Abstract
Wildfires are increasing in frequency, size, and intensity, and increasingly affect highly populated areas. Wildfire smoke impacts cardiorespiratory health; children are at increased risk due to smaller airways, a higher metabolic rate and ongoing development. The objective of this systematic review was to describe the risk of pediatric respiratory symptoms and healthcare visits following exposure to wildfire smoke. Medical and scientific databases and the grey literature were searched from inception until December 2020. Included studies evaluated pediatric respiratory-related healthcare visits or symptoms associated with wildfire smoke exposure. Prescribed burns, non-respiratory symptoms and non-pediatric studies were excluded. Risk of bias was evaluated using the National Toxicology Program’s Office of Health Assessment and Translation Risk of Bias Rating Tool. Data are presented narratively due to study heterogeneity. Of 2138 results, 1167 titles and abstracts were screened after duplicate removal; 65 full text screens identified 5 pre-post and 11 cross-sectional studies of rural, urban and mixed sites from the USA, Australia, Canada and Spain. There is a significant increase in respiratory emergency department visits and asthma hospitalizations within the first 3 days of exposure to wildfire smoke, particularly in children < 5 years old.
1. Introduction
Climate change influences extreme weather events, contributing to global natural disasters, including wildfires. Heatwaves, changes to precipitation leading to increased incidence of flooding and drought, as well as increased intensity of windstorms all increase the risk of uncontrolled fires [1]. Historically, the driving force of wildfires was precipitation level; however, modelling predicts that a shift to temperature-driven wildfires has begun to occur and will continue throughout the 21st century [2]. Wildfires have been increasing in frequency, size and intensity [3], with the number of unmanageable crown fires projected to continue increasing throughout the remainder of the 21st century [4,5,6]. The wildfire burning season is also expected to increase, with more days of uncontrolled burning in which the intensity exceeds the ability to suppress the fire [4]. Nearly 4000 wildfires burned in Canada in 2019, with the area burned exceeding 1.8 million hectares [6].
Wildfires negatively impact the environment, climate, economy and importantly, human health. Smoke produced by wildfires is composed of small particulate matter and toxic gases that are harmful to human health when inhaled. Composition of the smoke is dependent on many factors including: temperature of the fire, how long it burns, the fuel source/vegetation burned, the weather, and how far the smoke has travelled from the fire source [7]. In any type of air pollution, of most concern is the small particulate matter (PM2.5) that can be inhaled into bronchioles and alveoli, where it causes local irritation and damage [8,9]. Systemic impacts are also observed, including effects on pregnancy outcomes such as preterm birth [10]. Wildfire smoke significantly increases PM2.5 levels in the air, spreading long distances from the source and remaining elevated for weeks [11,12]. Regardless of the source, increased levels of PM2.5 in the air have adverse health effects such as increased cardiorespiratory morbidity and mortality and public health burdens [13,14], including cost and increased numbers of healthcare visits. This adds a significant financial burden to the healthcare system from potentially preventable use of resources.
Previous systematic reviews that included participants of all ages found that exposure to wildfire smoke significantly increased respiratory morbidity. A small number of studies have investigated the risk of respiratory-related healthcare utilization specifically in children [15,16,17]. It has been suggested that children may be at an increased risk of negative respiratory effects from wildfire smoke due to their smaller airway size and developing lungs [18,19]. Additionally, parents of young children may be more likely to access healthcare for their child’s respiratory symptoms compared to the average adult. The primary objective of this systematic review was to synthesize the data from studies investigating the risk of respiratory-related healthcare visits specifically among children aged 0–18 years old following exposure to wildfire smoke. The secondary objective was to pool data from primary studies reporting respiratory (both upper and lower respiratory tract) symptoms in children following exposure to wildfire smoke. We hypothesized that respiratory-related symptoms and healthcare visits will increase significantly in children following wildfire smoke exposure, and that younger children (<5) will demonstrate increased risk of healthcare visits compared to older children and teenagers.
2. Materials and Methods
2.1. Protocol, Registration and Search Strategy
This systematic review follows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [20]; Appendix A Table A1. The study protocol was registered with PROSPERO (CRD: 188705). A health sciences librarian (LD) conducted comprehensive searches of biomedical databases from database inception to December 2020: Medline (Ovid MEDLINE(R) ALL), Embase (Ovid interface), CINAHL Plus with Full Text (EBSCOhost interface), Greenfile (EBSCOhost interface), Web of Science (Indexes = SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH, BKCI-S, BKCI-SSH, ESCI, CCR-EXPANDED, IC), CABI: CAB Abstracts and Global Health (Clarivate Analytics interface), Proquest Earth, Atmospheric & Aquatic Science Database, Scopus, and HERO- Health and Environmental Research Online from database inception until 21 December 2020. The search combined a list of keyword synonyms for wildfires with a modified search hedge for pediatric studies [21]. No date, language, or study design limits were used. Google and Google Scholar searches and contacts with experts in the field were conducted and reference lists of reviews and included articles were reviewed to identify additional studies. The full details of the search strategy can be found in Supplement S1.
2.2. Eligibility Criteria and Study Selection
Peer-reviewed primary research on wildfires and pediatric respiratory health published up to 21 December 2020 was reviewed; the inclusion criteria are described using the Population, Exposure, Comparison, Outcome, Study Design (PECOS) framework for environmental health reviews [22]. To be included, studies must have included and independently described a population of children between 0–20 years old (increased from 0–18 years old described in the PROSPERO protocol to include additional studies). Exposures were characterized as specifically exposure to smoke produced by wildfires burning any vegetation. Measures of exposure were direct, through air sampling devices deployed for the study or standardized reporting from existing local air quality monitors, or indirect through satellite imagery, visibility index or self-reported perception of exposure, and the exposed population was designated by postal or zip code, county, or address of residence. Outcomes included respiratory-related ambulatory, Emergency Department (ED) and hospitalization-related healthcare visits and/or respiratory symptoms. Comparison populations included: similar populations during the same time period that were not exposed, the same population at a different time point when the exposure was not present, or healthcare visits in the exposed population during the exposure time that were not attributable to wildfire exposure (e.g., fractures). Studies regarding prescribed burning, indoor/outdoor controlled wood burning, or wildfires resulting from a separate primary disaster (e.g., volcanic eruption) were excluded. As this review intended to focus on wildfire smoke and healthcare utilization, studies that focused solely on non-smoke-related outcomes (e.g., burns) and mortality/fatality were excluded. Studies that exclusively reported pregnancy and birth-related outcomes were also excluded. Included study designs were observational studies (prospective and retrospective cohort, cross-sectional, case–control, ecological and time series). Case-reports and case-series, reviews, simulation studies, letters to the editor and commentaries were excluded. Studies with a high risk of bias in all domains of the PECOS question were excluded. We included all papers that had full text available in English or translatable by Google Translate, as translation services were not feasible for this review. For duplicate or overlapping studies the most recent article was used and the rest excluded. Full texts of relevant articles were retrieved and screened independently by the same reviewers for inclusion; disagreements regarding study inclusion were resolved by discussion between reviewers (SH and AH) until consensus was reached [20]. All studies underwent title and abstract screening for relevance, followed by a full-text review and risk of bias assessment conducted in duplicate by two independent reviewers (SH, AH). Titles with inconclusive title/abstract results or disagreements were retrieved for full text review. Disagreements about full-text study inclusion were resolved by consensus [20]. Complete references of excluded studies and reasons for exclusion are available on request.
2.3. Data Collection
Data were extracted independently by one reviewer (SH) with a second reviewer (AH) verifying accuracy. Study authors were contacted for additional information as required. Information regarding study characteristics (i.e., study date, duration, design, location), population characteristics (sample size, sex, age at assessment), exposure characteristics (exposure measures including number of smoke days, PM2.5, PM10, ozone, visibility and perceived smoke exposure and measurement strategies including type and location of ground-based sampling devices, satellite imagery and data sources) and outcome assessment (symptoms, outpatient clinic, ED visits or hospitalizations for respiratory presentations) were extracted from individual studies using a data collection form designed prior to the literature search. Quantitative data were extracted when studies reported outcomes as odds ratio (OR), risk ratio (RR), or the number of excess health care visits attributable to the exposure. For one study, the original data were not available [23]; the principal author recommended measuring the graph to estimate OR and confidence intervals. The published graph was magnified to 400% and measured; estimated results were obtained by comparing data points to the y axis. Results were described using mean, standard deviation for continuous data and proportions and percentages for categorical data. Google Sheets was used to track data. Meta-analysis was not conducted due to significant heterogeneity in study design, exposure and outcome evaluation and reporting between included studies [24].
2.4. Risk of Bias, Evidence Synthesis and Certainty
Risk of bias of individual studies was evaluated by two independent reviewers (SH and AH) using the National Toxicology Program’s Office of Health Assessment and Translation (OHAT) Risk of Bias Rating Tool for Human and Animal Studies [25,26,27]. The OHAT tool evaluates six domains for bias at the outcome level using 11 questions that address selection, confounding, attrition/exclusion, performance, detection and selective reporting bias, without excluding low quality studies [27]. For this review of observational studies, participant selection, confounding, exposure measurement, outcome assessment, follow up and completeness of outcome reporting were assessed as: probably or possibly low, possibly high or probably high risk of bias based on consensus; discrepancies were resolved through discussion.
The Systematic Review Without Meta-analysis (SWIM) guidelines were used to report data and for evidence synthesis (Table A2). Study characteristics and risk of bias were described narratively and summarized in tables and figures. An effect direction plot was developed to present an overview of the information, using a vote-counting approach supported by the tabular data to summarize the direction of identified associations [10]. All reported results were considered to have no significant association unless the confidence interval did not cross an OR or RR of 1.0; significant numbers below 1.0 were reported as a negative association and above 1.0 a positive association. We used the adapted Grading of Recommendations Assessment, Development and Evaluation (GRADE) framework for environmental health reviews [28] to assess the certainty of the evidence as high, moderate, low, or very low. Parameters that increased certainty were evidence of a dose–response relationship and larger effect size; higher risk of bias, small sample or effect sizes, wide confidence intervals and poor relevance of the study to the PECO questions were criteria used to downgrade the certainty of evidence. Disagreements were resolved by consensus.
3. Results
3.1. Search Results
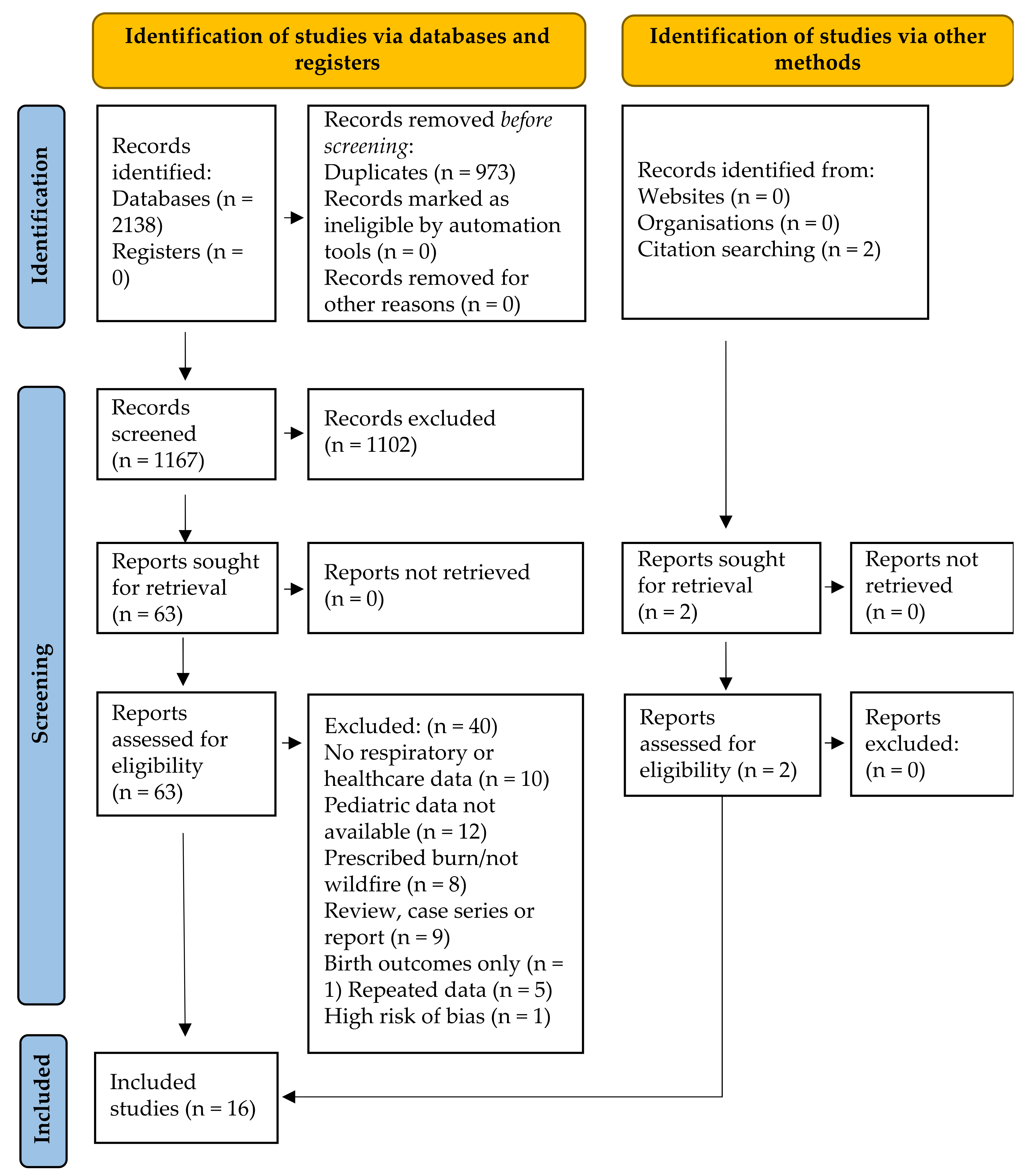
The electronic and grey literature search identified 2138 studies; 1165 after duplicate removal. No grey literature studies were identified. The detailed study selection process is outlined in Figure 1. After title and abstract screening, 1102 of the 1165 identified studies were excluded. No grey literature studies were identified. Of the 65 studies included in full-text screening, 10 were excluded due to no respiratory or healthcare outcomes, 12 due to no pediatric (or undifferentiated) data, 8 due to prescribed burn or other non-wildfire exposure, 9 due to study methodology (case-series, case report or review), 3 because no full text was available, 1 only assessed birth outcomes and 5 repeated data presented in included studies. One of the 17 remaining studies was excluded for high risk of bias [29] in all domains. The 16 studies included in this review are summarized in Table 1.
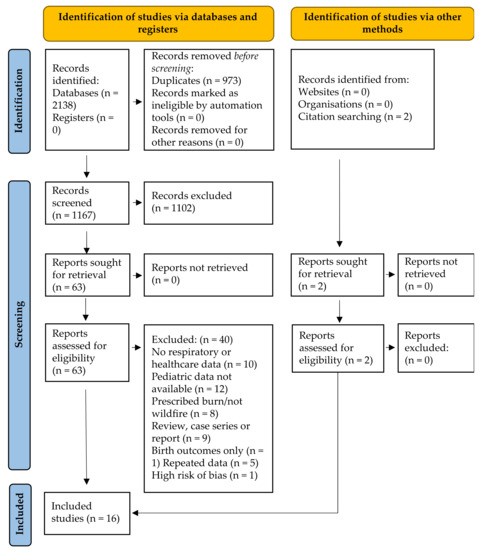
Figure 1.
Modified PRISMA flow diagram [20] for pediatric outcomes associated with wildfire smoke exposure resulting from searches of databases, registers and other sources.

Table 1.
Characteristics of included studies. Values that reach significance are in bold.
3.2. Study and Population Characteristics
Included studies encompassing data collected between 1996 and 2017 were published between 2006 and 2020. Four pre/post [30,35,38,39], three cross-sectional case crossover [15,34,40] and nine cross-sectional [16,23,31,33,36,37,40,41,42] studies were included. They represented North America and Australia; while some studies on pediatric exposure to biomass smoke from South America and Asia were screened, they were excluded due to a focus on seasonal controlled agricultural biomass burns, not wildfires. Most of the included studies focused on a single city (Albuquerque, NM, USA [39], Sydney, AU [34], Darwin, AU [32], Valencia, SP [16]) or region (Northern California, USA [38], Hoopa Valley, California, USA [35], Southern California, USA [15,30,33,36], Colorado, USA [40], North Carolina, USA [42], Washington State, USA [31], Victoria, AU [41], British Columbia, Canada [23]); one evaluated 10 years of data across the United States, using US Centers for Disease Control data for medical visits [37]. Four were exclusively urban [16,32,34,39], one covered only an Indigenous reserve site [35] and the remainder included mixed urban and rural populations [15,23,30,31,33,36,37,38,40,41,42].
Data for 565,321 children under the age of 20 years was reported in 13 of the 16 included studies [15,16,23,30,31,33,35,36,38,39,40,42,43]; the remaining three did not report their results in terms of population numbers [32,37,41]. Only three studies evaluated preschool age categories separately [23,30,33]. Two studies surveyed existing pediatric cohorts (age 5 in Spain [16], ages 6–7 and 17–18 in Southern California [15]) regarding symptoms during wildfire smoke exposure; the others were population-level and relied on government and/or medical care provider system databases focusing on respiratory or cardiorespiratory causes for hospitalization or ED visit [23,30,31,32,33,35,36,37,38,39,40,41,42]. International Classification of Diseases and Related Health Problems, 9th Revision (ICD-9) codes were used to identify outcomes in all of the studies except one, which relied on self-report data [15]. Some were reported as “all respiratory visits”, while others also reported specific diagnostic codes including asthma, bronchiolitis, bronchitis, pneumonia and upper respiratory tract infection. Several studies included chronic obstructive pulmonary disease, with no pediatric cases recorded. The most reported outcomes were ED visits in eight studies [23,33,34,36,38,39,40,42], hospitalizations in four [30,31,32,33] and outpatient clinic visits in three [33,35]. One of two studies that captured individual symptoms also reported physician visits for smoke-related symptoms [15]. Three studies reported trends in healthcare presentations rather than OR or RR [32,37,41].
Different comparison groups, and different approaches to comparison between groups, were reported. Some before and after studies compared healthcare visits during the period immediately preceding and/or after a wildfire [15,16,30,32,33,38,39,41], others during previous months or years [23,35,36], in some cases matching the month and day of the week to days during the exposure period [23]. Fractures were assumed to be non-wildfire-associated injuries and used as a stable baseline for comparison to cardiorespiratory visits attributable to wildfire smoke exposure [31,42]. An alternative study design was to compare populations from a single medical data source by exposure status, typically designated by postal or zip code on the medical record between exposed and unexposed areas [37].
3.3. Exposure Characterization
Wildfire smoke exposure reporting differed between studies. Particulate matter was most common, with PM2.5 [30,31,33,34,36,37,38,39,40,41,42] or PM10 [15,23,32,35,41] measured mainly using locally deployed sampling devices, often through access to government- or agency-based air quality monitoring programs. Different types of system were used; studies that deployed their own air quality monitoring devices typically employed tapered element oscillating microbalance devices [32,35,41], although in the 1990s, gravimetric stacked filter units were also used [32]. Several studies added satellite data specifically focused on particulate matter analyses associated with wildfires [23,31,33,36,38,40]. Weather characteristics including temperature, barometric pressure and humidity were added to modeling methods employed in one study [30]. Other measures of exposure included ozone [37,38,41], visibility [32] and the Air Quality Index (AQI) [33], often as adjuncts within modeling algorithms that employed PM as a primary measure of exposure. Population exposure was determined by the postal or zip code on the medical record, although three studies used addresses [16,23,41], one county-level data [42] and one country-wide data [37]. Exposures were reported as a daily average in most studies [15,23,30,31,33,34,36,38,39,40,41,42]. Lag times between reported exposure and medical visit data varied; some studies evaluated same-day results [23,30,35,36] while others reported a range of lag times from 0 to 21 days after peak smoke events [15,31,32,33,38,39,41,42]. One reported only a lag time of three days [40] and two did not clarify the lag time between exposure and outcome [16,37]. In one of the studies, perceived symptoms were greater when the subjects reported being able to smell smoke, but no comparison of measured and perceived exposure level was reported [16].
3.4. Outcomes
Reported outcomes are summarized in Table 2. The most frequently reported outcome was ED visits for any respiratory cause (nine studies) [23,33,34,36,38,39,40,41,42] or for asthma (nine studies) [23,33,34,37,38,39,40,41,42]. Hospitalizations for asthma or any respiratory cause were reported in four studies [30,31,32,33], outpatient medical clinic visits in three [15,33,35] and symptoms in two [15,16].

Table 2.
Effect direction plot (sorted by alphabetical order).
3.5. Risk of Bias Assessment
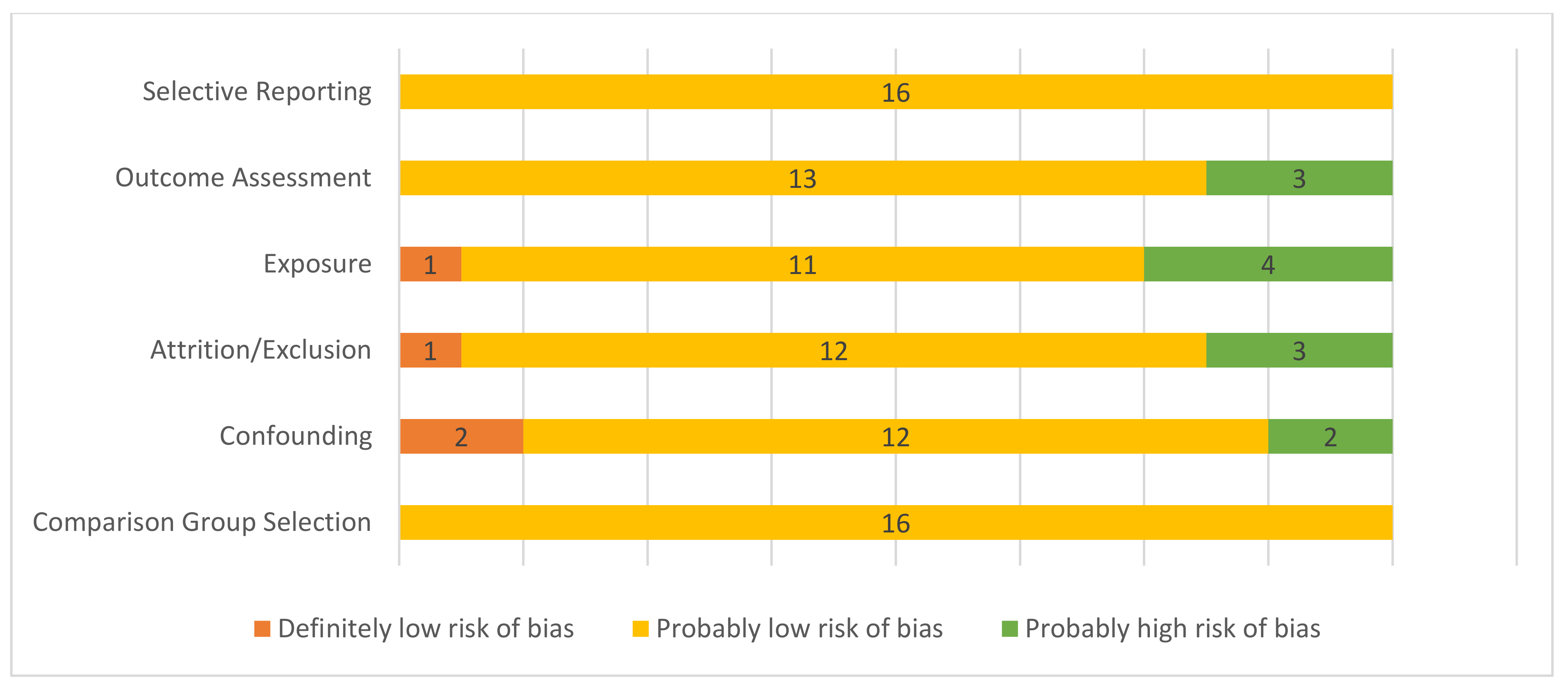
A summary of the risk of bias assessment can be found in Figure 2. Of the 17 studies evaluated, one was determined to be at high risk of selection bias, adjusting for potential confounders, and exposure misclassification bias, so was excluded from the analysis. There was an increased risk of detection bias due to exposure characterization in three studies that used indirect means to estimate exposure: visibility index [32], although correlated with PM10 evaluation at a nearby site, and perceived exposure [15,16] and one estimated exposure based on modeling and did not adjust for seasonal trends [37]. There was a high risk of bias due to attrition in two population-level studies [32,35]: one because some residents, particularly those at high risk of respiratory disease, evacuated from the area [35], and in one cohort study [15] with limited response rates for the wildfire symptoms survey in a subset of the cohort. Two studies expressed data as trends [32,41]; however, the rest provided data with confidence intervals.
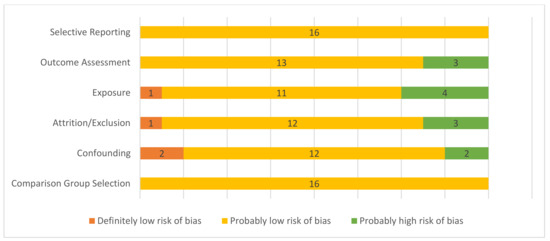
Figure 2.
Risk of bias summary for included studies. Definitely low risk of bias = direct evidence of low risk-of-bias practices; probably low risk of bias = indirect evidence of low risk-of bias practices or deviations would not appreciably bias results; probably high risk of bias = indirect evidence of high risk-of-bias practices or insufficient information provided for evaluation [25,26].
3.6. Association between Wildfire Smoke Exposure and Healthcare Visits
Health outcomes associated with wildfire visits are summarized in Table 3. Three studies encompassing 9977 participants noted a positive association between wildfire smoke exposure and outpatient clinic visits for any respiratory problem [15,33,35]; all were observational, and due to potential risk of bias the certainty of the evidence (GRADE) was low [28], whereas a larger number of participants (557,454 participants across 8 studies) demonstrated a positive association between respiratory visits to the ED and wildfire smoke exposure [23,33,36,38,39,40,42,44] with moderate certainty [28]. The four studies that demonstrated a positive association between hospitalization for any respiratory cause and wildfire smoke in the pediatric age group encompassed 13,258 participants [30,31,33,38], with moderate certainty and evidence of a dose effect [28]. The same studies showed a moderate certainty of evidence [28] in the positive association between ED visits and hospitalizations for asthma and exposure to wildfire smoke [23,30,31,33,34,36,38,39,40,42]. No significant association was noted for asthma-related clinic visits, with only one [33] of the three studies evaluating this outcome [15,16,33] showing a positive association and a low certainty of evidence [28].

Table 3.
Summary of findings with certainty of evidence (GRADE [28]).
3.7. Association between Wildfire Smoke Exposure and Symptoms
Only two studies assessed wildfire smoke-related symptoms [15,16]. There was a positive association between respiratory symptoms, with a very low certainty of evidence (GRADE) mainly due to risk of bias in exposure characterization and outcome assessment [28] in both studies and only one respiratory symptom (dry cough) being reported in one study [16]. Itchy or watery eyes, sneezing, sore throat and rhinitis had a positive association with smoke in both [15,16], with a dose effect and increased impact on participants with existing asthma or rhinitis reported in one study [16] and a low certainty of evidence [28].
3.8. Special Populations
Two studies addressed marginalized populations [32,35] and one specifically reported the difference between a marginalized Indigenous subpopulation and the total exposed group [32]. The impact on marginalized populations was not reported in one of the two studies, since the only non-Indigenous patients seen in the clinic during the wildfire period were firefighting personnel and only Indigenous patients typically attended the clinic in comparison periods [35]. One study did compare Indigenous to non-Indigenous participants, although this part of the study did not distinguish between children and adults. In this study, Indigenous patients were 15.02% (95% confidence interval 3.73%, 27.54%) more likely to be admitted to hospital for wildfire-attributable respiratory causes than non-Indigenous patients with the same level of PM10 exposure [32].
4. Discussion
4.1. Summary of Evidence
This review used a descriptive approach to summarize and evaluate the existing evidence on the impact wildfire smoke has on healthcare utilization in the pediatric population. All of the studies included in the review were observational, with either a pre–post or cross-sectional design. They encompassed urban or mixed urban and rural settings, other than one that focused on mainly rural exposures [42]. It was not possible to combine study data due to the significant heterogeneity in study design as well as differences between populations, comparison groups, exposures and outcomes [24]. Although outcome measures (healthcare visits, symptoms) were similar, there were differences in lag times between exposure and outcomes as well as reporting.
There is some evidence suggesting a positive association between wildfire smoke exposure and outpatient ED visits or hospitalizations for any respiratory diagnoses in the pediatric population, with no significant association specifically between asthma and ED visits. Eye itchiness, nasal congestion, rhinitis and sore throat were positively associated with wildfire smoke exposure with a low grade of certainty; respiratory symptoms such as wet or dry cough, asthma exacerbation, bronchitis or sneezing showed an increase that did not reach significance, with a very low grade of certainty (Table 3) [15,16]. Overall, there were no significant associations found between wildfire smoke exposure and pediatric outpatient visits or hospitalizations specifically for asthma (Table 2 and Table 3). It is possible that children with asthma spend more time indoors when air quality is poor and are more likely to increase their asthma medication proactively or in response to increased symptoms; in at least one of the included studies, higher-risk people were evacuated from the affected area [35].
Only two of the studies that recruited participants from an existing pediatric cohort focused solely on pediatric data [15,16]; the rest were population-level studies that included separately reported pediatric data but did not focus specifically on pediatric outcomes. In this context, four broke pediatric data into age-specific subgroups [23,30,33,36] while the rest reported results for participants < 15–20 years of age. Specifically in younger children, two studies found no significant association between wildfire smoke and respiratory ED visits [23,36]; however, the other two did note significant associations specifically in children less than 4 years of age, with no [30] or weaker [33] but significant associations between respiratory-related ED visits and wildfire smoke exposure in older children. While the two studies that specifically surveyed children from existing research cohorts did provide pediatric data, the age groups, total study numbers and outcomes were limited and using these pre-existing cohorts may have introduced selection bias. Given the significant differences in typical activities, airway size, respiratory reserve and developmental stage in young children [18], particularly in comparison with older children and adult-sized teens, this review highlights the paucity of existing data and the need for focused research on the response to wildfire smoke exposure in these very important age groups. As well, the importance of considering pediatric age groups as separate entities during population-level data analysis is evident from the studies that do show differences between infant, toddler, child and teen presentations [33].
Wildfire smoke composition is complex and dynamic [7]. The nature of exposure would be drastically variable within and across studies, as many factors including physical activity levels, length of time exposed, access to well-ventilated housing and weather trends all impact the true amount of exposure among participants. Few studies described the type of vegetation and other materials burned (e.g., houses, industrial materials and sites, type of trees and other plant matter), most only mentioning whether the smoke resulted from controlled, uncontrolled or mixed types of burning. The chemical composition of the smoke will vary depending on the material burned and the stage of burning, making it difficult to fully understand what chemical exposures were present across included studies.
More than half of the studies used PM2.5 as a primary measure of exposure; as a common component of air pollution whose role in pulmonary disease is well characterized, this is an option that should allow data from different studies to be compared and combined, provided other study characteristics are sufficiently similar. As well, PM10, another commonly measured component of air pollution that has already been associated with health outcomes, was employed by several more studies; while not directly comparable to PM2.5, reporting it in air pollution studies would likely be beneficial. Unfortunately, the means by which exposures were measured and reported, the length of time of exposure and lag time between exposure and outcome measurement varied considerably between studies. Other exposures that have been implicated in respiratory disease, including ozone, were also reported by some studies but not others. The heterogeneity between studies is likely at least in part due to differences in local measurement and reporting standards, since many relied on local, regional, or national air quality monitoring systems for information. Some studies added modeling to account for more detail in localizing which sites were impacted by wildfire smoke that included weather conditions and satellite imagery. While this may have improved accuracy in estimating exposed populations, it was not comparable between studies. Given the importance of air quality monitoring for studies evaluating any form of air pollution impacts on population health as well as increasing importance of and interest in monitoring exposures attributable to wildfire smoke, this review demonstrates that a simple universal system of exposure reporting, even if some studies also include more complex modeling, would promote data integration and collaborative efforts to understand the true impact of these exposures.
4.2. Challenges Associated with Synthesis
The study of exposure to wildfire smoke, like other exposure-related studies, tends to rely on observational data. Although some areas frequently impacted by wildfires can potentially be prepared for a more rigorous study design, for the most part it would not be feasible to entertain alternate research options. Study design is often influenced by the availability of data, including population and exposure characterization. Pooling data and meta-analysis were not possible in this review due to heterogeneity in study design, exposure characterization, population and comparison group definition, outcome assessment and reporting. Additionally, the lack of information available regarding pre-existing conditions among participants that may have affected the outcomes of the included studies limits the interpretation of these results.
4.3. Strengths and Limitations
We followed the Cochrane guidelines for conducting a systematic review and PRISMA reporting guidelines [20,24,45]. A detailed protocol outlining methodology, data extraction and data synthesis was published in advance of the review on PROSPERO. Comprehensive searches conducted by a health sciences librarian identified literature discussing pediatric healthcare utilization associated with wildfires. Two independent reviewers conducted the screening and risk of bias assessments as per the Cochrane guidelines [45]. These are all strengths of this study that added thoroughness to the approach used to detect eligible research studies and rigor in selecting them. However, there were also limitations. This review is limited by an inability to complete a quantitative analysis or accurate calculation of risk of bias due to the large heterogeneity in study design across included studies [24]. The review focused specifically on wildfires, excluding prescribed vegetation burns, to decrease heterogeneity between types of exposure; however, this excluded some studies with mixed contributors to smoke, as well as particularly excluding several studies from Asia and Brazil, where yearly prescribed burning was identified as a significant contributor to overall smoke days and smoke-related PM2.5 impacting air quality [43]. Few studies reported pediatric outcomes, and the small number of studies that broke down data by age did not allow for adequate comparison between infants, preschool children and teenagers, although physiologically and developmentally they are distinct populations. In one study, original data were not available from the publication or from the author, so numbers were estimated based on measuring points and confidence intervals on a graph in the original publication [23], which may have impacted the results.
5. Conclusions
A limited number of observational studies available in the literature suggest that children have an increased risk of respiratory-related healthcare visits associated with wildfire smoke exposure. With the increasing quantity and severity of wildfires in some regions, it is imperative to investigate the respiratory health implications of wildfire smoke at both an individual and population level. Future research should include longitudinal observational studies investigating the long-term impact of wildfire smoke exposure on children, as well as break down the impact of exposure by age. Additionally, promoting a standard means for reporting wildfire smoke exposures and outcomes will promote data integration in the future.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ijerph18168799/s1, Detailed search strategy for systematic review.
Author Contributions
Conceptualization, S.H. and A.H.; methodology, S.H., A.H., M.B.O. and L.D.; validation, S.H., M.B.O. and A.H.; formal analysis, S.H., A.H. and M.B.O.; investigation, S.H., A.H. and L.D.; data curation, S.H., A.H., M.B.O. and L.D.; writing—original draft preparation, S.H. and A.H.; writing—review and editing, S.H., A.H., M.B.O. and L.D.; visualization, S.H., M.B.O. and A.H.; supervision, M.B.O. and A.H.; project administration, S.H., A.H. and M.B.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by an Alberta Heath Services Respiratory Health Strategic Clinical Network (RHSCN) Summer Studentship Award (2020) for S.H.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
We would like to thank Matthew Hicks for advice regarding analysis and presentation of the data.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Appendix A

Table A1.
PRISMA 2020 Checklist for Systematic Reviews [20].
Table A1.
PRISMA 2020 Checklist for Systematic Reviews [20].
| Section and Topic | Item # | Checklist Item | Location Where Item is Reported |
|---|---|---|---|
| TITLE | |||
| Title | 1 | Identify the report as a systematic review. | Title Page 1 |
| ABSTRACT | |||
| Abstract | 2 | See the PRISMA 2020 for Abstracts checklist. | Abstract Page 1 |
| INTRODUCTION | |||
| Rationale | 3 | Describe the rationale for the review in the context of existing knowledge. | Section 1 Page 2, line 28 |
| Objectives | 4 | Provide an explicit statement of the objective(s) or question(s) the review addresses. | Section 1 Page 2, line 34 |
| METHODS | |||
| Eligibility criteria | 5 | Specify the inclusion and exclusion criteria for the review and how studies were grouped for the syntheses. | Eligibility: Section 2.3 Page 3 line 66 Grouping: Section 2.3 Page 3, line 84 |
| Information sources | 6 | Specify all databases, registers, websites, organisations, reference lists and other sources searched or consulted to identify studies. Specify the date when each source was last searched or consulted. | Section 2.2 Page 2, line 49 |
| Search strategy | 7 | Present the full search strategies for all databases, registers and websites, including any filters and limits used. | Section 2.2 Page 2, line 48; Supplementary Materials S1 Page 26, line 423 |
| Selection process | 8 | Specify the methods used to decide whether a study met the inclusion criteria of the review, including how many reviewers screened each record and each report retrieved, whether they worked independently, and if applicable, details of automation tools used in the process. | Section 2.4 Page 3, line 93 |
| Data collection process | 9 | Specify the methods used to collect data from reports, including how many reviewers collected data from each report, whether they worked independently, any processes for obtaining or confirming data from study investigators, and if applicable, details of automation tools used in the process. | Section 2.5 Page 3, line 100 |
| Data items | 10a | List and define all outcomes for which data were sought. Specify whether all results that were compatible with each outcome domain in each study were sought (e.g., for all measures, time points, analyses), and if not, the methods used to decide which results to collect. | Section 2.5 Page 3, line 103 |
| 10b | List and define all other variables for which data were sought (e.g., participant and intervention characteristics, funding sources). Describe any assumptions made about any missing or unclear information. | Section 2.5 Page 3, line 103 | |
| Study risk of bias assessment | 11 | Specify the methods used to assess risk of bias in the included studies, including details of the tool(s) used, how many reviewers assessed each study and whether they worked independently, and if applicable, details of automation tools used in the process. | Section 2.6 Page 4, line 121 |
| Effect measures | 12 | Specify for each outcome the effect measure(s) (e.g., risk ratio, mean difference) used in the synthesis or presentation of results. | Section 2.5 Page 3, line 110 |
| Synthesis methods | 13a | Describe the processes used to decide which studies were eligible for each synthesis (e.g., tabulating the study intervention characteristics and comparing against the planned groups for each synthesis (item #5)). | Section 2.7 Page 4, line 136 |
| 13b | Describe any methods required to prepare the data for presentation or synthesis, such as handling of missing summary statistics, or data conversions. | Section 2.7 Page 4, line 137 | |
| 13c | Describe any methods used to tabulate or visually display results of individual studies and syntheses. | Section 2.7 Page 4, lines 137 and 149 | |
| 13d | Describe any methods used to synthesize results and provide a rationale for the choice(s). If meta-analysis was performed, describe the model(s), method(s) to identify the presence and extent of statistical heterogeneity, and software package(s) used. | Section 2.7 Page 4, line 135 | |
| 13e | Describe any methods used to explore possible causes of heterogeneity among study results (e.g., subgroup analysis, meta-regression). | NA | |
| 13f | Describe any sensitivity analyses conducted to assess robustness of the synthesized results. | Section 2.7 Page 4, line 142 | |
| Reporting bias assessment | 14 | Describe any methods used to assess risk of bias due to missing results in a synthesis (arising from reporting biases). | Section 2.6 Page 4, line 123 |
| Certainty assessment | 15 | Describe any methods used to assess certainty (or confidence) in the body of evidence for an outcome. | Section 2.7 Page 4, line 142 |
| RESULTS | |||
| Study selection | 16a | Describe the results of the search and selection process, from the number of records identified in the search to the number of studies included in the review, ideally using a flow diagram. | Section 3.1 Page 4, line 154 and Figure 1 |
| 16b | Cite studies that might appear to meet the inclusion criteria, but which were excluded, and explain why they were excluded. | Section 3.1 Page 4, line 163 | |
| Study characteristics | 17 | Cite each included study and present its characteristics. | Section 3.2 Page 19, line 176 and Table 1 |
| Risk of bias in studies | 18 | Present assessments of risk of bias for each included study. | Section 3.7 Page 22, line 252 |
| Results of individual studies | 19 | For all outcomes, present, for each study: (a) summary statistics for each group (where appropriate) and (b) an effect estimate and its precision (e.g., confidence/credible interval), ideally using structured tables or plots. | Section 3.2 Page 19, line 176 and Table 1 |
| Results of syntheses | 20a | For each synthesis, briefly summarise the characteristics and risk of bias among contributing studies. | Section 3.6–3.10 Page 21, line 241 and Table 2 |
| 20b | Present results of all statistical syntheses conducted. If meta-analysis was done, present for each the summary estimate and its precision (e.g., confidence/credible interval) and measures of statistical heterogeneity. If comparing groups, describe the direction of the effect. | NA | |
| 20c | Present results of all investigations of possible causes of heterogeneity among study results. | NA | |
| 20d | Present results of all sensitivity analyses conducted to assess the robustness of the synthesized results. | NA | |
| Reporting biases | 21 | Present assessments of risk of bias due to missing results (arising from reporting biases) for each synthesis assessed. | Section 3.7 Page 22, line 251 and Figure 2 |
| Certainty of evidence | 22 | Present assessments of certainty (or confidence) in the body of evidence for each outcome assessed. | Section 3.8 Page 22, line 268 and Table 3 |
| DISCUSSION | |||
| Discussion | 23a | Provide a general interpretation of the results in the context of other evidence. | Section 4.1 Page 24, line 309 |
| 23b | Discuss any limitations of the evidence included in the review. | Section 4.2 Page 25, line 379 | |
| 23c | Discuss any limitations of the review processes used. | Section 4.3 Page 25, line 390 | |
| 23d | Discuss implications of the results for practice, policy, and future research. | Section 5 line 416 | |
| OTHER INFORMATION | |||
| Registration and protocol | 24a | Provide registration information for the review, including register name and registration number, or state that the review was not registered. | Section 2.1 Page 2, line 46 |
| 24b | Indicate where the review protocol can be accessed, or state that a protocol was not prepared. | Section 2.1 Page 2, line 46 | |
| 24c | Describe and explain any amendments to information provided at registration or in the protocol. | Section 2.3 Page 3, lines 64 and 67 | |
| Support | 25 | Describe sources of financial or non-financial support for the review, and the role of the funders or sponsors in the review. | Funding: Page 26, line 431 |
| Competing interests | 26 | Declare any competing interests of review authors. | Conflicts of Interest: Page 26, line 436 |
| Availability of data, code and other materials | 27 | Report which of the following are publicly available and where they can be found: template data collection forms; data extracted from included studies; data used for all analyses; analytic code; any other materials used in the review. | Supplemental Materials |
Appendix B

Table A2.
Systematic Review Without Meta-analysis (SWIM) checklist [24].
Table A2.
Systematic Review Without Meta-analysis (SWIM) checklist [24].
| SWiM Reporting Item | Item Description | Page in Manuscript Where Item Is Reported |
|---|---|---|
| (1a) Provide a description of, and rationale for, the groups used in the synthesis (e.g., groupings of populations, interventions, outcomes, study design) | Section 2.7 Page 4, line 134 |
| (1b) Detail and provide rationale for any changes made subsequent to the protocol in the groups used in the synthesis | Section 2.3 Page 3, lines 64 and 67 | |
| Describe the standardised metric for each outcome. Explain why the metric(s) was chosen, and describe any methods used to transform the intervention effects, as reported in the study, to the standardised metric, citing any methodological guidance consulted | Section 2.3 Page 3, line 68 |
| Describe and justify the methods used to synthesise the effects for each outcome when it was not possible to undertake a meta-analysis of effect estimates | Section 2.7 Page 3, line 134 |
| Where applicable, provide the criteria used, with supporting justification, to select the particular studies, or a particular study, for the main synthesis or to draw conclusions from the synthesis (e.g., based on study design, risk of bias assessments, directness in relation to the review question) | Section 2.7 Page 3, line 142 |
| State the method(s) used to examine heterogeneity in reported effects when it was not possible to undertake a meta-analysis of effect estimates and its extensions to investigate heterogeneity | Section 2.7 Page 3, line 137 |
| Describe the methods used to assess certainty of the synthesis findings | Section 2.7 Page 3, line 142 |
| Describe the graphical and tabular methods used to present the effects (e.g., tables, forest plots, harvest plots). Specify key study characteristics (e.g., study design, risk of bias) used to order the studies, in the text and any tables or graphs, clearly referencing the studies included | Section 2.6, Section 2.7 Page 3; Section 2.3 Page 2, Table 2 Page 21, Figure 2 Page 22 |
| For each comparison and outcome, provide a description of the synthesised findings, and the certainty of the findings. Describe the result in language that is consistent with the question the synthesis addresses, and indicate which studies contribute to the synthesis | Table 3 Page 23, Sections 3.8–3.10 Page 22 |
| Discussion | ||
| Report the limitations of the synthesis methods used and/or the groupings used in the synthesis, and how these affect the conclusions that can be drawn in relation to the original review question | Section 4.2 and Section 4.3 Page 25 |
References
- Anderson, J.; Bausch, C. Climate Change and Natural Disasters: Scientific Evidence of a Possible Relation between Recent Natural Disasters and Climate Change (IP/A/ENVI/FWC/2005-35); Institute for European Environmental Policy: Brussels, Belgium, 2005; Volume 35. [Google Scholar]
- Pechony, O.; Shindell, D.T. Driving forces of global wildfires over the past millennium and the forthcoming century. Proc. Natl. Acad. Sci. USA 2010, 107, 19167–19170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Westerling, A.L.; Hidalgo, H.G.; Cayan, D.R.; Swetnam, T.W. Warming and earlier spring increase Western U.S. forest wildfire activity. Science 2006, 313, 940–943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Parisien, M.A.; Taylor, S.W.; Candau, J.N.; Stralberg, D.; Marshall, G.A.; Little, J.M.; Flannigan, M.D. Projected changes in daily fire spread across Canada over the next century. Environ. Res. Lett. 2017, 12, 025005. [Google Scholar] [CrossRef]
- Dupuy, J.-L.; Fargeon, H.; Martin-StPaul, N.; Pimont, F.; Ruffault, J.; Guijarro, M.; Hernando, C.; Madrigal, J.; Fernandes, P. Climate change impact on future wildfire danger and activity in southern Europe: A review. Ann. For. Sci. 2020, 77, 1–24. [Google Scholar] [CrossRef]
- Abatzoglou, J.T.; Kolden, C.A. Climate change in Western US deserts: Potential for increased wildfire and invasive annual grasses. Rangel. Ecol. Manag. 2011, 64, 471–478. [Google Scholar] [CrossRef]
- Liu, J.C.; Peng, R.D. The impact of wildfire smoke on compositions of fine particulate matter by ecoregion in the Western US. J. Expo. Sci. Environ. Epidemiol. 2019, 29, 765–776. [Google Scholar] [CrossRef]
- Xing, Y.F.; Xu, Y.H.; Shi, M.H.; Lian, Y.X. The impact of PM2.5 on the human respiratory system. J. Thorac. Dis. 2016, 8, E69–E74. [Google Scholar] [CrossRef]
- Matz, C.J.; Egyed, M.; Xi, G.; Racine, J.; Pavlovic, R.; Rittmaster, R.; Henderson, S.B.; Stieb, D.M. Health impact analysis of PM2.5 from wildfire smoke in Canada (2013–2015, 2017–2018). Sci. Total Environ. 2020, 725, 138506. [Google Scholar] [CrossRef] [PubMed]
- Amjad, S.; Chojecki, D.; Osornio-vargas, A.; Ospina, M.B. Wildfire exposure during pregnancy and the risk of adverse birth outcomes: A systematic review. Environ. Int. 2021, 156, 106644. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Lin, M.; Horowitz, L.W. Summer PM2.5 Pollution Extremes Caused by Wildfires Over the Western United States During 2017–2018. Geophys. Res. Lett. 2020, 47, 1–11. [Google Scholar] [CrossRef]
- Shi, H.; Jiang, Z.; Zhao, B.; Li, Z.; Chen, Y.; Gu, Y.; Jiang, J.H.; Lee, M.; Liou, K.N.; Neu, J.L.; et al. Modeling Study of the Air Quality Impact of Record-Breaking Southern California Wildfires in December 2017. J. Geophys. Res. Atmos. 2019, 124, 6554–6570. [Google Scholar] [CrossRef] [PubMed]
- Nolte, C.G.; Dolwick, P.D.; Fann, N.; Horowitz, L.W.; Naik, V.; Pinder, R.W.; Spero, T.L.; Winner, D.A.; Ziska, L.H. Air Qulality. In Impacts, Risks and Adaptation in the United States: Fourth National Climate Assessment; United States Global Change Research Program: Washington, DC, USA, 2018; Volume 2, pp. 1–24. ISBN 978-1-46658-445-7. [Google Scholar]
- Lu, F.; Xu, D.; Cheng, Y.; Dong, S.; Guo, C.; Jiang, X.; Zheng, X. Systematic review and meta-analysis of the adverse health effects of ambient PM2.5 and PM10 pollution in the Chinese population. Environ. Res. 2015, 136, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Künzli, N.; Avol, E.; Wu, J.; Gauderman, W.J.; Rappaport, E.; Millstein, J.; Bennion, J.; McConnell, R.; Gilliland, F.D.; Berhane, K.; et al. Health effects of the 2003 Southern California wildfires on children. Am. J. Respir. Crit. Care Med. 2006, 174, 1221–1228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vicedo-Cabrera, A.M.; Esplugues, A.; Iñíguez, C.; Estarlich, M.; Ballester, F. Health effects of the 2012 Valencia (Spain) wildfires on children in a cohort study. Environ. Geochem. Health 2016, 38, 703–712. [Google Scholar] [CrossRef]
- Black, C.; Tesfaigzi, Y.; Bassein, J.A.; Miller, L.A. Wildfire smoke exposure and human health: Significant gaps in research for a growing public health issue. Environ. Toxicol. Pharmacol. 2017, 55, 186–195. [Google Scholar] [CrossRef]
- Dietert, R.R.; Etzel, R.A.; Chen, D.; Halonen, M.; Holladay, S.; Jarabek, A.M.; Landreth, K.; Peden, E.B.; Pinkerton, K.; Smialowicz, R.J.; et al. Workshop to identify critical windows of exposure for children’s health: Reproductive health in children and adolescents work group summary. Environ. Health Perspect. 2000, 108, 483–490. [Google Scholar] [CrossRef] [Green Version]
- Mirabelli, M.C.; Künzli, N.; Avol, E.; Gilliland, F.D.; Gauderman, W.J.; McConnell, R.; Peters, J.M. Respiratory symptoms following wildfire smoke exposure: Airway size as a susceptibility factor. Epidemiology 2009, 20, 451–459. [Google Scholar] [CrossRef] [Green Version]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Tjosvold, L.; Campbell, S.; Dorgan, M. Filter to Retrieve Pediatrics Articles in OVID Medline; John W. Scott Health Sciences Library, University of Alberta: Edmonton, AB, Canada, 2015; p. 14026652. [Google Scholar] [CrossRef]
- Morgan, R.L.; Whaley, P.; Thayer, K.A.; Shunemann, H.J. Identifying the PECO: A framework for formulating good questions to explore the association of environmental and other exposures with health outcomes. Environ. Int. 2018, 121, 1027–1031. [Google Scholar] [CrossRef]
- Henderson, S.B.; Johnston, F.H. Measures of forest fire smoke exposure and their associations with respiratory health outcomes. Curr. Opin. Allergy Clin. Immunol. 2012, 12, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.; McKenzie, J.E.; Sowden, A.; Katikireddi, S.V.; Brennan, S.E.; Ellis, S.; Hartmann-Boyce, J.; Ryan, R.; Shepperd, S.; Thomas, J.; et al. Synthesis without meta-analysis (SWiM) in systematic reviews: Reporting guideline. BMJ 2020, 368, 1–6. [Google Scholar] [CrossRef] [Green Version]
- NTP (National Toxicology Program). Handbook for Conducting a Literature-Based Health Assessment Using OHAT Approach for Systemic Review and Evidence Integration; NTP: Research Triangle Park, NC, USA, 2015; pp. 1–98. [Google Scholar]
- National Toxicology Program. OHAT Risk of Bias Rating Tool for Human and Animal Studies Organization of This Document Indirectness, Timing, and Other Factors Related to Risk of Bias. Available online: https://ntp.niehs.nih.gov/ntp/ohat/pubs/riskofbiastool_508.pdf (accessed on 14 August 2020).
- Eick, S.M.; Goin, D.E.; Chartres, N.; Lam, J.; Woodruff, T.J. Assessing risk of bias in human environmental epidemiology studies using three tools: Different conclusions from different tools. Syst. Rev. 2020, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Morgan, R.L.; Beverly, B.; Ghersi, D.; Schunemann, H.J.; Rooney, A.A.; Whaley, P.; Zhu, Y.-G.; Thayer, K.A. GRADE guidelines for environmental and occupational health: A new series of articles in Environment International. Environ. Int. 2019, 128, 11–12. [Google Scholar] [CrossRef] [PubMed]
- Mott, J.A.; Mannino, D.M.; Alverson, C.J.; Kiyu, A.; Hashim, J.; Lee, T.; Falter, K. Cardiorespiratory hospitalizations associated with smoke exposure during the 1997 Southeast Asian forest fires. Int. J. Hyg. Environ. Health 2005, 208, 75–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delfino, R.J.; Brummel, S.; Wu, J.; Stern, H.; Ostro, B.; Lipsettt, M.; Winer, A.; Street, D.H.; Zhang, L.; Tjoa, T.; et al. The relationship of respiratory and cardiovascular hospital admissions ot the southern California wildfires of 2003. Occup. Environ. Med. 2009, 66, 189–197. [Google Scholar] [CrossRef] [Green Version]
- Gan, R.W.; Ford, B.; Lassman, W.; Gabriele, P.; Vaidyanathan, A.; Fischer, E.; Volckens, J.; Pierce, J.R.; Magzamen, S. Comparison of wildfire smoke estimation methods and associations with cardiopulmonary-related hospital admissions. GeoHealth 2017, 1, 122–136. [Google Scholar] [CrossRef]
- Hanigan, I.C.; Johnston, F.H.; Morgan, G.G. Vegetation fire smoke, indigenous status and cardio-respiratory hospital admissions in Darwin, Australia, 1996–2005: A time-series study. Environ. Health Glob. Access Sci. Source 2008, 7, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Hutchinson, J.A.; Vargo, J.; Milet, M.; French, N.H.F.; Billmire, M.; Johnson, J.; Hoshiko, S. The San Diego 2007 wildfires and Medi-Cal emergency department presentations, inpatient hospitalizations, and outpatient visits: An observational study of smoke exposure periods and a bidirectional case-crossover analysis. PLoS Med. 2018, 15, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Johnston, F.H.; Purdie, S.; Jalaludin, B.; Martin, K.L.; Henderson, S.B.; Morgan, G.G. Air pollution events from forest fires and emergency department attendances in Sydney, Australia 1996–2007: A case-crossover analysis. Environ. Health Glob. Access Sci. Source 2014, 13, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Lee, T.S.; Falter, K.; Meyer, P.; Mott, J.; Gwynn, C. Risk factors associated with clinic visits during the 1999 forest fires near the Hoopa Valley Indian Reservation, California, USA. Int. J. Environ. Health Res. 2009, 19, 315–327. [Google Scholar] [CrossRef]
- Leibel, S.; Nguyen, M.; Brick, W.; Parker, J.; Ilango, S.; Aguilera, R.; Gershunov, A.; Benmarhnia, T. Increase in pediatric respiratory visits associated with Santa ana wind–driven wildfire smoke and PM2.5 levels in San Diego County. Ann. Am. Thorac. Soc. 2020, 17, 313–320. [Google Scholar] [CrossRef]
- Pratt, J.R.; Gan, R.W.; Ford, B.; Brey, S.; Pierce, J.R.; Fischer, E.V.; Magzamen, S. A national burden assessment of estimated pediatric asthma emergency department visits that may be attributed to elevated ozone levels associated with the presence of smoke. Environ. Monit. Assess. 2019, 191, 269. [Google Scholar] [CrossRef]
- Reid, C.E.; Jerrett, M.; Tager, I.B.; Petersen, M.L.; Mann, J.K.; Balmes, J.R. Differential respiratory health effects from the 2008 northern California wildfires: A spatiotemporal approach. Environ. Res. 2016, 150, 227–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Resnick, A.; Woods, B.; Krapfl, H.; Toth, B. Health outcomes associated with smoke exposure in Albuquerque, New Mexico, during the 2011 Wallow fire. J. Public Health Manag. Pract. 2015, 21, S55–S61. [Google Scholar] [CrossRef] [PubMed]
- Stowell, J.D.; Geng, G.; Saikawa, E.; Chang, H.H.; Fu, J.; Yang, C.E.; Zhu, Q.; Liu, Y.; Strickland, M.J. Associations of wildfire smoke PM2.5 exposure with cardiorespiratory events in Colorado 2011–2014. Environ. Int. 2019, 133, 105151. [Google Scholar] [CrossRef] [PubMed]
- Tham, R.; Erbas, B.; Akram, M.; Dennekamp, M.; Abramson, M.J. The impact of smoke on respiratory hospital outcomes during the 2002-2003 bushfire season, Victoria, Australia. Respirology 2009, 14, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Tinling, M.A.; West, J.J.; Cascio, W.E.; Kilaru, V.; Rappold, A.G. Repeating cardiopulmonary health effects in rural North Carolina population during a second large peat wildfire. Environ. Health Glob. Access Sci. Source 2016, 15, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Johnston, F.H.; Webby, R.J.; Pilotto, L.S.; Bailie, R.S.; Parry, D.L.; Halpin, S.J. Vegetation fires, particulate air pollution and asthma: A panel study in the Australian monsoon tropics. Int. J. Environ. Health Res. 2006, 16, 391–404. [Google Scholar] [CrossRef] [PubMed]
- Bowman, D.M.J.S.; Johnston, F.H. Wildfire smoke, fire management, and human health. Ecohealth 2005, 2, 76–80. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions; Version 6; John Wiley & Sons: Hoboken, NJ, USA, 2021. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).