Abstract
Chemical investigation of a Chinese collection of marine red alga Symphyocladia latiuscula yielded two new highly brominated phenols. The structures of the new compounds were elucidated by detailed spectroscopic analysis, including HRMS, 1D and 2D NMR and MS methods. Compounds 1 and 2 were evaluated for radical scavenging capability by 1,1-diphenyl-2-picrylhydrazuyl (DPPH) radical with the IC50 value of 14.5 and 20.5 μg/mL, respectively.
1. Introduction
Symphyocladia latiuscula (Harvey) Yamada is a marine red alga distributed along the coasts of Northern China, Korea, and Japan [1]. This red alga is a rich source of bromophenols with high chemical diversity and various biological activities. Previous chemical studies on this species have resulted in the characterization of 25 monoaryl and diaryl bromophenols with a variety of bioactivities, such as antibacterial [2,3], antifungal [4,5], free-radical-scavenging [6,7], aldose reductase inhibitory [8], antiviral [9], anticancer [10] and Taq DNA polymerase inhibitory activities [11]. During the course of our continuing search for new biologically active bromophenols from this marine red alga, by mass spectrum guided fractionation, two new bromophenols (1 and 2) with radical scavenging activity were characterized (Figure 1). Herein, we report the isolation, structure elucidation and bioactivity evaluation of these bromophenols.
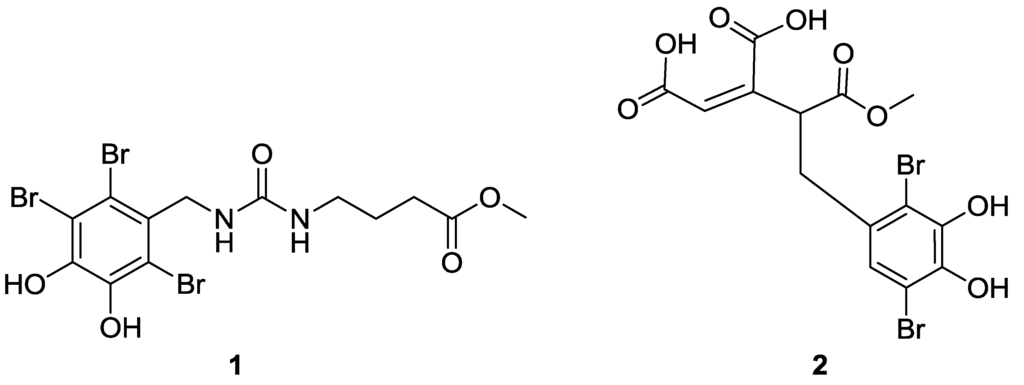
Figure 1.
Structures of compounds 1 and 2.
2. Results and Discussion
Compound 1 was obtained as a light brown amorphous powder. The positive ESIMS of 1 gave a pseudo molecular ion peak cluster for a tribrominated molecule at 517/519/521/523(1:3:3:1) [M + H]+. The molecular formula was determined to be C13H15Br3N2O5 by analysis of its HRESIMS (m/z 516.8613 [M + H]+). The 1H NMR spectrum of 1 showed three multiplets assignable to a 1,3-disubstituted propane unit at δ 2.99 (2H, quartet, J = 6.6 Hz, H2-4), 1.60 (2H, quintet, J = 6.6 Hz, H2-3), and 2.28 (2H, t, J = 6.6 Hz, H2-2), one doublet sp3 methylene at δ 4.51 (2H, d, H-7′) and an ester methoxyl singlet at δ 3.58 (3H, s, OCH3), and two exchangeable broad triplets assigned to amino protons at δ 5.85 (1H, brt, J = 4.8 Hz, N′-H) and 5.84 (1H, brt, J = 6.6 Hz, N–H). The 13C NMR data for 1 revealed the carbon signals associated with the above structural units (Table 1) as well as a set of resonances for the 2,3,6-tribromo-4,5-dihydroxybenzyl unit [2,3,4,5,6,7,8] and two additional signals associated to ester carbonyl carbon at δ 173.2 (C-1) and one sp2-hybridized quaternary carbon at δ 157.4 (C-5). The NMR signals of protons and corresponding carbons were assigned by the 1H–1H COSY and HSQC experiments (Table 1). The structure of 1 was unambiguously established by 1H−1H COSY, HSQC, and HMBC experiments. In the HMBC spectrum, the correlations (Figure 2) from H2-7′ to C-1′, C-2′, and C-6′, in combination with chemical shift values of brominated quaternary carbons (δ < 120) and oxygenated quaternary carbons (δ > 140), demonstrated the existence of the 2,3,6-tibromo-4,5-dihydroxybenzyl unit in 1. The HMBC correlations from H2-2, H2-3, and the methoxyl protons to C-1, H2-2 and H2-3 to C-4, H2-4 to C-5, together with the 1H–1H COSY signals between H2-4 and H–N and between H2-3 and H2-2 and H2-4 and between H2-7′ and H-N′, revealed the presence of a methyl γ-ureidobutyrate moiety in 1. In addition, HMBC correlations from H2-7′, H-N′, H-N and H2-4 to C-5 revealed that C-5 connected across N to C-7 and across N′ to C-7′. Therefore, 1 was determined as methyl N′-(2,3,6-tibromo-4,5-dihydroxybenzyl)-γ-ureidobutyrate. It is very interesting that the similar compound methyl N′-(2,3-dibromo-4,5-dihydroxybenzyl)-γ-ureidobutyrate was isolated from the same family marine red alga Rhodomela confervoidesc [12]. The specific halogenase may play an important role in the biosynthesis of these bromophenols.

Table 1.
NMR data for compounds 1 (600 MHz, DMSO-d6) and 2 (600 MHz, Methanol-d4).
| Compound 1 | Compound 2 | |||||
|---|---|---|---|---|---|---|
| pos | C mult | (J in Hz) | HMBC a | C mult | (J in Hz) | HMBC a |
| 1 | 173.2, C | 168.4, C | ||||
| 2 | 30.7, CH2 | 2.28, t (6.6) | 1, 3, 4 | 131.5, CH | 6.76, s | |
| 3 | 25.5, CH2 | 1.60, quint (6.6) | 1, 2, 4 | 144.2, C | ||
| 4 | 38.5, CH2 | 2.99, quart (6.6) | 2, 3, 5 | 44.2, CH2 | 5.06, dd (10.8, 4.2) | 2, 3, 5, 6, 7′ |
| N–H | 5.84, brt (6.6) | |||||
| 5 | 157.4, C | 174.2, C | ||||
| 6 | 169.1, C | |||||
| OMe | 51.2, CH3 | 3.58, s | 1 | 52.7, CH3 | 3.68, s | 5 |
| 1′ | 129.7, C | 132.0, C | ||||
| 2′ | 116.8, C | 126.3, CH | 6.73, s | 3′, 4′, 6′, 7′ | ||
| 3′ | 113.8, C | 109.6, C | ||||
| 4′ | 144.7, C | 143.7, C | ||||
| 5′ | 143.7, C | 145.4, C | ||||
| 6′ | 113.7, C | 113.5, C | ||||
| 7′ | 46.7, CH2 | 4.51, d (4.8) | 1′, 2′, 6′ | 36.9, CH2 | 3.60, dd (13.8, 4.2 ) | 3, 4, 5, 1′, 2′, 6′ |
| 5 | 2.98, dd (13.8, 10.8 ) | 3, 4, 5, 1′, 2′, 6′ | ||||
| N–H′ | 5.85, brt (4.8) | |||||
a HMBC correlations, optimized for 8 Hz, are from proton(s) stated to the indicated carbon.
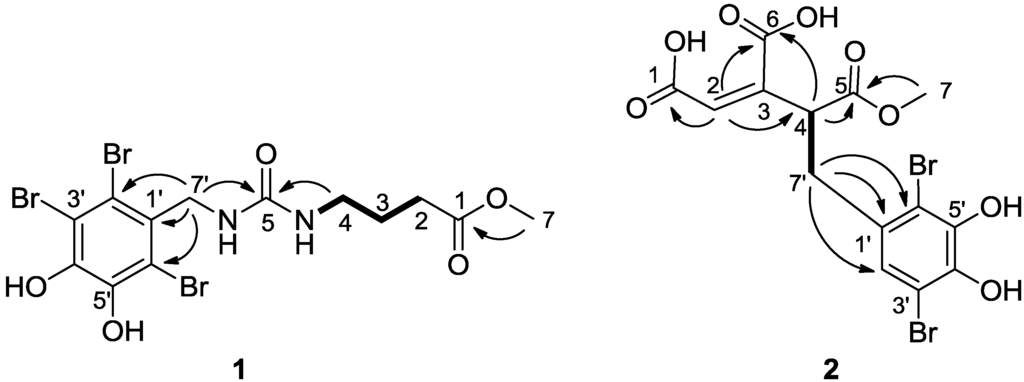
Figure 2.
Key HMBC correlations (H→C) and 1H–1H COSY (bold line) for compounds 1 and 2.
Compound 2 was obtained as a light brown amorphous powder. The positive ESIMS of 1 gave a pseudo molecular ion peak cluster for a tribrominated molecule at 467/469/471(1:2:1) [M + H]+. The molecular formula was determined to be C14H12Br2O8 by analysis of its HRESIMS (m/z 466.8985 [M + H]+). The 1H NMR spectrum of 2 displayed two alkenyl protons at δ 6.76 (H, s, H-2) and 6.73 (H, s, H-2′), one sp3methylene at δ 3.60 (1H, dd, 13.8, 4.2, H-7′a) and 2.98 (1H, dd, 13.8, 10.8, H-7′b), one doublet doublets at δ 5.06 (1H, dd, 10.8, 4.2, H-4) and an ester methoxyl singlet at δ 3.68 (3H, s, OCH3). The 13C NMR data for 2 revealed a cis-aconitic acid methyl ester moiety which was reported from the same species in our previous studies [4], two oxygenated quaternary at downfield (δ > 140) and two brominated quaternary carbons at high field (δ < 120). The cis-aconitic acid methyl ester moiety was confirmed by the HMBC cross peaks from H-2 to C-1, C-4 and C-6, from H-4 to C-5 and C-6 and from H3-7 to C-5. Meanwhile, the HMBC correlations from H-2′ (δ 6.73, s) to C-3′, C-4′, C-6′ and -7′ and from H2-7′ to C-1′, C-2′ and C-6′ revealed that this compound contains a 3,6-dibromo-4,5-dihydroxybenzyl unit. Finally, the connection between C-4 and C-7′ was assigned by the COSY signals between H-4 and H-7′a and 7′b. The ROESY correlation between H-2 and H-4 established the Z configuration about ∆2,3. The absolute configuration of C-4 was not assigned. Although methanol was used during the isolation, only mono-methyl ester of the tri-acid compound was detected from these samples. These data indicated that compound 2 could be a natural product. Microbial reductive dechlorination of polychlorinated biphenyls can occur in aquatic sediments [13], which suggested that the 2′-debrominated analogues 2 might be derived from the reductive debromination of the hexa-substituted compound.
Compounds 1 and 2 were evaluated for radical scavenging capability on 1,1-diphenyl-2-picrylhydrazuyl (DPPH) radical. Compounds 1 and 2 exhibited moderate radical scavenging ability with IC50 value of 14.5 and 20.5 μg/mL, respectively. The IC50 of positive control vitamin C is 7.82 μg/mL.
3. Experimental Section
3.1. General Experimental Procedures
NMR spectra were recorded on a Varian Inova 600 MHz spectrometer at 600 MHz for 1H and 125 MHz for 13C in DMSO-d6 and Methanol-d4 using solvent signals (DMSO: δH 2.50/δC 39.51; Methanol: δH 3.31/δC 49.15) as reference; the coupling constants were in Hz. ESIMS spectra were recorded with a ABI Mariner ESI-TOF. Column chromatography was performed with silica gel (200–300 mesh, Qingdao Haiyang Chemical Factory) and Sephadex LH-20 (Pharmacia Co., Sweden) columns. HPLC was performed using an Agilent 1100 Series separations module equipped with Agilent 1100 Series diode array detector and performed using an Agilent Zorbax Eclipse XDB-C8 (5 μm) semipreparative column (9.4 × 250 mm).
3.2. Algal Material
Symphyocladia latiuscula was collected on the coast of Qingdao, Shandong Province, China, in May 2004. The specimen identification was verified by Dr. Kui-Shuang Shao (Institute of Oceanology, Chinese Academy of Sciences, Qingdao 266071, China). A voucher specimen (No. 2004X16) was deposited at the Herbarium of the Institute of Oceanology, Chinese Academy of Sciences, Qingdao 266071, China.
3.3. Extraction and Isolation
The air-dried red alga Symphyocladia latiuscula (4.3 kg) was extracted with 95% EtOH at room temperature (3 × 72 h). After the solvent was removed under reduced pressure at <40 °C, a dark residue (610 g) was obtained. The residue was partitioned between EtOAc and H2O and the EtOAc-soluble partition (320 g) was chromatographed over silica gel, eluting with a gradient of 0%–100% Me2CO/petroleum ether [4]. The fraction eluted by 30% Me2CO/petroleum ether was further fractionated over Sephadex LH-20 using petroleum ether–CHCl3–MeOH (5:5:1) to afford 18 fractions. The ninth fraction from the LH-20 column was further fractionated by ODS column, which was eluted with a stepwise gradient of 0%–100% MeOH/H2O to afford 11 subfractions. The forth ODS subfraction was subjected to HPLC fractionation (Zorbax Eclipse XDB-C8 5 μm 250 × 9.4 mm column) to yield compound 1. The 13th fraction from the LH-20 column was further fractionated by ODS column, which was eluted with a stepwise gradient of 0%–100% MeOH/H2O to afford 11 subfractions. The third ODS subfraction was subjected to HPLC fractionation (Zorbax Eclipse XDB-C8 5 μm 250 × 9.4 mm column) to yield compound 2.
Compound 1: Light brown amorphous powder; IR νmax 3335, 2953, 1702, 1627, 1548, 1452, 1398, 1365, 1168, 1117, 1067, 1031, 968, 942, 910, 882, 831, 766, 702 cm−1;1H and 13C NMR data, see Table 1; ESIMS m/z 517/519/521/523(1:3:3:1) [M + H]+; HRESIMS m/z by analysis of its HRESIMS (m/z 516.8613 [M + H]+) (calcd for C13H1679Br3N2O5, 516.8604).
Compound 2: Light brown amorphous powder; [α]20D +10.0 (c 0.05, MeOH); IR νmax 3367, 3296, 2954, 2615, 1766, 1715, 1643, 1568, 1472, 1439, 1423, 1264, 1137, 1018, 933, 910, 816, 643, cm−1; 1H and 13C NMR data, see Table 1; ESIMS m/z 467/469/471(1:2:1) [M + H]+; HRESIMS m/z 466.8985 [M]+ (calcd for C14H1379Br2O8, 466.8972).
3.4. Scavenging Ability on 1,1-Diphenyl-2-picrylhydrazuyl (DPPH) Radical
Each power (0.1–20 mg/mL, 4.0 mL) in deionized water was mixed with 1.0 mL of methanolic solution containing DPPH (Sigma) radicals, resulting in a final concentration of 0.2 mM DPPH. The mixture was shaken vigorously and left to stand for 30 min in the dark, and the absorbance was then measured at 517 nm against a blank [14]. The scavenging ability was calculated as follows:
scavenging ability (%) = [(A517 of control − A517 of sample)/A517 of control] × 100
Vitamin C was used for positive control.
4. Conclusions
Symphyocladia latiuscula is a rich source of bromophenols with specific subunit of 2,3,6-tribromo-4,5-dihydroxybenzene. During the course of our systematic search for new biologically active bromophenols from this marine red alga, two new bromophenols (1 and 2) with radical scavenging activity were characterized by mass spectrum guided fractionation. Compounds 1 and 2 exhibited radical scavenging capability on 1,1-diphenyl-2-picrylhydrazuyl (DPPH) radical with the IC50 value of 14.5 and 20.5 μg/mL, respectively.
Acknowledgments
This study was supported by the Fundamental Research Funds for the Central Universities, Key Lab of Marine Bioactive Substance and Modern Analytical Technique, SOA and the National Natural Science Foundation of China (41106111/D0608).
References
- Tseng, C.K. Common Seaweeds of China; Science Press: Beijing, China, 1983; p. 160. [Google Scholar]
- Kurata, K.; Amiya, T. Bis(2,3,6-tribromo-4,5-dihydroxybenzyl) ether from the red alga, Symphyocladia latiuscula. Phytochemistry 1980, 19, 141–142. [Google Scholar]
- Xu, X.L.; Song, F.H.; Fan, X.; Fang, N.Q.; Shi, J.G. A novel bromophenol from marine red alga Symphyocladia latiuscula. Chem. Nat. Compd. 2009, 45, 811–813. [Google Scholar] [CrossRef]
- Xu, X.L.; Piggott, A.M.; Yin, L.Y.; Capon, R.J.; Song, F.H. Symphyocladins A–G: Bromophenol adducts from a Chinese marine red alga, Symphyocladia latiuscula. Tetrahedron Lett. 2012, 53, 2103–2106. [Google Scholar] [CrossRef]
- Xu, X.L.; Yin, L.Y.; Wang, Y.H.; Wang, S.Y.; Song, F.H. A new bromobenzyl methyl sulphoxide from marine red alga Symphyocladia latiuscula. Nat. Prod. Res. 2012. [Google Scholar] [CrossRef]
- Choi, J.S.; Park, H.J.; Jung, H.A.; Chung, H.Y.; Jung, J.H.; Choi, W.C. A cyclohexanonyl bromophenol from the red alga Symphyocladia latiuscula. J. Nat. Prod. 2000, 63, 1705–1706. [Google Scholar] [CrossRef]
- Duan, X.J.; Li, X.M.; Wang, B.G. Chemical constituents of the red alga Symphyocladia latiuscula. J. Nat. Prod. 2007, 70, 1210–1213. [Google Scholar] [CrossRef]
- Wang, W.; Okada, Y.; Shi, H.B.; Wang, Y.Q.; Okuyama, T. Structures and aldose reductase inhibitory effects of bromophenols from the red alga Symphyocladia latiuscula. J. Nat. Prod. 2005, 68, 620–622. [Google Scholar] [CrossRef]
- Park, H.J.; Kurokawa, M.; Shiraki, K.; Nakamura, N.; Choi, J.S.; Hattori, M. Antiviral activity of the marine alga Symphyocladia latiuscula against herpes simplex virus (HSV-1) in vitro and its therapeutic efficacy against HSV-1 infection in mice. Biol. Pharm. Bull. 2005, 28, 2258–2262. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, S.E.; Hossain, M.A.; Kim, M.Y.; Kim, M.; Chung, H.Y.; Choi, J.S.; Yoo, Y. 2,3,6-Tribromo-4,5-dihydroxybenzyl methyl ether induces growth inhibition and apoptosis in MCF-7 human breast cancer cells. Arch. Pharm. Res. 2007, 30, 1132–1137. [Google Scholar] [CrossRef]
- Jin, H.J.; Oh, M.Y.; Jin, D.H.; Hong, Y.K. Identification of a Taq DNA polymerase inhibitor from the red seaweed Symphyocladia latiuscula. J. Eviron. Biol. 2008, 29, 475–478. [Google Scholar]
- Ma, M.; Zhao, J.L.; Wang, S.J.; Li, S.; Yang, Y.C.; Shi, J.G.; Fan, X.; He, L. Bromophenols coupled with methyl γ-ureidobutyrate and bromophenol sulfates from the red alga Rhodomela confervoides. J. Nat. Prod. 2006, 69, 206–210. [Google Scholar] [CrossRef]
- Wiegel, J.; Wu, Q. Microbial reductive dehalogenation of polychlorinated biphenyls. FEMS Microbiol. Ecol. 2000, 32, 1–15. [Google Scholar] [CrossRef]
- Shimada, K.; Fujikawa, K.; Yahara, K.; Nakamura, T. Antioxidative properties of xanthan on the antioxidation of soybean oil in cyclodextrin emulsion. J. Agric. Food Chem. 1992, 40, 945–948. [Google Scholar] [CrossRef]
- Samples Availability: Available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).