Association of CT HU Values with Adjacent Vertebral Fractures After Balloon Kyphoplasty
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Data Collection
2.3. Statistical Analysis
3. Results
4. Discussion
4.1. Incidence of AVF and the Importance of Early Surgery
4.2. Fracture Location
4.3. Bone Density Assessment
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADL | Activities of Daily Living |
| AVF | Adjacent Vertebral Fracture |
| AUC | Area Under the Curve |
| BKP | Balloon Kyphoplasty |
| BMD | Bone Mineral Density |
| CI | Confidence Interval |
| CT | Computed Tomography |
| DISH | Diffuse Idiopathic Skeletal Hyperostosis |
| DEXA | Dual-Energy X-ray Absorptiometry |
| HU | Hounsfield Unit |
| OR | Odds Ratio |
| OVF | Osteoporotic Vertebral Fracture |
| PVP | Percutaneous Vertebroplasty |
| ROC | Receiver Operating Characteristic |
| ROI | Region of Interest |
| SD | Standard Deviation |
| YAM | Young Adult Mean |
References
- Yoshimura, N.; Muraki, S.; Oka, H.; Mabuchi, A.; En-Yo, Y.; Yoshida, M.; Saika, A.; Yoshida, H.; Suzuki, T.; Yamamoto, S.; et al. Prevalence of knee osteoarthritis, lumbar spondylosis, and osteoporosis in Japanese men and women: The research on osteoarthritis/osteoporosis against disability study. J. Bone Miner. Metab. 2009, 27, 620–628. [Google Scholar] [CrossRef]
- Buchbinder, R.; Osborne, R.H.; Ebeling, P.R.; Wark, J.D.; Mitchell, P.; Wriedt, C.; Graves, S. A randomized trial of vertebroplasty for painful osteoporotic vertebral fractures. N. Engl. J. Med. 2009, 361, 557–568. [Google Scholar] [CrossRef]
- Wardlaw, D.; Cummings, S.R.; Meirhaeghe, J.V.; Bastian, L.; Tillman, J.B.; Ranstam, J.; Eastell, R.; Shabe, P.; Talmadge, K.; Boonen, S. Efficacy and safety of balloon kyphoplasty compared with nonsurgical care for vertebral compression fracture (FREE): A randomised controlled trial. Lancet 2009, 373, 1016–1024. [Google Scholar] [CrossRef] [PubMed]
- Minamide, A.; Maeda, T.; Yamada, H.; Murakami, K.; Okada, M.; Enyo, Y.; Nakagawa, Y.; Iwasaki, H.; Tsutsui, S.; Takami, M.; et al. Early versus delayed kyphoplasty for thoracolumbar osteoprotic verterbral fractures: The effect of timing on clinical and radiographic outcomes and subsequent compression fractures. Clin. Neurol. Neurosurg. 2018, 173, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Trout, A.T.; Kallmes, D.F.; Lane, J.I.; Layton, K.F.; Marx, W.F. Subsequent vertebral fractures after vertebroplasty association with intraosseous clefts. Am. J. Neuroradial. 2006, 27, 1586–1591. [Google Scholar]
- Frankel, B.M.; Monroe, T.; Wang, C. Percutaneous vertebral augmentation: An elevation in adjacent-level fracture risk in kyphoplasty as compared with vertebroplasty. Spine J. 2007, 7, 575–582. [Google Scholar] [CrossRef]
- Fribourg, D.; Tang, C.; Sra, P.; Delamarter, R.; Bae, H. Incidence of subsequent vertebral fracture after kyphoplasty. Spine 2004, 29, 2270–2276. [Google Scholar] [CrossRef]
- McKiernan, F.; Faciszewski, T.; Jensen, R. Reporting Height Restoration in Osteoporotic Compression Fractures. Spine 2003, 28, 2517–2521. [Google Scholar] [CrossRef]
- Schreiber, J.J.; Anderson, P.A.; Rosas, H.G.; Buchholz, A.L.; Au, A.G. Hounsfield units for assessing bone mineral density and strength: A tool for osteoporosis management. J. Bone Jt. Surg. Am. 2011, 93, 1057–1063. [Google Scholar] [CrossRef]
- Zou, D.; Li, W.; Deng, C.; Du, G.; Xu, N. The use of CT Hounsfield unit values to identify the undiagnosed spinal osteoporosis in patients with lumbar degenerative diseases. Eur. Spine J. 2019, 28, 1758–1766. [Google Scholar] [CrossRef]
- Lin, W.C.; Cheng, T.T.; Lee, Y.C.; Wang, T.N.; Cheng, Y.F.; Lui, C.C.; Yu, C.Y. New vertebral osteoporotic compression fractures after percutaneous vertebroplasty: Retrospective analysis of risk factors. J. Vasc. Interv. Radiol. 2008, 19, 225–231. [Google Scholar] [CrossRef]
- Phillips, F.M.; Ho, E.; Campbell-Hupp, M.; McNally, T.; Wetzel, F.T.; Gupta, P. Early radiographic and clinical results of balloon kyphoplasty for the treatment of osteoporotic vertebral compression fractures. Spine 2003, 28, 2260–2265. [Google Scholar] [CrossRef]
- Takahashi, S.; Hoshino, M.; Terai, H.; Toyoda, H.; Suzuki, A.; Tamai, K.; Watanabe, K.; Tsujio, T.; Yasuda, H.; Kono, H.; et al. Differences in short-term clinical and radiological outcomes depending on timing of balloon kyphoplasty for painful osteoporotic vertebral fracture. J. Orthop. Sci. 2018, 23, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Sakai, T. Is early stage balloon kyphoplasty for osteoporotic vertebral fracture appropriate? J. Spine Res. 2018, 9, 1719–1722. [Google Scholar]
- Shawn, J.S.; Mark, H.Y.; Jun-Hao, T.; Hwee Weng, D.H. Early cement augmentation may be a good treatment option for pain relief for osteoporotic compression fractures: A systematic review and meta-analysis. Eur. Spine J. 2023, 32, 1751–1762. [Google Scholar] [CrossRef]
- Takano, H.; Nojiri, H.; Shimura, A.; Teramoto, J.; Sugawara, Y.; Ishijima, M. Early balloon kyphoplasty treatment for osteoporotic vertebral fracture reduces adjacent vertebral fractures. Medicina 2024, 60, 1097. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wang, Y.; Zhang, P.; Xue, F.; Zhang, D.; Jiang, B. What are the risk factors for adjacent vertebral fracture after vertebral augmentation? A meta-analysis of published studies. Glob. Spine J. 2022, 12, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, R.; Silverman, S.L.; Cooper, C.; Hanley, D.A.; Barton, I.; Broy, S.B.; Licata, A.; Benhamou, L.; Geusens, P.; Flowers, K.; et al. Risk of new vertebral fracture in the year following a fracture. JAMA 2001, 285, 320–323. [Google Scholar] [CrossRef]
- Takano, H.; Yonezawa, I.; Todo, M.; Mazlan, M.H.; Sato, T.; Kaneko, K. Biomechanical study of the effects of balloon kyphoplasty on the adjacent vertebrae. J. Biomed. Sci. Eng. 2016, 9, 478–487. [Google Scholar] [CrossRef]
- Martin, J.B.; Jean, B.; Sugiu, K.; San Millán Ruíz, D.; Piotin, M.; Murphy, K.; Rüfenacht, M.; Muster, M.; Rüfenacht, D.A. Vertebroplasty: Clinical experience and follow-up results. Bone 1999, 25, 11S–15S. [Google Scholar] [CrossRef]
- Lin, Y.; Zhang, X.; Chen, H.; Li, W.; Zhao, J. Risk factors for adjacent vertebral fractures after balloon kyphoplasty in osteoporotic vertebral compression fractures. Pain Physician 2022, 25, E205–E212. [Google Scholar]
- Oxland, T.R. Fundamental biomechanics of the spine-What we have learned in the past 25 years and future directions. J. Biomech. 2016, 49, 817–832. [Google Scholar] [CrossRef]
- Rand, T.; Seidl, G.; Kainberger, F.; Resch, A.; Hittmair, K.; Schneider, B.; Glüer, C.C.; Imhof, H. Impact of spinal degenerative changes on the evaluation of bone mineral density with dual energy X-ray absorptiometry(DXA). Calcif. Tissue Int. 1997, 60, 430–433. [Google Scholar] [CrossRef]
- Pickhardt, P.J.; Pooler, B.D.; Lauder, T.; Muñoz del Rio, A.; Bruce, R.J.; Binkley, N. Opportunistic screening for osteoporosis using abdominal computed tomography scans obtained for other indications. Ann. Intern. Med. 2013, 158, 588–595. [Google Scholar] [CrossRef]
- Gausden, E.B.; Nwachukwu, B.U.; Schreiber, J.J.; Lorich, F.G.; Lane, J.M. Opportunistic use of CT imaging for osteoporosis screening and bone density assessment. J. Bone Jt. Surg. Am. 2017, 99, 1580–1590. [Google Scholar] [CrossRef]
- Mi, J.; Li, K.; Zhao, X.; Zhao, C.Q.; Li, H.; Zhao, J. Vertebral body compressive strength evaluated by dual-energy X-ray absorptiometry and Hounsfield units in vitro. J. Clin. Densitom. 2018, 21, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Xi, Z.; Mummaneni, P.V.; Wang, M.; Ruan, H.; Burch, S.; Deviren, V.; Clark, A.J.; Berven, S.H.; Chou, D. The association between lower Hounsfield units on computed tomography and cage subsidence after lateral lumbar interbody fusion. Neurosurg. Focus 2020, 49, E8. [Google Scholar] [CrossRef] [PubMed]
- Bredow, J.; Boese, C.K.; Werner, C.M.L.; Siewe, J.; Löhrer, L.; Zarghooni, K.; Eysel, P.; Scheyerer, M.J. Predictive validity of preoperative CT scans and the risk of pedicle screw loosening in spinal surgery. Arch. Orthop. Trauma Surg. 2016, 136, 1063–1067. [Google Scholar] [CrossRef] [PubMed]
- Schwaiger, B.J.; Behr, M.; Gersing, A.S.; Meyer, B.; Zimmer, C.; Kirschke, J.S.; Ryang, Y.M.; Ringel, F. Computed tomography findings associated with clinical outcome after dynamic posterior stabilization of the lumbar spine. World Neurosurg. 2016, 93, 306–314. [Google Scholar] [CrossRef]
- Meredith, D.S.; Schreiber, J.J.; Taher, F.; Cammisa, F.P., Jr.; Girardi, F.P. Lower preoperative Hounsfield unit measurements are associated with adjacent segment fracture after spinal fusion. Spine 2013, 38, 415–418. [Google Scholar] [CrossRef]
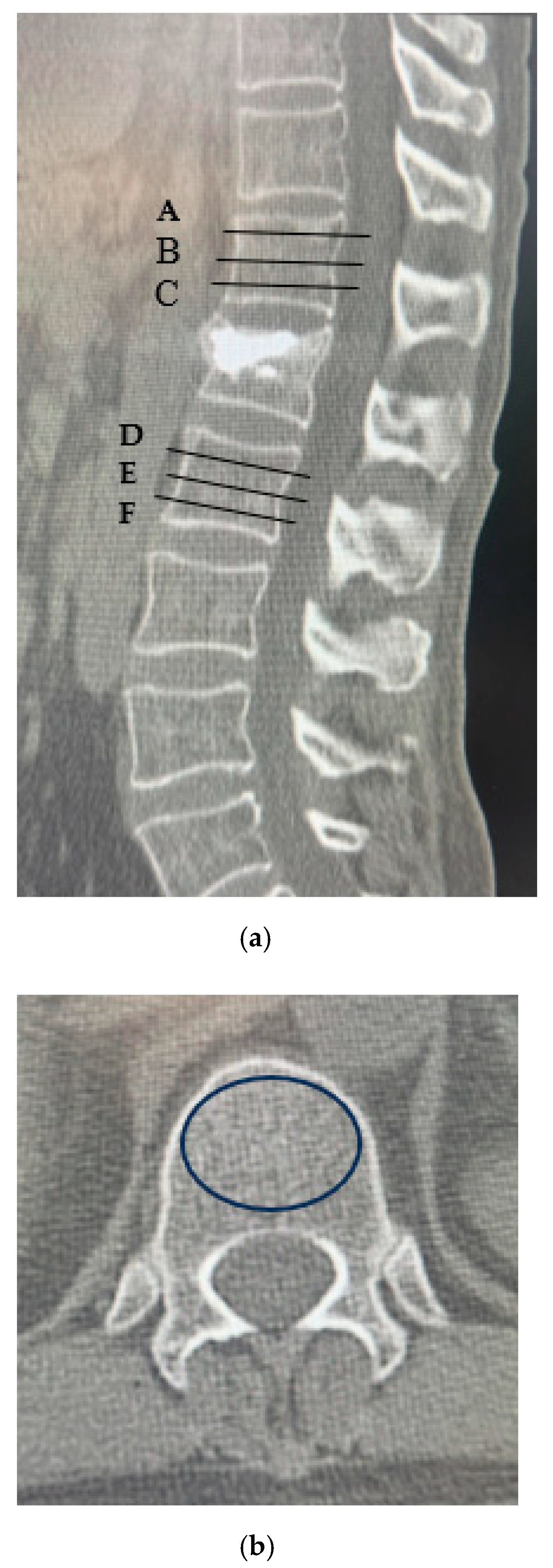

| AVF Group (n = 31) | Non-AVF Group (n = 149) | p-Value | |
|---|---|---|---|
| Age (years) | 79.9 ± 0.6 | 82.0 ± 1.3 | N.S. |
| Sex (male/female) | M8/F23 | M37/F112 | N.S. |
| Time from injury to surgery (days) | 22.9 ± 3.88 | 16.5 ± 1.77 | N.S. |
| YAM (%) | 68.0 ± 2.89 | 66.2 ± 1.37 | N.S. |
| Fracture Level | |||
| Thoracic spine (-T10) | 0 (0%) | 0 (0%) | N.S. |
| Thoracolumbar spine (T11-L2) | 29 (93.5%) | 115 (77.2%) | p < 0.05 |
| Lumbar spine (L3-L5) | 2 (6.5%) | 34 (22.8%) | p < 0.05 |
| Old vertebral fracture | 0.90 ± 0.18 | 0.59 ± 0.09 | p < 0.05 |
| Posterior wall injury (%) | 48.4% (15/31) | 32.9% (49/149) | N.S. |
| Intravertebral cleft | 35.5% (11/31) | 26.9% (40/149) | N.S. |
| Vacuum phenomenon in the adjacent intervertebral disc | 25.8% (8/31) | 11.4% (17/149) | N.S. |
| Cement volume (mL) | 7.3 ± 0.27 | 7.3 ± 0.12 | N.S. |
| Preoperative vertebral kyphosis angle | −10.7 ± 0.91 | −9.5 ± 0.41 | N.S. |
| Immediately postoperative vertebral kyphosis angle | −4.7 ± 0.59 | −4.4 ± 0.27 | N.S. |
| Final postoperative vertebral kyphosis angle | −7.9 ± 0.86 | −6.6 ± 0.39 | N.S. |
| Preoperative local kyphosis angle | −11.3 ± 2.54 | −6.3 ± 1.16 | N.S. |
| Immediately postoperative local kyphosis angle | −0.9 ± 3.05 | −1.1 ± 1.39 | N.S. |
| Final postoperative local kyphosis angle | −11.2 ± 2.74 | −5.2 ± 1.23 | p < 0.05 |
| Preoperative vertebral wedge ratio (%) | 69.5 ± 2.49 | 76.2 ± 1.13 | p < 0.05 |
| Immediately postoperative vertebral wedge ratio (%) | 86.5 ± 1.58 | 88.6 ± 0.72 | N.S. |
| Final postoperative vertebral wedge ratio (%) | 78.7 ± 2.33 | 82.1 ± 1.05 | N.S. |
| Upper Vertebral | Lower Vertebral | p-Value | |
|---|---|---|---|
| AVF levels | 22/31 (71.0%) | 9/31 (29.0%) | p < 0.05 |
| AVF Group | Non-AVF Group | p-Value | |
|---|---|---|---|
| A | 53.6 ± 68.9 | 94.0 ± 79.5 | p < 0.05 |
| B | 57.3 ± 72.3 | 76.7 ± 81.0 | p < 0.05 |
| C | 62.9 ± 67.2 | 86.2 ± 76.2 | p < 0.05 |
| D | 50.7 ± 69.7 | 94.3 ± 79.6 | p < 0.05 |
| E | 46.8 ± 71.2 | 72.2 ± 85.7 | p < 0.05 |
| F | 47.9 ± 68.1 | 83.0 ± 83.4 | p < 0.05 |
| AVF Group (n = 31) | Non-AVF Group (n = 149) | p-Value | |
|---|---|---|---|
| Upper vertebral CT values (A + B + C/3) | 61.1 ± 6.03 | 84.7 ± 2.75 | p < 0.05 |
| Lower vertebral CT values (D + E + F/3) | 51.5 ± 8.44 | 81.0 ± 3.85 | p < 0.05 |
| Risk Factor | Odds Ratio (95% Confidence Interval) | p-Value |
|---|---|---|
| Old vertebral fracture | 1.12 (0.73–1.64) | 0.571 |
| Final postoperative local kyphosis angle | 0.97 (0.93–1.01) | 0.188 |
| Preoperative vertebral wedge ratio | 0.97 (0.94–1.01) | 0.250 |
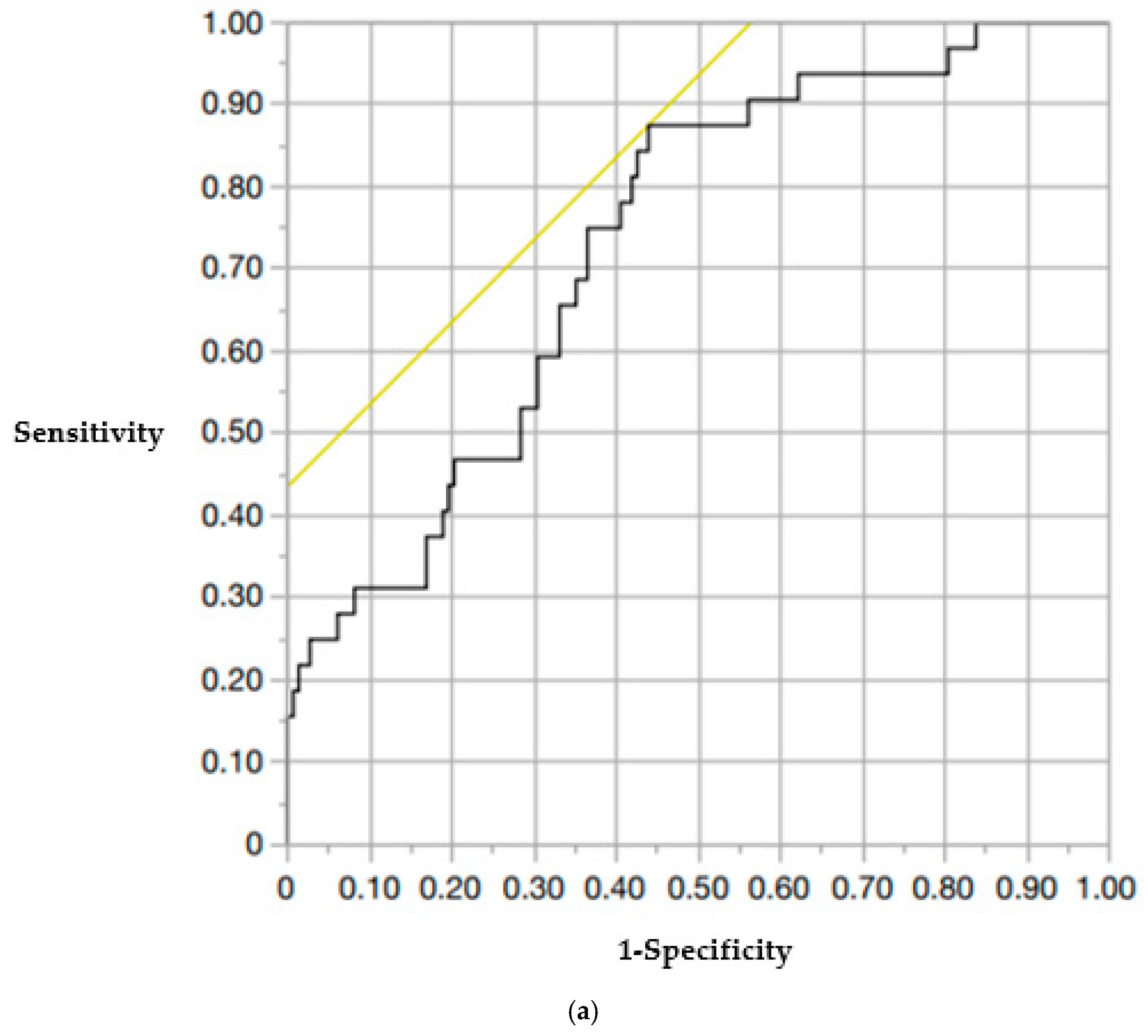
| Upper vertebral CT values (A + B + C/3) | 0.97 (0.95–0.99) | 0.043 |
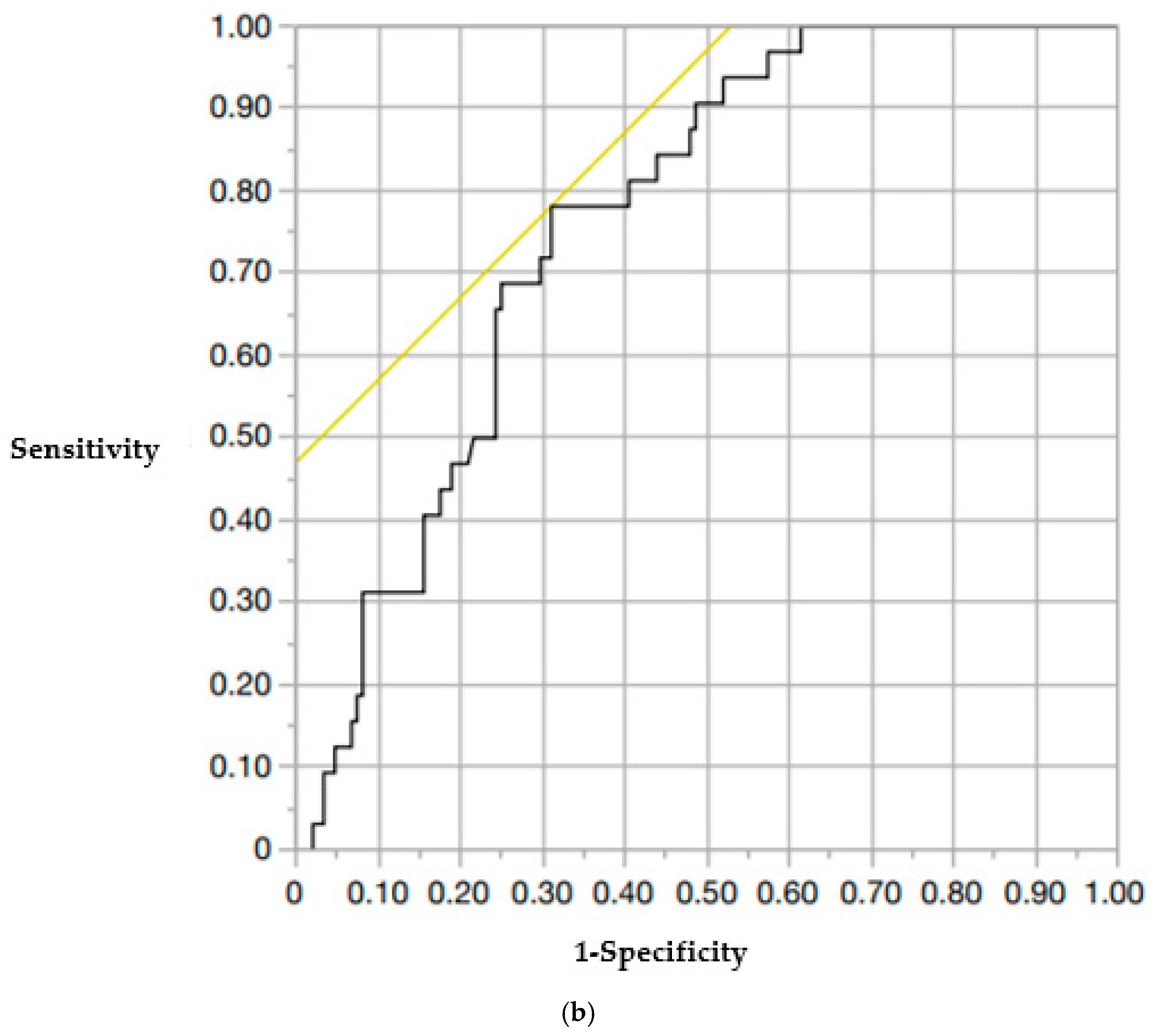
| Lower vertebral CT values (D + E + F/3) | 0.98 (0.96–0.99) | 0.048 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takano, H.; Nojiri, H.; Tamagawa, S.; Shimura, A.; Teramoto, J.; Ishibashi, H.; Sugawara, Y.; Nakai, K.; Ishijima, M. Association of CT HU Values with Adjacent Vertebral Fractures After Balloon Kyphoplasty. Medicina 2025, 61, 1517. https://doi.org/10.3390/medicina61091517
Takano H, Nojiri H, Tamagawa S, Shimura A, Teramoto J, Ishibashi H, Sugawara Y, Nakai K, Ishijima M. Association of CT HU Values with Adjacent Vertebral Fractures After Balloon Kyphoplasty. Medicina. 2025; 61(9):1517. https://doi.org/10.3390/medicina61091517
Chicago/Turabian StyleTakano, Hiromitsu, Hidetoshi Nojiri, Shota Tamagawa, Arihisa Shimura, Juri Teramoto, Hisashi Ishibashi, Yuta Sugawara, Kazuki Nakai, and Muneaki Ishijima. 2025. "Association of CT HU Values with Adjacent Vertebral Fractures After Balloon Kyphoplasty" Medicina 61, no. 9: 1517. https://doi.org/10.3390/medicina61091517
APA StyleTakano, H., Nojiri, H., Tamagawa, S., Shimura, A., Teramoto, J., Ishibashi, H., Sugawara, Y., Nakai, K., & Ishijima, M. (2025). Association of CT HU Values with Adjacent Vertebral Fractures After Balloon Kyphoplasty. Medicina, 61(9), 1517. https://doi.org/10.3390/medicina61091517








