Abstract
Background: Transvenous lead extraction (TLE) in patients with implantable cardioverter defibrillator (ICD) and cardiac resynchronization therapy (CRT) devices is considered as more risky. The aim of this study was to assess the safety and effectiveness of TLE in patients with infected CRT systems. Methods: Data of 3468 patients undergoing TLE in a single high-volume center in years 2006–2021 were analyzed. The clinical and procedural parameters as well as the efficacy and safety of TLE were compared between patients with infected CRT and pacemakers (PM) and ICD systems. Results: Infectious indications for TLE occurred in 1138 patients, including 150 infected CRT (112 CRT-D and 38 CRT-P). The general health condition of CRT patients was worse with higher Charlson’s comorbidity index. The number of extracted leads was higher in the CRT group, but implant duration was significantly longer in the PM than in the ICD and CRT groups (98.93 vs. 55.26 vs. 55.43 months p < 0.01). The procedure was longer in duration, more difficult, and more complex in patients with pacemakers than in those in the CRT group. The occurrence of major complications and clinical and procedural success as well as procedure-related death did not show any relationship to the type of CIED device. Mortality at more than one-year follow-up after TLE was significantly higher among patients with CRT devices (22.7% vs. 8.7%) than among those in the PM group. Conclusion: Despite the greater burden of lead and comorbidities, the complexity and efficiency of removing infected CRT systems is no more dangerous than removing other infected systems. The duration of the implant seems to play a dominant role.
1. Introduction
In recent years, the number of patients with cardiac implantable electronic devices (CIED) has increased significantly. A special group are patients with dyssynchronous heart failure, in whom the use of cardiac resynchronization therapy (CRT) such as CRT-defibrillators (CRT-D) and pacemakers (CRT-P) improves symptoms and reduces mortality, as confirmed in many randomized clinical trials [1,2,3]. With the increase in the number of cardiac implantable electronic devices in patients with heart failure, a significant increase in the number of infections associated with CIEDs has been observed. This is probably due to the presence of comorbidities such as diabetes mellitus, chronic renal failure and more frequent replacement procedures [4]. Olsen et al. reported that the incidence of device-related infections over the lifetime of the device was 2.18% (1.78–2.64) for cardiac resynchronization therapy (CRT)-pacemakers, and 3.35% (2.92–3.83) for CRT-defibrillators [5]. Transvenous lead extraction (TLE) is an integral part of the lead management strategy and the gold standard for treatment of CIED infections and lead failure [6,7,8,9,10,11]. The effectiveness of TLE is high (more than 90% in general) but rates of major complications vary between studies from 0.4 to 3.4%, whereas mortality risk is 0.00–1.86% [8,9,10,11,12,13].
Transvenous lead extraction of permanently implanted coronary sinus (CS) leads and ICD leads is widely believed to present greater risks than the removal of other leads [12,13,14,15,16,17]. The increased difficulty in removing the left ventricular lead is explained by the thin wall of the coronary sinus and the smaller diameter of the electrode body, but there are limited data to support this hypothesis. Most studies of lead extraction provide information on leads from the right atrium and right ventricle, but only a few studies investigated the extraction of CS leads [18,19,20,21,22,23]. In this study, we analyzed our experience with cardiac resynchronization lead extraction due to infection from the perspectives of efficacy, safety, and complication rate.
2. Materials and Methods
2.1. Study Population
All transvenous lead extraction procedures performed between March 2006 and July 2021 at a single high-volume center were screened. Patient and lead data were retrospectively analyzed from a computerized database. All participants provided written informed consent prior to study enrollment, and before the TLE procedure as medically indicated. Multiple parameters including patient demographics, comorbidities, procedural success, device type, major complications, and mortality were incorporated into the database prospectively. In patients with non-infective TLE indications, antibiotic prophylaxis was based on a bolus of a first-generation cephalosporin administered 1 h before TLE. In patients with an infective indication for TLE, the antibiotic regimen was culture-guided. In these patients, a targeted antibiotic regimen was then continued for at least 2 weeks in the case of pocket infection and for more than 4 weeks in the case of endocarditis or systemic bacteriaemia. Reimplantation was performed once targeted antibiotic therapy was effective and blood cultures after TLE were negative [9,10,11].
The study groups were formed on the basis of the different types of infected devices extracted: all pacemakers (n = 756), all ICD (n = 232), all CRT systems (n = 150) including CRT-P (n = 38) and CRT-D (n = 112). The results for the different groups were analyzed and compared.
2.2. Lead Extraction Procedure
Indications for TLE, procedure effectiveness, and complications were assessed according to the 2009 and 2017 HRS consensus and 2018 EHRA guidelines [8,9,10,11]. The efficacy of TLE was determined based on the percentage of procedural success and clinical success including complete and partial radiographic success. Procedural success was defined as the removal of all targeted leads and lead material from the vascular space with the absence of any permanently disabling complication or procedure-related death. Clinical success was defined as the removal of all targeted leads or retention of a small portion (<4 cm) of the lead that did not negatively impact the outcome goals of the procedure (i.e., residual lead did not increase the risk of perforation, embolic events, perpetuation of infection, or cause any undesired outcome), absence of any permanently disabling complication or procedure-related death [8,9,10,11].
The complications of TLE were also defined as major complications such as those that were life threatening, resulted in significant or permanent disability or death, or required surgical intervention [8,9,10].
A CRT TLE (CRT group) was defined as a TLE in a patient with a CRT system incorporating a CS lead, including both CRT defibrillators (CRT-D) and pacemakers (CRT-P). A non-CRT TLE (non-CRT group) was defined as all other system and lead extractions in patients without a CRT system.
In most procedures, standard stylets were used to stiffen the leads. Locking stylets (Liberator Locking Stylet, Cook Medical Inc., Bloomington, IN, USA) were used only for extraction of the oldest leads when estimated risk of lead fracture was high. Simple traction or traction on a locking stylet with insulation-bound suture was very rarely applied (usually in patients with infection, when prolonged temporary pacing was not planned). Lead extraction was performed using mainly non-powered mechanical telescoping polypropylene sheaths (Byrd Dilator Sheaths, Cook Medical Inc., Bloomington, IN, USA) of all diameters and lengths, and using various stylets. When the polypropylene telescoping sheaths appeared ineffective, powered mechanical sheath systems (Evolution Mechanical Dilator Sheath, Cook Medical Inc., USA; TightRail Rotating Dilator Sheath, Spectranetics, Colorado Springs, Co, USA) were used. A combined approach, using two or more different (jugular, subclavian, femoral) access sites, was selected when conventional methods were insufficient. Laser and electrosurgical dissection sheaths were not used.
All extraction procedures were performed following different organizational models spanning 15 years of experience. At the beginning of lead extraction, the procedures were performed in the electrophysiology laboratory using intravenous analgesia/sedation [24]; then, the recommended safety precautions were observed to perform more complex and risky procedures in the operating theater, and finally in the hybrid room under general anesthesia. Over the past 6 years, the core extraction team has consisted of the same highly experienced TLE operator, experienced echocardiographer and dedicated cardiac surgeon [25,26,27].
2.3. Dataset and Statistical Methods
Statistical analyses were carried out using Statistica v. 13.3 (TIBCO Software Inc., Palo Alto, CA, USA). Categorical variables were expressed as counts and percentages, and continuous variables as either the mean and standard deviation (SD) or median. The variables were compared using the nonparametric Chi2 test with Yates correction (dichotomous data) or the unpaired Mann–Whitney U test (continuous data), as appropriate. A p-value of less than 0.05 was considered statistically significant.
2.4. Approval of the Bioethics Committee
All patients gave their informed written consent to undergo TLE and use anonymous data from their medical records, approved by the Bioethics Committee at the Regional Chamber of Physicians in Lublin no. 288/2018/KB/VII. The study was carried out in accordance with the ethical standards of the 1964 Declaration of Helsinki.
3. Results
A total of 3546 patients underwent lead extraction procedures (61%male), age 5–94 (66.7 ± 14.96). Indications for TLE included: systemic infection in 22.4% of patients, local isolated pocket infection in 9.6%, and non-infective indications in 67.9% of patients. Among patients with infection, 150 were patients with CRT, representing 4% of all patients with TLE and 13% of all patients with infection. The mean dwell time of the oldest infective lead in one patient was 91.58 ± 69.23 months; the time from last CIED procedure in one patient was 35.18 ± 32.15 months.
The annual number of TLEs due to infectious reasons varied from year to year. Most CRT systems were removed in 2014–2018 (Figure 1).
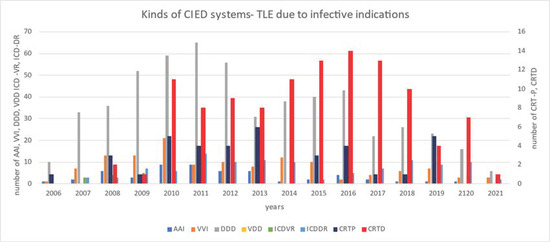
Figure 1.
Annual number of transvenous lead extraction procedures procedures, taking into account the type of devices.
For the purposes of analysis, the study population with infective CIED was divided into five groups: 1—all pacemakers (AAI, VVI, DDD, VDD), 756 patients, 2—ICDs all (VVI, DDD), 232 patients, 3—CRT-P, 38 patients, 4—CRT-D, 112 patients, and 5—all CRT systems, 150 patients. Tables summarize the indications for the initial implantation of devices and present the specific patient-, system- and procedure-related risk factors as well as analyze complexity, efficacy, complications of the procedures and long-term mortality after TLE.
Table 1 presents detailed indications for the implantation of particular types of devices in the study population.

Table 1.
Indications for initial implantation of CIED.
Analysis of the clinical factors showed that CRT-group patients were slightly younger, there were more male patients, with worse functional NYHA class, decreased LVEF, more frequent renal failure, diabetes mellitus and finally, higher Charlson’s comorbidity index. However, the type of infection (infective endocarditis or pocket infection) did not show any relationship to the type of CIED system (Table 2).

Table 2.
Clinical characteristics of patients with different types of CIED system.
The number of leads in the heart before TLE, presence of ≥ 4 leads in the heart and number of procedures before lead extraction were more frequent in the CRT system groups. Similarly, the number of extracted leads in one patient and extraction of three or more leads were more frequent in CRT groups. Implant duration expressed as the oldest extracted lead dwell time in patient, average extracted lead dwell time in patient, average lead duration in analyzed group, and cumulative dwell time of extracted lead in the patient was significantly longer in PM (AAI, VVI, DDD, VDD) than in the CRT and ICD groups. In the group of CRT patients, the highest percentage of passive fixation leads was found. The risk of infectious complications according to the PADIT [28] scale was highest in patients with CRT-D. Estimated risk of major complication using SAFeTY-TLE calculator [29] (expressed in points and as probability percentage) was lower in the CRT and ICD groups. Multiple leads to be removed seemed to be a less important risk factor than implant duration (Table 3.)

Table 3.
System and history of pacing, TLE procedure and potential risk factors for major TLE complications and technical problems.
A comparison of the TLE complexity of different CIED systems showed that the duration of the procedure was longer in the "all pacemakers" group than in the CRT group. Additionally, the appearance of most technical difficulties (problems) was less frequent in the CRT group than among all pacemaker carriers. Differences did not reach statistical significance, but the direction of the trend was visible. (Table 4).

Table 4.
TLE complexity in compared groups of patients with different CIED systems.
The occurrence of any major complications, the need for rescue cardiac surgery, damage to the tricuspid valve during TLE, the complete clinical success and complete procedural success, and deaths related to the procedure (intra-, postoperative) did not show any relationship with the type of CIED removed (Table 5).

Table 5.
TLE efficacy and complications in compared groups of patients with different CIED systems.
The prognosis analysis after TLE for infectious reasons showed that the percentage of deaths in the CRT group was higher than that in the pacemaker group (64% vs. 45.9% p < 0.001), but no association was shown for the 48 h and 1 month mortality with the type of device removed. However, mortality in more than 1 year of follow-up after TLE was significantly higher in patients with CRT (22.7%) than in the group with pacemakers (8.7%) p < 0.001, as was the mortality at 3 years after TLE (30.7%) vs. (10.8%) p < 0.001 (Table 6, Figure 2).

Table 6.
Prognosis in short-, mean- and long-term follow-up in compared groups of patients.
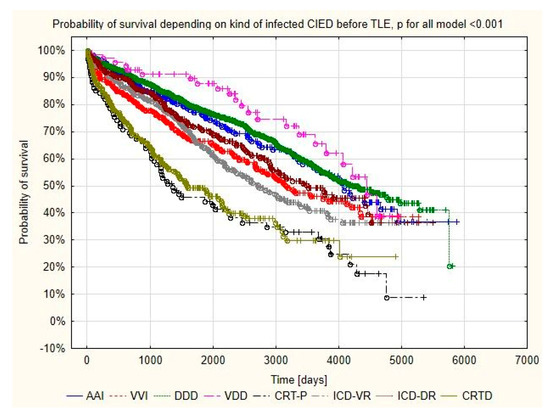
Figure 2.
Kaplan–Meier survival curves of patients with the different types of infected CIED.
4. Discussion
Transvenous lead extraction of permanently implanted coronary sinus (CS) leads and ICD leads is widely believed to present greater risks than the removal of other leads [12,13,14,15,16,17]. The greater difficulty in removing the left ventricular lead is explained by the thin wall of the coronary sinus and the smaller diameter of the electrode body, but there are limited data to support this hypothesis. Most reports provide information on TLE for leads from the right atrium and right ventricle, but only a few relate to the extraction of CS electrodes [19,20,21,22,23]. The present study showed that despite worse general health condition, higher lead burden and number of extracted leads in the CRT group, the complexity of the procedure, complication rate, the effectiveness of TLE, and the mortality associated with the procedure were not worse than in the PM and ICD groups. However, it should be emphasized that the lead dwell time in the CRT group was significantly shorter compared to that in the PM and ICD groups. Thus, multiple leads appear to be a less significant risk factor than the implant duration. Additionally, the old models of the double-coil ICD lead represented an accepted risk factor for serious complications of TLE [12,13,14,15,16,17], but the latest models do not generate additional risk [30,31,32], similar to the extraction of modern CS leads [23,30]. A separate, important problem is sudden temporary loss of cardiac resynchronization, which may lead to severe circulatory deterioration in good CRT responders [33,34,35], but this phenomenon is not considered a complication of TLE.
The current study also found that simple, cheap and conventional tools (non-powered polypropylene mechanical sheaths) used as first-line support help to achieve excellent results in CRT patients. The procedure-related major complications for all infected patients was 2.6%, and was higher than reported in the ELECTRa (1.7%) [36] and 5000 lead extracted Cleveland Clinic Series (1.8%) [17] and in the study by Gould et al. (1.4%) [37]; however, no major complication- and procedure-related deaths (0%) were found in the CRT group. The all-cause 30-day mortality rate was 2.7% with no statistically significant difference between the two groups (CRT 4.6%, n = 7 vs. non-CRT 2.4%, n = 24, p = 0.247). These results confirmed that the serious complications associated with the TLE procedure and the mortality rate in patients with CRT are not higher compared to those in the PM / ICD groups, despite the greater number of comorbidities and the greater number of leads removed in each case. The specificity of postoperative management involving the extraction of an infected CRT system during antibiotic therapy is associated with frequent deterioration of the hemodynamic status of patients [33,34,35] and more difficult, more complicated reimplantation of CRT [38].
5. Conclusions
- In spite of the higher lead and co-morbidity burdens, TLE of infected CRT systems is no more dangerous or difficult than removing infected pacemaker and ICD systems. The main factor influencing the effectiveness of the procedure remains implant duration.
- Long-term survival after removal of infected CRT systems is worse than that after removal of other systems, but short term mortality is comparable with that of non-CRT patients. It is related to the worse clinical presentation of CRT patients at baseline.
Author Contributions
P.S., writing—original draft preparation; D.N., investigation, data curation; A.P., investigation; Ł.T., data curation; K.T., data curation; W.J., methodology, statistical study; E.L., investigation; A.T., data curation; A.K., supervision, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was approved by Bioethics Committee at the Regional Chamber of Physicians in Lublin no. 288/2018/KB/VII.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Abraham, W.T.; Hayes, D.L. Cardiac resynchronization therapy for heart failure. Circulation 2003, 108, 2596–2603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saxon, L.A.; Bristow, M.R.; Boehmer, J.; Krueger, S.; Kass, D.A.; De Marco, T.; Carson, P.; Di Carlo, L.; Feldman, A.M.; Galle, E.; et al. Predictors of sudden cardiac death and appropriate shock in the Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION) Trial. Circulation 2006, 114, 2766–2772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moss, A.J.; Hall, W.J.; Cannom, D.S.; Klein, H.; Brown, M.W.; Daubert, J.P.; Estes, N.A., 3rd; Foster, E.; Greenberg, H.; Higgins, S.L.; et al. Cardiac-resynchronization therapy for the prevention of heart-failure events. N. Engl. J. Med. 2009, 361, 1329–1338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mittal, S.; Shaw, R.E.; Michel, K.; Palekar, R.; Arshad, A.; Musat, D.; Preminger, M.; Sichrovsky, T.; Steinberg, J.S. Cardiac implantable electronic device infections: Incidence, risk factors, and the effect of the AigisRx antibacterial envelope. Heart Rhythm 2014, 11, 595–601. [Google Scholar] [CrossRef]
- Olsen, T.; Jorgensen, O.D.; Nielsen, J.C.; Thogersen, A.M.; Philbert, B.T.; Johansen, J.B. Incidence of device-related infection in 97 750 patients: Clinical data from the complete Danish device-cohort (1982–2018). Eur. Heart J. 2019, 40, 1862–1869. [Google Scholar] [CrossRef] [Green Version]
- Tarakji, K.G.; Wilkoff, B.L. Management of cardiac implantable electronic device infections: The challenges of understanding the scope of the problem and its associated mortality. Expert Rev. Cardiovasc. Ther. 2013, 11, 607–616. [Google Scholar] [CrossRef]
- Rao, A.; Garner, D.; Starck, C.; Kirkfeldt, R.E.; Dagres, N.; Didier, K.; Montano, N.; Heidbuchel, H. Knowledge gaps, lack of confidence, and system barriers to guideline implementation among European physicians managing patients with CIED lead or infection complications: A European Heart Rhythm Association/European Society of Cardiology educational needs assessment survey. Europace 2020, 22, 1743–1753. [Google Scholar]
- Kusumoto, F.M.; Schoenfeld, M.H.; Wilkoff, B.; Berul, C.I.; Birgersdotter-Green, U.M.; Carrillo, R.; Cha, Y.M.; Clancy, J.; Deharo, J.C.; Ellenbogen, K.A.; et al. 2017 HRS expert consensus statement on cardiovascular implantable electronic device lead management and extraction. Heart Rhythm 2017, 14, e503–e551. [Google Scholar] [CrossRef] [Green Version]
- Wilkoff, B.L.; Love, C.J.; Byrd, C.L.; Bongiorni, M.G.; Carrillo, R.G.; Crossley, G.H., 3rd; Epstein, L.M.; Friedman, R.A.; Kennergren, C.E.; Mitkowski, P.; et al. Transvenous lead extraction: Heart Rhythm Society expert consensus on facilities, training, indications, and patient management: This document was endorsed by the American Heart Association (AHA). Heart Rhythm 2009, 6, 1085–1104. [Google Scholar] [CrossRef]
- Bongiorni, M.G.; Burri, H.; Deharo, J.C.; Starck, C.; Kennergren, C.; Saghy, L.; Rao, A.; Tascini, C.; Lever, N.; Kutarski, A.; et al. 2018 EHRA expert consensus statement on lead extraction: Recommendations on definitions, endpoints, research trial design, and data collection requirements for clinical scientific studies and registries: Endorsed by APHRS/HRS/LAHRS. Europace 2018, 20, 1217. [Google Scholar] [CrossRef]
- Deharo, J.C.; Bongiorni, M.G.; Rozkovec, A.; Bracke, F.; Defaye, P.; Fernandez-Lozano, I.; Golzio, P.G.; Hansky, B.; Kennergren, C.; Manolis, A.S.; et al. Pathways for training and accreditation for transvenous lead extraction: A European Heart Rhythm Association position paper. Europace 2012, 14, 124–134. [Google Scholar]
- Byrd, C.L.; Schwartz, S.J.; Hedin, N.B.; Goode, L.B.; Fearnot, N.E.; Smith, H.J. Intravascular lead extraction using locking stylets and sheaths. Pacing Clin. Electrophysiol. 1990, 13, 1871–1875. [Google Scholar] [CrossRef]
- Wilkoff, B.L.; Byrd, C.L.; Love, C.J.; Hayes, D.L.; Sellers, T.D.; Schaerf, R.; Parsonnet, V.; Epstein, L.M.; Sorrentino, R.A.; Reiser, C. Pacemaker lead extraction with the laser sheath: Results of the pacing lead extraction with the excimer sheath (PLEXES) trial. J. Am. Coll. Cardiol. 1999, 33, 1671–1676. [Google Scholar] [CrossRef] [Green Version]
- Saad, E.B.; Saliba, W.I.; Schweikert, R.A.; Al-Khadra, A.S.; Abdul-Karim, A.; Niebauer, M.J.; Wilkoff, B.L. Nonthoracotomy implantable defibrillator lead extraction: Results and comparison with extraction of pacemaker leads. PACE 2003, 26, 1944–1950. [Google Scholar] [CrossRef]
- Neuzil, P.; Taborsky, M.; Rezek, Z.; Vopalka, R.; Sediva, L.; Niederle, P.; Reddy, V. Pacemaker and ICD lead extraction with electrosurgical dissection sheaths and standard transvenous extraction systems: Results of a randomized trial. Europace 2007, 9, 98–104. [Google Scholar] [CrossRef]
- Byrd, C.L.; Wilkoff, B.L.; Love, C.J.; Sellers, T.D.; Turk, K.T.; Reeves, R.; Young, R.; Crevey, B.; Kutalek, S.P.; Freedman, R.; et al. Intravascular extraction of problematic or infected permanent pacemaker leads: 1994-1996. U.S. Extraction Database, MED Institute. Pacing Clin. Electrophysiol. 1999, 22, 1348–1357. [Google Scholar] [CrossRef]
- Brunner, M.P.; Cronin, E.M.; Duarte, V.E.; Yu, C.; Tarakji, K.G.; Martin, D.O.; Callahan, T.; Cantillon, D.J.; Niebauer, M.J.; Saliba, W.I.; et al. Clinical predictors of adverse patient outcomes in an experience of more than 5000 chronic endovascular pacemaker and defibrillator lead extractions. Heart Rhythm 2014, 11, 799–805. [Google Scholar] [CrossRef]
- Crossley, G.H.; Sorrentino, R.A.; Exner, D.V.; Merliss, A.D.; Tobias, S.M.; Martin, D.O.; Augostini, R.; Piccini, J.P.; Schaerf, R.; Li, S.; et al. Extraction of chronically implanted coronary sinus leads active fixation vs passive fixation leads. Heart Rhythm 2016, 13, 1253–1259. [Google Scholar] [CrossRef]
- Rickard, J.; Tarakji, K.; Cronin, E.; Brunner, M.P.; Jackson, G.; Baranowski, B.; Borek, P.P.; Martin, D.O.; Wazni, O.; Wilkoff, B.L. Cardiac venous left ventricular lead removal and reimplantation following device infection: A large single-center experience. J. Cardiovasc. Electrophysiol. 2012, 23, 1213–1216. [Google Scholar] [CrossRef]
- di Cori, A.; Bongiorni, M.G.; Zucchelli, G.; Segreti, L.; Viani, S.; de Lucia, R.; Paperini, L.; Soldati, E. Large, single-center experience in transvenous coronary sinus lead extraction: Procedural outcomes and predictors for mechanical dilatation. Pacing Clin. Electrophysiol. 2012, 35, 215–222. [Google Scholar] [CrossRef]
- Cronin, E.M.; Ingelmo, C.P.; Rickard, J.; Wazni, O.M.; Martin, D.O.; Wilkoff, B.L.; Baranowski, B. Active fixation mechanism complicates coronary sinus lead extraction and limits subsequent reimplantation targets. J. Interv. Card. Electrophysiol. 2013, 36, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, S.; Friedman, P.A.; Hayes, D.L.; Osborn, M.J.; Cha, Y.M.; Rea, R.F.; Asirvatham, S.J. Outcomes and predictors of difficulty with coronary sinus lead removal. J. Interv. Card. Electrophysiol. 2012, 35, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Kutarski, A.W.; Jacheć, W.; Tułecki, Ł.; Tomków, K.; Stefańczyk, P.; Borzęcki, W.; Nowosielecka, D.; Czajkowski, M.; Polewczyk, M.; Polewczyk, A. Safety and effectiveness of coronary sinus leads extraction-single high-volume centre experience. Postepy Kardiol Interwencyjnej 2019, 15, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Kutarski, A.; Czajkowski, M.; Pietura, R.; Obszanski, B.; Polewczyk, A.; Jachec, W.; Polewczyk, M.; Mlynarczyk, K.; Grabowski, M.; Opolski, G. Effectiveness, safety, and long-term outcomes of non-powered mechanical sheaths for transvenous lead extraction. Europace 2018, 20, 1324–1333. [Google Scholar] [CrossRef]
- Tułecki, Ł.; Polewczyk, A.; Jacheć, W.; Nowosielecka, D.; Tomków, K.; Stefańczyk, P.; Kosior, J.; Duda, K.; Polewczyk, M.; Kutarski, A. Study of Major and Minor Complications of 1500 Transvenous Lead Extraction Procedures Performed with Optimal Safety at Two High-Volume Referral Centers. Int. J. Env. Res. Public Health 2021, 18, 10416. [Google Scholar] [CrossRef]
- Stefańczyk, P.; Nowosielecka, D.; Tułecki, Ł.; Tomków, K.; Polewczyk, A.; Jacheć, W.; Kleinrok, A.; Borzęcki, W.; Kutarski, A. Transvenous Lead Extraction without Procedure-Related Deaths in 1000 Consecutive Patients: A Single-Center Experience. Vasc. Health Risk Manag. 2021, 17, 445–459. [Google Scholar] [CrossRef]
- Nowosielecka, D.; Jacheć, W.; Polewczyk, A.; Tułecki, Ł.; Tomków, K.; Stefańczyk, P.; Tomaszewski, A.; Brzozowski, W.; Szcześniak-Stańczyk, D.; Kleinrok, A.; et al. Transesophageal Echocardiography as a Monitoring Tool During Transvenous Lead Extraction-Does It Improve Procedure Effectiveness? J. Clin. Med. 2020, 9, 1382. [Google Scholar] [CrossRef]
- Birnie, D.H.; Wang, J.; Alings, M.; Philippon, F.; Parkash, R.; Manlucu, J.; Angaran, P.; Rinne, C.; Coutu, B.; Low, R.A.; et al. Risk factors for infections involving cardiac implanted electronic devices. J. Am. Coll. Cardiol. 2019, 10, 2845–2854. [Google Scholar] [CrossRef]
- Jacheć, W.; Polewczyk, A.; Polewczyk, M.; Tomasik, A.; Kutarski, A. Transvenous Lead Extraction SAFeTY Score for Risk Stratification and Proper Patient Selection for Removal Procedures Using Mechanical Tools. J. Clin. Med. 2020, 9, 361. [Google Scholar] [CrossRef] [Green Version]
- Jacheć, W.; Polewczyk, A.; Polewczyk, M.; Tomasik, A.; Janion, M.; Kutarski, A. Risk Factors Predicting Complications of Transvenous Lead Extraction. Biomed Res. Int. 2018, 2018, e201. [Google Scholar] [CrossRef]
- Jacheć, W.; Tomasik, A.; Polewczyk, A.; Kutarski, A. Impact of ICD lead on the system durability, predictors of long-term survival following ICD system extraction. Pacing Clin. Electrophysiol. 2017, 40, 1139–1146. [Google Scholar] [CrossRef]
- Małecka, B.; Kutarski, A.; Grabowski, M. Is the transvenous extraction of cardioverter-defibrillator leads more hazardous than that of pacemaker leads? Kardiol. Pol. 2010, 68, 884–890. [Google Scholar]
- Lisy, M.; Schmid, E.; Kalender, G.; Stock, U.A.; Doernberger, V.; Khalil, M.; Kornberger, A. Coronary sinus lead extraction in CRT patients with CIED-related infection: Risks, implications and outcomes. Minerva Cardioangiol. 2015, 63, 91–98. [Google Scholar]
- Williams, S.E.; Arujuna, A.; Whitaker, J.; Shetty, A.K.; Bostock, J.; Patel, N.; Mobb, M.; Cooklin, M.; Gill, J.; Blauth, C.; et al. Percutaneous lead and system extraction in patients with cardiac resynchronization therapy (CRT) devices and coronary sinus leads. Pacing Clin. Electrophysiol. 2011, 34, 1209–1216. [Google Scholar] [CrossRef]
- Nishii, N.; Nishimoto, T.; Mizuno, T.; Masuda, T.; Asada, S.; Miyamoto, M.; Kawada, S.; Nakagawa, K.; Nakamura, K.; Morita, H.; et al. Prognosis of patients with severe left ventricular dysfunction after transvenous lead extraction and the need for additional hemodynamic support in the perioperative period. Heart Rhythm 2021, 18, 962–969. [Google Scholar] [CrossRef]
- Bongiorni, M.G.; Kennergren, C.; Butter, C.; Deharo, J.C.; Kutarski, A.; Rinaldi, C.A.; Romano, S.L.; Maggioni, A.P.; Andarala, M.; Auricchio., A.; et al. The European Lead Extraction ConTRolled (ELECTRa) study: A European Heart Rhythm Association (EHRA) Registry of Transvenous Lead Extraction Outcomes. Eur. Heart J. 2017, 38, 2995–3005. [Google Scholar] [CrossRef]
- Gould, J.; Sidhu, B.S.; Porter, B.; Sieniewicz, B.J.; Teall, T.; Williams, S.E.; Shetty, A.; Bosco, P.; Blauth, C.; Gill, J.; et al. Prolonged lead dwell time and lead burden predict bailout transfemoral lead extraction. Pacing Clin. Electrophysiol. 2019, 42, 1355–1364. [Google Scholar] [CrossRef]
- Zucchelli, G.; Bongiorni, M.G.; Di Cori, A.; Soldati, E.; Solarino, G.; Fabiani, I.; Segreti, L.; De Lucia, R.; Viani, S.; Coluccia, G.; et al. Cardiac resynchronization therapy after coronary sinus lead extraction: Feasibility and mid-term outcome of transvenous reimplantation in a tertiary referral centre. Europace 2012, 14, 515–521. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).