsicum annuum. J. Toxicol. Environ. Health—Part A Curr. 2017, 80, 145–154. [Google Scholar] [CrossRef]
- Falconer, I.; Humpage, A. Health risk assessment of cyanobacterial (blue-green algal) toxins in drinking water. Int. J. Environ. Res. Public Health 2005, 2, 43–50. [Google Scholar] [CrossRef] [Green Version]
- Florczyk, M.; Łakomiak, A.; Woźny, M.; Brzuzan, P. Neurotoxicity of cyanobacterial toxins. Environ. Biotechnol. 2014, 10, 26–43. [Google Scholar] [CrossRef] [Green Version]
- Codd, G.A.; Testai, E.; Funari, E.; Svirčev, Z. Cyanobacteria, Cyanotoxins, and Human Health. In Water Treatment for Purification from Cyanobacteria and Cyanotoxins; Hiskia, A.E., Triantis, T.M., Antoniou, M.G., Kaloudis, T., Dionysiou, D.D., Eds.; Wiley: West Sussex, UK, 2020; Volume 2, pp. 37–68. [Google Scholar] [CrossRef]
- Šuput, D.; Zorc-Pleskovič, R.; Petrovič, D.; Milutinovič, A. Cardiotoxic injury caused by chronic administration of microcystin-YR. Folia Biol. 2010, 56, 14–18. [Google Scholar]
- Pham, T.; Utsumi, M. An overview of the accumulation of microcystins in aquatic ecosystems. J. Environ. Manag. 2018, 213, 520–529. [Google Scholar] [CrossRef]
- Wu, J.; Huang, H.; Yang, L.; Zhang, X.; Zhang, S.; Liu, H.; Wang, Y.; Yuan, L.; Cheng, X.; Zhuang, D. Gastrointestinal toxicity induced by microcystins. World J. Clin. Cases. 2018, 6, 344. [Google Scholar] [CrossRef]
- Hinojosa, M.G.; Gutiérrez-Praena, D.; Prieto, A.I.; Guzmán-Guillén, R.; Jos, A.; Cameán, A.M. Neurotoxicity induced by microcystins and cylindrosper-mopsin: A review. Sci. Total Environ. 2019, 668, 547–565. [Google Scholar] [CrossRef]
- Alosman, M.; Cao, L.; Massey, I.Y.; Yang, F. The lethal effects and determinants of microcystin-LR on heart: A mini review. Toxin Rev. 2020, 40, 517–526. [Google Scholar] [CrossRef]
- Li, X.; Xu, L.; Zhou, W.; Zhao, Q.; Wang, Y. Chronic exposure to microcystin-LR affected mitochondrial DNA maintenance and caused pathological changes of lung tissue in mice. Environ Pollut. 2016, 210, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Tokodi, N.; Drobac, D.; Lazić, G.; Petrović, T.; Marinović, Z.; Lujić, J.; Malešević, T.P.; Meriluoto, J.; Svirčev, Z. Screening of cyanobacterial cultures originating from different environments for cyanotoxicity and cyanotoxins. Toxicon 2018, 154, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, Y.; Mohammadipanah, F.; Te, S.H.; You, L.; Gin, K.Y.H. Biodiversity, phylogeny and toxin production profile of cyanobacterial strains isolated from lake Latyan in Iran. Harmful Algae 2021, 106, 102054. [Google Scholar] [CrossRef] [PubMed]
- Baralla, Ε.; Varoni, M.V.; Sedda, T.; Pasciu, V.; Floris, A.; Demontis, M.D. Microcystins presence in mussels (M. galloprovincialis) and water of two productive mediterranean’s Lagoons (Sardinia, Italy). Biomed. Res. Int. 2017, 2017, 3769245. [Google Scholar] [CrossRef]
- Vareli, K.; Zarali, E.; Zacharioudakis, G.S.A.; Vagenas, G.; Varelis, V.; Pilidis, G.; Briasoulis, E.; Sainis, I. Microcystin producing cyanobacterial communities in Amvrakikos Gulf (Mediterranean Sea, NW Greece) and toxin accumulation in mussels (Mytilus galloprovincialis). Harmful Algae 2012, 15, 109–118. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).