Abstract
Over the years, anthropogenic sources have increasingly affected food quality. One of the most sensitive and nutritional matrices affected by chemical contamination is honey, due to the use of acaricides. Recently, the attention has moved to the presence of phthalates (PAEs) and bisphenol A (BP-A), molecules present in plastic materials used both in the production phase and in the conservation of honey. In this study, an analytical method for the simultaneous determination of PAEs (dimethyl phthalate DMP, diethyl phthalate DEP, diisobutyl phthalate DiBP, dibutyl phthalate DBP, bis(2-ethylhexyl) phthalate DEHP, and di-n-octyl-phthalate DnOP) and BP-A was developed. The extraction technique is the ultrasound-vortex-assisted dispersive liquid–liquid microextraction (UVA-DLLME), using 150 µL of toluene as an extraction solvent, followed by the gas chromatography coupled with ion trap mass spectrometry analysis (GC–IT/MS). The developed method is sensitive, reliable, and reproducible: it shows high correlation coefficients (R > 0.999); limits of detection (LODs) less than 11 ng·g−1; limits of quantification (LOQs) less than 16 ng·g−1; repeatability below 3.6%, except BP-A (11.6%); and accuracy below 4.8%, except BP-A (17.6%). The method was applied to 47 nectar honey samples for evidencing similarities among them. The chemometric approach based on Hierarchical Cluster Analysis and Principal Component Analysis evidenced some similitudes about sample origin as well as marked differences between PAE and BP-A sources.
1. Introduction
Honey, always considered “The Food of the Gods”, is a natural sweet substance that bees (Apis mellifera) produce from the nectar of plants which they forge, transform, combine with their own specific substances, deposit, dehydrate, store, and let mature in the honeycombs of the hive [1]. Honey has multiple properties, the first of which is certainly nutritional. It is very important in children’s and athletes’ diets due to the absence of potentially harmful artificial sugars. There are also beneficial properties, especially for the respiratory tract (decongestant and calming of coughs) and gastroenteric apparatus [2] as well as antioxidant properties due to the presence of enzymatic antioxidants [3].
In the beekeeping sector, we have witnessed in recent years the invasion of the market with non-EU produced honey not suitable for consumption in the EU, because they come from hives treated with pesticides that have been prohibited in the EU for years now. In this regard, European legislation allows the placing on the market of honey intended for human consumption which “as far as possible must be free of organic and inorganic substances extraneous to its composition” [1]. In fact, the honey produced by Apis mellifera is subject to possible contaminated residues used for the fight against parasites such as Varroa distructor [4,5,6,7]. Both through the pollen and the nectar foraged from flowers, as well as the water used, bees collect daily the residues of contaminants present in the environment and themselves carry out a first concentration step. Often, precisely for these reasons, the bee products and, even if to a lesser extent, the honey, are considered indicators of environmental contamination [8,9,10,11]. Among the most important food contaminants, phthalates (PAEs) and bisphenol A (BP-A) play a really important role (Table 1).

Table 1.
Chemical and legislation data of the compounds investigated in this study.
Phthalates, ubiquitous substances, are esters of phthalic acid, essentially used in the production of plastics to which they give flexibility and resilience [12,13]. The presence of these molecules in plastic polymers (e.g., polyvinyl chloride, PVC) facilitates the flow of monomers, making the material soft and moldable even at room temperature. The possible alarm is because PAEs are not chemically linked to the plastic polymer and, due to thermal and mechanical stress, can also be released in polar matrices such as water [13]. PAEs can come into contact with the human body (by ingestion, inhalation, or contact), generating a health risk. Foods can be contaminated during growth, production, processing, or packaging; fat matrix foods are most at risk. The effects of these molecules on human health have been controversial for years. In particular, low acute toxicity but a worrying chronic toxicity on rats has been highlighted which has led to the development of liver cancer and teratogenicity. However, this symptomatology was not subsequently found in the experimentation carried out on non-human primates [14]. Alterations of the hormonal system with contrasting effects (endocrine disruptors) have been highlighted both on rats and on primates [15,16,17,18]. Finally, it should be considered that plasticizers such as phthalates are largely involved in packaging process [19,20] and have been found in different food matrices [21,22,23,24]. The other very interesting compound is BP-A, an organic synthetic compound, poorly soluble in water (0.344% at 83 °C), used for the production of epoxy resins and polycarbonates for food packaging; it can also be present in water bottles [25,26,27,28,29] as well as in the internal linings of cans. Based on scientific studies, it can be said that BP-A can pose a risk to the health of the consumer, above all due to its cytotoxicity [30,31]. Containers unfortunately, the knowledge of the toxicokinetics is still incomplete and interventions are being considered to eliminate this compound from the human body [32]. A recent study verified the relative concentration of BP-A in three body fluids: blood, urine, and sweat. The BP-A concentration was higher in sweat than in urine and blood; BP-A was found in sweat even when it was not present in the other two body fluids. This implies that induced sweating can be an intervention to facilitate the elimination of this compound and also that the analysis of sweat can be placed as an indicator of contamination [33]. The consumption of canned food by consumers found a 1600% increase in the concentration of BP-A in the urine compared to glass containers. After taking these drinks, blood pressure was recorded again, which recorded a significant and systematic increase in values; the contamination of the human body by BP-A causes an increase in blood pressure [34]. A study, through cross-analysis between the presence of BP-A in the urine and the percentage of fat mass, found that contamination with BP-A is responsible for a higher incidence of obesity and an abnormal girth-height ratio in children [35].
In the absence of legal limits, specific migration limits (SMLs) are applicable to food contact materials containing food additives or polymer production aids that have the potential of leaching into food. It is the maximum amount of substance permissible per kg of food. SMLs have been set up by the Commission Regulation (EU) in regulations No 10/2011 for some PAEs [36] and No 213/2918 for BP-A [37] (Table 1).
The possible presence of PAEs and BP-A in this matrix is not only related to (raw) honey, but also to the remaining production processes which may involve direct contact with unsuitable plastic. In addition, in recent years, the use of plastic honeycombs has spread to reduce the risk of melting the wax itself, with consequent loss of the crop, during the hottest summer seasons. Furthermore, even if marginal, single-dose, it should be considered that plastic packages may be left under the sun for long periods. Ultimately, the critical point is certainly the type and quality of plastic used.
From an analytical point of view, the determination of PAEs and BP-A content is not trivial: the papers regarding their analysis are increasing in these last decade. Specifically, just one paper deals with the determination of PAEs/BP-A in honey samples [22], whereas different papers deal with the analysis of such compounds in human fluids. Recently, three interesting papers were published in this field. Babu-Rajendran et al. [38] investigated the PAE levels in human urine using a GC–MS analysis: limits of quantification (LOQs) range between 0.8-3.9 ng mL−1, recoveries between 99% and 104%, and precision below 7.8 (calculated as relative standard deviation, RSD). Pinguet et al. [39] analyzed 22 metabolites of bis(2-ethylhexyl) phthalate (DEHP) in urine by means of turbulent flow online extraction technology coupled with high-performance liquid chromatography–tandem mass spectrometry (HPLC–MS/MS): the analytical validation allowed LOQs to reach 0.01 to 0.1 ng mL−1, accuracy between 86% and 117% and interday and intraday precisions < 20%. Finally, Wang et al. studied a multiresidue method for determining 36 endocrine disrupting chemicals, including 8 bisphenols and 14 phthalates, in human serum [40]. They compared Liquid-Liquid Extraction (LLE) and Solid Phase Extraction (SPE) followed by ultraperformance liquid chromatography coupled to tandem mass spectrometry (UPLC–MS/MS): they obtained recoveries ranging between 45.8% and 120% with LOQs between 0.002 and 0.532 ng mL−1 and intraday (0.1%–12.7%) and interday (0.2%–13.3%) calculated as RSD.
The aim of this paper is to develop a fast, sensitive, and reproducible method for the analytical determination of phthalates and bisphenol A in honey matrix. The determination of these molecules shows considerable difficulties mainly related to their low concentration, which is difficult to determine even for very sensitive instruments. To overcome this problem, it is necessary to develop analytical methods that provide for extraction and preconcentration steps. In this paper, dispersive liquid–liquid microextraction (DLLME), slightly modified, is used as the extraction technique, followed by gas chromatography combined with ion trap mass spectrometry (GC–IT/MS) as the analytical procedure. The method developed has been validated, all the analytical parameters influencing the extraction efficiency of the analytes are studied and discussed. Forty-seven nectar honey samples, sampled in the Central Italy, are investigated for determining the concentration of plastic residues. A chemometric approach is applied for identifying the clusters and the possible unique source of the considered compounds.
2. Materials and Methods
2.1. Chemicals and Reagents
Standards of PAEs investigated in this study, such as dimethyl phthalate (DMP; C10H10O4), diethyl phthalate (DEP; C12H14O4), diisobutyl phthalate (DiBP; C16H22O4), dibutyl phthalate (DBP; C16H22O4), bis(2-ethylhexyl) phthalate (DEHP; C24H38O4), di-n-octyl-phthalate (DnOP); C24H34O4), and bisphenol A (BPA); C15H16O2), were obtained from Sigma-Aldrich (Milan, Italy). In Table 1, analytical data (i.e., Chemical Abstracts Service (CAS) number, molecular weight (MW), selected ion monitoring (SIM), octanol–water partition coefficient (Kow)) are reported. n-Hexane, n-heptane, iso-octane, ethyl acetate, and toluene were of pesticide grade (Carlo Erba, Milan, Italy), whereas sodium chloride (Carlo Erba) was of analytical reagent grade. A standard solution of phenanthrene 10 µg mL−1 (C14H10; LabService Analytica, Anzola Emilia, Bologna, Italy) was added as internal standard (I.S.) to each sample before being processed.
Solutions of each phthalate and bisphenol A were prepared at concentrations of 1 mg mL−1. Further, PAE and BP-A mix solutions at different concentrations (0.005, 0.01, 0.1, 0.25, 0.5, 1.0, 5.0 µg mL−1) were prepared by dilution. The solutions were stored in vials at −20 °C.
For avoiding cross-contamination due to reagents, materials, and laboratory equipment, a severe cleaning procedure was performed: the glassware was soaked and washed in acetone, dried at 140 °C for at least 4 h; NaCl was heated for 4 h at 140 °C and kept in a tightly sealed glass vial. For the PAE standard solutions (0.1 mg mL−1 of each PAE), absolute ethanol was used.
2.2. Ultrasound-Vortex-Assisted Dispersive Liquid–Liquid Microextraction Procedure
The study of this analytical approach (i.e., the ultrasound-vortex-assisted dispersive liquid–liquid microextraction (UVA-DLLME) procedure) can be divided into several phases. The choice of the best extraction solvent among different solvents, such as n-hexane (density 0.66 g cm−3), n-heptane (0.68 g cm−3), iso-octane (0.69 g cm−3), and toluene (0.867 g cm−3), is the first step. All the solvents tested had a lower density than water. Taking into account the absence of the dispersive solvent, it was necessary to use other methods to achieve efficient emulsification. Among the different possibilities, ultrasound-vortex-assisted was tested: 5 min of vortex and 6 min in the ultrasonic bath (100 W power) allowed us to create a stable and homogeneous emulsion. Second, 10 g L−1 of NaCl addition was necessary to break the emulsion.
Following this scheme, 2.5 g of honey and 2 µL of phenanthrene were brought to 10 g of aqueous solution, pH 4. Then 150 µL of toluene was added, identified as the best extraction solvent. Subsequently, the sample was subjected to 5 min stirring and ultrasounds for 6 min and NaCl 10 g L−1 was added. The solution was centrifuged for 30 min at 4000 rpm to break the emulsion, then 1 μL was injected into the GC–IT/MS instrument. All experimental conditions were applied to study the analytical parameters of the PAE and BP-A extraction.
2.3. GC–IT/MS Analysis
A TraceGC gas chromatograph (GC) coupled with an ion trap mass spectrometry (IT/MS) PolarisQ (ThermoFischer, Milan, Italy) was used for the analysis. The data acquisition and process were performed by specific software (Xcalibur, version 1.4.1, ThermoFischer). A fused-silica capillary column (SE-54, 5% phenyl–95% dimethylpolysiloxane, 30 m×0.25 mm×0.25 μm; Teknokroma, Rome, Italy) was used. Helium 5.5 was used as carrier gas at flow rate of 1.0 mL min−1. Inside the ion trap, helium as dumping gas was flowed at a rate of 0.3 mL min−1.
A programmable temperature vaporization (PTV) injector in splitless mode was used: 10 s after the injection, the PTV was heated from 100 °C to 330 °C at 800 °C min−1; the splitter valve was opened after 150 s. The oven temperature program was 100 °C for 1 min, increased up to 330 °C at a rate of 10 °C min−1 and kept at this temperature for 3 min.
The acquisition was made in full scan in a range of atomic mass units between m/z 75 and 400 a.m.u. Selected ion monitoring (SIM) mode was used for the analysis of the different phthalate compounds (Table 1). All the samples were determined in triplicate.
3. Results
Among the different variants of the DLLME technique developed recently [41,42,43,44,45], the authors optimized the ultrasound-vortex-assisted dispersion liquid–liquid microextraction (UVA-DLLME). In fact, already in other studies carried out by this research group [46,47,48,49], the authors have focused their attention on the elimination of the dispersive solvent in favor of reducing the solvents used. To create an optimal analytes extraction, the vortex was used for 5 min first and then the ultrasonic bath for 6 min. By means of the reduction of the interfacial tension, this coupling helps to determine the microdispersion of the extractant in the aqueous solution obtaining an optimal size of the microdrops with consequent high extraction efficiency. The use of vortex helps the extraction solvent in the initial dispersion within the aqueous sample. Subsequently, the ultrasounds supply adequate energy for obtaining a microdispersive phase in order to achieve quantitative extraction of the molecules. The methodological evaluation was performed on solutions containing the real matrix, adding 10 g L−1 of NaCl to standardize the ionic strength of all the samples and to favor a better breaking of the emulsion. The quantification of the analytes for the optimization of the method was carried out by percentage comparison with a standard solution.
First, the PAE/BP-A recoveries by each organic solvent were investigated in order to determine the solvent that had the highest percentage of recovery of the analytes present in the solution. As for the extraction solvent, a volume as low as possible to reach good preconcentration factors should have been preferred, but too low volumes generate practical problems in recovery. At the same time, too high volumes decrease the system performance due to dilution problems. Among the different solvents studied (such as n-heptane, n-hexane, iso-octane, ethyl acetate, toluene, and toluene:ethyl acetate 1:1), toluene has allowed to obtain the best recoveries (Table 2).

Table 2.
Recoveries (% ± standard deviation) related to the effect of different solvents on nectar honey samples spiked with a mixed PAE/BP-A standard solution. The conditions were as follows: each PAE/BP-A at 50 ng mL−1 and 150 μL of extraction solvent.
Operative parameters for vortexing and ultrasounds were established on the basis of previous studies carried out in this laboratory [26,50]. To better optimize the PAE/BP-A recoveries, experiments were carried out with the selected solvent (toluene) by varying the pH of the aqueous solution containing the analytes. Figure 1 shows the different PAE/BP-A recoveries at different pH values; as it can be seen, the maximum extraction efficiency was at pH 4.
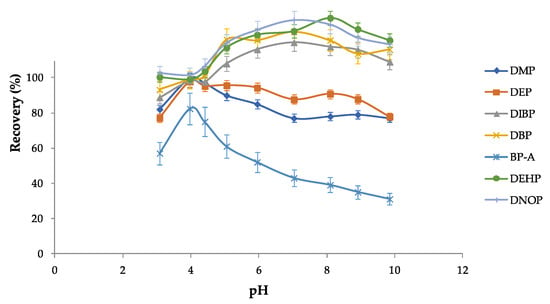
Figure 1.
Effect of the pH on the recoveries of PAEs/BP-A investigated in this study. The conditions were as follows: each PAE/BP-A at 50 ng mL−1, 150 μL of toluene, 10 g L−1 of NaCl, and 6 min of ultrasounds.
For determining the repeatability and the accuracy of the developed analytical method, the intraday and interday errors were studied, respectively. Table 3 shows the results obtained: intraday errors ranged between 2.2 and 11.6 whereas the interday errors ranged between 2.6 and 17.6. Further, the limit of detection (LOD) and the limit of quantification (LOQ) of each compound were investigated. In particular, LOD and LOQ are defined as the quantities of analyte that produce a signal equal to three and seven times the standard deviation of the gross blank signal, respectively [51]. Table 3 shows the limits for investigating PAEs and BP-A in honey matrix using the analytical conditions developed: LODs ranged between 2 and 6 ng·g−1 for the 7 PAEs and 11 ng·g−1 for BP-A, whereas LOQs ranged between 5–11 ng·g−1 for the 7 PAEs and 16 ng·g−1 for BP-A. It should be underlined that LODs and LOQs were directly determined on the honey matrix. These limits are sufficient for determining PAEs and BP-A in such matrices according to the SMLs reported in the regulations. Finally, the correlation coefficients were above 0.998 in the range 20–2000 ng·g−1. Eight calibration points were considered at 20, 50, 75, 100, 200, 500, 1000 and 2000 ng·g−1, directly on the honey matrix.

Table 3.
Retention time (tr, expressed as min), intraday and interday precision calculated as relative standard deviation (RSD) %, limit of detection (LOD) (ng·g−1), limit of quantification (LOQ) (ng g−1), and recovery (%) in blank and honey samples of each PAE and BP-A investigated in this study.
As some plastic can migrate from food contact materials, the EU Commission has defined the presence and the levels of small amounts of additives in food up to a food is still assumed safe for the human intake. Particularly, according to the EU Commission No. 10/2011 and 213/2018, the safety limit, defined by each SML in food, for DMP, DEP, DiBP, and DnOP is 60 mg·kg−1, whereas they are 0.3, 0.05, and 1.5 mg·kg−1 for DBP, BP-A, and DEHP, respectively. The limit of 60 mg·kg−1 deserves a consideration: this high value means that the additive is permitted to be used in the polymer production for food packaging and there are no restrictions provided. The LOQs are lower than the SML provided by the EU Commission: this means that the method investigated is sensitive enough to analyze the threshold limits of the different compounds in the collected honey samples.
Figure 2 shows the chromatograms both of the standard solution (a) and of the nectar honey sample (b). The peaks are well-solved; the PAE/BP-A determinations are precise and accurate.
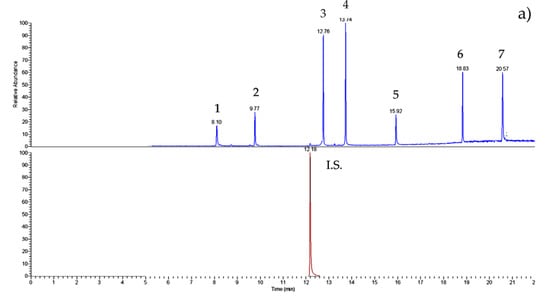
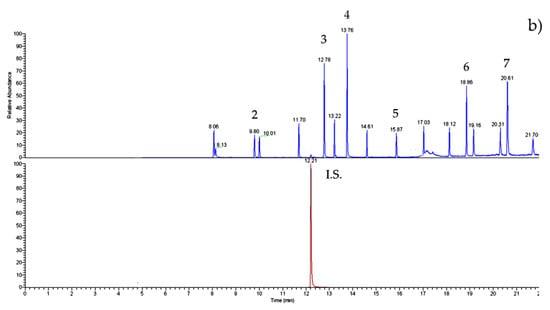
Figure 2.
GC–IT/MS chromatograms of (a) PAE/BP-A (50 ng mL−1 of each) standard solution and (b) nectar honey sample (#27). For experimental conditions, see text. Peak list: 1—DMP; 2—DEP; internal standard (I.S.)—phenanthrene; 3—DiBP; 4—DBP; 5—BP-A; 6—DEHP; 7—DnOP.
It should be pointed out that no clean-up step was necessary: this occurrence was just evidenced in our previous paper on the honey matrix for determining acaricides [5] and in this case it is another confirmation.
Using the analytical protocol developed (briefly resuming: 10 mL of water solution at pH 4 containing 2.5 g of nectar honey sample and I.S., addition of 150 μL toluene as extraction solvent, vortex for 5 min, ultrasounds for 6 min, addition of 10 g L−1 of NaCl, centrifugation for 30 min at 4000 rpm, 1 μL injection into GC–IT/MS, see Figure 3; in Figure S1 the MS spectrum of each PAE and BP-A investigated in this study is reported), 47 samples of nectar honey collected in Central Italy were analyzed. The samples were collected in four different places. Table 4 reports the measurement (ng g−1) performed in all the samples.
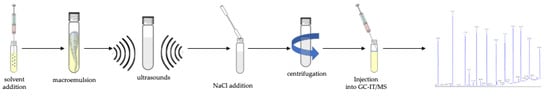
Figure 3.
Workflow from sample preparation to data analysis of the entire analytical procedure.

As it can been seen, the DMP level was below the relative LOQ in all the samples, except in sample # 36 (12 ng g−1) whereas BP-A, DEHP, and DnOP showed interesting levels in some samples. Interesting levels were detected for samples #22, #33, and #35. Sample #44 showed relevant levels of BP-A and DEHP, while #45 showed relevant levels of DEHP and DnOP. Finally, the very high Pearson’s correlation coefficient, 0.880, between DEHP and DnOP, the most detected compounds, should be noted. From a toxicological point of view, these levels were always below the lethal dose (LD50) for each element, reported in Table 1, as well as below the relative SMLs, except for DBP and BP-A in some samples (#32 for DBP and #21, #32, #34 and #43 for BP-A). It should be underlined that the samples #32 and #34 showed the highest DBP and BP-A concentration levels of all the samples analyzed: the reason could be due to the presence of a large industrial area in the proximity of both beehives. Over the SMLs, it should be also taken into account the octanol–water partition ratio (Kow) of each compound: it is the most common way of expressing the lipophilicity of a compound, and it is defined as the ratio of the concentration of a solute in a water-saturated octanolic phase to its concentration in an octanol-saturated aqueous phase [52]. A high octanol–water partition ratio therefore indicates a highly lipophilic substance, whereas one with a very low coefficient (<< 1) indicates a highly hydrophilic substance. The Kow values can be directly used for the evaluation of the potential bioaccumulation: according to the screening assessment for organic compounds with a Kow < 4.5, it is considered that the affinity for the lipid layer of an organism is such that the substance is not considered bioaccumulative. In this case, DMP, DEP, DiBP, DBP, and BP-A showed coefficients below 4.5 whereas DEHP and DnOP coefficients were above 4.5 (7.27 and 8.10, respectively). Luckily, the LD50 of DEHP and the SML of DnOP are very high (10,000 mg kg−1 and 60 mg kg−1, respectively). These considerations mean that the PAE presence in the 47 samples (and the relative concentration levels) should not be of concern from a health point of view.
For a deeper knowledge about the correlations among the different samples, a chemometric approach was tested based on the Hierarchical Cluster Analysis (HAC) and the Principal Component Analysis (PCA) for evidencing eventual similarities. The method was applied to overall the data by the use of the Tanagra open-source software [53], by means of the centroid merge method and the Euclidean distance as a proximity measure [54,55,56]. First, the HAC evidenced the presence of four different clusters: two formed by one sample (cluster 1 by sample #32 and cluster 2 by sample #34), one by two samples (cluster 3 by samples #27 and #43), and the fourth by 43 samples (i.e., 91.5% of overall samples). Figure 4 shows the dendrogram and the relative similarities among samples.
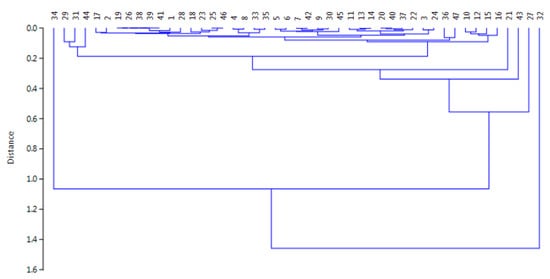
Figure 4.
Dendrogram showing the similarities among the samples investigated in this study.
Starting from this consideration, the authors applied the PCA to the data. First, three parameters (i.e., BP-A, DEHP, and DnOP) could be considered sufficient to describe 96% of the overall data. Figure 5 shows the factor analysis applied to overall the samples. For the case under investigation, the authors determined Factor 1 (F1) and Factor 2 (F2):
F1 = 0.047 DEP + 0.228 DiBP + 0.203 DBP - 0.559 BP-A + 1.735 DEHP + 1.607 DnOP
F2 = 0.812 DEP − 0.474 DiBP + 1.376 DBP + 1.118 BP-A − 0.891 DEHP + 1.086 DnOP
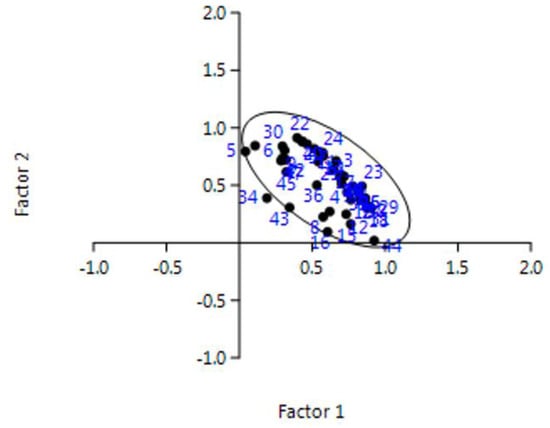
Figure 5.
Factor analysis applied to overall data. For the F1 and F2 meaning, see the text.
As it can be seen in the figure, all the samples cluster in one group, including samples #5, #16 and #43, except only the sample #34 which is out of the cluster (sample #32 is in as well as sample #27). This analysis confirms that the samples come from the same area (Central Italy), characterized by some differences which however do not affect the similarities among samples yet. Finally, Figure 6 shows how PAEs and BP-A were related between them: the six phthalates appear as a unique cluster, whereas BP-A is quite different.
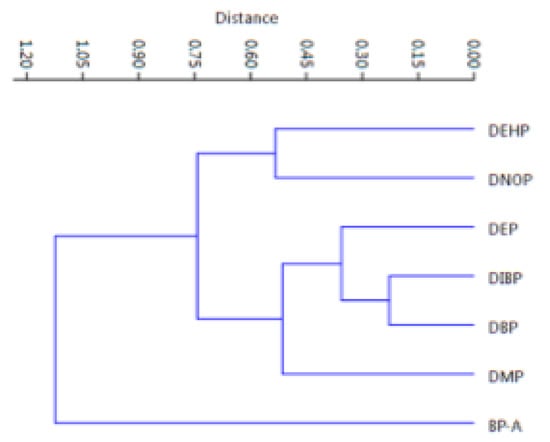
Figure 6.
Hierarchical Cluster Analysis for evidencing the correlations among the six PAEs and the BP-A.
This confirms the difference between these two classes of compounds both in origin and in their different widespread usage: PAEs, esters of phthalic anhydride, are mainly used as plasticizers, whereas BP-A, belonging to the group of diphenylmethane derivatives and bisphenols, is a plastic precursor, primarily polycarbonates and epoxy resins. In 2015, the global volume of BP-A consumption was estimated at 7.7 million tons, making it one of the highest volumes of chemicals produced worldwide. It is forecast to be 10.6 million tons in 2022, at an annual growth rate of almost 5% [57].
4. Conclusions
An increasing amount of plastic is ending up on our plates. Consumers do not notice it because they are very small particles, between 1 nm and 5 mm, called microplastics or nanoplastics. Their effects on human health are now not quantifiable. They derive from waste and, through various paths, they enter the food chain and go as far as food. Recent studies [58] have shown the wide scope of the phenomenon. It is wrong to think that fish is the only contaminated food. In 19 honey samples, taken in Germany, France, Italy, Spain, and Mexico, microplastics were found [59] and the sources are still unknown. This paper evidences plastic residues in nectar honey samples measuring different compounds characterizing such material, i.e. phthalates and bisphenol A. Although their presence and performance in plastics are well-studied, their determination is not a trivial issue: the authors developed a rapid and accurate method based on UVA-DLLME and GC–IT/MS for analyzing 7 among them (i.e., DMP, DEP, DiBP, DBP, BP-A, DEHP, DnOP). It should be underlined that the proposed method does not require any clean-up procedure as evidenced in a previous paper on the honey matrix. The entire protocol, validated for all the analytical parameters, was applied to the determination of such residues in 47 nectar honeys sampled: a chemometric approach allowed us to demonstrate both how all the samples clustered in a single group, except one sample, and how the PAEs came from the same source whereas BP-A showed a different provenance.
Supplementary Materials
The following are available online at https://www.mdpi.com/1660-4601/17/5/1692/s1, Figure S1: Mass spectra of (a) DMP, (b) DEP, (c) DiBP, (d) DBP, (e) DEHP, (f) DnOP, and (g) BP-A.
Author Contributions
Conceptualization, P.A. and M.V.R.; methodology, I.N. and P.A.; software, S.P. and G.I.; validation, I.N., S.P., and G.I.; formal analysis, C.D.F. and G.I.; investigation, S.P.; data curation, C.D.F. and G.I.; writing—original draft preparation, P.A.; writing—review and editing, P.A.; visualization, M.V.R.; supervision, M.V.R.; project administration, I.N.; funding acquisition, P.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors wish to thank Ettore Guerriero for the useful information on the honey samples.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Legislative Decree. Implementation of the Directive 2001/110/CE regarding the production and the marketing of honey. Gazz. Uff. It. 2004, 168, 29–34. [Google Scholar]
- Abdulrhman, M.A.; Mekawy, M.A.; Awadalla, M.M.; Mohamed, A.H. Bee honey added to the oral rehydration solution in treatment of gastroenteritis in infants and children. J. Med. Food 2010, 13, 605–609. [Google Scholar] [CrossRef]
- Montenegro, G.; Mejias, E. Biological applications of honeys produced by Apis mellifera. Biol. Res. 2013, 46, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Calderone, N.W.; Lin, S. Rapid determination of the numbers of Varroa destructor, a parasitic mite of the honey bee, Apis mellifera, on sticky-board collection devices. Apidologie 2003, 34, 11–17. [Google Scholar] [CrossRef]
- Notardonato, I.; Avino, P.; Cinelli, G.; Russo, M.V. Trace determination of acaricides in honey samples using XAD-2 adsorbent and gas chromatography coupled with an ion trap mass spectrometer detector. RSC Adv. 2014, 4, 42424–42431. [Google Scholar] [CrossRef]
- Ruffinengo, S.R.; Maggi, M.D.; Marcangeli, J.A.; Eguaras, M.J.; Principal, J.; Barrios, C.; De Piano, F.; Giullia, M. Integrated pest management to control Varroa destructor and its implications to Apis mellifera colonies. Zootec. Trop. 2014, 32, 149–168. [Google Scholar]
- Notardonato, I.; Avino, P.; Cinelli, G.; Russo, M.V. Rapid and reliable method for analyzing acaricides in honey-based products. Food Anal. Methods 2016, 9, 1675–1685. [Google Scholar] [CrossRef]
- Corredera, L.; Bayarri, S.; Pérez-Arquillué, C.; Lázaro, R.; Molino, F.; Herrera, A. Evaluation of heavy metals and polycyclic aromatic hydrocarbons in honeys from different origins. J. Food Prot. 2014, 77, 504–509. [Google Scholar] [CrossRef]
- de Andrade, C.K.; dos Anjos, V.E.; Felsner, M.L.; Torres, Y.R.; Quináia, S.P. Relationship between geographical origin and contents of Pb, Cd, and Cr in honey samples from the state of Paraná (Brazil) with chemometric approach. Environ. Sci. Pollut. Res. 2014, 21, 12372–12381. [Google Scholar] [CrossRef]
- Russo, M.V.; Avino, P.; Notardonato, I. PAH residues in honey by ultrasound-vortex-assisted liquid-liquid micro-extraction followed by GC-FID/IT-MS. Food Anal. Methods 2017, 10, 2132–2142. [Google Scholar] [CrossRef]
- Goretti, E.; Pallottini, M.; Rossi, R.; La Porta, G.; Gardi, T.; Cenci Goga, B.T.; Elia, A.C.; Galletti, M.; Moroni, B.; Petroselli, C.; et al. Heavy metal bioaccumulation in honey bee matrix, an indicator to assess the contamination level in terrestrial environments. Environ. Pollut. 2020, 256, 113388. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.V.; Notardonato, I.; Cinelli, G.; Avino, P. Determination of phthalate esters at trace levels in light alcoholic drinks and soft drinks by XAD-2 adsorbent and gas chromatography coupled with ion trap-mass spectrometry detection. Anal. Methods 2014, 6, 7030–7037. [Google Scholar] [CrossRef]
- Russo, M.V.; Avino, P.; Perugini, L.; Notardonato, I. Extraction and GC-MS analysis of phthalate esters in food matrices: A review. RSC Adv. 2015, 5, 37023–37043. [Google Scholar] [CrossRef]
- Barrett, J.R. Attention worthy: Prenatal phthalate exposure and subsequent ADHD diagnosis. Environ. Health Perspect. 2019, 127, 34001. [Google Scholar] [CrossRef]
- Shoaff, J.R.; Calafat, A.M.; Schantz, S.L.; Korrick, S.A. Endocrine disrupting chemical exposure and maladaptive behavior during adolescence. Environ. Res. 2019, 172, 231–241. [Google Scholar] [CrossRef]
- Huang, H.B.; Kuo, P.H.; Su, P.H.; Sun, C.W.; Chen, W.J.; Wang, S. Prenatal and childhood exposure to phthalate diesters and neurobehavioral development in a 15-year follow-up birth cohort study. Environ. Res. 2019, 172, 569–577. [Google Scholar] [CrossRef]
- Ran, D.; Luo, Y.; Gan, Z.; Liu, J.; Yang, J. Neural mechanisms underlying the deficit of learning and memory by exposure to di(2-ethylhexyl) phthalate in rats. Ecotoxicol. Environ. Saf. 2019, 174, 58–65. [Google Scholar] [CrossRef]
- Shen, C.; Wei, J.; Wang, T.; Wang, Y. Acute toxicity and responses of antioxidant systems to dibutyl phthalate in neonate and adult Daphnia magna. PeerJ 2019, 7, e6584. [Google Scholar] [CrossRef]
- Vera, P.; Aznar, M.; Mercea, P.; Nerín, C. Study of hotmelt adhesives used in food packaging multilayer laminates. Evaluation of the main factors affecting migration to food. J. Mater. Chem. 2011, 21, 420–431. [Google Scholar] [CrossRef]
- Aznar, M.; Vera, P.; Canellas, E.; Nerín, C.; Mercea, P.; Störmer, A.J. Composition of the adhesives used in food packaging multilayer materials and migration studies from packaging to food. Mater. Chem. 2011, 21, 4358–4370. [Google Scholar] [CrossRef]
- Di Bella, G.; Saitta, M.; La Pera, L.; Alfa, M.; Dugo, G. Pesticide and plasticizer residues in bergamot essential oils from Calabria (Italy). Chemosphere 2004, 56, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Lo Turco, V.; Di Bella, G.; Potortì, A.G.; Tropea, A.; Casale, E.K.; Fede, M.R.; Dugo, G. Determination of plasticisers and BPA in Sicilian and Calabrian nectar honeys by selected ion monitoring GC/MS. Food Addit. Contam. A 2016, 33, 1693–1699. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.V.; Avino, P.; Notardonato, I. Fast analysis of phthalates in freeze-dried baby foods by ultrasound-vortex-assisted liquid-liquid microextraction coupled with gas chromatography-ion trap/mass spectrometry. J. Chromatogr. A 2016, 1474, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Notardonato, I.; Protano, C.; Vitali, M.; Avino, P. Phthalates and bisphenol A determination and release from different beverage plastic containers by dispersive liquid-liquid microextraction and GC-IT/MS analysis. Food Anal. Methods 2019, 12, 2562–2571. [Google Scholar] [CrossRef]
- Santhi, V.A.; Sakai, N.; Ahmad, E.D.; Mustafa, A.M. Occurrence of bisphenol A in surface water, drinking water and plasma from Malaysia with exposure assessment from consumption of drinking water. Sci. Total Environ. 2012, 427, 332–338. [Google Scholar] [CrossRef]
- Zhang, Z.; Hu, Y.; Guo, J.; Yu, T.; Sun, L.; Xiao, X.; Zhu, D.; Nakanishi, T.; Hiromori, Y.; Li, J.; et al. Fluorene-9-bisphenol is anti-oestrogenic and may cause adverse pregnancy outcomes in mice. Nat. Commun. 2017, 8, 14585. [Google Scholar] [CrossRef]
- Notardonato, I.; Russo, M.V.; Avino, P. Phthalates and bisphenol-A residues in water samples: An innovative analytical approach. Rend. Licei. 2018, 29, 831–840. [Google Scholar] [CrossRef]
- Notardonato, I.; Protano, C.; Vitali, M.; Bhattacharya, B.; Avino, P. A method validation for simultaneous determination of phthalates and bisphenol A released from plastic water containers. Appl. Sci. 2019, 9, 2945. [Google Scholar] [CrossRef]
- Adebola, A.A.; Babalola, B.A. Bisphenol-A (BPA) in Foods commonly consumed in Southwest Nigeria and its human health risk. Sci. Rep. 2019, 9, 17458. [Google Scholar]
- Wang, H.X.; Zhou, Y.; Tang, C.X.; Wu, J.G.; Chen, Y.; Jiang, Q.W. Association between bisphenol A exposure and body mass index in Chinese school children: A cross-sectional study. Environ. Health 2012, 11, 79. [Google Scholar] [CrossRef]
- Shen, Y.; Zheng, Y.; Jiang, J.; Liu, Y.; Luo, X.; Shen, Z.; Chen, X.; Wang, Y.; Dai, Y.; Zhao, J.; et al. Higher urinary bisphenol A concentration is associated with unexplained recurrent miscarriage risk: Evidence from a case-control study in eastern China. PLoS ONE 2015, 10, e012788. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.; Rider, C.V.; Hwang, G.S.; Hoy, H.; LeBlanc, G.A. Covert signal disruption: Anti-ecdysteroidal activity of bisphenol A involves cross talk between signaling pathways. Environ. Toxicol. Chem. 2005, 24, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Genuis, S.J.; Beesoon, S.; Birkholz, D.; Lobo, R.A. Human excretion of bisphenol A: Blood, urine, and sweat (BUS) study. J. Environ. Public Health 2012. [Google Scholar] [CrossRef]
- Bae, S.; Hong, Y.C. Exposure to bisphenol A from drinking canned beverages increases blood pressure: Randomized crossover trial. Hypertension 2015, 65, 313–319. [Google Scholar] [CrossRef]
- Eng, D.S.; Lee, J.M.; Gebremariam, A.; Meeker, J.D.; Peterson, K.; Padmanabhan, V. Bisphenol A and chronic disease risk factors in US children. Pediatrics 2013, 132, e637–e645. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EU) No 10/2011 of 14 January 2011 on plastic materials and articles intended to come into contact with food. Off. J. Eur. Union 2011, 12, 1–89. [Google Scholar]
- European Commission. Commission Regulation (EU) 2018/213 of 12 February 2018 on the use of bisphenol A in varnishes and coatings intended to come into contact with food and amending Regulation (EU) No 10/2011 as regards the use of that substance in plastic food contact materials. Off. J. Eur. Union 2018, 41, 6–12. [Google Scholar]
- Babu-Rajendran, R.; Preethi, G.; Poopal, R.K.; Nikhil, N.P.; Vimalkumar, K.; Subramanian, A.; Krishna-Kumar, S. GC-MS determination of phthalate esters in human urine: A potential biomarker for phthalate bio-monitoring. J. Chromatogr. B 2018, 1079, 15–24. [Google Scholar] [CrossRef]
- Pinguet, J.; Kerckhove, N.; Eljezi, T.; Lambert, C.; Moreau, E.; Bernard, L.; Boeuf, B.; Decaudin, B.; Genay, S.; Masse, M.; et al. New SPE-LC-MS/MS method for the simultaneous determination in urine of 22 metabolites of DEHP and alternative plasticizers from PVC medical devices. Talanta 2019, 198, 377–389. [Google Scholar] [CrossRef]
- Wang, Y.; Li, G.; Zhu, Q.; Liao, C. A multi-residue method for determination of 36 endocrine disrupting chemicals in human serum with a simple extraction procedure in combination of UPLC-MS/MS analysis. Talanta 2019, 205, 120144. [Google Scholar] [CrossRef]
- Mousavi, L.; Tamiji, Z.; Khoshayand, M.R. Applications and opportunities of experimental design for the dispersive liquid-liquid microextraction method—A review. Talanta 2018, 190, 335–356. [Google Scholar] [CrossRef] [PubMed]
- Rutkowska, M.; Płotka-Wasylka, J.; Sajid, M.; Andruch, V. Liquid-phase microextraction: A review of reviews. Microchem. J. 2019, 149, 103989. [Google Scholar] [CrossRef]
- Li, G.; Row, K.H. Utilization of deep eutectic solvents in dispersive liquid-liquid micro-extraction. TrAC-Trend. Anal. Chem. 2019, 120, 115651. [Google Scholar] [CrossRef]
- Kalschne, D.L.; Canan, C.; Beato, M.O.; Leite, O.D.; Moraes Flores, E.L. A new and feasible analytical method using reversed-phase dispersive liquid-liquid microextraction (RP-DLLME) for further determination of Nickel in hydrogenated vegetable fat. Talanta 2020, 208, 120409. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Xing, X.; Du, Z. Application of dispersive liquid-liquid microextraction based on solidification of floating organic drop for the determination of extractables from pharmaceutical packaging materials. Talanta 2020, 209, 120540. [Google Scholar] [CrossRef] [PubMed]
- Cinelli, G.; Avino, P.; Notardonato, I.; Centola, A.; Russo, M.V. Rapid analysis of six phthalate esters in wine by ultrasound-vortex-assisted dispersive liquid-liquid micro-extraction coupled with gas chromatography-flame ionization detector or gas chromatography-ion trap mass spectrometry. Anal. Chim. Acta 2013, 769, 72–78. [Google Scholar] [CrossRef]
- Russo, M.V.; Notardonato, I.; Avino, P.; Cinelli, G. Fast determination of phthalate ester residues in soft drinks and light alcoholic beverages by ultrasound/vortex assisted dispersive liquid-liquid microextraction followed by gas chromatography-ion trap mass spectrometry. RSC Adv. 2014, 4, 59655–59663. [Google Scholar] [CrossRef]
- Cinelli, G.; Avino, P.; Notardonato, I.; Russo, M.V. Ultrasound-vortex-assisted dispersive liquid-liquid microextraction coupled with gas chromatography with a nitrogen-phosphorus detector for simultaneous and rapid determination of organophosphorus pesticides and triazines in wine. Anal. Methods 2014, 6, 782–790. [Google Scholar] [CrossRef]
- Cinelli, G.; Avino, P.; Notardonato, I.; Centola, A.; Russo, M.V. Study of XAD-2 adsorbent for the enrichment of trace levels of phthalate esters in hydroalcoholic food beverages and analysis by gas chromatography coupled with flame ionization and ion-trap mass spectrometry detectors. Food Chem. 2014, 146, 181–187. [Google Scholar] [CrossRef]
- Notardonato, I.; Salimei, E.; Russo, M.V.; Avino, P. Simultaneous determination of organophosphorus pesticides and phthalates in baby food simples by ultrasound-vortex-assisted liquid-liquid microextraction and GC–IT/MS. Anal. Bioanal. Chem. 2018, 410, 3285–3296. [Google Scholar] [CrossRef]
- Knoll, J.E. Estimation of the limit of detection in chromatography. J. Chromatogr. Sci. 1985, 23, 422–425. [Google Scholar] [CrossRef]
- Amézqueta, S.; Subirats, X.; Fuguet, E.; Rosés, M.; Ràfols, C. Octanol-water partition constant. In Liquid-Phase Extraction; Poole, C.F., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 183–208. [Google Scholar]
- Tanagra. Available online: http://eric.univ-lyon2.fr/~ricco/tanagra/en/tanagra.html (accessed on 25 January 2020).
- Escofier, B.; Pagès, J. Analyses Factorielles Multiples; Dunod: Paris, France, 1988. [Google Scholar]
- Hohnson, R.A.; Wichern, D.W. Applied Multivariate Statistical Analysis; Prentice-Hill: Upper Saddle River, NJ, USA, 2002. [Google Scholar]
- Avino, P.; Capannesi, G.; Renzi, L.; Rosada, A. Instrumental neutron activation analysis and statistical approach for determining baseline values of essential and toxic elements in hairs of high school students. Ecotoxicol. Environ. Saf. 2013, 92, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Almeida, S.; Raposo, A.; Almeida-González, M.; Carrascosa, C. Bisphenol A: Food exposure and impact on human health. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1503–1517. [Google Scholar] [CrossRef]
- Lusher, A.L.; McHugh, M.; Thompson, R.C. Occurrence of microplastics in the gastrointestinal tract of pelagic and demersal fish from the English Channel. Mar. Pollut. Bull. 2013, 67, 94–99. [Google Scholar] [CrossRef]
- Liebezeit, G.; Liebezeit, E. Non-pollen particulates in honey and sugar. Food Addit. Contam. A 2013, 30, 2136–2140. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).