Abstract
Background: Neurological dysfunction due to stroke affects not only the extremities and trunk muscles but also the respiratory muscles. Aim: to synthesise the evidence available about the effectiveness of respiratory muscle training (RMT) to improve respiratory function parameters and functional capacity in poststroke patients. Methods: a systematic electronic search was performed in the MEDLINE, EMBASE, SPORTDiscus, PEDro and Web of Science databases, from inception to May 2020. Study selection and data extraction: randomised controlled trials (RCTs) that examined the effects of RMT versus non-RMT or sham RMT in poststroke patients. We extracted data about respiratory function, respiratory muscle strength and functional capacity (walking ability, dyspnea, balance, activities of daily life), characteristics of studies and features of RMT interventions (a type of RMT exercise, frequency, intensity and duration). Two reviewers performed study selection and data extraction independently. Results: nineteen RCTs met the study criteria. RMT improved the first second forced expiratory volume (FEV1), forced vital capacity (FVC), peak expiratory flow (PEF), maximal expiratory pressure (MEP), maximal inspiratory pressure (MIP) and walking ability (6 min walking test), but not Barthel index, Berg balance scale, and dyspnea. Conclusions: RMT interventions are effective to improve respiratory function and walking ability in poststroke patients.
1. Introduction
Stroke is a loss of focal neurological function due to infarction or haemorrhage in an essential part of the brain [1]. This neurological damage affects not only peripheral but also respiratory muscles, causing respiratory weakness, changes in the respiratory pattern and decreases in respiratory volumes and flows [2,3]. These breathing alterations are related to a decrease in physical activity and therefore, to a reduction in the ability to carry out the activities of daily life [4].
Respiratory muscles respond to training similarly to any other skeletal muscle, so just as the upper and lower limb muscles are trained in stroke patients, the respiratory muscles must be trained. In this regard, respiratory muscle training (RMT) consists of repetitive breathing exercises with hand-held respiratory trainer devices to provide pressure threshold or flow-dependent resistance against inhalation (inspiratory muscle training (IMT)) and/or exhalation (expiratory muscle training (EMT)) [5,6] to stimulate this musculature and to produce changes in the muscles’ structure.
Previous systematic reviews and meta-analyses have synthesised the available evidence about the effectiveness of RMT exercises on improving respiratory function [3,7,8,9,10], even exercise tolerance in poststroke patients; however, some weaknesses of these reviews were that they did not only include randomised controlled trials (RCTs) [10], and that they included a limited number of studies [3,8]. Moreover, since the last systematic review and meta-analysis [7], many studies aiming at analysing the effect of RMT on respiratory function and functional capacity have been published.
For these reasons, this systematic review and meta-analysis aimed to synthetize the most novel evidence about the effectiveness of RMT to improve respiratory function, respiratory muscle strength and functional capacity in poststroke patients.
2. Materials and Methods
This meta-analysis was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [11] and following the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions [12]. Additionally, this systematic review and meta-analysis was registered through PROSPERO (awaiting register number; ID: 182082).
2.1. Search Strategy
Two researchers (DPP-C and AIC-C) independently searched MEDLINE (via Pubmed), EMBASE (via Scopus), SPORTDiscus, PEDro and Web of Science databases from inception to May 2020; disagreements were solved by consensus or involving a third researcher (JAL-A). The search strategy used was as follow: (“inspiratory muscle training” OR “IMT” OR “expiratory muscle training” OR “respiratory therapy” OR “chest physiotherapy” OR “respiratory exercise” OR “breathing exercises” AND (“stroke” OR “acute stroke” OR “cerebral stroke”) AND (“pulmonary function” OR “respiratory function” OR “exercise tolerance” OR “walking ability” OR “gait ability”) AND (random* control* trials). The literature search was complemented by scanning the reference list of the included articles, and the list of references of previous systematic reviews was reviewed for additional relevant studies.
2.2. Study Selection
Reviewers were not blinded to authors, journals, or institutions. Included articles were RCTs studies that analysed the effectiveness of RMT (IMT and/or EMT) on improving pulmonary function, respiratory muscle strength and functional capacity parameters in patients with stroke.
The inclusion criteria were: (1) Patients: adults with stroke (haemorrhagic or ischaemic); (2) Intervention: IMT, EMT or both; (3) Control: no respiratory training or sham respiratory muscle training (without any resistance); (4) Outcomes: variables of pulmonary function such as the first second forced expiratory volume (FEV1), forced vital capacity (FVC), and peak expiratory flow (PEF); parameters of respiratory muscle weakness: maximal expiratory pressure (MEP) and maximal inspiratory pressure (MIP); Functional capacity including 6 min walking test (6-MWT), Barthel index (MBI), Berg balance scale (BBS), and dyspnea (Borg scale); (5) type of studies: RCTs.
Exclusion criteria were: (1) studies not written in English or Spanish; (2) studies not reporting the outcome variables; (3) non-RCTs; (4) insufficient data.
2.3. Data Extraction
For data extraction, two authors (DPP-C and AIC-C), independently, used a standardised data collection form. From each selected study, the following data were collected: (1) first author’s name and year of publication; (2) country; (3) period of data collection; (4) characteristics of study sample: sample size, age mean, gender; (5) data concerning inclusion/exclusion criteria; (6) main study outcomes. We emailed the corresponding author requesting the data when some information was lacking.
2.4. Risk of Bias Assessment
While in the Prospero register we stated that the intent to use the Cochrane Collaboration tool to assess the risk of bias (RoB2) [13], we observed that all previous systematic reviews and meta-analysis on this topic had used the PEDro Scale to assess the quality of included studies. Thus, with the aim of increase the homogeneity and the comparability, we decided to use this scale instead of RoB2.
PEDro Scale is a useful tool for assessing the quality of physical therapy trials [14]. This scale has 11 items designed for rating the methodological quality of RCTs assessing the internal validity (e.g., random and concealed allocation, baseline similarity, blinding, and intention-to-treat analysis) and statistical reporting. The total PEDro score ranges from 0 to 10 points (the first item, eligibility criteria, is not included in the total score); higher score means better study’s quality [15].
Data extraction and risk of bias assessment were independently completed by two reviewers (DPP-C and AIC-C) if appeared inconsistencies were solved by consensus or involving a third researcher (JAL-A).
2.5. Statistical Analysis
For each of the main outcomes (FEV1, MIP, MEP, PEF, FVC, 6-MWT, dyspnea, MBI and BBS), a separate pooled estimate of effect size (ES) and their respective 95% confidence intervals (95% CI) was calculated. The ES parameters from preintervention to postintervention between groups (exercise intervention vs control) [16] in each study were calculated assuming a correlation coefficient of 0.5 and using a random-effects model based on the DerSimonian and Laird method [17]. Statistical heterogeneity was analysed by the I2 statistic. Heterogeneity was considered as not important (I2: 0% to 40%), moderate (I2: 30% to 60%), substantial (I2: 50% to 90%) or considerable (I2: 75% to 100%); the corresponding p-values were considered [12]. When a study reported results separately for IMT and EMT, we calculated a combined estimate.
According to the Cochrane Handbook recommendations, when mean and standard deviation (SD) were lacking, and available statistics were median and interquartile range (IQR), the IQR was divided by 1.35 to transform these estimates on CI [18]. Finally, when studies were scaled inversely (i.e., lower values indicated worse outcomes), the mean in each group was multiplied by −1.
Subgroup analyses were conducted when it was possible due to the number of studies, to assess whether the intervention of RMT (IMT/EMT) or only IMT allowed better results on outcome variables. Sensitivity analyses were performed by removing studies one by one to assess the robustness of the summary estimates and to detect whether any study accounted for a large proportion of heterogeneity among RMT and ES pooled estimations. Meaningful results of the sensitivity analyses were considered when the resulting estimates were modified beyond the CIs of the original summary estimate.
Finally, Egger regression asymmetry method and visual inspection of funnel plots were used to assess publication bias. Statistical analyses were performed using Stata Statistical software, version 16.0 (Stata, College Station, TX, USA).
3. Results
The electronic search retrieved 826 studies, and we identified two new studies through manual search. After removing duplicated studies, the title and abstract of 800 studies were revised. Of these, 754 were excluded by irrelevancy and 46 were full-text revised. Finally, nineteen studies aimed to assess the effectiveness of RMT on pulmonary function and functional capacity variables in patients with stroke were included in the meta-analysis [19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37]. Figure 1 shows the flow of study selection. The reasons for the full-text studies excluded are summarised in Supplementary File S1.
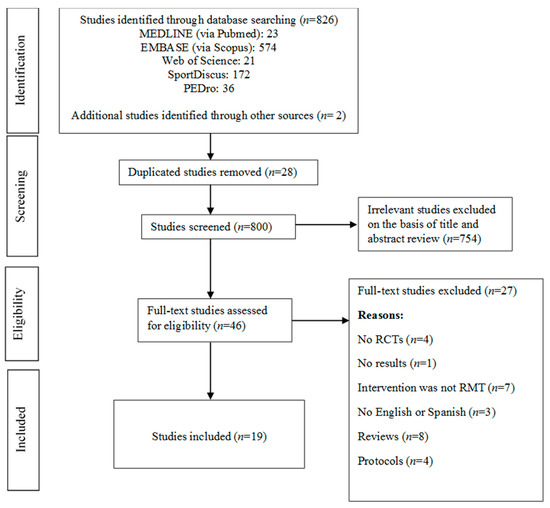
Figure 1.
Literature search: Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) consort diagram. RMT, Respiratory muscle training; RCTs, randomized controlled trials.
Table 1 depicts the characteristics of the included studies and the participants. Studies were conducted in Korea [20,22,23,24,25,26,27,33,34,36], Turkey, Spain [28,30], Brazil [21,35], Germany [32], United Kingdom [31] and Taiwan [19,29].

Table 1.
Characteristics of studies included.
The nineteen studies included involved 643 patients with stroke, with the sample size varying between 12 and 82 participants. The mean age of participants was 61.3 years.
All included studies performed RMT; nevertheless, ten studies carried out IMT and EMT [19,20,21,23,28,30,31,32,33,36], and nine studies only performed IMT [22,24,25,26,27,29,34,35,37]. Regarding the threshold devices used in the RMT interventions, they were very varied: Respironics, Respifit-S, Orygen Dual Valve, SpiroTiger, Threshold, PowerBreath, tri-ball incentive spirometer and Dofin Breathing (Table 1).
The interventions were very diverse; the number of sets per session varied between two and ten sets; the repetitions in each set ranged between five and 30. Other studies listed time rather than the number of repetitions; the load of RMT in the majority of studies was at 30% of MIP/MEP at the beginning, increasing with the intervention. However, some studies used higher loads at 40% or 50% at the beginning of the intervention.
The length of the interventions ranged between three and ten weeks. In the control group, most studies conducted the conventional stroke rehabilitation program, however, four out of twenty studies performed sham respiratory training without resistance and/or progression [21,30,31,35].
3.1. Risk of Bias Assessment
The risk of bias of included studies was assessed using the PEDro Scale. All RCTs included complied with the following items: random allocation, to report between-group differences and in to report point estimate and variability (Table 2). The highest score was eight points for two studies [21,30]. Four studies scored seven [19,31,35,37], mainly due to the non-blinding of therapist and assessors and the non-presentation of an intention-to-treat analysis. Five studies scored six and four studies obtained five points. The lowest score was three points for the study of the NJ Jung et al. [25]. The three resting studies obtained four points [24,33,34]. Thus, fifteen studies obtained higher or equal to five points, a moderate quality.

Table 2.
Risk of bias and study quality on the PEDro Scale.
3.2. Effect of Intervention: Pooled Estimates
3.2.1. Pulmonary Function
Among nineteen studies included, eleven displayed results about FEV1 [19,20,23,25,27,29,32,33,34,36,37]. When all RMT interventions (IMT/EMT and IMT) were jointly analysed, the summary ES was 0.57 (95% CI: 0.24 to 0.90; I2 = 42.9%, p = 0.064) (Figure 2). The subgroup analysis by type of intervention showed a slightly greater effect in the pooled of the studies that performed only IMT [ES= 0.89 (95% CI: 0.19 to 1.58; I2 = 66.3%, p = 0.018)] compared with the IMT/EMT subgroup [ES= 0.38 (95% CI: 0.06 to 0.69; I2 = 0.0%, p = 0.650)].
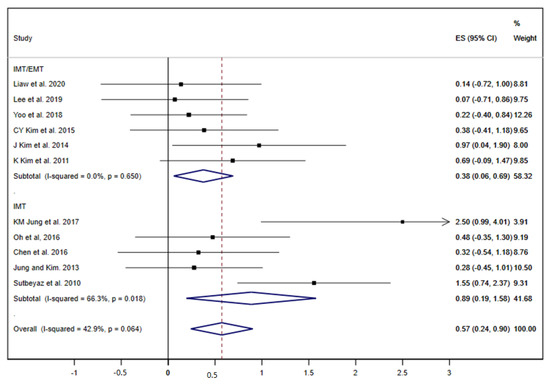
Figure 2.
Forest plot showing the effect size (ES) of respiratory muscle training (RMT) on first second forced expiratory volume (FEV1) between intervention and control groups for each study. IMT, inspiratory muscle training; EMT, expiratory muscle training.
The FVC was analysed in nine studies [19,23,25,27,29,32,34,36,37]. The pooled ES was 0.32 (95% CI: 0.06 to 0.58; I2 = 0.0%, p = 0.507) (Figure 3). The subgroup analysis by type of RMT exercises did not show great differences between the ES of both groups, although without statistical significance in any group (IMT and IMT/EMT).
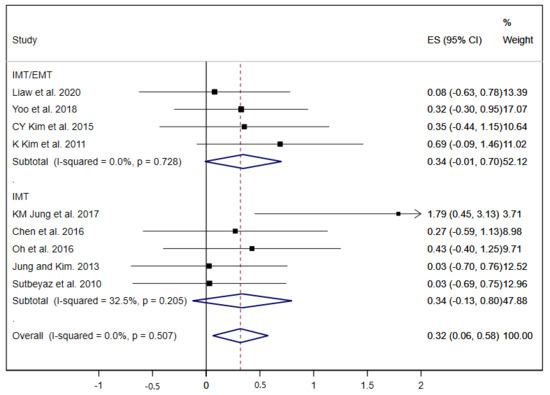
Figure 3.
Forest plot showing the effect size (ES) of respiratory muscle training (RMT) on forced vital capacity (FVC) between intervention and control groups for each study. IMT, inspiratory muscle training; EMT, expiratory muscle training.
Other pulmonary function parameters such as PEF and the strength of inspiratory and expiratory muscles (MIP and MEP) were analysed in the studies included. Seven studies showed results about PEF and the pooled estimated ES was 0.48 (95% CI: 0.20 to 0.77; I2 = 0.0%, p = 0.823) (Figure 4) [20,23,27,33,34,36,37], nevertheless, the subgroup analysis displayed a statistically significant ES in the IMT/EMT group (ES = 0.55; 95% CI: 0.17 to 0.92) but not in the IMT group (ES = 0.40; 95% CI: −0.04 to 0.84).
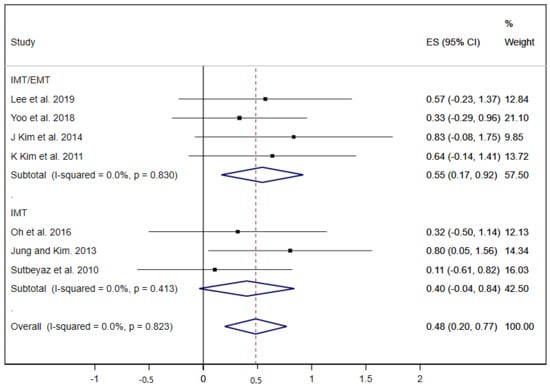
Figure 4.
Forest plot showing the effect size (ES) of respiratory muscle training (RMT) on peak expiratory flow (PEF) between intervention and control groups for each study. IMT, inspiratory muscle training; EMT, expiratory muscle training.
Equally, MEP was analysed in seven studies [19,20,21,28,30,31,37], and only one of these performed exclusively IMT exercises [37]; thus, the overall ES was 0.55 (95% CI: 0.12 to 0.98; I2 = 71.9%, p = 0.002) (Figure 5).
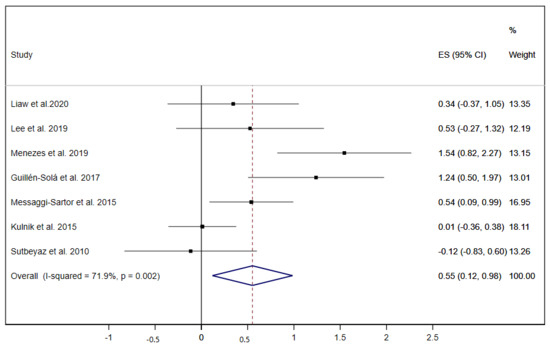
Figure 5.
Forest plot showing the effect size (ES) of respiratory muscle training (RMT) on maximal expiratory pressure (MEP) between intervention and control groups for each study. IMT, inspiratory muscle training; EMT, expiratory muscle training.
The MIP was examined in nine studies [19,20,21,22,28,30,31,35,37], and obtained a pooled ES= 0.84 (95% CI: 0.44 to 1.24; I2 = 69.2%, p = 0.001) (Figure 6); three of these studies performed only IMT exercises [22,35,37], and six studies carried out both IMT and EMT exercises, showing statistically significant improvements for both groups of interventions.
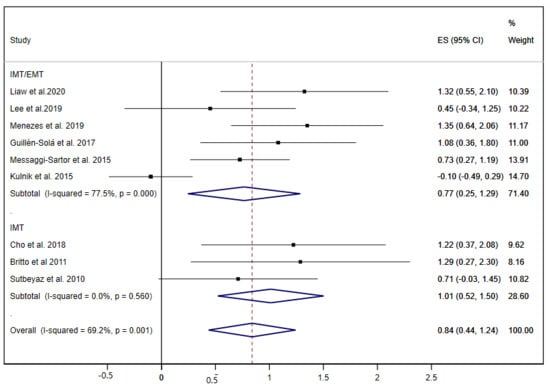
Figure 6.
Forest plot showing the effect size (ES) of respiratory muscle training (RMT) on maximal inspiratory pressure (MIP) between intervention and control groups for each study. IMT, inspiratory muscle training; EMT, expiratory muscle training.
Sensitivity analyses for all pulmonary function variables showed that the pooled ES estimates were not significantly modified in magnitude or direction when individual study data were removed from the analysis one at a time. The exception was the FVC analysis, which lost the statistical significance when the studies of KM Jung et al. [25] and K Kim et al. [36] were removed from the analysis.
There was no evidence of publication bias by funnel plot asymmetry and Egger’s test in all pulmonary function variables studied.
3.2.2. Functional Capacity
Six out of nineteen studies evaluated the 6-MWT [21,22,24,25,26,33]. Of these, two studies only performed IMT exercises [25,26]; for this, a subgroup analysis by type of RMT exercises was not performed. The overall ES for the 6-MWT was 0.39 (95% CI: 0.05 to 0.74; I2 = 0.0%, p = 0.919) (Figure 7). When the studies were removed one by one, the pooled ES analysis showed a loss of statistical significance when the study of NJ Jung et al. [26] was removed.
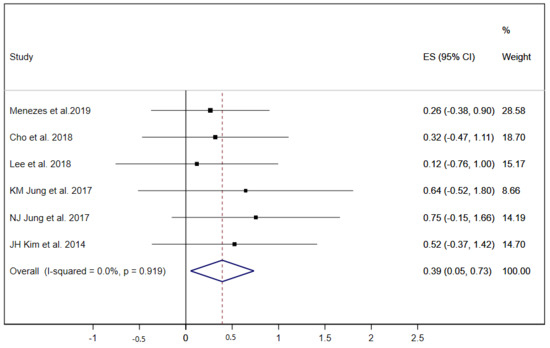
Figure 7.
Forest plot showing the effect size (ES) of respiratory muscle training (RMT) on 6 min walking test (6-MWT) between intervention and control groups for each study. IMT, inspiratory muscle training; EMT, expiratory muscle training.
Dyspnea was evaluated in five studies [19,21,29,33,37]; due to the limited number of studies, the subgroup analysis by type of RMT exercise was not performed. The pooled ES of RMT interventions on dyspnea was 0.47 (95% CI: −0.30 to 1.23; I2 = 77%, p = 0.002) (Figure 8), showing a positive trend of improvement which was not statistically significant.
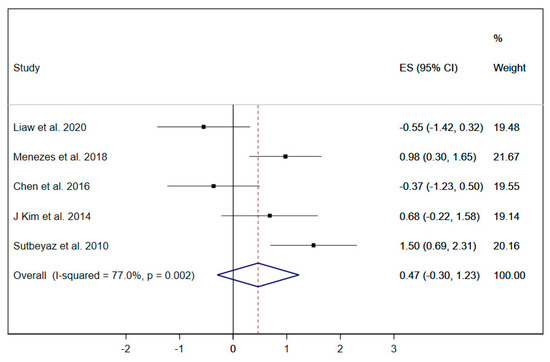
Figure 8.
Forest plot showing the effect size (ES) of respiratory muscle training (RMT) on dyspnea between intervention and control groups for each study. IMT, inspiratory muscle training; EMT, expiratory muscle training.
The MBI [19,23,29] and BBS [23,24,27] were only evaluated in three studies. In both, the RMT interventions did not show a statistically significant improvement (MBI: 0.22 (95% CI: −0.31 to 0.75; I2 = 37.6%, p = 0.201; and BBS: 0.06 (95% CI: −0.37 to 0.49; I2 = 0.0%, p = 0.953).
Publication bias was not assessed by funnel plot and Egger test, due to the reduced number of studies that evaluated these variables of functional capacity [38].
4. Discussion
In the present study, the results show a significant positive relationship between the RMT interventions on the improvement of pulmonary function parameters (FEV1, FVC, PEF, MEP, MIP) and the functional capacity such as walking ability (6-MWT) but not in balance (BBS), MBI and dyspnea, in patients with stroke.
The subgroup analyses by type of RMT exercises did not show differences between the exclusive IMT interventions and the IMT/EMT interventions, except in the PEF, in which the combined IMT and EMT exercises showed a greater effect than only IMT exercises on the improvements of this pulmonary parameter.
A previously published meta-analysis that included eleven trials also obtained similar results [7]. Nevertheless, the main difference was found in dyspnea. Contrary to this study, our meta-analysis did not find a positive effect of RMT on dyspnea in patients with stroke. These differences could be due to several reasons: first, the previous study only included two moderate methodological quality RCTs, a reduced number of studies to provide consistent conclusions about the effectiveness of RMT on dyspnea; second, patients with stroke usually have a low perception of dyspnea, due to their dissociation between respiratory effort and dyspnea [39].
Regarding the pulmonary function parameters, our results showed positive effectiveness of RMT interventions to improve FEV1, FVC, PEF, MEP, and MIP in patients with stroke. These results are in line with previous systematic reviews and meta-analyses results [7,8]. Patients who have suffered a stroke present abdominal and diaphragm dysfunction, causing a decrease of respiratory muscle strength [2,40,41]. This weakness of respiratory muscles is usually associated with reduced lung volumes, flows and restrictive ventilatory patterns [42]. Thus, it seems obvious, and the results of this meta-analysis corroborate this, that the training of the respiratory muscle will improve the lung volumes and flows, noting an increase of FEV1, FVC, PEF, MEP and MIP.
Aiming to facilitate the clinical interpretation of these results, we expressed the pooled effect estimate of each pulmonary function parameter on clinical measurement improvements, using methods recommended by the Cochrane Collaboration [43]. Thus, our data show that RMT interventions increase baseline FEV1 values in 12.2% of predicted-FEV1 by age and sex, FVC baseline values improve in 6.75% of predicted FVC, PEF baseline measure increases in 46.97 litres per second. Finally, MEP and MIP improved their baseline values in 10.05 and 22.40 cm H20, respectively. These results align with previously published meta-analysis [7,8,9,10].
The improvement of inspiratory and expiratory muscle strength had significant implications for the functional capacity of poststroke patients. The weakness of respiratory muscles, secondary to stroke, can lead to a reduced tolerance to exercise [8,44]. Thus, the benefits gained by the increase of respiratory muscle strength and lung function could improve the tolerance to exercise [8].
Our results showed that RMT achieved an improvement of walking ability measured by 6-MWT, with an ES= 0.39, which when translated in terms of clinical measure improvement, using methods endorsed by the Cochrane Collaboration [43], is equal to an increase of 25 m travelled in 6 min. Although it could be seen as little progress, walking ability is a significant predictor of physical activity and social and community participation after stroke [45,46], so even small improvements could imply immense benefits for the physical and social health of stroke patients. They can help these patients to carry out daily activities more efficiently.
In line with this, three studies reported results about the Barthel index, a scale used to measure disability or dependence in activities of daily living [47]. The pooled estimate showed a positive but not statistically significant trend. The same occurred with the BBS [48]. Nevertheless, the limited number of studies that reported these variables do not allow for robust conclusions about it.
Despite the wide variety of characteristics of RMT interventions, the previous meta-analysis concluded that 30 min of RMT, five times/week, for five weeks could be sufficient to increase respiratory muscle strength in very weak poststroke patients [3].
This study has several limitations that must be considered, some of them, such as the heterogeneity, are inherent to meta-analyses. First, although a higher number of studies than the previous meta-analysis was included, not all of them report the same variables, so some variables such as MBI or BBS only were informed in three studies. Second, it is known that patients with more significant respiratory muscle weakness usually respond better to RMT [8], and in the present work around the half studies [19,20,21,22,28,29,30,31,37] included poststroke patients with respiratory muscle weakness (MIP < 50 cm H2O) which could have influenced the results. Third, the inclusion/exclusion criteria were different in the studies included, equally to the RMT characteristics of interventions. Nevertheless, the subgroup analysis by type of RMT exercises allowed for the comparison of the effect of IMT alone with the IMT and EMT exercises. Fourth, despite the number of studies included, the total sample of participants included was scarce.
5. Conclusions
The RMT interventions are effective in improving pulmonary function parameters (FEV1, FVC, PEF), strength of expiratory and inspiratory muscles (MEP and MIP), and walking ability in poststroke patients. More well-designed RCTs with larger sample sizes are needed to examine the most appropriate features of interventions: IMT, EMT or both, duration, frequency, and intensity to establish the highest clinical efficacy.
Supplementary Materials
The following are available online at https://www.mdpi.com/1660-4601/17/15/5356/s1, File S1: Articles excluded and reasons.
Author Contributions
Conceptualization, D.P.P.-C. and A.I.C.-C.; methodology, D.P.P.-C. and A.I.C.-C.; software, D.P.P.-C.; validation, D.P.P.-C., J.A.L.-A., J.M.C.-T. and A.I.C.-C.; formal analysis, D.P.P.-C. and A.I.C.-C.; data curation, D.P.P.-C. and A.I.C.-C.; Writing—Original Draft preparation, D.P.P.-C., and A.I.C.-C.; Writing—Review and Editing, D.P.P.-C., J.M.C.-T., J.A.L.-A., P.Á.L.-R., J.A.P.-M. and A.I.C.-C.; visualization, D.P.P.-C., J.M.C.-T., J.A.L.-A., P.Á.L.-R., J.A.P.-M. and A.I.C.-C.; supervision, D.P.P.-C.; project administration, D.P.P.-C. All authors have read and agreed to the published version of the manuscript.
Funding
Diana P. Pozuelo Carrascosa is contracted by FEDER funds (2019/7375). This research was funded by a grant from the European Regional Development Fund (ERDF) [Fondo Europeo de Desarrollo Regional (FEDER) (DOCM 19/02/20)].
Conflicts of Interest
The authors have declared no conflict of interest. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Hankey, G.J. Stroke. Lancet 2017, 389, 641–654. [Google Scholar] [CrossRef]
- Jandt, S.R.; Caballero, R.M.D.S.; Junior, L.A.F.; Dias, A.S. Correlation between trunk control, respiratory muscle strength and spirometry in patients with stroke: An observational study. Physiother. Res. Int. 2010, 16, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Menezes, K.K.P.; Nascimento, L.R.; Ada, L.; Polese, J.C.; Avelino, P.R.; Teixeira-Salmela, L.F. Respiratory muscle training increases respiratory muscle strength and reduces respiratory complications after stroke: A systematic review. J. Physiother. 2016, 62, 138–144. [Google Scholar] [CrossRef]
- Polese, J.C.; Pinheiro, M.B.; Faria, C.D.C.M.; Britto, R.R.; Parreira, V.F.; Teixeira-Salmela, L.F. Strength of the respiratory and lower limb muscles and functional capacity in chronic stroke survivors with different physical activity levels. Braz. J. Phys. Ther. 2013, 17, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, E.; Fulgenzi, A.; Ferrero, M.E. Rationale of the combined use of inspiratory and expiratory devices in improving maximal inspiratory pressure and maximal expiratory pressure of patients with chronic obstructive pulmonary disease. Arch. Phys. Med. Rehabil. 2009, 90, 913–918. [Google Scholar] [CrossRef] [PubMed]
- Forbes, S.; Game, A.; Syrotuik, D.; Jones, R.; Bell, G.J. The effect of inspiratory and expiratory respiratory muscle training in rowers. Res. Sports Med. 2011, 19, 217–230. [Google Scholar] [CrossRef] [PubMed]
- De Menezes, K.K.P.; Nascimento, L.R.; Avelino, P.R.; Alvarenga, M.T.M.; Teixeira-Salmela, L.F. Efficacy of interventions to improve respiratory function after stroke. Respir. Care 2018, 63, 920–933. [Google Scholar] [CrossRef]
- Gomes-Neto, M.; Saquetto, M.B.; Silva, C.M.; Carvalho, V.O.; Ribeiro, N.; Conceição, C.S. Effects of respiratory muscle training on respiratory function, respiratory muscle strength and exercise tolerance in post-stroke patients: A systematic review with meta-analysis. Arch. Phys. Med. Rehabil. 2016, 97, 1994–2001. [Google Scholar] [CrossRef]
- Xiao, Y.; Luo, M.; Wang, J.; Luo, H. Inspiratory muscle training for the recovery of function after stroke (Cochrane review). Cochrane Database Syst. Rev. 2012, 5. [Google Scholar] [CrossRef]
- Martín-Valero, R.; Almeida, M.D.L.C.; Casuso-Holgado, M.J.; Heredia-Madrazo, A. Systematic Review of Inspiratory Muscle Training after Cerebrovascular Accident. Respir. Care 2015, 60, 1652–1659. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; John Wiley & Sons: Chichester, UK, 2019. [Google Scholar]
- Higgins, J.P.; Thomas, S.J.; Page, M.J.; Elbers, R.G.; Sterne, J.A.C. Chapter 8: Assessing risk of bias in a randomized trial. In Cochrane Handbook for Systematic Reviews of Interventions Version 60 (Updated July 2019): Cochrane; Higgins, J.P., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; John Wiley & Sons: Chichester, UK, 2019. [Google Scholar]
- Olivo, S.A.; Macedo, L.; Gadotti, I.C.; Fuentes, J.; Stanton, T.R.; Magee, D.J. Scales to Assess the Quality of Randomized Controlled Trials: A Systematic Review. Phys. Ther. 2008, 88, 156–175. [Google Scholar] [CrossRef] [PubMed]
- Maher, C.G.; Sherrington, C.; Herbert, R.D.; Moseley, A.M.; Elkins, M.R. Reliability of the PEDro Scale for Rating Quality of Randomized Controlled Trials. Phys. Ther. 2003, 83, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Morris, S.B. Estimating Effect Sizes From Pretest-Posttest-Control Group Designs. Organ. Res. Methods 2007, 11, 364–386. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Li, T.; Deeks, J.J. Chapter 6: Choosing effect measures and computing estimates of effect. In Cochrane Handbook for Systematic Reviews of Interventions Version 60 (Updated July 2019): Cochrane; Higgins, J.P., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; John Wiley & Sons: Chichester, UK, 2019. [Google Scholar]
- Liaw, M.Y.; Hsu, C.H.; Leong, C.P.; Liao, C.Y.; Wang, L.Y.; Lu, C.H.; Lin, M.C. Respiratory muscle training in stroke patients with respiratory muscle weakness, dysphagia, and dysarthria—A prospective randomized trial. Medicine (Baltimore) 2020, 99, e19337. [Google Scholar] [CrossRef]
- Lee, K.; Park, D.; Lee, G. Progressive Respiratory Muscle Training for Improving Trunk Stability in Chronic Stroke Survivors: A Pilot Randomized Controlled Trial. J. Stroke Cerebrovasc. Dis. 2019, 28, 1200–1211. [Google Scholar] [CrossRef]
- De Menezes, K.K.P.; Nascimento, L.R.; Ada, L.; Avelino, P.R.; Polese, J.C.; Alvarenga, M.T.M.; Barbosa, M.H.; Teixeira-Salmela, L.F. High-Intensity Respiratory Muscle Training Improves Strength and Dyspnea Poststroke: A Double-Blind Randomized Trial. Arch. Phys. Med. Rehabil. 2018, 100, 205–212. [Google Scholar] [CrossRef]
- Cho, J.E.; Lee, H.J.; Kim, M.K.; Lee, W.H. The improvement in respiratory function by inspiratory muscle training is due to structural muscle changes in patients with stroke: A randomized controlled pilot trial. Top. Stroke Rehabil. 2017, 25, 37–43. [Google Scholar] [CrossRef]
- Yoo, H.J.; Pyun, S.B. Efficacy of Bedside Respiratory Muscle Training in Patients with Stroke: A Randomized Controlled Trial. Am. J. Phys. Med. Rehabil. 2018, 97, 691–697. [Google Scholar] [CrossRef]
- Lee, H.J.; Kang, T.W.; Kim, B.R. Effects of diaphragm and deep abdominal muscle exercise on walking and balance ability in patients with hemiplegia due to stroke. J. Exerc. Rehabil. 2018, 14, 648–653. [Google Scholar] [CrossRef]
- Jung, K.M.; Bang, D.H. Effect of inspiratory muscle training on respiratory capacity and walking ability with subacute stroke patients: A randomized controlled pilot trial. J. Phys. Ther. Sci. 2017, 29, 336–339. [Google Scholar] [CrossRef] [PubMed]
- Jung, N.J.; Na, S.S.; Kim, S.K.; Hwangbo, G. The effect of the inspiratory muscle training on functional ability in stroke patients. J. Phys. Ther. Sci. 2017, 29, 1954–1956. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.; Kim, G.; Lee, W.; Shin, M.M.S. Effects of inspiratory muscle training on balance ability and abdominal muscle thickness in chronic stroke patients. J. Phys. Ther. Sci. 2016, 28, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Guillen-Sola, A.; Sartor, M.M.; Soler, N.B.; Duarte, E.; Barrera, M.C.; Marco, E. Respiratory muscle strength training and neuromuscular electrical stimulation in subacute dysphagic stroke patients: A randomized controlled trial. Clin. Rehabil. 2016, 31, 761–771. [Google Scholar] [CrossRef]
- Chen, P.C.; Liaw, M.Y.; Wang, L.Y.; Tsai, Y.C.; Hsin, Y.J.; Chen, Y.C. Inspiratory muscle training in stroke patients with congestive heart failure A CONSORT-compliant prospective randomized single-blind controlled trial. Medicine 2016, 95, e4856. [Google Scholar] [CrossRef]
- Messaggi-Sartor, M.; Guillen-Sola, A.; Depolo, M.; Duarte, E.; Rodríguez, D.A.; Barrera, M.-C.; Barreiro, E.; Escalada, F.; Orozco-Levi, M.; Marco, E. Inspiratory and expiratory muscle training in subacute stroke: A randomized clinical trial. Neurology 2015, 85, 564–572. [Google Scholar] [CrossRef]
- Kulnik, S.T.; Birring, S.S.; Moxham, J.; Rafferty, G.F.; Kalra, L. Does Respiratory Muscle Training Improve Cough Flow in Acute Stroke? Pilot Randomized Controlled Trial. Stroke 2015, 46, 447–453. [Google Scholar] [CrossRef]
- Kim, C.Y.; Lee, J.S.; Kim, H.D.; Kim, I.S. Effects of the combination of respiratory muscle training and abdominal drawing-in maneuver on respiratory muscle activity in patients with post-stroke hemiplegia: A pilot randomized controlled trial. Top. Stroke Rehabil. 2015, 22, 262–270. [Google Scholar] [CrossRef]
- Yim, J.; Kim, J.; Park, J. Effects of Respiratory Muscle and Endurance Training Using an Individualized Training Device on the Pulmonary Function and Exercise Capacity in Stroke Patients. Med. Sci. Monit. 2014, 20, 2543–2549. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.-H.; Kim, N.-S. Effects of Inspiratory Muscle Training on Diaphragm Thickness, Pulmonary Function, and Chest Expansion in Chronic Stroke Patients. J. Korean Soc. Phys. Med. 2013, 8, 59–69. [Google Scholar] [CrossRef]
- Britto, R.R.; Rezende, N.R.; Marinho, K.C.; Torres, J.L.; Parreira, V.F.; Teixeira-Salmela, L.F. Inspiratory Muscular Training in Chronic Stroke Survivors: A Randomized Controlled Trial. Arch. Phys. Med. Rehabil. 2011, 92, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Fell, D.W.; Lee, J.H. Feedback Respiratory Training to Enhance Chest Expansion and Pulmonary Function in Chronic Stroke: A Double-Blind, Randomized Controlled Study. J. Phys. Ther. Sci. 2011, 23, 75–79. [Google Scholar] [CrossRef]
- Sutbeyaz, S.T.; Koseoglu, F.; Inan, L.; Coskun, O. Respiratory muscle training improves cardiopulmonary function and exercise tolerance in subjects with subacute stroke: A randomized controlled trial. Clin. Rehabil. 2010, 24, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.; Sutton, A.J.; Ioannidis, J.P.A.; Terrin, N.; Jones, D.R.; Lau, J.; Carpenter, J.R.; Rücker, G.; Harbord, R.; Schmid, C.H.; et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011, 343, d4002. [Google Scholar] [CrossRef] [PubMed]
- Lanini, B.; Gigliotti, F.; Coli, C.; Bianchi, R.; Pizzi, A.; Romagnoli, I.; Grazzini, M.; Stendardi, L.; Scano, G. Dissociation between respiratory effort and dyspnoea in a subset of patients with stroke. Clin. Sci. 2002, 103, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Pollock, R.D.; Rafferty, G.F.; Moxham, J.; Kalra, L.; Rafferty, G.F. Respiratory Muscle Strength and Training in Stroke and Neurology: A Systematic Review. Int. J. Stroke 2012, 8, 124–130. [Google Scholar] [CrossRef]
- De Almeida, I.C.L.; Clementino, A.C.C.R.; Rocha, E.H.T.; Brandão, D.C.; De Andrade, A.D. Effects of hemiplegy on pulmonary function and diaphragmatic dome displacement. Respir. Physiol. Neurobiol. 2011, 178, 196–201. [Google Scholar] [CrossRef]
- Tomczak, C.R.; Jelani, A.; Haennel, R.G.; Haykowsky, M.; Welsh, R.; Manns, P. Cardiac Reserve and Pulmonary Gas Exchange Kinetics in Patients With Stroke. Stroke 2008, 39, 3102–3106. [Google Scholar] [CrossRef]
- Schünemann, H.J.; Vist, G.E.; Higgins, J.P.; Santesso, N.; Deeks, J.J.; Glasziou, P.; Akl, E.A.; Guyatt, G.H. Interpreting results and drawing conclusions. In Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Chichester, UK, 2019; pp. 403–431. [Google Scholar]
- Reyes, A.; Ziman, M.; Nosaka, K.; Nosaka, K. Respiratory Muscle Training for Respiratory Deficits in Neurodegenerative Disorders. Chest 2013, 143, 1386–1394. [Google Scholar] [CrossRef]
- Field, M.J.; Gebruers, N.; Shanmuga Sundaram, T.; Nicholson, S.; Mead, G. Physical Activity after Stroke: A Systematic Review and Meta-Analysis. ISRN Stroke 2013, 2013, 1–13. [Google Scholar] [CrossRef]
- Michaelsen, S.M.; Ovando, A.C.; Romaguera, F.; Ada, L. Effect of Backward Walking Treadmill Training on Walking Capacity after Stroke: A Randomized Clinical Trial. Int. J. Stroke 2014, 9, 529–532. [Google Scholar] [CrossRef] [PubMed]
- Sulter, G.; Steen, C.; De Keyser, J. Use of the Barthel index and modified Rankin scale in acute stroke trials. Stroke 1999, 30, 1538–1541. [Google Scholar] [CrossRef] [PubMed]
- Blum, L.; Korner-Bitensky, N. Usefulness of the Berg Balance Scale in Stroke Rehabilitation: A Systematic Review. Phys. Ther. 2008, 88, 559–566. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).