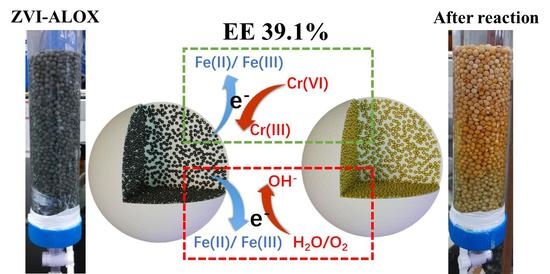
Improved Electron Efficiency of Zero-Valent Iron towards Cr(VI) Reduction after Sequestering in Al2O3 Microspheres
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Preparation of ZVI and ZVI-ALOX
2.3. Characterizations and Measurements
2.4. Batch Experiments
2.5. Column Flow Experiments
2.6. Luminous Bacteria Toxicity Assay
2.7. Electron Efficiency Measurements
3. Results and Discussion
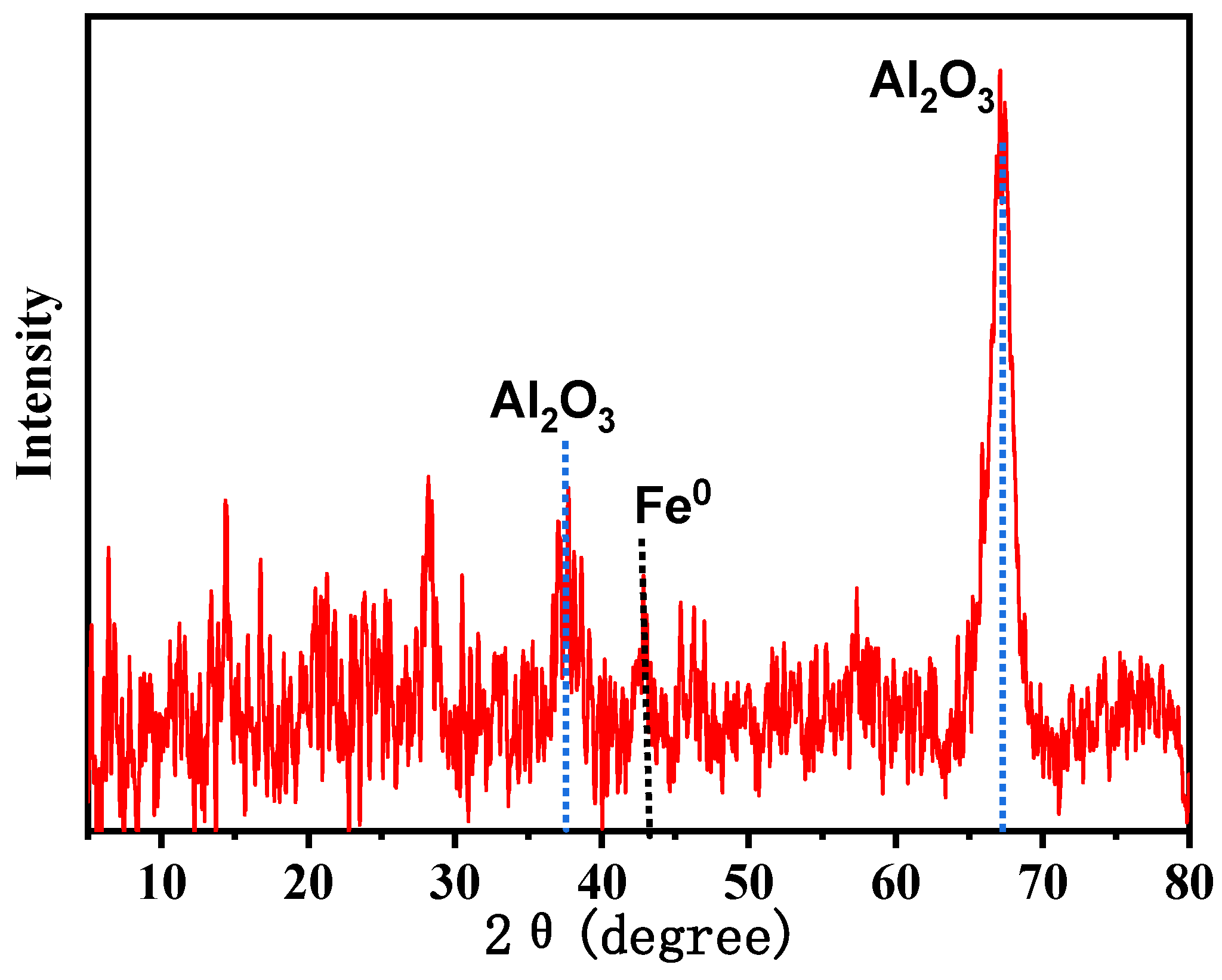
3.1. Characterization
3.2. Batch Experiments
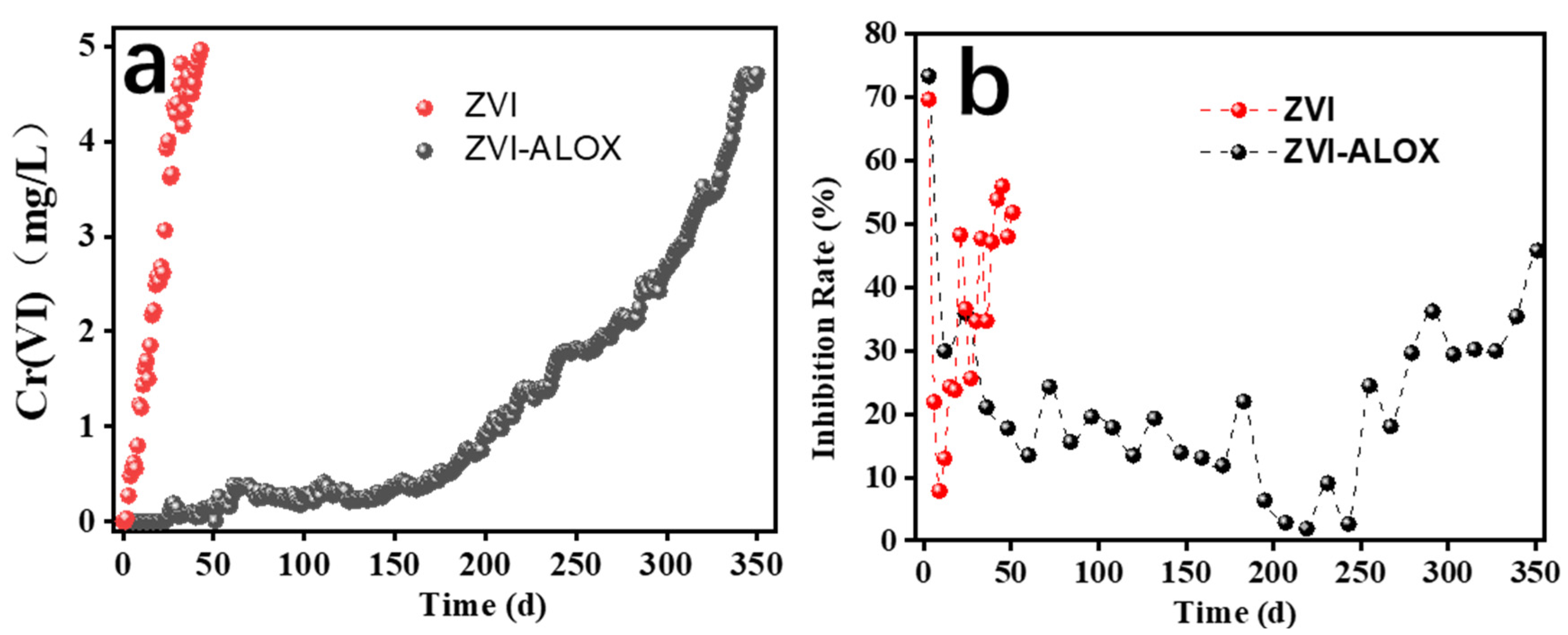
3.3. Column Flow Experiments and Toxicity Studies
3.4. Transformation of Cr and Fe Species in Reactions of Cr(VI) with ZVI-ALOX
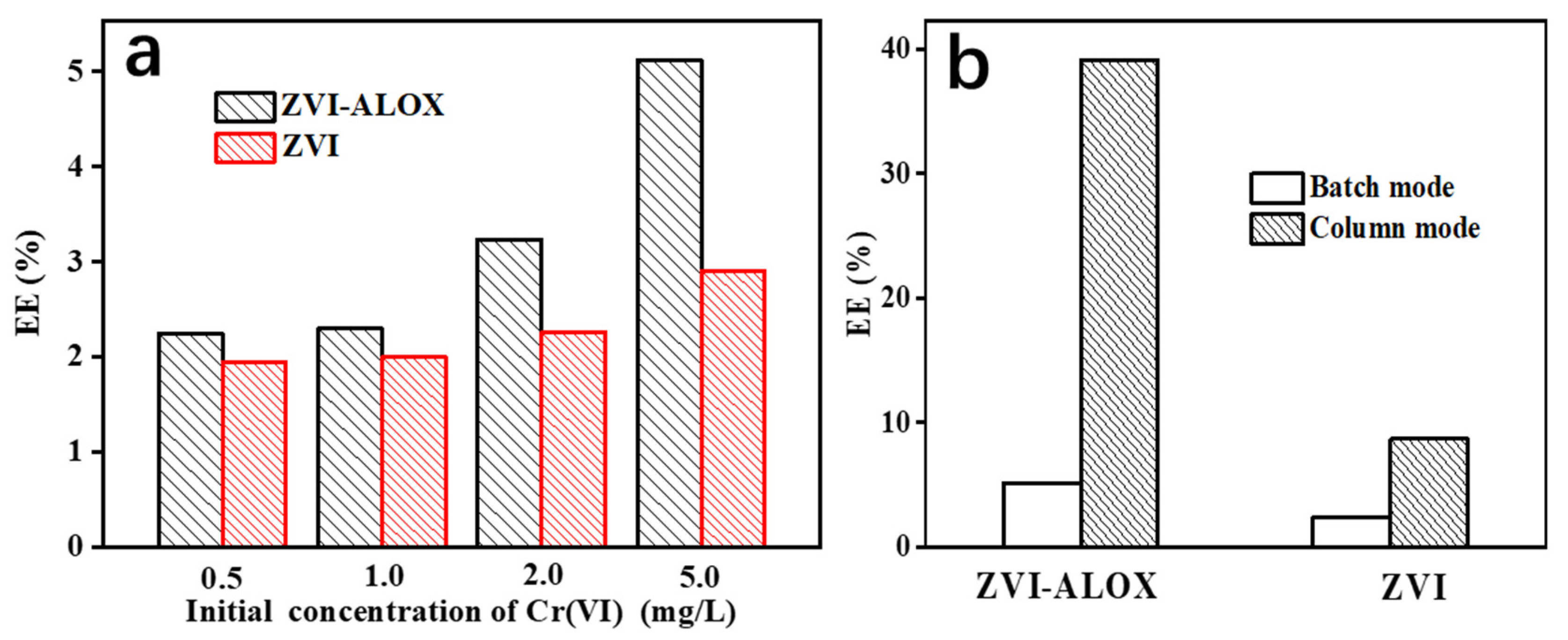
3.5. EE Measurements
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tratnyek, P. Permeable Reactive Barriers of Iron and Other Zero- Valent Metals. In Chemical Degradation Methods for Wastes and Pollutants: Environmental and Industrial Applications; Marcel Dekker: New York, NY, USA, 2003. [Google Scholar]
- Mondal, A.; Dubey, B.K.; Arora, M.; Mumford, K. Porous media transport of iron nanoparticles for site remediation application: A review of lab scale column study, transport modelling and field-scale application. J. Hazard. Mater. 2021, 403, 123443. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ji, Q.Q.; Lai, L.D.; Yao, G.; Lai, B. Degradation of p-nitrophenol (PNP) in aqueous solution by mFe/Cu-air-PS system. Chin. Chem. Lett. 2019, 30, 1129–1132. [Google Scholar] [CrossRef]
- Xu, J.; Wang, Y.; Weng, C.; Bai, W.; Jiao, Y.; Kaegi, R.; Lowry, G.V. Reactivity, Selectivity, and Long-Term Performance of Sulfidized Nanoscale Zerovalent Iron with Different Properties. Environ. Sci. Technol. 2019, 53, 5936–5945. [Google Scholar] [CrossRef]
- Alowitz, M.J.; Scherer, M.M. Kinetics of Nitrate, Nitrite, and Cr(VI) Reduction by Iron Metal. Environ. Sci. Technol. 2002, 36, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, Q.; Wang, C.; Li, X.-z. Electron efficiency of zero-valent iron for groundwater remediation and wastewater treatment. Chem. Eng. J. 2013, 215-216, 90–95. [Google Scholar] [CrossRef]
- Latif, A.; Sheng, D.; Sun, K.; Si, Y.; Azeem, M.; Abbas, A.; Bilal, M. Remediation of heavy metals polluted environment using Fe-based nanoparticles: Mechanisms, influencing factors, and environmental implications. Environ. Pollut. 2020, 264, 114728. [Google Scholar] [CrossRef]
- Chen, R.; Yin, H.; Peng, H.; Wei, X.; Yu, X.; Xie, D.; Lu, G.; Dang, Z. Removal of triphenyl phosphate by nanoscale zerovalent iron (nZVI) activated bisulfite: Performance, surface reaction mechanism and sulfate radical-mediated degradation pathway. Environ. Pollut. 2020, 260, 113983. [Google Scholar] [CrossRef]
- Noubactep, C. A critical review on the process of contaminant removal in Fe0-H2O systems. Environ. Technol. 2008, 29, 909–920. [Google Scholar] [CrossRef] [Green Version]
- Guan, X.; Sun, Y.; Qin, H.; Li, J.; Lo, I.M.; He, D.; Dong, H. The limitations of applying zero-valent iron technology in contaminants sequestration and the corresponding countermeasures: The development in zero-valent iron technology in the last two decades (1994-2014). Water Res. 2015, 75, 224–248. [Google Scholar] [CrossRef]
- Ji, H.; Zhu, Y.; Duan, J.; Liu, W.; Zhao, D. Reductive immobilization and long-term remobilization of radioactive pertechnetate using bio-macromolecules stabilized zero valent iron nanoparticles. Chin. Chem. Lett. 2019, 30, 2163–2168. [Google Scholar] [CrossRef]
- Xu, W.; Li, Z.; Shi, S.; Qi, J.; Cai, S.; Yu, Y.; O’Carroll, D.M.; He, F. Carboxymethyl cellulose stabilized and sulfidated nanoscale zero-valent iron: Characterization and trichloroethene dechlorination. Appl. Catal. B Environ. 2020, 262, 118303. [Google Scholar] [CrossRef]
- Xu, J.; Liu, X.; Cao, Z.; Bai, W.; Shi, Q.; Yang, Y. Fast degradation, large capacity, and high electron efficiency of chloramphenicol removal by different carbon-supported nanoscale zerovalent iron. J. Hazard. Mater. 2020, 384, 121253. [Google Scholar] [CrossRef] [PubMed]
- Perraki, M.; Vasileiou, E.; Bartzas, G. Tracing the origin of chromium in groundwater: Current and new perspectives. Curr. Opin. Environ. Sci. Health. 2021, 22, 100267. [Google Scholar] [CrossRef]
- Costa, M.; Klein, C.B. Toxicity and carcinogenicity of chromium compounds in humans. Crit. Rev. Toxicol. 2006, 36, 155–163. [Google Scholar] [CrossRef]
- Galdames, A.; Ruiz-Rubio, L.; Orueta, M.; Sanchez-Arzalluz, M.; Vilas-Vilela, J.L. Zero-Valent Iron Nanoparticles for Soil and Groundwater Remediation. Int. J. Environ. Res. Public Health. 2020, 17, 5817. [Google Scholar] [CrossRef]
- Wang, W.; Hu, B.; Wang, C.; Liang, Z.; Cui, F.; Zhao, Z.; Yang, C. Cr(VI) removal by micron-scale iron-carbon composite induced by ball milling: The role of activated carbon. Chem. Eng. J. 2020, 389, 122633. [Google Scholar] [CrossRef]
- He, R.; Yuan, X.; Huang, Z.; Wang, H.; Jiang, L.; Huang, J.; Tan, M.; Li, H. Activated biochar with iron-loading and its application in removing Cr (VI) from aqueous solution. Colloids Surf. A. 2019, 579, 123642. [Google Scholar] [CrossRef]
- Yi, Y.; Wang, X.; Ma, J.; Ning, P. An efficient Egeria najas-derived biochar supported nZVI composite for Cr(VI) removal: Characterization and mechanism investigation based on visual MINTEQ model. Environ. Res. 2020, 189, 109912. [Google Scholar] [CrossRef]
- Yin, Y.; Shen, C.; Bi, X.; Li, T. Removal of hexavalent chromium from aqueous solution by fabricating novel heteroaggregates of montmorillonite microparticles with nanoscale zero-valent iron. Sci. Rep. 2020, 10, 12137. [Google Scholar] [CrossRef]
- Silva-Calpa, L.; Correia, T.; Netto-Ferreira, J.C.; Kuriyama, S.N.; Letichevsky, S.; de Avillez, R.R. Stable and highly active zero-valent iron-nickel nanofilaments/silica for the hexavalent chromium reduction. Environ. Nanotechnol. Monit. Manag. 2020, 14, 100332. [Google Scholar] [CrossRef]
- Guo, Y.; Zhao, Y.; Yang, T.; Gong, B.; Chen, B. Highly efficient nano-Fe/Cu bimetal-loaded mesoporous silica Fe/Cu-MCM-41 for the removal of Cr(VI): Kinetics, mechanism and performance. J. Hazard. Mater. 2021, 418, 126344. [Google Scholar] [CrossRef] [PubMed]
- Mondal, A.; Arora, M.; Kumar Dubey, B.; Mumford, K. Comparative assessment of the characteristics and Cr(VI) removal activity of the bimetallic Fe/Cu nanoparticles pre- and post-coated with carboxymethyl cellulose. Chem. Eng. J. 2022, 444, 136343. [Google Scholar] [CrossRef]
- Seynnaeve, B.; Lauwaert, J.; Vermeir, P.; Van Der Voort, P.; Verberckmoes, A. Model-based control of iron- and copper oxide particle distributions in porous γ-Al2O3 microspheres through careful tuning of the interactions during impregnation. Mater. Chem. Phys. 2022, 276, 125428. [Google Scholar] [CrossRef]
- Deryabin, D.G.; Aleshina, E.S. Natural and recombinant luminescent microorganisms in biotoxicity testing of mineral waters. Appl. Biochem. Microbiol. 2008, 44, 378–381. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, G.; Wang, X.; Li, S.; Liu, Y.; Yang, G. Removal of hexavalent chromium by bentonite supported organosolv lignin-stabilized zero-valent iron nanoparticles from wastewater. J. Clean. Prod. 2020, 267, 1–11. [Google Scholar] [CrossRef]
- Fu, F.; Cheng, Z.; Dionysiou, D.D.; Tang, B. Fe/Al bimetallic particles for the fast and highly efficient removal of Cr(VI) over a wide pH range: Performance and mechanism. J. Hazard. Mater. 2015, 298, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Fu, R.; Zhang, X.; Xu, Z.; Guo, X.; Bi, D.; Zhang, W. Fast and highly efficient removal of chromium (VI) using humus-supported nanoscale zero-valent iron: Influencing factors, kinetics and mechanism. Sep. Purif. Technol. 2017, 174, 362–371. [Google Scholar] [CrossRef]




| Sample | Specific Surface Area (m2/g) | V(cm3/g) |
|---|---|---|
| Al2O3 microspheres | 287.20 | 0.42 |
| ZVI-ALOX | 165.16 | 0.33 |
| ZVI-ALOX after Cr(VI) reduction (used in continuous tests) | 225.47 | 0.37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Wang, S.; Song, C.; Liu, H.; Yang, J. Improved Electron Efficiency of Zero-Valent Iron towards Cr(VI) Reduction after Sequestering in Al2O3 Microspheres. Int. J. Environ. Res. Public Health 2022, 19, 8367. https://doi.org/10.3390/ijerph19148367
Wang C, Wang S, Song C, Liu H, Yang J. Improved Electron Efficiency of Zero-Valent Iron towards Cr(VI) Reduction after Sequestering in Al2O3 Microspheres. International Journal of Environmental Research and Public Health. 2022; 19(14):8367. https://doi.org/10.3390/ijerph19148367
Chicago/Turabian StyleWang, Chuan, Sha Wang, Cheng Song, Hong Liu, and Jingxin Yang. 2022. "Improved Electron Efficiency of Zero-Valent Iron towards Cr(VI) Reduction after Sequestering in Al2O3 Microspheres" International Journal of Environmental Research and Public Health 19, no. 14: 8367. https://doi.org/10.3390/ijerph19148367
APA StyleWang, C., Wang, S., Song, C., Liu, H., & Yang, J. (2022). Improved Electron Efficiency of Zero-Valent Iron towards Cr(VI) Reduction after Sequestering in Al2O3 Microspheres. International Journal of Environmental Research and Public Health, 19(14), 8367. https://doi.org/10.3390/ijerph19148367