Abstract
Background: The separation between oral and systemic health is increasingly challenged. Globally prevalent inflammatory diseases such as gastritis, often caused by Helicobacter pylori (H. pylori), and oral pathologies like periodontitis may be interconnected through microbial and inflammatory pathways. Objective: This review synthesizes evidence on the dental-gastric link, examining mechanistic pathways and clinical implications. Methods: A structured literature search identified key studies from 2000 to 2025, prioritizing systematic reviews and high-quality human research. Findings: Three key mechanistic pathways link oral dysbiosis with gastric pathology: (1) the direct translocation of oral pathogens to the stomach, including H. pylori and the broader dysbiotic oral microbiome; (2) the systemic inflammatory spillover from the periodontium, which primes the host immune system and exacerbates gastric inflammation; and (3) ancillary mechanisms such as the disruption of beneficial nitrate-nitrite-nitric oxide metabolism. Epidemiological studies show strong associations, and initial interventional trials indicate periodontal therapy may improve H. pylori eradication rates and reduce recurrence. However, the evidence is tempered by methodological limitations, including profound confounding by shared risk factors (e.g., smoking, socioeconomic status), the challenge of reverse causality, and inconsistent results from interventional studies. Conclusion: While confounding factors require consideration, oral health is a promising modifiable risk factor for gastritis. Interdisciplinary collaboration between dentistry and gastroenterology is essential to advance research and integrate oral care into gastrointestinal disease management.
1. Introduction: The Oral-Gastric Axis in Health and Disease
For centuries, the medical and dental professions have operated in relative silos, with the oral cavity often considered a separate entity from the rest of the human body. This artificial dichotomy is rapidly dissolving under the weight of compelling evidence establishing oral health as a critical determinant of systemic well-being [1,2,3,4]. The concept of the “oral-systemic link” has evolved from a hypothesis into a cornerstone of modern pathophysiology, implicating oral infections in a spectrum of diseases including cardiovascular ailments, diabetes mellitus, rheumatoid arthritis, and adverse pregnancy outcomes [5,6,7,8]. Within this expansive landscape, the intricate connection between the oral cavity and the gastrointestinal tract represents one of the most direct and physiologically plausible pathways, yet it remains underexplored in its complexity [9,10]. This review focuses on a specific and clinically significant component of this pathway: the potential bidirectional relationship between dental pathologies, primarily periodontitis and caries, and gastritis. While some frameworks approach this topic by detailing each site’s microbiome separately, this review is structured around the functional pathways of their interaction to provide a mechanistic perspective on the oral-gastric axis.
Gastritis, the inflammation of the gastric mucosa, represents a global health burden of staggering proportions. It is primarily driven by chronic infection with Helicobacter pylori (H. pylori), a Gram-negative bacterium that colonizes the stomachs of over half the world’s population [11]. While many infections are asymptomatic, chronic H. pylori-induced gastritis is a well-established precursor to peptic ulcer disease, gastric mucosa-associated lymphoid tissue (MALT) lymphoma, and gastric adenocarcinoma, the latter remaining the fifth most common cancer and the fourth leading cause of cancer-related deaths worldwide [12,13,14]. The pathogenesis of H. pylori is complex, involving bacterial virulence factors (e.g., CagA, VacA), host immune responses, and environmental co-factors [15]. Despite the efficacy of combination antibiotic therapies, eradication failure and recurrence remain significant clinical challenges, suggesting the existence of reservoirs outside the gastric niche that can facilitate reinfection [16,17,18].
The Oral Ecosystem: The oral ecosystem in health is characterized by a diverse and balanced community of commensal bacteria, maintained in equilibrium by host immunity. Nevertheless, in states of dysbiosis such as periodontitis and caries, this symbiosis collapses. Periodontitis, in particular, represents a canonical shift to a pathogenic state, driven by ecological pressures that select for a less diverse, more virulent microbiota [19,20]. This dysbiotic community is dominated by proteolytic, pro-inflammatory pathobionts like Porphyromonas gingivalis and Tannerella forsythia (the “red complex”), which manipulate host immunity, leading to chronic inflammation and the destruction of periodontal tissues [21,22,23,24]. This creates a state of perpetual oral dysbiosis and a compromised ecological barrier [25,26].
The Gastric Ecosystem: In contrast, the gastric environment presents a formidable challenge for microbial colonization due to its acidic pH. Despite this, it hosts a unique microbiome and is the primary niche for Helicobacter pylori (H. pylori), the major driver of gastric pathology [11]. Chronic H. pylori infection induces gastritis through a complex interplay of bacterial virulence factors (e.g., CagA, VacA) and the host immune response, establishing a well-defined pathway to peptic ulcer disease, gastric MALT lymphoma, and gastric adenocarcinoma [12,13,14,15]. The persistent challenges of eradication failure and recurrence suggest the existence of extra-gastric reservoirs that can facilitate reinfection [16,17,18].
The theoretical and physiological basis for a link between these two prevalent conditions is robust. The gastrointestinal tract begins in the oral cavity, and the continuous swallowing of saliva (approximately 1.5 L per day) ensures a constant influx of oral microorganisms and inflammatory mediators into the stomach [27,28,29]. This establishes a direct “oral-gastric axis” where the oral environment can directly influence the gastric ecosystem. The historical and most straightforward hypothesis posits the oral cavity, particularly dental plaque, as a potential extra-gastric reservoir for H. pylori [30,31,32]. Numerous studies have detected H. pylori DNA and specific antigens in dental plaque and saliva using polymerase chain reaction (PCR), culture, and immunohistochemistry, with prevalence rates varying widely, often higher in individuals with poor oral hygiene and periodontal disease [33,34]. This reservoir theory is clinically significant, as it could explain instances of failed eradication therapy and recurrent gastric infection, hypothesizing that oral H. pylori can recolonize the stomach after antibiotic clearance [35,36].
However, to confine the investigation to H. pylori alone is to overlook a far more complex and potentially broader mechanistic landscape. The advent of high-throughput sequencing technologies has revolutionized our understanding of human-associated microbiomes, revealing that the oral and gastric microbiomes, while distinct, are intimately connected [37,38,39]. Periodontitis is not merely an infection by a few select pathogens but a state of profound microbial dysbiosis where the diversity of the oral microbiome collapses, and pathobionts thrive [40,41]. The swallowing of this dysbiotic microbiota represents a daily inoculation of the stomach with a complex consortium of pro-inflammatory pathogens and their virulence factors (e.g., lipopolysaccharides, gingipains). While the stomach’s acidic environment is a formidable barrier, many oral bacteria, including periodontopathogens, possess acid tolerance mechanisms and can survive transit, potentially acting as pathobionts that could disrupt gastric homeostasis and exacerbate local inflammation [42,43,44].
Beyond the direct translocation of bacteria, a second, parallel pathway involves systemic inflammation. Periodontitis is a significant source of low-grade, chronic systemic inflammation. The local inflammatory process in the periodontium leads to increased serum levels of pro-inflammatory cytokines (e.g., C-reactive protein, IL-1β, IL-6, TNF-α) and inflammatory mediators [45,46,47,48]. This systemic “inflammatory priming” can alter the host’s immune status, potentially lowering the threshold for inflammatory responses in other tissues, including the gastric mucosa. This could render the stomach more susceptible to damage from H. pylori or other pathogenic bacteria, amplifying the severity of gastritis [49,50].
Historically, the investigation of oral-gastric connections has been dominated by a pathogen-centric model, focusing almost exclusively on Helicobacter pylori and the hypothesis of the oral cavity as an extra-gastric reservoir. This paradigm, while foundational, offers a limited perspective by isolating a single pathogen from the complex microbial ecosystems of both sites. This review aims to contextualize and then move beyond this established model by synthesizing evidence for a broader holistic microbiome paradigm. This emerging perspective considers the collective impact of the entire oral microbial community—its state of dysbiosis, its diverse metabolic output, and its role as a continuous inoculum for the gut—as a critical factor influencing gastric health and disease. The following chapters will first examine the evidence for the classic H. pylori reservoir hypothesis (Section 3) before expanding the discussion to explore the modern, multi-faceted oral-gastric axis in the microbiome era (Section 4, Section 5 and Section 6).
Therefore, the objective of this narrative review is to move beyond the established reservoir hypothesis and synthesize the current evidence investigating the multifactorial link between dental pathologies and gastritis. It evaluates the epidemiological evidence connecting periodontitis and caries to gastritis and its sequelae. It delves into the modern microbiome perspective, exploring the concept of oral-gut microbiome dysbiosis and its consequences for gastric health. Furthermore, it dissects the mechanisms spanning direct bacterial translocation and systemic inflammation. By integrating evidence from microbiology, immunology, gastroenterology, and dentistry, this review aims to provide a comprehensive up-to-date assessment, highlight critical knowledge gaps, and propose future research directions to validate this compelling oral-gastric axis. Establishing this link could have profound implications for the interdisciplinary management of patients, positioning oral health intervention as a novel adjunctive strategy in preventing and managing gastritis and its devastating consequences (Figure 1).
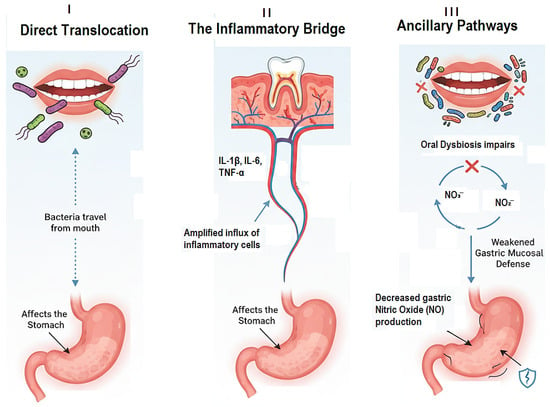
Figure 1.
The Three Core Mechanistic Pathways Linking Oral Dysbiosis to Gastritis.
2. Materials and Methods
To ensure a comprehensive synthesis of the current evidence, an extensive literature search and analysis strategy was employed. This methodology was designed to capture the highest quality and most relevant evidence linking dental pathologies to gastritis.
2.1. Search Strategy
A comprehensive research of the literature was conducted using the electronic databases PubMed/MEDLINE, Web of Science, and Scopus for articles published from January 2000 to 2025. Key search concepts included (“periodontitis” OR “oral microbiome”) AND (“gastritis” OR “Helicobacter pylori” OR “gastric cancer”) to capture studies on association and mechanism. The search was iterative, and reference lists of retrieved articles were screened to ensure comprehensive coverage.
2.2. Study Selection and Eligibility Criteria
The focus was on identifying human studies published in English. The selection process prioritized evidence in the following hierarchy:
- Systematic Reviews and Meta-Analyses: Given their role in synthesizing high-level evidence.
- Randomized Controlled Trials (RCTs): Particularly for evaluating interventional evidence (e.g., impact of periodontal therapy on H. pylori eradication).
- Prospective Cohort and Case–Control Studies: For assessing temporal relationships and risk.
- Large Cross-Sectional Studies: For establishing associations, acknowledging the inherent limitations of this design.
Studies were excluded if they were editorials, case reports, conference abstracts only, or focused on animal or in vitro models without a clear link to human clinical outcomes.
3. The H. pylori Paradigm: The Oral Cavity as an Extra-Gastric Reservoir
The most established and extensively researched hypothesis linking the oral cavity to gastritis revolves around the role of Helicobacter pylori (H. pylori) [51,52,53]. While the gastric mucosa is the primary ecological niche for this pathogen, the persistent challenges of eradication failure, recrudescence, and reinfection following apparently successful triple or quadruple therapy have long suggested the existence of sanctuary sites outside the stomach [54,55,56]. The oral cavity, particularly the complex microbial biofilms of dental plaque and periodontal pockets, has emerged as the most plausible candidate for this extra-gastric reservoir [57,58,59], (Figure 2).
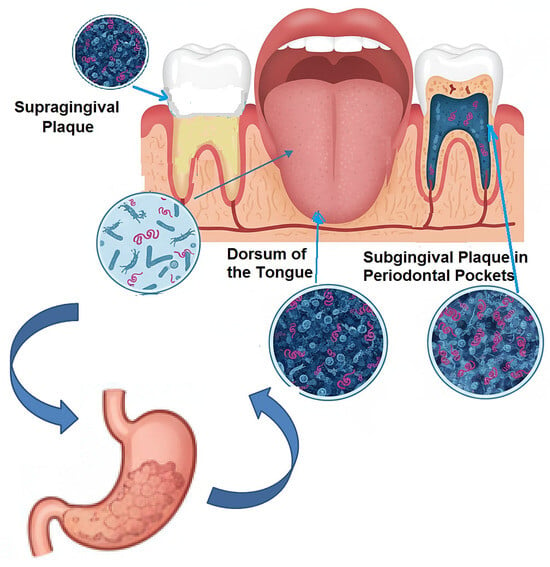
Figure 2.
The Oral Cavity as an Extra-Gastric Reservoir.
The initial evidence for this paradigm was built on the detection of H. pylori within the oral environment. Early studies utilizing culture techniques frequently isolated H. pylori from dental plaque and saliva, though with variable success rates, likely due to the fastidious nature of the bacterium and its potential transition to a viable-but-non-culturable (VBNC) state outside the stomach [60,61]. The advent of polymerase chain reaction (PCR) technology, targeting specific H. pylori genes (e.g., ureA, glmM, 16S rRNA, vacA), provided more sensitive and conclusive evidence. A multitude of PCR-based studies have consistently detected H. pylori DNA in supragingival and subgingival plaque, saliva, and even the dorsum of the tongue, with prevalence rates ranging from 20% to over 80% in individuals with gastric infection [62,63,64,65]. This wide variation is attributable to differences in sampling techniques, DNA extraction methods, primer specificity, and the population studied.
Critically, the presence of H. pylori in the mouth is not merely incidental; it is significantly associated with oral health status. A compelling body of evidence indicates that the detection rate of oral H. pylori is markedly higher in individuals with periodontitis compared to those with healthy periodontium [30,66,67]. The subgingival plaque within periodontal pockets provides an ideal micro-environment: it is relatively protected from salivary flow and mechanical disruption, exhibits a reduced oxygen tension that may favor microaerophilic bacteria, and possesses a rich proteinaceous exudate (gingival crevicular fluid) that could serve as a nutrient source [68,69,70]. This association suggests that periodontal disease creates a conducive habitat for H. pylori colonization and persistence.
The clinical significance of the oral reservoir is profoundly illustrated in the context of eradication therapy. The standard triple therapy, comprising a proton pump inhibitor and two antibiotics, is highly effective against gastric H. pylori but achieves poor bioavailability in saliva and may not effectively penetrate mature dental biofilm [71,72,73,74]. This creates a therapeutic dilemma: while gastric colonization is cleared, the oral reservoir remains untouched. Several studies have demonstrated that the presence of H. pylori in dental plaque prior to treatment is a significant predictor of eradication failure [51,68,75]. The proposed mechanism is that following the cessation of antibiotics, the orally resident bacteria can be swallowed, successfully recolonizing the now-vacant gastric niche and leading to recrudescence of the infection. This hypothesis is supported by studies showing identical H. pylori strains, as determined by random amplified polymorphic DNA (RAPD) or whole-genome sequencing, in the plaque and stomach of the same patient pre- and post-treatment failure [17,36,76,77,78].
Further strengthening the reservoir hypothesis is interventional evidence. While still an emerging area of research, some studies have investigated the effect of adjunctive oral hygiene interventions on H. pylori eradication rates. For instance, a systematic review from 2025 that included clinical studies demonstrated that patients who received non-surgical periodontal treatment (NSPT), such as scaling and root planing, in conjunction with standard antibiotic therapy for H. pylori had a higher gastric eradication rate and a significantly lower recurrence rate than those who received antibiotic therapy alone [79]. This suggests that reducing the bacterial load in the oral cavity, a potential reservoir for H. pylori, can directly improve the long-term success of gastric treatment, providing a causal link between oral health and therapeutic outcomes.
However, the oral H. pylori paradigm is not without its controversies and complexities. A primary critique is the difficulty in culturing the bacterium from oral samples, leading some to argue that detected DNA may originate from dead cells or non-viable fragments shed from the stomach [80,81,82]. Furthermore, some highly sensitive studies have failed to find a significant oral presence, suggesting that the reservoir’s importance may vary between populations and individuals [83]. There is also ongoing debate about whether H. pylori is a true colonizer of the mouth or a transient passenger continually re-seeded from the stomach. Nevertheless, the cumulative weight of epidemiological, molecular, and clinical intervention evidence presents a persuasive case for the oral cavity, particularly in the context of periodontal disease, acting as a clinically relevant reservoir that compromises the long-term management of H. pylori-associated gastritis.
This classic paradigm provides an essential foundation, but it also serves as a springboard into a more complex and holistic understanding of the oral-gastric axis, which extends far beyond this single pathogen.
4. Beyond H. pylori: The Oral–Gut Axis in the Microbiome Era
While the H. pylori reservoir model provides a strongly supported direct link, it represents a pathogen-centric view of a far more complex ecological dialog. The advent of culture-independent, high-throughput sequencing technologies has dismantled the notion of isolated microbiomes, revealing the existence of a continuous oral–gut axis wherein the oral cavity acts as a primary microbial inoculum for the entire gastrointestinal tract [84]. This paradigm shift moves the focus from a single pathogen to the collective impact of the entire oral microbial community, its state of dysbiosis, and its profound potential to influence gastric homeostasis and inflammation (Figure 3).
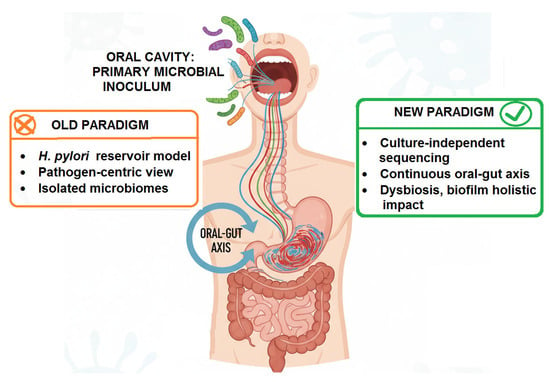
Figure 3.
A Conceptual View of the Paradigm Shift in Oral–Gut Axis.
4.1. Oral Dysbiosis: More than Just Pathogen Overgrowth
Periodontitis and dental caries are no longer viewed simply as infections by specific bacteria but as canonical examples of dysbiosis, a pathological disruption of the symbiotic microbiome-host relationship [24,40,85]. In periodontal health, a diverse community of commensal bacteria is maintained in balance by host immunity [86]. The shift to dysbiosis is driven by ecological pressures (e.g., diet, inflammation) that select for a less diverse, more virulent microbiota [87,88,89,90]. Key periodontopathogens like Porphyromonas gingivalis, Tannerella forsythia, and Treponema denticola (the “red complex”) are not mere passengers but “keystone pathogens” that manipulate the host immune response (e.g., via subversion of complement and toll-like receptor signaling) to create a state of destructive inflammation that benefits themselves and other inflammophilic pathobionts [91,92].
This dysbiotic community is characterized by:
- Loss of beneficial taxa and overall diversity.
- Overgrowth of pro-inflammatory and proteolytic bacteria.
- Increased production of virulence factors (e.g., lipopolysaccharide (LPS), gingipains, fimbriae).
- A breached epithelial barrier in the periodontal pocket, facilitating the systemic dissemination of both bacteria and inflammatory mediators.
It is this entire dysbiotic consortium, not just H. pylori, that is swallowed continuously into the stomach [93,94].
4.2. Translocation and Survival: The Journey to the Stomach
The gastric acid barrier is a formidable first line of defense, but evidence suggests that oral microbes are far from passive victims of this harsh environment. Many oral bacteria possess inherent acid tolerance mechanisms. Fusobacterium nucleatum, a bridging organism in periodontal dysbiosis, can survive at a pH as low as 3.0 for several hours [95,96]. More importantly, bacteria within biofilms or embedded in food debris are afforded significant protection from acid shock [42,97]. The constant influx—approximately 1.5 L of saliva containing over 108 bacteria per milliliter—ensures a substantial daily microbial inoculum that can overcome gastric clearance through sheer numbers [27,98].
Metagenomic sequencing studies have confirmed that the gastric fluid and mucosa of individuals, even in the absence of H. pylori, contain a detectable microbial signature of oral taxa, including Streptococcus, Veillonella, Prevotella, and Granulicatella [38,99,100,101]. This “oralization” of the gastric microbiome is significantly more pronounced in individuals with poor oral health, suggesting that a diseased oral cavity directly seeds the stomach with its dysbiotic microbiota [37,102,103,104].
4.3. Ecological Impact: Disrupting the Gastric Niche
The ecological impact of this constant seeding of oral bacteria on the gastric environment is an area of intense and novel research. The image below delineates the pathogenic interplay between oral microbiota and gastric mucosa, encompassing direct competition with niche modification, exacerbated inflammation, and synergistic interactions with H. pylori that collectively disrupt homeostasis and drive disease (Figure 4).
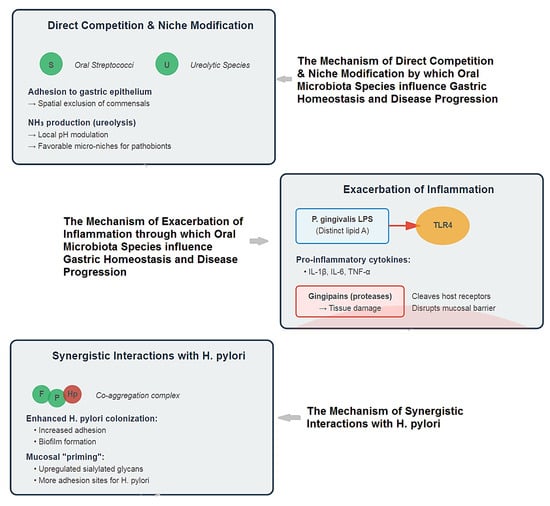
Figure 4.
Multifaceted Mechanisms of Oral Microbiota in Gastric Pathogenesis.
Thus, the proposed mechanisms extend far beyond simple colonization:
- Direct Competition and Niche Modification: The influx of oral bacteria can compete with resident gastric microbes for space and nutrients. Some oral streptococci are adept at binding to gastric epithelial cells, potentially excluding beneficial commensals [105,106,107]. Furthermore, the metabolic activity of oral bacteria (e.g., production of ammonia by ureolytic species) could locally modulate pH, creating micro-niches that favor the growth of other acid-sensitive pathobionts [108] (Figure 3).
- Exacerbation of Inflammation: This is perhaps the most significant mechanism. The gastric mucosa is in a constant state of low-grade exposure to swallowed oral microbes. In health, this may contribute to immune homeostasis. However, the swallowing of a dysbiotic, inflammation-primed oral microbiome delivers a heightened load of potent immunostimulatory molecules [109,110].
- LPS from oral Gram-negative bacteria (e.g., P. gingivalis), which has distinct lipid A structures compared to enteric LPS, can activate Toll-like receptor 4 (TLR4) on gastric epithelial and immune cells, triggering the production of pro-inflammatory cytokines (IL-1β, IL-6, TNF-α) [111,112].
- Bacterial virulence factors like gingipains from P. gingivalis are potent proteases that can directly damage tissue and cleave host cell surface receptors, further dysregulating immune responses and potentially disrupting gastric mucosal integrity [113,114,115], (Figure 3).
- Synergistic Interactions with H. pylori: The oral-gastric axis may critically modulate H. pylori pathogenicity. Co-culture studies show that oral bacteria like F. nucleatum and P. gingivalis can enhance the adhesion and biofilm formation of H. pylori [108,116,117]. The inflammatory environment created by oral pathobionts could “prime” the gastric mucosa, upregulating adhesion receptors (e.g., sialylated glycans) that H. pylori exploits for colonization and amplifying the subsequent destructive host immune response to the gastric pathogen [43,118], (Figure 3).
This creates a vicious cycle where oral dysbiosis worsens gastric inflammation, which in turn may feedback to alter the oral environment. The table below outlines the key mechanisms by which the seeding of oral bacteria into the stomach can impact the gastric environment, moving beyond simple colonization to complex ecological and immunological interactions (Table 1).

Table 1.
Oral-gastric axis as an active driver of gastric ecology and disease [105,106,107,108,109,110,111,112,113,114,115,116,117,118].
This table positions the “oral-gastric axis” not as a passive conduit, but as an active driver of gastric ecology and disease. The state of the oral microbiome—symbiotic vs. dysbiotic—directly influences gastric health through these interconnected mechanisms, establishing oral health as a critical, modifiable factor in gastrointestinal disease prevention and management.
In conclusion, the microbiome era compels us to view the link between dental pathologies and gastritis not as a simple highway for a single pathogen, but as a complex, bustling waterway constantly transporting a microbial and inflammatory cargo. The state of that cargo, whether it is a balanced symbiotic community or a dysbiotic, inflammatory one, fundamentally shapes the ecology and immune landscape of the stomach. Critically, this constant seeding of a dysbiotic oral microbiome not only directly impacts the gastric niche but also serves as the primary instigator of the systemic inflammatory cascade, which we will explore in the following section. This broader perspective positions oral health not merely as a dental concern, but as a modifiable upstream factor in gastrointestinal health, with profound implications for preventive and therapeutic strategies.
5. The Inflammatory Bridge: Systemic Inflammation as a Mechanistic Link
Beyond the direct physical translocation of microbes, a second, potent pathway connects dental pathologies to gastritis: the dissemination of inflammatory mediators from the periodontium into the systemic circulation [119,120]. Periodontitis is not a localized infection but a chronic inflammatory disease with measurable systemic repercussions [48]. This low-grade, persistent inflammatory state can “prime” the host’s immune system, thereby lowering the threshold for inflammatory responses in distant organs, including the gastric mucosa [121]. This systemic inflammatory bridge provides a powerful mechanism by which oral dysbiosis can exacerbate gastric inflammation, even in the absence of direct bacterial colonization [122].
5.1. The Periodontium as a Factory of Inflammatory Mediators
The dysbiotic biofilm in periodontitis triggers a sustained and dysregulated host immune response. Resident cells (gingival epithelial cells, fibroblasts) and recruited immune cells (primarily neutrophils, macrophages, and lymphocytes) release a storm of pro-inflammatory cytokines, chemokines, and acute-phase proteins in an attempt to control the bacterial challenge [123,124].
Key players include:
- Interleukin-1β (IL-1β) and Tumor Necrosis Factor-α (TNF-α): Potent pro-inflammatory cytokines that drive bone resorption, activate endothelial cells, and stimulate the production of other cytokines [125,126].
- Interleukin-6 (IL-6): A pleiotropic cytokine that induces hepatic production of C-reactive protein (CRP) and promotes Th17 differentiation [127,128].
- Prostaglandin E2 (PGE2): A key mediator of pain, vasodilation, and bone destruction [129].
In healthy periodontium, this response is controlled and localized. In periodontitis, the response becomes chronic and excessive, leading to the collateral damage of periodontal tissues. Importantly, these inflammatory mediators enter the systemic circulation via the sulcular epithelium—an ulcerated, permeable tissue that is continuous with the gingival crevice and has a rich capillary network [130].
5.2. Measurable Systemic Inflammatory Burden
The systemic spillover from periodontitis is well-documented. Individuals with periodontitis consistently exhibit higher serum levels of CRP, IL-6, and other inflammatory markers compared to periodontal healthy controls [45,46,131,132]. This is not merely an association; successful periodontal treatment (e.g., scaling and root planning) has been shown to significantly reduce these systemic levels of inflammation, demonstrating a direct causal link [133,134].
This chronic, low-grade systemic inflammation creates a heightened state of immune alertness throughout the body. Circulating monocytes and neutrophils from periodontitis patients display a “primed” phenotype, characterized by enhanced production of reactive oxygen species (ROS) and cytokines upon secondary stimulation [135,136].
5.3. Exacerbation of Gastric Inflammation: Mechanisms of Action
This systemically disseminated inflammatory burden can exacerbate gastritis through several interconnected mechanisms, which prime the host immune response, amplify recruitment of inflammatory cells, and compromise gastric mucosal defense (summarized in Table 2).

Table 2.
The Systemic Inflammatory Bridge: Mechanisms Linking Periodontitis to Exacerbated Gastritis.
The following paragraphs detail the evidence for these pathways.
Priming of Gastric Mucosal Immune Cells: The gastric mucosa is populated by resident immune cells, including macrophages and dendritic cells. Constant exposure to elevated levels of systemic IL-1β, TNF-α, and IL-6 can prime these cells, altering their response threshold. When these pre-activated cells encounter a gastric trigger—such as H. pylori, Non-Steroidal Anti-Inflammatory Drugs (NSAIDs), or even swallowed oral pathobionts—they mount an exaggerated inflammatory response. This leads to increased local production of inflammatory cytokines, greater tissue damage, and more severe gastritis [137,138,139].
Endothelial Activation and Leukocyte Recruitment: Systemic inflammatory cytokines, particularly TNF-α and IL-1β, activate the vascular endothelium throughout the body. In the gastric microvasculature, this activation upregulates adhesion molecules (e.g., E-selectin, ICAM-1, VCAM-1), facilitating the enhanced recruitment of circulating primed neutrophils and monocytes into the gastric tissue [140,141]. This amplified influx of inflammatory cells directly contributes to tissue damage through the release of proteolytic enzymes (e.g., matrix metalloproteinases) and ROS [142].
Synergy with H. pylori Pathogenesis: The systemic inflammatory milieu can directly interact with H. pylori infection. H. pylori itself induces a pro-inflammatory response in the stomach, primarily through the cag Pathogenicity Island (cagPAI) which activates NF-κB signaling. Systemic inflammation from periodontitis can synergize with this pathway. For instance, TNF-α is a powerful activator of NF-κB. The combined effect of H. pylori virulence factors and systemically elevated TNF-α could lead to a hyper-activation of NF-κB, resulting in a massive overproduction of gastric IL-8 and a significantly more robust neutrophilic infiltrate, thereby amplifying the severity of H. pylori-induced gastritis [137,143,144].
Impairment of Mucosal Defense and Repair: Chronic systemic inflammation can compromise the intrinsic defense mechanisms of the gastric mucosa. TNF-α, for example, can inhibit the proliferation of gastric epithelial cells and impair mucosal healing, leaving the tissue more vulnerable to injury and delaying repair processes [145,146]. This condition renders the gastric mucosa more susceptible to injury.
In summary, the inflammatory bridge model posits that the oral cavity acts as an endocrine-like source of inflammation. The chronic inflammatory lesion in the periodontium systemically elevates key mediators that alter the host’s immune set-point. This, in turn, predisposes the gastric mucosa to more severe inflammatory outcomes upon encountering any challenge, be it infectious, chemical, or dietary [147,148,149]. This mechanism operates in parallel to and can synergize with the direct microbial translocation pathways, and may even be exacerbated by the concurrent loss of protective, microbiome-derived signaling molecules such as nitric oxide. This mechanism operates in parallel to and can synergize with the direct microbial translocation pathways, providing a comprehensive explanation for the observed epidemiological links between periodontitis and gastritis. It underscores that treating oral inflammation may be a viable strategy for modulating systemic inflammatory burden and mitigating its impact on gastrointestinal health.
6. Other Potential Mechanisms: Nitrate Metabolism, Molecular Mimicry, and Salivary Factors
While microbial translocation and systemic inflammation represent the primary mechanistic pathways, several other intriguing mechanisms may contribute to the link between oral health and gastritis. These ancillary pathways highlight the profound complexity of the oral-gastric axis and offer additional avenues for research.
6.1. Nitrate Metabolism and Gastric Mucosal Defense
The nitrate-nitrite-nitric oxide (NO) pathway is a fundamental signaling and defense system, and the oral microbiome plays an indispensable role as its primary activator. Dietary nitrate (from leafy green vegetables) is absorbed and concentrated in saliva. Oral commensal bacteria, particularly on the dorsum of the tongue, possess nitrate reductase enzymes that reduce nitrate (NO3−) to nitrite (NO2−) [150,151]. Upon swallowing, this nitrite encounters the acidic gastric environment, where it is non-enzymatically reduced to potent vasodilator nitric oxide (NO) [152], (Figure 5).

Figure 5.
Dietary Nitrate Metabolism Pathway.
The figure above is illustrating the enterosalivary nitrate-nitrite-NO pathway:
- Dietary Nitrate—Intake from leafy greens and beetroot
- Salivary Gland—Active concentration of nitrate (10–20× plasma levels) via the sialin transporter
- Oral Commensals—Bacterial conversion of NO3− to NO2− by nitrate-reducing bacteria in tongue biofilm, with an “X” mark indicating disruption by dysbiosis
- Stomach—Gastric acid-mediated conversion of NO2− to NO and other nitrogen oxides
- Physiological Effects—Increased mucosal blood flow and enhanced mucosal defense mechanisms
The red “X” over the oral step emphasizes how antiseptic mouthwash or oral dysbiosis can disrupt this critical bacterial conversion step, thereby reducing downstream NO production and its beneficial cardiovascular and gastrointestinal effects. This pathway demonstrates the important symbiotic relationship between dietary nitrate, oral microbiota, and systemic health.
This pathway is critically important for gastric health:
- Mucosal Blood Flow: NO induces vasodilation, increasing blood flow to the gastric mucosa, which is essential for maintaining the mucosal barrier, delivering oxygen and nutrients, and supporting repair mechanisms [153,154].
- Mucosal Defense: NO exhibits antimicrobial properties against a range of pathogens and can modulate immune responses [155].
The Periodontitis Disruption: Periodontal dysbiosis can drastically alter this beneficial process. The shift in the oral microbiome from a health-associated, nitrate-reducing community to a disease-associated, proteolytic one may impair the oral reduction of nitrate to nitrite. This reduction in bioavailable nitrite could lead to decreased gastric NO production, compromising mucosal blood flow and defense, and thereby increasing susceptibility to injury and inflammation, including H. pylori-associated damage [156,157]. Thus, poor oral health may not just add harmful elements, but also subtract an essential protective one.
6.2. Molecular Mimicry and Autoimmunity
Molecular mimicry occurs when microbial antigens share structural similarities with host self-antigens, potentially leading to the production of cross-reactive antibodies that attack host tissues. This mechanism is well-established in rheumatic heart disease following streptococcal infection [158].
There is preliminary evidence to suggest a similar process could play a role in certain forms of gastritis, particularly autoimmune gastritis (AIG). AIG is characterized by autoantibodies against parietal cell components, including the H+/K+ ATPase proton pump. Some oral bacteria possess antigens that may mimic these host structures. For instance, antibodies generated against H. pylori or other oral pathogens might cross-react with gastric epithelial cells, initiating or perpetuating an autoimmune inflammatory response [159,160,161].
While this link is more speculative and requires further validation, it represents a fascinating potential mechanism by which oral infections could trigger specific autoimmune responses against the stomach.
6.3. The Role of Saliva and Its Constituents
Saliva is far more than a simple transport medium; it is a complex biologic fluid whose composition is altered by oral disease, and these changes can directly influence the gastric environment [162,163]:
- Altered Buffering Capacity: Saliva is a primary buffer for gastric acid refluxed into the esophagus and oral cavity. Hyposalivation or altered composition in individuals with poor oral health may impair this neutralizing capacity, potentially prolonging acid contact time with esophageal and oropharyngeal tissues [27]. While this more directly impacts Gastroesophageal Reflux Disease (GERD), esophageal inflammation can have downstream effects on the gastric cardia.
- Immunological Components: Saliva contains numerous antimicrobial and immunomodulatory factors, including immunoglobulins (e.g., secretory IgA), lactoferrin, lysozyme, and histatins. The quality and quantity of these components can be affected by oral inflammation. For example, levels of certain protective proteins may decrease, while inflammatory mediators like cytokines (IL-1β, IL-6) from the gingival crevicular fluid increase in the saliva of individuals with periodontitis [29,164,165]. The swallowing of this “inflammatory saliva” provides a direct route for these mediators to contact the gastric mucosa.
- Matrix Metalloproteinases (MMPs): Periodontitis is associated with dramatically elevated levels of active MMPs (e.g., MMP-8, MMP-9) in saliva, released by neutrophils and fibroblasts to degrade collagen in periodontal tissues [166,167,168]. Upon swallowing, these proteolytic enzymes could theoretically contribute to the degradation of the gastric epithelial basement membrane and extracellular matrix, weakening mucosal integrity and promoting inflammation.
The figure below aims to represent the impact of saliva and its constituents on the gastric environment (Figure 6).
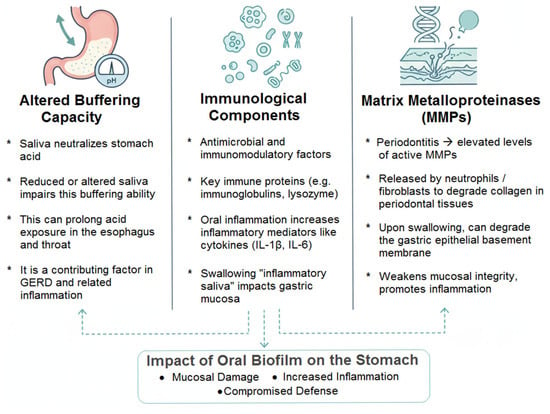
Figure 6.
The Role of Saliva and its Constituents.
In conclusion, these ancillary mechanisms—the impairment of a protective nitrate cycle, the potential triggering of autoimmunity, and the alteration of saliva’s protective properties—collectively paint a picture of an oral-gastric relationship that is multifaceted. They suggest that the impact of oral biofilm on the stomach is not monolithic but may occur through a symphony of direct, indirect, metabolic, and immunological effects, further strengthening the argument for integrative management of oral and gastrointestinal health.
7. Discussion: The Oral-Gastric Axis: A Causal Link or a Plausible Illusion? A Critical Debate
The mechanistic pathways linking the oral and gastric environments are compelling, yet the ultimate clinical significance hinges on robust human evidence. This section moves beyond a simple synthesis to engage in the central, unresolved debate: Is the oral-gastric axis a modifiable causal pathway or merely an epiphenomenon of shared risk factors? It thoroughly evaluates the evidence for both positions, aiming to provide a balanced perspective on this evolving paradigm.
7.1. The Case for Causality
Proponents of a causal relationship point to converging lines of evidence from epidemiology, microbiology, and intervention that form a coherent, multi-faceted narrative. It is important to note that these mechanistic pathways—direct translocation, systemic inflammation, and ancillary metabolic functions—are not isolated but are deeply interconnected and often act in concert to drive gastric pathology.
7.1.1. Consistent Epidemiological Association
A growing body of evidence from large-scale, prospective cohorts underscores a link between poor oral health and an elevated risk of gastrointestinal cancers, though the specific associations vary by cancer site and population. A nationwide cohort study in Sweden by Ruan et al. provides some of the strongest evidence to date, demonstrating that periodontitis was associated with an 11% increased risk of gastric cancer and a 25% increased risk of cardia gastric cancer specifically [169]. This study further identified a clear dose–response relationship, where having fewer remaining teeth was progressively associated with a higher risk of both gastric cancer subtypes. The findings were bolstered by sibling-controlled analyses, which reinforced the association while accounting for shared genetic and environmental familial factors. However, the relationship appears to be cancer-specific, as illustrated by a prospective analysis of the UK Biobank by Jordão et al. [170]. That study found no overall association between self-reported poor oral health (painful gums, bleeding gums, loose teeth) and the risk of most gastrointestinal cancers, including esophageal, gastric, and colorectal cancers. Notably, it did identify a significant, site-specific increase in risk for hepatobiliary cancers, with the association being strongest for hepatocellular carcinoma (HR = 1.75). Complementing these findings, a recent case–control study from Southwest China by Luo et al. confirmed significant associations between periodontitis and upper gastrointestinal cancers, identifying it as an independent risk factor for esophageal cancer (OR = 2.810), colon cancer (OR = 2.330), and rectal cancer (OR = 2.730) even after extensive adjustment for confounders [171]. Importantly, Luo et al. also demonstrated that periodontitis was significantly associated with distant metastasis in rectal cancer, with severe periodontitis conferring a markedly higher risk (aHR = 10.138), suggesting a role for oral health in cancer progression.
7.1.2. The Oral H. pylori Reservoir Hypothesis: A Reinforced Causal Argument
A key pillar of the causal argument is the oral cavity acting as a significant reservoir for H. pylori, which has profound implications for gastric infection and reinfection. The most compelling evidence comes from a recent systematic review and meta-analysis by Anand et al. (2025), which synthesized data from 27 observational studies and 2408 participants [59]. Their work conclusively demonstrated that the presence of gastric H. pylori infection was significantly higher among patients with H. pylori in their dental plaque, with a pooled odds ratio of 3.80 (95% CI: 2.24–6.43). This robust statistical association strongly suggests that dental plaque can serve as an extra-gastric reservoir from which the microorganism can recolonize the gastric mucosa after eradication therapy. A critical advance in validating this reservoir hypothesis was the successful culturing of viable H. pylori from the oral cavity. As reviewed by Zhang et al. (2022), since the initial isolation by Krajden et al. in 1989, a limited number of studies have successfully cultured H. pylori from dental plaque, saliva, and even dental pulp samples [33,172]. The ability to culture the bacterium confirms its metabolic activity and viability in the oral environment, moving beyond mere DNA detection. Furthermore, research into the broader oral ecosystem suggests that specific biofilms and inter-microbial interactions may facilitate H. pylori colonization and persistence. Zhang et al. (2022) elaborate that the oral cavity is not an ideal habitat for H. pylori on its own; however, the bacterium can survive by integrating into dental plaque biofilms [33]. Within these structures, bacteria like Fusobacterium nucleatum can act as a bridge for co-aggregation, and streptococci may consume oxygen to create a more favorable microaerophilic environment. Moreover, a remarkable survival strategy involves H. pylori invading yeast cells such as Candida albicans, which provides an intracellular niche that protects it from environmental stresses and antibiotics. This complex interplay with the oral microbiome helps explain the persistence of H. pylori in the mouth and its role in recalcitrant gastric infections.
7.1.3. Direct Translocation of Oral Pathobionts
Beyond H. pylori, the broader oral and gastric microbiome is implicated in gastric pathogenesis. Metagenomic sequencing studies consistently show that oral microbes, including members of the genera Peptostreptococcus, Streptococcus, and particularly Fusobacterium nucleatum, can colonize the gastric mucosa, with their abundance often increased in gastric cancer tissues [173,174]. F. nucleatum has emerged as a prime candidate for a pro-tumorigenic role. Work by Coker et al. demonstrated its association with microbial dysbiosis in gastric carcinogenesis, while Sorino et al. provided direct mechanistic evidence showing that F. nucleatum promotes gastric cancer progression by modulating the tumor microenvironment, specifically by activating immune checkpoint pathways to suppress anti-tumor immunity [175]. This mechanism mirrors its extensively documented role in other gastrointestinal cancers [176,177].
7.1.4. The Systemic Inflammation Pathway
A compelling, though still evolving, body of evidence suggests that periodontitis is not merely a localized oral issue but a significant contributor to systemic disease, particularly cancer. The chronic, low-grade inflammation it seeds throughout the body creates a fertile ground for tumorigenesis at distant sites. This is not a settled consensus but a hypothesis strengthened by key findings. Researchers like Slade et al. and D’Aiuto et al. have provided foundational evidence, demonstrating that periodontal disease directly elevates systemic inflammatory markers like CRP and IL-6, and that treating the oral infection mitigates this response [45,178]. The critical link between this inflammatory milieu and cancer is powerfully articulated by Grivennikov et al., who detail how cytokines precisely like those elevated in periodontitis can drive protumorigenic processes [179]. The most provocative evidence, however, comes from work like that of Salazar et al. and reviews by Zhou et al., which move beyond correlation to implicate specific periodontal pathogens, such as Porphyromonas gingivalis, in the pathogenesis of gastric precancerous lesions themselves [180,181]. While questions of absolute causality remain, the collective weight of this research positions periodontal health as a plausible and modifiable risk factor in the broader landscape of cancer prevention.
7.1.5. Preliminary Interventional Support
The proposition that periodontal therapy can directly influence gastrointestinal health finds its most compelling, yet nuanced, support in interventional studies. Proponents of this oral-systemic link often cite the robust prospective randomized trial by Tongtawee et al., which demonstrated that while adjunctive periodontal therapy did not significantly boost the initial H. pylori eradication rate, it profoundly and significantly reduced the recurrence of gastric infection [182]. This is an important distinction, as it strongly implicates the oral cavity as a reservoir for reinfection, a theory bolstered by their finding of a close relationship between H. pylori in saliva and in the stomach. Synthesizing the available evidence, the systematic review by Inchingolo et al. concludes that there is indeed evidence that patients with H. pylori infection benefit from non-surgical periodontal treatment (NSPT) and even suggests it might be included in future eradication guidelines [79]. However, this is where the debate sharpens. A skeptical view must highlight the caveats that Inchingolo et al. [79] themselves underscore: the current body of evidence is limited by small sample sizes, short follow-up periods, and a scarcity of large-scale RCTs. Therefore, while the findings from Tongtawee et al. [182] on preventing recurrence are powerful and mechanistically clear, the scientific community largely agrees with the systematic review’s conclusion that more evidence is required before this becomes a standard clinical mandate. The relationship is modifiable in theory, but its universal application is not yet fully supported by the evidence.
7.2. The Case for Skepticism
Despite the compelling narrative, skeptics rightly argue that the evidence for causality is overstated and vitiated by methodological limitations that are difficult to overcome.
7.2.1. The Intractable Problem of Confounding
The most potent counterargument against a causal link between periodontitis and gastric cancer is the profound and likely insurmountable challenge of confounding. The established risk factors for periodontitis—low socioeconomic status, smoking, poor diet, and alcohol consumption—are, in many cases, the identical risk factors for gastric cancer. This creates a perfect storm for spurious conclusions in observational studies. A critical appraisal of the literature confirms that residual confounding is a near certainty. This pervasive confounding is likely responsible for the inconsistent findings across large studies. For instance, the prospective analysis of the UK Biobank by Jordão et al. found no overall association between self-reported poor oral health and the risk of gastric cancer, highlighting how the relationship may not be robust when examined in different populations and with different exposure measurements [170]. As Meyer et al. (2017) highlight in their systematic review, while studies show an association, it remains difficult to exclude confounding and bias as explanations for the findings due to these shared risk factors [183]. This is not a minor issue; it is paramount. The work of Ahn et al. (2012) correctly underscores that oral and GI cancers share a ‘common soil’ of powerful environmental risk factors, particularly smoking [184]. Therefore, until data is presented that fully and rigorously accounts for this pervasive confounding, which even the most sophisticated multivariate models can only partially alleviate, any claimed direct, causal link between the oral microbiome and gastric carcinogenesis remains speculative, not proven.
7.2.2. The Cross-Sectional Conundrum and Reverse Causality
The claim that oral bacteria directly cause gastric cancer is critically weakened by the Cross-Sectional Conundrum and the strong likelihood of Reverse Causality. Most human evidence comes from cross-sectional studies: single snapshots comparing cancer patients to healthy controls. This design is fundamentally unable to determine if oral bacteria are the cause or the consequence of the disease. The compelling alternative is reverse causality. Gastric carcinogenesis follows a known sequence: from healthy tissue to chronic gastritis, atrophy, and hypochlorhydria (low stomach acid). This dismantles the stomach’s primary microbial barrier. It is highly probable that this loss of acid allows for retrograde colonization by oral bacteria. This is not just speculation; it is supported by evidence:
- Hypochlorhydria Comes First: Ferreira et al. (2018) showed that patients with precancerous hypochlorhydria already have a stomach microbiome enriched with oral taxa, proving this shift occurs before cancer [174].
- Animal Model Proof: Lofgren et al. (2011) demonstrated that the loss of acid-secreting cells directly enables gastric colonization by commensal bacteria, establishing a clear cause-and-effect [185].
- Correlation with Stage: Coker et al. (2018) and others find that oral bacteria increase step-wise with disease severity, peaking in advanced precancerous states, marking them as passengers in a permissive environment [173].
Therefore, the evidence aligns perfectly with the interpretation that altered stomach physiology is the primary event, and the influx of oral bacteria is a secondary effect. Therefore, the temporal sequence essential for establishing causality remains unproven, and the observed enrichment of oral bacteria in gastric tumors may be a consequence of the diseased gastric environment rather than its cause.
7.2.3. Microbiome Complexity and the “Passenger” Hypothesis
The complexity of the microbiome is a challenge, but modern sequencing is clarifying the picture. While the overall abundance of oral taxa in a healthy stomach is low, specific pathogens like Fusobacterium nucleatum are consistently enriched within gastric tumor tissue, suggesting a non-random, tropic relationship. A growing body of evidence from mechanistic studies demonstrates that these bacteria are not passive ‘passengers’ but active ‘drivers’ of disease, capable of promoting tumorigenesis and chemoresistance by modulating host signaling pathways [174,186,187]. The difficulty in culturing these bacteria from early-stage lesions does not negate their role; it may reflect technical limitations or support a ‘hit-and-run’ model of pathogenesis where an initial trigger sets off a lasting oncogenic cascade.
7.2.4. Inconsistent Interventional Evidence
The interventional evidence is critically weak and marked by directly conflicting outcomes. The trial by Tongtawee et al. found that periodontal therapy significantly reduced H. pylori recurrence but did not improve the initial eradication rate [182]. This contrasts with the conclusion of the systematic review by Inchingolo et al., which suggests a benefit for NSPT but itself highlights the scarcity of large-scale RCTs and the low quality of the existing evidence [79]. This inconsistency suggests the relationship is not straightforward and may depend on intervention intensity, timing, and patient population. The claim that improving oral health directly improves gastric outcomes is not robustly supported by high-level evidence, which is dominated by small, underpowered pilot studies and conflicting results from randomized controlled trials. This inconsistency suggests the relationship is not straightforward and may depend on intervention intensity, timing, and patient population.
7.2.5. Limitations in Measuring Exposure
The measurement of periodontal disease exposure is hampered by significant methodological heterogeneity and potential for misclassification. Key limitations include the use of variable case definitions, ranging from self-report to clinical measurement, and the reliance on tooth loss as a proxy. Furthermore, the application of the 2018 Classification of Periodontitis has been inconsistent, a problem exacerbated by variability in examiner training, experience, and the use of diagnostic aids [188]. Consequently, methodological reviews consistently emphasize the need for standardized training and implementation tools to improve diagnostic accuracy and reliability.
7.3. Synthesis and Future Research: Resolving the Debate
The current evidence paints a complex picture, insufficient to declare a definitive victory for either side. The sheer volume of consistent epidemiological signals, coupled with plausible mechanisms and positive interventional trends, is too compelling to dismiss outright. However, the skepticism grounded in confounding, microbiome complexity, and inconsistent trials is valid and necessary for scientific rigor. The path to resolving this debate lies in future research designed to address these criticisms directly.
- Next-Generation Targeted RCTs: Large, multi-center, double-blind RCTs are needed, comparing intensive, subgingival periodontal therapy against a sham control. These trials must use long-term H. pylori recurrence and gastric histologic improvement as primary endpoints, moving beyond short-term eradication rates.
- Deep Mechanistic Embedding: These trials must embed correlative studies using multi-omics approaches (e.g., metagenomics, metatranscriptomics, and metabolomics) to track the flux of oral bacteria to the stomach and their functional impact. This will help move beyond correlation to mechanism and identify which patients are most likely to benefit.
- Sophisticated Observational Analyses: Employing methods like Mendelian randomization could help triangulate causality by using genetic instruments for periodontitis susceptibility to assess its effect on gastric cancer risk, thereby mitigating confounding.
Conclusion of the Debate: While the “plausible illusion” of confounding remains a serious concern, the weight of evidence is gradually shifting towards a causal, and more importantly, a modifiable relationship. The oral-gastric axis, particularly involving H. pylori and specific pathobionts like F. nucleatum, should be considered a highly plausible and promising frontier for intervention. The burden of proof now lies with researchers to rigorously demonstrate through mechanistic trials outlined above that improving oral health translates into a tangible, long-term benefit for gastric health.
7.4. Limitations of This Review
While this review has sought to provide a comprehensive and critical synthesis, it is subject to several inherent limitations. Firstly, as a narrative review, it carries a potential for selection bias, unlike a protocol-driven systematic review. Secondly, the conclusions are constrained by the limitations pervasive in the primary literature, including a preponderance of cross-sectional data, inconsistent definitions of exposure and outcome, and inadequate control for confounding. Thirdly, while the mechanistic pathways were significantly discussed, their direct clinical translation remains inferential, and the current interventional evidence is too limited to firmly bridge this gap. Finally, the rapid evolution of microbiome science means this review represents a snapshot in time, and new studies with advanced technologies will undoubtedly refine the current understanding.
7.5. Clinical Implications
Despite the need for more definitive evidence, the current body of knowledge carries significant implications for clinical practice, advocating for a paradigm shift towards integrated care.
- Interdisciplinary Collaboration and Screening: The management of patients with chronic gastritis, particularly cases of H. pylori recurrence, should involve collaboration between gastroenterologists and dental professionals. A simple oral health assessment, including inquiries about gum bleeding, tooth mobility, and the time since the last dental examination, should be considered in the gastroenterological history-taking for high-risk patients. This low-cost step can identify individuals who may benefit from a formal dental referral.
- Oral Health as a Modifiable Risk Factor in H. pylori Management: For patients with concurrent H. pylori infection and periodontitis, non-surgical periodontal therapy could be considered a potential adjunct to standard eradication therapy. Based on emerging evidence, a pragmatic approach could be to schedule NSPT either shortly before or concurrently with the initiation of H. pylori eradication therapy. This timing aims to reduce the oral bacterial load and potential reservoir effect, potentially improving long-term eradication success and reducing recurrence. Promoting good oral hygiene may be a simple, cost-effective strategy to reduce systemic inflammatory burden.
- Patient Education and Empowerment: Patients should be educated about the oral-systemic connection, empowering them to take an active role in their overall well-being and improving adherence to both dental and medical treatments.
8. Conclusions
The intricate dialog between the oral cavity and the stomach, once a speculative concept, is now supported by a compelling, if not yet definitive, convergence of evidence. This review has synthesized the multifaceted pathways linking dental pathologies to gastritis. While definitive proof of causality awaits more rigorous data, the existing evidence presents a compelling narrative. The consistent epidemiological signals and plausible biological mechanisms are counterbalanced by significant methodological challenges, including confounding and the possibility of reverse causality. Therefore, the state of the oral microbiome is best considered a plausible and modifiable contributor to gastric health, rather than a definitive causative agent.
Author Contributions
Conceptualization, D.T., R.-I.V. and A.M.D.; methodology, D.C.G., I.M.E., I.-C.L. and L.B.; software, R.-I.V., O.S. and A.M.D.; validation, R.-I.V., D.T., D.C.G., O.S. and A.M.D.; formal analysis, L.I., M.A. and M.D.; investigation, D.T., I.M.E., I.-C.L. and L.B.; resources, R.-I.V., D.C.G., D.T. and A.M.D.; data curation, R.-I.V., L.B., I.-C.L. and A.M.D.; writing—original draft preparation, R.-I.V., D.T. and A.M.D.; writing—review and editing, R.-I.V., D.T., L.B., I.-C.L., D.C.G. and A.M.D.; visualization, I.M.E., D.C.G. and O.S.; supervision, R.-I.V., D.T. and A.M.D.; project administration, R.-I.V., D.T. and A.M.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
During the preparation of this work, the authors used [ChatGPT-4, OpenAI] in order to improve readability and language. Following the use of this tool, the authors reviewed and edited the content as needed and take full responsibility for the content of the publication. AI was not used to generate, interpret, or synthesize scientific content. The authors confirm that all figures included in the manuscript were fully conceived, designed, and created by themselves. Artificial intelligence tools were used solely for esthetic adjustments and did not contribute to the creation of new content or to any substantive modification of the scientific information presented. Full responsibility for the originality and accuracy of the figures belongs to the authors.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| H. pylori | Helicobacter pylori |
| NSPT | non-surgical periodontal treatment |
| PCR | Polymerase Chain Reaction |
| NSAIDs | Non-Steroidal Anti-Inflammatory Drugs |
| NO | nitric oxide |
| GERD | Gastroesophageal Reflux Disease |
| AIG | autoimmune gastritis |
References
- Natarajan, P.; Madanian, S.; Marshall, S. Investigating the Link between Oral Health Conditions and Systemic Diseases: A Cross-Sectional Analysis. Sci. Rep. 2025, 15, 10476. [Google Scholar] [CrossRef]
- Petersen, P.E.; Ogawa, H. The Global Burden of Periodontal Disease: Towards Integration with Chronic Disease Prevention and Control. Periodontology 2000 2012, 60, 15–39. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Marco Del Castillo, A.; Jepsen, S.; Gonzalez-Juanatey, J.R.; D’Aiuto, F.; Bouchard, P.; Chapple, I.; Dietrich, T.; Gotsman, I.; Graziani, F.; et al. Periodontitis and Cardiovascular Diseases: Consensus Report. J. Clin. Periodontol. 2020, 47, 268–288. [Google Scholar] [CrossRef] [PubMed]
- Kapila, Y.L. Oral Health’s Inextricable Connection to Systemic Health: Special Populations Bring to Bear Multimodal Relationships and Factors Connecting Periodontal Disease to Systemic Diseases and Conditions. Periodontology 2000 2021, 87, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.L.; Mark Welch, J.L.; Kauffman, K.M.; McLean, J.S.; He, X. The Oral Microbiome: Diversity, Biogeography and Human Health. Nat. Rev. Microbiol. 2024, 22, 89–104. [Google Scholar] [CrossRef]
- Willis, J.R.; Gabaldón, T. The Human Oral Microbiome in Health and Disease: From Sequences to Ecosystems. Microorganisms 2020, 8, 308. [Google Scholar] [CrossRef]
- Ma, Z.; Zuo, T.; Frey, N.; Rangrez, A.Y. A Systematic Framework for Understanding the Microbiome in Human Health and Disease: From Basic Principles to Clinical Translation. Signal Transduct. Target. Ther. 2024, 9, 237. [Google Scholar] [CrossRef]
- Upadhyay, M.; Swaroop, A.; Sinhal, V.K.; Srivastava, A.; Garg, S.K.; Singh, V.P.; Arora, P.K. Role of Human Oral Microbiome in Diseases. J. Pure Appl. Microbiol. 2024, 18, 168–176. [Google Scholar] [CrossRef]
- Zarco, M.; Vess, T.; Ginsburg, G. The Oral Microbiome in Health and Disease and the Potential Impact on Personalized Dental Medicine. Oral Dis. 2012, 18, 109–120. [Google Scholar] [CrossRef]
- Barboza-Solís, C.; Acuña-Amador, L.A. The Oral Microbiota: A Literature Review for Updating Professionals in Dentistry. Part I. Odovtos—Int. J. Dent. Sci. 2020, 22, 143–152. [Google Scholar] [CrossRef]
- Li, Y.; Choi, H.; Leung, K.; Jiang, F.; Graham, D.Y.; Leung, W.K. Global Prevalence of Helicobacter pylori Infection between 1980 and 2022: A Systematic Review and Meta-Analysis. Lancet Gastroenterol. Hepatol. 2023, 8, 553–564. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Plummer, M.; Franceschi, S.; Vignat, J.; Forman, D.; De Martel, C. Global Burden of Gastric Cancer Attributable to Helicobacter pylori: Helicobacter pylori in Gastric Cancer. Int. J. Cancer 2015, 136, 487–490. [Google Scholar] [CrossRef] [PubMed]
- Herreros-Pomares, A.; Hervás, D.; Bagan-Debon, L.; Proaño, A.; Garcia, D.; Sandoval, J.; Bagan, J. Oral Cancers Preceded by Proliferative Verrucous Leukoplakia Exhibit Distinctive Molecular Features. Oral Dis. 2024, 30, 1072–1083. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.; Yamaoka, Y. Helicobacter pylori Virulence Factors Exploiting Gastric Colonization and Its Pathogenicity. Toxins 2019, 11, 677. [Google Scholar] [CrossRef]
- Garcia-Maset, R.; Chu, V.; Yuen, N.; Blumgart, D.; Yoon, J.; Murray, B.O.; Joseph, A.A.; Rohn, J.L. Effect of Host Microenvironment and Bacterial Lifestyles on Antimicrobial Sensitivity and Implications for Susceptibility Testing. npj Antimicrob. Resist. 2025, 3, 42. [Google Scholar] [CrossRef]
- Miyabayashi, H.; Furihata, K.; Shimizu, T.; Ueno, I.; Akamatsu, T. Influence of Oral Helicobacter pylori on the Success of Eradication Therapy Against Gastric Helicobacter pylori. Helicobacter 2000, 5, 30–37. [Google Scholar] [CrossRef]
- Agarwal, M.; Shiau, S.; Larson, E.L. Repeat Gram-Negative Hospital-Acquired Infections and Antibiotic Susceptibility: A Systematic Review. J. Infect. Public Health 2018, 11, 455–462. [Google Scholar] [CrossRef]
- Kassebaum, N.J.; Bernabé, E.; Dahiya, M.; Bhandari, B.; Murray, C.J.L.; Marcenes, W. Global Burden of Severe Periodontitis in 1990-2010: A Systematic Review and Meta-Regression. J. Dent. Res. 2014, 93, 1045–1053. [Google Scholar] [CrossRef]
- Frencken, J.E.; Sharma, P.; Stenhouse, L.; Green, D.; Laverty, D.; Dietrich, T. Global Epidemiology of Dental Caries and Severe Periodontitis—A Comprehensive Review. J. Clin. Periodontol. 2017, 44, S94–S105. [Google Scholar] [CrossRef]
- Socransky, S.S.; Haffajee, A.D.; Cugini, M.A.; Smith, C.; Kent, R.L. Microbial Complexes in Subgingival Plaque. J. Clin. Periodontol. 1998, 25, 134–144. [Google Scholar] [CrossRef]
- Hajishengallis, G. Periodontitis: From Microbial Immune Subversion to Systemic Inflammation. Nat. Rev. Immunol. 2015, 15, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Yoneda, M.; Hirofuji, T. Mixed Red-Complex Bacterial Infection in Periodontitis. Int. J. Dent. 2013, 2013, 587279. [Google Scholar] [CrossRef] [PubMed]
- Abdulkareem, A.A.; Al-Taweel, F.B.; Al-Sharqi, A.J.B.; Gul, S.S.; Sha, A.; Chapple, I.L.C. Current Concepts in the Pathogenesis of Periodontitis: From Symbiosis to Dysbiosis. J. Oral Microbiol. 2023, 15, 2197779. [Google Scholar] [CrossRef] [PubMed]
- Pitts, N.B.; Zero, D.T.; Marsh, P.D.; Ekstrand, K.; Weintraub, J.A.; Ramos-Gomez, F.; Tagami, J.; Twetman, S.; Tsakos, G.; Ismail, A. Dental Caries. Nat. Rev. Dis. Primers 2017, 3, 17030. [Google Scholar] [CrossRef]
- Spatafora, G.; Li, Y.; He, X.; Cowan, A.; Tanner, A.C.R. The Evolving Microbiome of Dental Caries. Microorganisms 2024, 12, 121. [Google Scholar] [CrossRef]
- Dawes, C.; Pedersen, A.M.L.; Villa, A.; Ekström, J.; Proctor, G.B.; Vissink, A.; Aframian, D.; McGowan, R.; Aliko, A.; Narayana, N.; et al. The Functions of Human Saliva: A Review Sponsored by the World Workshop on Oral Medicine VI. Arch. Oral Biol. 2015, 60, 863–874. [Google Scholar] [CrossRef]
- Schmidt, T.S.; Hayward, M.R.; Coelho, L.P.; Li, S.S.; Costea, P.I.; Voigt, A.Y.; Wirbel, J.; Maistrenko, O.M.; Alves, R.J.; Bergsten, E.; et al. Extensive Transmission of Microbes along the Gastrointestinal Tract. eLife 2019, 8, e42693. [Google Scholar] [CrossRef]
- Lynge Pedersen, A.M.; Belstrøm, D. The Role of Natural Salivary Defences in Maintaining a Healthy Oral Microbiota. J. Dent. 2019, 80, S3–S12. [Google Scholar] [CrossRef]
- Gebara, E.C.E.; Faria, C.M.; Pannuti, C.; Chehter, L.; Mayer, M.P.A.; Lima, L.A.P.A. Persistence of Helicobacter pylori in the Oral Cavity after Systemic Eradication Therapy. J. Clin. Periodontol. 2006, 33, 329–333. [Google Scholar] [CrossRef]
- Liu, S.; Wang, S.; Zhang, N.; Li, P. The Oral Microbiome and Oral and Upper Gastrointestinal Diseases. J. Oral Microbiol. 2024, 16, 2355823. [Google Scholar] [CrossRef]
- Cuba, E.; Sánchez, M.C.; Ciudad, M.J.; Collado, L. Association of Helicobacter pylori as an Extragastric Reservoir in the Oral Cavity with Oral Diseases in Patients with and Without Gastritis—A Systematic Review. Microorganisms 2025, 13, 1955. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, X.; Ren, B.; Zhou, X.; Cheng, L. Helicobacter pylori in the Oral Cavity: Current Evidence and Potential Survival Strategies. Int. J. Mol. Sci. 2022, 23, 13646. [Google Scholar] [CrossRef]
- Costa, L.C.M.C.; Carvalho, M.D.G.; Vale, F.F.; Marques, A.T.; Rasmussen, L.T.; Chen, T.; Barros-Pinheiro, M. Helicobacter pylori in Oral Cavity: Current Knowledge. Clin. Exp. Med. 2024, 24, 209. [Google Scholar] [CrossRef] [PubMed]
- Kayali, S.; Manfredi, M.; Gaiani, F.; Bianchi, L.; Bizzarri, B.; Leandro, G.; Mario, F.D.; de’Angelis, G.L. Helicobacter pylori, Transmission Routes and Recurrence of Infection: State of the Art. Acta Biomed. Atenei Parm. 2018, 89, 72–76. [Google Scholar] [CrossRef]
- Li, R.; Zhang, P.; Hu, Z.; Yi, Y.; Chen, L.; Zhang, H. Helicobacter pylori Reinfection and Its Risk Factors after Initial Eradication: A Protocol for Systematic Review and Meta-Analysis. Medicine 2021, 100, e25949. [Google Scholar] [CrossRef] [PubMed]
- Segata, N.; Haake, S.K.; Mannon, P.; Lemon, K.P.; Waldron, L.; Gevers, D.; Huttenhower, C.; Izard, J. Composition of the Adult Digestive Tract Bacterial Microbiome Based on Seven Mouth Surfaces, Tonsils, Throat and Stool Samples. Genome Biol. 2012, 13, R42. [Google Scholar] [CrossRef]
- Zhang, C.; Powell, S.E.; Betel, D.; Shah, M.A. The Gastric Microbiome and Its Influence on Gastric Carcinogenesis. Hematol./Oncol. Clin. N. Am. 2017, 31, 389–408. [Google Scholar] [CrossRef]
- Bhandary, R.; Venugopalan, G.; Ramesh, A.; Tartaglia, G.; Singhal, I.; Khijmatgar, S. Microbial Symphony: Navigating the Intricacies of the Human Oral Microbiome and Its Impact on Health. Microorganisms 2024, 12, 571. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Lamont, R.J. Beyond the Red Complex and into More Complexity: The Polymicrobial Synergy and Dysbiosis (PSD) Model of Periodontal Disease Etiology. Mol. Oral Microbiol. 2012, 27, 409–419. [Google Scholar] [CrossRef]
- Sulaiman, Y.; Pacauskienė, I.M.; Šadzevičienė, R.; Anuzyte, R. Oral and Gut Microbiota Dysbiosis Due to Periodontitis: Systemic Implications and Links to Gastrointestinal Cancer: A Narrative Review. Medicina 2024, 60, 1416. [Google Scholar] [CrossRef] [PubMed]
- Olsen, I.; Yamazaki, K. Can Oral Bacteria Affect the Microbiome of the Gut? J. Oral Microbiol. 2019, 11, 1586422. [Google Scholar] [CrossRef]
- Kitamoto, S.; Nagao-Kitamoto, H.; Hein, R.; Schmidt, T.M.; Kamada, N. The Bacterial Connection between the Oral Cavity and the Gut Diseases. J. Dent. Res. 2020, 99, 1021–1029. [Google Scholar] [CrossRef]
- Xu, Q.; Wang, W.; Li, Y.; Cui, J.; Zhu, M.; Liu, Y.; Liu, Y. The Oral-Gut Microbiota Axis: A Link in Cardiometabolic Diseases. npj Biofilms Microbiomes 2025, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- D’Aiuto, F.; Parkar, M.; Andreou, G.; Suvan, J.; Brett, P.M.; Ready, D.; Tonetti, M.S. Periodontitis and Systemic Inflammation: Control of the Local Infection Is Associated with a Reduction in Serum Inflammatory Markers. J. Dent. Res. 2004, 83, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Paraskevas, S.; Huizinga, J.D.; Loos, B.G. A Systematic Review and Meta-analyses on C-reactive Protein in Relation to Periodontitis. J. Clin. Periodontol. 2008, 35, 277–290. [Google Scholar] [CrossRef]
- Iwasaki, M.; Shirobe, M.; Ohara, Y.; Motokawa, K.; Shida, T.; Motohashi, Y.; Edahiro, A.; Kawai, H.; Fujiwara, Y.; Ihara, K.; et al. Periodontal Inflammation and Serum Inflammatory Markers in Community-Dwelling Older Adults in Japan: The Otassha Study. J. Clin. Periodontol. 2025, 52, 1099–1107. [Google Scholar] [CrossRef]
- Hasan, F.; Tandon, A.; AlQallaf, H.; John, V.; Sinha, M.; Gibson, M.P. Inflammatory Association between Periodontal Disease and Systemic Health. Inflammation 2025. [Google Scholar] [CrossRef]
- Verma, J.; Anwar, M.T.; Linz, B.; Backert, S.; Pachathundikandi, S.K. The Influence of Gastric Microbiota and Probiotics in Helicobacter pylori Infection and Associated Diseases. Biomedicines 2024, 13, 61. [Google Scholar] [CrossRef]
- Padoan, A.; Musso, G.; Contran, N.; Basso, D. Inflammation, Autoinflammation and Autoimmunity in Inflammatory Bowel Diseases. Curr. Issues Mol. Biol. 2023, 45, 5534–5557. [Google Scholar] [CrossRef]
- Alkhaldi, N.K.; Alghamdi, W.K.; Alharbi, M.H.; Almutairi, A.S.; Alghamdi, F.T. The Association Between Oral Helicobacter pylori and Gastric Complications: A Comprehensive Review. Cureus 2022, 14, e24703. [Google Scholar] [CrossRef]
- Adler, I. Helicobacter pylori and Oral Pathology: Relationship with the Gastric Infection. World J. Gastroenterol. 2014, 20, 9922. [Google Scholar] [CrossRef]
- Sgamato, C.; Rocco, A.; Compare, D.; Priadko, K.; Romano, M.; Nardone, G. Exploring the Link between Helicobacter pylori, Gastric Microbiota and Gastric Cancer. Antibiotics 2024, 13, 484. [Google Scholar] [CrossRef] [PubMed]
- Barakat, A.; Althahabi, R.; Aljazaf, H.; Hammadi, M.; Alabbasi, M.; Alserdieh, F.; Sharif, O.; Abubaker, F. The Efficacy of Quadruple Therapy Versus Triple Therapy in Helicobacter pylori Eradication. Cureus 2025, 17, e82255. [Google Scholar] [CrossRef] [PubMed]
- Bakiera, A.; Solarz, A.; Kowalczyk, M.; Cichoż-Lach, H.; Korona-Głowniak, I. Challenges and Prospects for Eradication of Helicobacter pylori: Targeting Virulence Factors, Metabolism, and Vaccine Innovation. Pathogens 2025, 14, 619. [Google Scholar] [CrossRef]
- Figura, N.; Franceschi, F.; Santucci, A.; Bernardini, G.; Gasbarrini, G.; Gasbarrini, A. Extragastric Manifestations of Helicobacter pylori Infection. Helicobacter 2010, 15, 60–68. [Google Scholar] [CrossRef]
- Al Sayed, A.; Anand, P.S.; Kamath, K.P.; Patil, S.; Preethanath, R.S.; Anil, S. Oral Cavity as an Extragastric Reservoir of Helicobacter pylori. ISRN Gastroenterol. 2014, 2014, 261369. [Google Scholar] [CrossRef]
- Navabi, N.; Nazeri, M. The Possible Role of Dental Plaque as Extra-Gastric Reservoir of Helicobacter pylori in Gastric Re-Infection: A Science-Metric Study. J. Oral Health Oral Epidemiol. 2019, 8, 1–8. [Google Scholar] [CrossRef]
- Anand, P.S.; Kamath, K.P.; Gandhi, A.P.; Shamim, M.A.; Padhi, B.K.; Das, S. Dental Plaque as an Extra-Gastric Reservoir of Helicobacter pylori: A Systematic Review and Meta-Analysis. Arch. Oral Biol. 2025, 170, 106126. [Google Scholar] [CrossRef]
- Bernander, S.; Dalén, J.; Gästrin, B.; Hedenborg, L.; Lamke, L.O.; Öhrn, R. Absence of Helicobacter pylori in Dental Plaques in Helicobacter pylori Positive Dyspeptic Patients. Eur. J. Clin. Microbiol. Infect. Dis. 1993, 12, 282–285. [Google Scholar] [CrossRef]
- Cellini, L. Helicobacter pylori: A Chameleon-like Approach to Life. World J. Gastroenterol. 2014, 20, 5575. [Google Scholar] [CrossRef] [PubMed]
- Tsimpiris, A.; Tsolianos, I.; Grigoriadis, A.; Moschos, I.; Goulis, D.G.; Kouklakis, G. Association of Chronic Periodontitis with Helicobacter pylori Infection in Stomach or Mouth: A Systematic Review and Meta-Analysis. Eur. J. Dent. 2023, 17, 270–282. [Google Scholar] [CrossRef] [PubMed]
- Tiveljung, A.; Borch, K.; Jonasson, J.; MaRrdh, S.; Petersson, F.; Monstein, H.-J. Identification of Helicobacter in Gastric Biopsies by PCR Based on 16S rDNA Sequences: A Matter of Little Significance for the Prediction of H. pylori-Associated Gastritis? J. Med. Microbiol. 1998, 47, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Akeel, M.; Shehata, A.; Elhafey, A.; Elmakki, E.; Aboshouk, T.; Ageely, H.; Mahfouz, M.S. Large-Scale Evaluation of ureC (glmM) and SSA Conventional PCR for Rapid Direct Detection of Helicobacter pylori in Gastric Biopsies as Compared to rpoB-Based Quantitative Real-Time PCR. Open Microbiol. J. 2022, 16, e187428582207210. [Google Scholar] [CrossRef]
- Moradi, Y.; Majidi, L.; Khateri, S.; Azh, N.; Gheshlagh, R.G.; Saniee, N.; Zarei, M.; Moradpour, F. The Association between Periodontal Diseases and Helicobacter pylori: An Updated Meta-Analysis of Observational Studies. BMC Oral Health 2023, 23, 523. [Google Scholar] [CrossRef]
- Wu, X.; Zhu, H.; Hu, Y.; Zhang, L.; Huang, L. Meta-Analysis of H. pylori and the Gut Microbiome Interactions and Clinical Outcomes. Front. Cell. Infect. Microbiol. 2025, 15, 1610523. [Google Scholar] [CrossRef]
- Bakhti, S.Z.; Latifi-Navid, S. Oral Microbiota and Helicobacter pylori in Gastric Carcinogenesis: What Do We Know and Where Next? BMC Microbiol. 2021, 21, 71. [Google Scholar] [CrossRef]
- Anand, P.S. Role of Dental Plaque, Saliva and Periodontal Disease in Helicobacter pylori Infection. World J. Gastroenterol. 2014, 20, 5639. [Google Scholar] [CrossRef]
- Shi, M.; Wei, Y.; Hu, W.; Nie, Y.; Wu, X.; Lu, R. The Subgingival Microbiome of Periodontal Pockets With Different Probing Depths in Chronic and Aggressive Periodontitis: A Pilot Study. Front. Cell. Infect. Microbiol. 2018, 8, 124. [Google Scholar] [CrossRef]
- Romano, F.; Iaderosa, G.; Corana, M.; Perotto, S.; Baima, G.; Di Scipio, F.; Abbadessa, G.; Mariani, G.M.; Aimetti, M.; Berta, G.N. Comparing Ionic Profile of Gingival Crevicular Fluid and Saliva as Distinctive Signature of Severe Periodontitis. Biomedicines 2022, 10, 687. [Google Scholar] [CrossRef]
- Oshowo, A.; Gillam, D.; Botha, A.; Tunio, M.; Holton, J.; Boulos, P.; Hobsley, M. Helicobacter pylori: The Mouth, Stomach, and Gut Axis. Ann. Periodontol. 1998, 3, 276–280. [Google Scholar] [CrossRef]
- Scaccianoce, G.; Hassan, C.; Panarese, A.; Piglionica, D.; Morini, S.; Zullo, A. Helicobacter pylori Eradication with Either Seven-Day or 10-Day Triple Therapies, and with a 10-Day Sequential Regimen. Can. J. Gastroenterol. 2006, 20, 113–117. [Google Scholar] [CrossRef]
- Bago, I.; Bago, J.; Plečko, V.; Aurer, A.; Majstorović, K.; Budimir, A. The Effectiveness of Systemic Eradication Therapy against Oral Helicobacter pylori. J. Oral Pathology Medicine 2011, 40, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Okwandu, C.A.; Amayaevbo, M.-A.O.; Oyiana, N.I.; Jaiyeola, D.; Nwajuaku, P.A.; Emele, M.B.; Elenwoke, P.I.; Morebise, O. Helicobacter pylori: Strategies to Mitigate Antimicrobial Resistance. J. Adv. Med. Med. Res. 2025, 37, 272–279. [Google Scholar] [CrossRef]
- López-Valverde, N.; Macedo De Sousa, B.; López-Valverde, A.; Suárez, A.; Rodríguez, C.; Aragoneses, J.M. Possible Association of Periodontal Diseases With Helicobacter pylori Gastric Infection: A Systematic Review and Meta-Analysis. Front. Med. 2022, 9, 822194. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Tay, A.; Benghezal, M.; Marshall, B.J.; Tang, H.; Li, H. Advances in Helicobacter pylori Lipopolysaccharide Structure and Function. FEMS Microbiol. Rev. 2025, 49, fuaf034. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bang, C.S.; Gong, E.J. Antibiotic Resistance of Helicobacter pylori: Mechanisms and Clinical Implications. J. Korean Med. Sci. 2024, 39, e44. [Google Scholar] [CrossRef]
- Elbehiry, A.; Abalkhail, A.; Anajirih, N.; Alkhamisi, F.; Aldamegh, M.; Alramzi, A.; AlShaqi, R.; Alotaibi, N.; Aljuaid, A.; Alzahrani, H.; et al. Helicobacter pylori: Routes of Infection, Antimicrobial Resistance, and Alternative Therapies as a Means to Develop Infection Control. Diseases 2024, 12, 311. [Google Scholar] [CrossRef]
- Inchingolo, A.M.; Inchingolo, A.D.; Fatone, M.C.; Ferrante, L.; Casamassima, L.; Trilli, I.; Inchingolo, F.; Palermo, A.; Dipalma, G. The Effect of Periodontal Treatment on Helicobacter pylori-Infection: A Systematic Review. Periodontal Implant Res. 2025, 9, 3. [Google Scholar] [CrossRef]
- Nocker, A.; Camper, A.K. Selective Removal of DNA from Dead Cells of Mixed Bacterial Communities by Use of Ethidium Monoazide. Appl. Environ. Microbiol. 2006, 72, 1997–2004. [Google Scholar] [CrossRef]
- Siqueira, J.F., Jr.; Rôças, I.N. As-yet-Uncultivated Oral Bacteria: Breadth and Association with Oral and Extra-Oral Diseases. J. Oral Microbiol. 2013, 5, 21077. [Google Scholar] [CrossRef] [PubMed]
- Mahdizade Ari, M.; Scholz, K.J.; Cieplik, F.; Al-Ahmad, A. Viable but Non-Cultivable State in Oral Microbiota: A Critical Review of an Underexplored Microbial Survival Strategy. Front. Cell. Infect. Microbiol. 2025, 15, 1533768. [Google Scholar] [CrossRef] [PubMed]
- Schulz, C.; Schütte, K.; Koch, N.; Vilchez-Vargas, R.; Wos-Oxley, M.L.; Oxley, A.P.A.; Vital, M.; Malfertheiner, P.; Pieper, D.H. The Active Bacterial Assemblages of the Upper GI Tract in Individuals with and without Helicobacter Infection. Gut 2018, 67, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Kunath, B.J.; De Rudder, C.; Laczny, C.C.; Letellier, E.; Wilmes, P. The Oral–Gut Microbiome Axis in Health and Disease. Nat. Rev. Microbiol. 2024, 22, 791–805. [Google Scholar] [CrossRef]
- Xi, M.; Ruan, Q.; Zhong, S.; Li, J.; Qi, W.; Xie, C.; Wang, X.; Abuduxiku, N.; Ni, J. Periodontal Bacteria Influence Systemic Diseases through the Gut Microbiota. Front. Cell. Infect. Microbiol. 2024, 14, 1478362. [Google Scholar] [CrossRef]
- Devine, D.A.; Marsh, P.D.; Meade, J. Modulation of Host Responses by Oral Commensal Bacteria. J. Oral Microbiol. 2015, 7, 26941. [Google Scholar] [CrossRef]
- Marsh, P.D. Are Dental Diseases Examples of Ecological Catastrophes? Microbiology 2003, 149, 279–294. [Google Scholar] [CrossRef]
- Randeni, N.; Bordiga, M.; Xu, B. A Comprehensive Review of the Triangular Relationship among Diet–Gut Microbiota–Inflammation. Int. J. Mol. Sci. 2024, 25, 9366. [Google Scholar] [CrossRef]
- Shen, Y.; Fan, N.; Ma, S.; Cheng, X.; Yang, X.; Wang, G. Gut Microbiota Dysbiosis: Pathogenesis, Diseases, Prevention, and Therapy. MedComm 2025, 6, e70168. [Google Scholar] [CrossRef]
- Cusumano, G.; Flores, G.A.; Venanzoni, R.; Angelini, P. The Impact of Antibiotic Therapy on Intestinal Microbiota: Dysbiosis, Antibiotic Resistance, and Restoration Strategies. Antibiotics 2025, 14, 371. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Liang, S.; Payne, M.A.; Hashim, A.; Jotwani, R.; Eskan, M.A.; McIntosh, M.L.; Alsam, A.; Kirkwood, K.L.; Lambris, J.D.; et al. Low-Abundance Biofilm Species Orchestrates Inflammatory Periodontal Disease through the Commensal Microbiota and Complement. Cell Host Microbe 2011, 10, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G.; Korostoff, J.M. Revisiting the Page & Schroeder Model: The Good, the Bad and the Unknowns in the Periodontal Host Response 40 Years Later. Periodontology 2000 2017, 75, 116–151. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Lamont, R.J. Polymicrobial Communities in Periodontal Disease: Their Quasi-organismal Nature and Dialogue with the Host. Periodontology 2000 2021, 86, 210–230. [Google Scholar] [CrossRef] [PubMed]
- Albush, A.; Yassine, F.; Abbas, H.; Hanna, A.; Saba, E.; Bilen, M. The Impact of Helicobacter pylori Infection and Eradication Therapies on Gut Microbiota: A Systematic Review of Microbial Dysbiosis and Its Implications in Gastric Carcinogenesis. Front. Cell. Infect. Microbiol. 2025, 15, 1592977. [Google Scholar] [CrossRef]
- Brennan, C.A.; Garrett, W.S. Fusobacterium nucleatum—Symbiont, Opportunist and Oncobacterium. Nat. Rev. Microbiol. 2019, 17, 156–166. [Google Scholar] [CrossRef]
- Li, X.; Zhang, S.; Sheng, H.; Zhen, Y.; Wu, B.; Li, Z.; Chen, D.; Zhou, H. Oral Fusobacterium nucleatum Resists the Acidic pH of the Stomach Due to Membrane Erucic Acid Synthesized via Enoyl-CoA Hydratase-Related Protein FnFabM. J. Oral Microbiol. 2025, 17, 2453964. [Google Scholar] [CrossRef]
- Sharif, S.; Yadav, A.K. Bacterial Biofilm and Its Role in Antibiotic Resistance. Microbe 2025, 7, 100356. [Google Scholar] [CrossRef]
- Surdu, A.; Foia, L.G.; Luchian, I.; Trifan, D.; Tatarciuc, M.S.; Scutariu, M.M.; Ciupilan, C.; Budala, D.G. Saliva as a Diagnostic Tool for Systemic Diseases—A Narrative Review. Medicina 2025, 61, 243. [Google Scholar] [CrossRef]
- Nasidze, I.; Li, J.; Quinque, D.; Tang, K.; Stoneking, M. Global Diversity in the Human Salivary Microbiome. Genome Res. 2009, 19, 636–643. [Google Scholar] [CrossRef]
- Nath, A.R.; Natarajan, J. Gut Metagenomic Analysis of Gastric Cancer Patients Reveals Akkermansia, Gammaproteobacteria, and Veillonella Microbiota as Potential Non-Invasive Biomarkers. Genom. Inform. 2024, 22, 1. [Google Scholar] [CrossRef]
- Xia, M.; Lei, L.; Zhao, L.; Xu, W.; Zhang, H.; Li, M.; Hu, J.; Cheng, R.; Hu, T. The Dynamic Oral–Gastric Microbial Axis Connects Oral and Gastric Health: Current Evidence and Disputes. npj Biofilms Microbiomes 2025, 11, 1. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.H.; Park, J.; Choi, J.K.; Lee, K.E.; Lee, W.H.; Yang, J.; Lee, J.Y.; Park, Y.J.; Oh, C.; Won, H.-R.; et al. Association between the Microbiomes of Tonsil and Saliva Samples Isolated from Pediatric Patients Subjected to Tonsillectomy for the Treatment of Tonsillar Hyperplasia. Exp. Mol. Med. 2020, 52, 1564–1573. [Google Scholar] [CrossRef] [PubMed]
- Lupu, A.; Adam-Raileanu, A.; Bozomitu, L.I.; Gimiga, N.; Forna, L.; Anton, C.R.; Sasaran, M.O.; Nedelcu, A.H.; Ghica, D.C.; Anton, E.; et al. Helicobacter pylori and Compositional Patterns of Digestive Tract Microbiome in Children: A Literature Review. Nutrients 2025, 17, 2711. [Google Scholar] [CrossRef]
- McCune, E.; Sharma, A.; Johnson, B.; O’Meara, T.; Theiner, S.; Campos, M.; Heditsian, D.; Brain, S.; Gilbert, J.A.; Esserman, L.; et al. Gut and Oral Microbial Compositional Differences in Women with Breast Cancer, Women with Ductal Carcinoma In Situ, and Healthy Women. mSystems 2024, 9, e01237-24. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Patnayak, R.L.; Khodiar, P.K.; Amle, D.; Pandey, A.; Tripathi, P. Molecular Detection of Streptococcus mutans and Streptococcus sobrinus in Dental Plaque Samples in Children Aged Six to Nine Years. Cureus 2022, 14, e32672. [Google Scholar] [CrossRef]
- Baty, J.J.; Stoner, S.N.; Scoffield, J.A. Oral Commensal Streptococci: Gatekeepers of the Oral Cavity. J. Bacteriol. 2022, 204, e00257-22. [Google Scholar] [CrossRef]
- Isenberg, R.Y.; Sackih, C.W.; Willett, J.L.E. Streptococcal Natural Products Mediate Interspecies Competition with Gram-Positive Bacterial Pathogens. bioRxiv 2025. [Google Scholar] [CrossRef]
- Bostanghadiri, N.; Kouhzad, M.; Taki, E.; Elahi, Z.; Khoshbayan, A.; Navidifar, T.; Darban-Sarokhalil, D. Oral Microbiota and Metabolites: Key Players in Oral Health and Disorder, and Microbiota-Based Therapies. Front. Microbiol. 2024, 15, 1431785. [Google Scholar] [CrossRef]
- Kleinstein, S.E.; Nelson, K.E.; Freire, M. Inflammatory Networks Linking Oral Microbiome with Systemic Health and Disease. J. Dent. Res. 2020, 99, 1131–1139. [Google Scholar] [CrossRef]
- Rajasekaran, J.J.; Krishnamurthy, H.K.; Bosco, J.; Jayaraman, V.; Krishna, K.; Wang, T.; Bei, K. Oral Microbiome: A Review of Its Impact on Oral and Systemic Health. Microorganisms 2024, 12, 1797. [Google Scholar] [CrossRef]
- Darveau, R.P. Periodontitis: A Polymicrobial Disruption of Host Homeostasis. Nat. Rev. Microbiol. 2010, 8, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Zou, Q.; Xie, H.; Yang, W.; Xu, J.; Ying, S.; Liao, X.; Xie, J.; Wu, X.; Meng, F. Advances in the Study of the Relationship between Porphyromonas gingivalis and Various Diseases. Front. Cell Dev. Biol. 2025, 13, 1480233. [Google Scholar] [CrossRef] [PubMed]
- Aleksijević, L.H.; Aleksijević, M.; Škrlec, I.; Šram, M.; Šram, M.; Talapko, J. Porphyromonas gingivalis Virulence Factors and Clinical Significance in Periodontal Disease and Coronary Artery Diseases. Pathogens 2022, 11, 1173. [Google Scholar] [CrossRef] [PubMed]
- Shahoumi, L.A.; Saleh, M.H.A.; Meghil, M.M. Virulence Factors of the Periodontal Pathogens: Tools to Evade the Host Immune Response and Promote Carcinogenesis. Microorganisms 2023, 11, 115. [Google Scholar] [CrossRef]
- Messina, B.M.; Grippaudo, C.; Polizzi, A.; Blasi, A.; Isola, G. The Key Role of Porphyromonas gingivalis in the Pathogenesis of Periodontitis Linked with Systemic Diseases. Appl. Sci. 2025, 15, 6847. [Google Scholar] [CrossRef]
- Yáñez, L.; Soto, C.; Tapia, H.; Pacheco, M.; Tapia, J.; Osses, G.; Salinas, D.; Rojas-Celis, V.; Hoare, A.; Quest, A.F.G.; et al. Co-Culture of P. gingivalis and F. nucleatum Synergistically Elevates IL-6 Expression via TLR4 Signaling in Oral Keratinocytes. Int. J. Mol. Sci. 2024, 25, 3611. [Google Scholar] [CrossRef]
- Fan, Y.; Chen, X.; Shan, T.; Wang, N.; Han, Q.; Ren, B.; Cheng, L. Polymicrobial Interactions of Helicobacter pylori and Its Role in the Process of Oral Diseases. J. Oral Microbiol. 2025, 17, 2469896. [Google Scholar] [CrossRef]
- Siddiqui, Z.R.; Rahman, S.; Verma, N.; Srivastava, A.; Jahan, N.; Mohanty, S.; Suman, S. Gut–Oral Microbial Dysbiosis: A Correlated Ecosystem. Adv. Gut Microbiome Res. 2025, 2025, 7351229. [Google Scholar] [CrossRef]
- Isola, G.; Santonocito, S.; Lupi, S.M.; Polizzi, A.; Sclafani, R.; Patini, R.; Marchetti, E. Periodontal Health and Disease in the Context of Systemic Diseases. Mediat. Inflamm. 2023, 2023, 1–19. [Google Scholar] [CrossRef]
- Zhu, J.; Jiang, Z.; Yu, F.; Gao, L.; Wang, X.; Wang, Q. Integrated Oral-Gut Microbiota Therapy: A Novel Perspective on Preventing Bacterial Translocation for Systemic Disease Management. Front. Cell. Infect. Microbiol. 2025, 15, 1641816. [Google Scholar] [CrossRef]
- Schenkein, H.A.; Papapanou, P.N.; Genco, R.; Sanz, M. Mechanisms Underlying the Association between Periodontitis and Atherosclerotic Disease. Periodontology 2000 2020, 83, 90–106. [Google Scholar] [CrossRef]
- Yamazaki, K.; Kamada, N. Exploring the Oral-Gut Linkage: Interrelationship between Oral and Systemic Diseases. Mucosal Immunol. 2024, 17, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Graves, D.T.; Corrêa, J.D.; Silva, T.A. The Oral Microbiota Is Modified by Systemic Diseases. J. Dent. Res. 2019, 98, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Wang, P.; Gao, W. Microbial Dysbiosis in Periodontitis and Peri-Implantitis: Pathogenesis, Immune Responses, and Therapeutic. Front. Cell. Infect. Microbiol. 2025, 15, 1517154. [Google Scholar] [CrossRef] [PubMed]
- Neurath, N.; Kesting, M. Cytokines in Gingivitis and Periodontitis: From Pathogenesis to Therapeutic Targets. Front. Immunol. 2024, 15, 1435054. [Google Scholar] [CrossRef]
- Guo, Q.; Jin, Y.; Chen, X.; Ye, X.; Shen, X.; Lin, M.; Zeng, C.; Zhou, T.; Zhang, J. NF-κB in Biology and Targeted Therapy: New Insights and Translational Implications. Signal Transduct. Target. Ther. 2024, 9, 53. [Google Scholar] [CrossRef]
- Grebenciucova, E.; VanHaerents, S. Interleukin 6: At the Interface of Human Health and Disease. Front. Immunol. 2023, 14, 1255533. [Google Scholar] [CrossRef]
- Webb, C.E.; Vautrinot, J.; Hers, I. IL-6 as a Mediator of Platelet Hyper-Responsiveness. Cells 2025, 14, 766. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, W.; Chen, Z. Dual Roles of Prostaglandin E2 (PGE2) in Bone Remodeling and Pain Management: Bridging the Gap in Osteoarthritis Research. Mediat. Inflamm. 2025, 2025, 8882429. [Google Scholar] [CrossRef]
- Yekani, M.; Dastgir, M.; Fattahi, S.; Shahi, S.; Maleki Dizaj, S.; Memar, M.Y. Microbiological and Molecular Aspects of Periodontitis Pathogenesis: An Infection-Induced Inflammatory Condition. Front. Cell. Infect. Microbiol. 2025, 15, 1533658. [Google Scholar] [CrossRef]
- Machado, V.; Botelho, J.; Escalda, C.; Hussain, S.B.; Luthra, S.; Mascarenhas, P.; Orlandi, M.; Mendes, J.J.; D’Aiuto, F. Serum C-Reactive Protein and Periodontitis: A Systematic Review and Meta-Analysis. Front. Immunol. 2021, 12, 706432. [Google Scholar] [CrossRef] [PubMed]
- Arregoces, F.M.E.; Roa, N.S.; Velosa-Porras, J.; Rodríguez, L.V.; Merchan, M.J.; Poveda, J.C.V.; Otero, L.; Ruiz, Á.J.; Uriza, C.L. Changes in Serum Inflammatory Markers and in Clinical Periodontal Condition After Non-Surgical Periodontal Treatment in Hypertensive Patients. Biomedicines 2025, 13, 374. [Google Scholar] [CrossRef]
- Vachhani, D.K.; Bhavsar, D.N. Effects of Non-Surgical Periodontal Therapy on Serum Inflammatory Factor High-Sensitive C-Reactive Protein, Periodontal Parameters and Renal Biomarkers in Patients with Chronic Periodontitis and Chronic Kidney Disease. Dent. Med. Probl. 2021, 58, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Popoca-Hernández, E.A.; Martínez-Martínez, R.E.; González-Amaro, R.F.; Niño-Moreno, P.D.C.; Ayala-Herrera, J.L.; Jerezano-Domínguez, A.V.; Espinosa-Cristóbal, L.F.; Márquez-Corona, M.D.L.; Espinosa-de Santillana, I.A.; Medina-Solís, C.E. Impact of Non-Surgical Periodontal Treatment on the Concentration and Level of MRP-8/14 (Calprotectin) as an Inflammatory Biomarker in Women with Periodontitis and Rheumatoid Arthritis: A Quasi-Experimental Study. Diseases 2024, 12, 12. [Google Scholar] [CrossRef]
- Matthews, J.B.; Wright, H.J.; Roberts, A.; Ling-Mountford, N.; Cooper, P.R.; Chapple, I.L.C. Neutrophil Hyper-Responsiveness in Periodontitis. J. Dent. Res. 2007, 86, 718–722. [Google Scholar] [CrossRef] [PubMed]
- Bassani, B.; Cucchiara, M.; Butera, A.; Kayali, O.; Chiesa, A.; Palano, M.T.; Olmeo, F.; Gallazzi, M.; Dellavia, C.P.B.; Mortara, L.; et al. Neutrophils’ Contribution to Periodontitis and Periodontitis-Associated Cardiovascular Diseases. Int. J. Mol. Sci. 2023, 24, 15370. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, Y.; Li, Z.; Yu, Y.; Kang, W.; Han, Y.; Geng, X.; Ge, S.; Sun, Y. Effect of Helicobacter pylori Infection on Chronic Periodontitis by the Change of Microecology and Inflammation. Oncotarget 2016, 7, 66700–66712. [Google Scholar] [CrossRef]
- Salehi, M.R.; Shah Aboei, M.; Naghsh, N.; Hajisadeghi, S.; Ajami, E. A Comparison in Prevalence of Helicobacter pylori in the Gingival Crevicular Fluid from Subjects with Periodontitis and Healthy Individuals Using Polymerase Chain Reaction. J. Dent. Res. Dent. Clin. Dent. Prospect. 2013, 7, 238–243. [Google Scholar] [CrossRef]
- Li, J.; Xiao, C.; Li, C.; He, J. Tissue-Resident Immune Cells: From Defining Characteristics to Roles in Diseases. Signal Transduct. Target. Ther. 2025, 10, 12. [Google Scholar] [CrossRef]
- Huang, N.; Gibson, F.C. Immuno-Pathogenesis of Periodontal Disease: Current and Emerging Paradigms. Curr. Oral Health Rep. 2014, 1, 124–132. [Google Scholar] [CrossRef]
- Wautier, J.-L.; Wautier, M.-P. Endothelial Cell Participation in Inflammatory Reaction. Int. J. Mol. Sci. 2021, 22, 6341. [Google Scholar] [CrossRef]
- Wolosowicz, M.; Prokopiuk, S.; Kaminski, T.W. The Complex Role of Matrix Metalloproteinase-2 (MMP-2) in Health and Disease. Int. J. Mol. Sci. 2024, 25, 13691. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M. Role of Toll-like Receptors in Helicobacter pylori Infection and Immunity. World J. Gastrointest. Pathophysiol. 2014, 5, 133. [Google Scholar] [CrossRef] [PubMed]
- Kareem, R.A.; Kadhim Baqer, L. Immunological Role Of Toll Like Receptor Markers (TLR2 and TLR4) In Patients With Helicobacter pylori Infection At Basrah, Iraq. Arch. Razi Inst. 2022, 78, 601–609. [Google Scholar] [CrossRef]
- Sonis, S.T. The Pathobiology of Mucositis. Nat. Rev. Cancer 2004, 4, 277–284. [Google Scholar] [CrossRef]
- Pateras, I.S.; Igea, A.; Nikas, I.P.; Leventakou, D.; Koufopoulos, N.I.; Ieronimaki, A.I.; Bergonzini, A.; Ryu, H.S.; Chatzigeorgiou, A.; Frisan, T.; et al. Diagnostic Challenges during Inflammation and Cancer: Current Biomarkers and Future Perspectives in Navigating through the Minefield of Reactive versus Dysplastic and Cancerous Lesions in the Digestive System. Int. J. Mol. Sci. 2024, 25, 1251. [Google Scholar] [CrossRef]
- Loos, B.G.; Van Dyke, T.E. The Role of Inflammation and Genetics in Periodontal Disease. Periodontology 2000 2020, 83, 26–39. [Google Scholar] [CrossRef]
- Bhuyan, R.; Bhuyan, S.K.; Mohanty, J.N.; Das, S.; Juliana, N.; Juliana, I.F. Periodontitis and Its Inflammatory Changes Linked to Various Systemic Diseases: A Review of Its Underlying Mechanisms. Biomedicines 2022, 10, 2659. [Google Scholar] [CrossRef]
- Zhu, L.; Tang, Z.; Hu, R.; Gu, M.; Yang, Y. Ageing and Inflammation: What Happens in Periodontium? Bioengineering 2023, 10, 1274. [Google Scholar] [CrossRef]
- Yang, Z.; Du, C.; Chang, Z.; Yang, Y.; Hu, L. Role of Oral and Gut Microbiomes in Enterosalivary Nitrate Metabolism and Their Effects on Systemic Disease. Front. Cell. Infect. Microbiol. 2025, 15, 1612223. [Google Scholar] [CrossRef]
- Rosier, B.T.; Johnston, W.; Carda-Diéguez, M.; Simpson, A.; Cabello-Yeves, E.; Piela, K.; Reilly, R.; Artacho, A.; Easton, C.; Burleigh, M.; et al. Nitrate Reduction Capacity of the Oral Microbiota Is Impaired in Periodontitis: Potential Implications for Systemic Nitric Oxide Availability. Int. J. Oral Sci. 2024, 16, 1. [Google Scholar] [CrossRef]
- Castiglione, N.; Rinaldo, S.; Giardina, G.; Stelitano, V.; Cutruzzolà, F. Nitrite and Nitrite Reductases: From Molecular Mechanisms to Significance in Human Health and Disease. Antioxid. Redox Signal. 2012, 17, 684–716. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, J.O.; Weitzberg, E.; Gladwin, M.T. The Nitrate–Nitrite–Nitric Oxide Pathway in Physiology and Therapeutics. Nat. Rev. Drug Discov. 2008, 7, 156–167. [Google Scholar] [CrossRef] [PubMed]
- De Souza, G.; Taladriz-Blanco, P.; Velloso, L.; De Oliveira, M. Nitric Oxide Released from Luminal S-Nitroso-N-Acetylcysteine Increases Gastric Mucosal Blood Flow. Molecules 2015, 20, 4109–4123. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wu, Y.; Zhu, Z.; Lu, C.; Zhang, C.; Zeng, L.; Xie, F.; Zhang, L.; Zhou, F. Mucosal Immune Response in Biology, Disease Prevention and Treatment. Signal Transduct. Target. Ther. 2025, 10, 7. [Google Scholar] [CrossRef]
- Ali, A.; AlHussaini, K.I. Helicobacter pylori: A Contemporary Perspective on Pathogenesis, Diagnosis and Treatment Strategies. Microorganisms 2024, 12, 222. [Google Scholar] [CrossRef]
- Wei, Y.-F.; Li, X.; Zhao, M.-R.; Liu, S.; Min, L.; Zhu, S.-T.; Zhang, S.-T.; Xie, S.-A. Helicobacter pylori Disrupts Gastric Mucosal Homeostasis by Stimulating Macrophages to Secrete CCL3. Cell Commun. Signal 2024, 22, 263. [Google Scholar] [CrossRef]
- Suliman, B.A. Potential Clinical Implications of Molecular Mimicry-induced Autoimmunity. Immun. Inflam. Dis. 2024, 12, e1178. [Google Scholar] [CrossRef]
- Conti, L.; Annibale, B.; Lahner, E. Autoimmune Gastritis and Gastric Microbiota. Microorganisms 2020, 8, 1827. [Google Scholar] [CrossRef]
- Jakob, B.; Birkholz, S.; Schneider, T.; Duchmann, R.; Zeitz, M.; Stallmach, A. Immune Response to Autologous and Heterologous Helicobacter pylori Antigens in Humans. Microsc. Res. Tech. 2001, 53, 419–424. [Google Scholar] [CrossRef]
- Santacroce, L.; Topi, S.; Cafiero, C.; Palmirotta, R.; Jirillo, E. The Role of the Immune Response to Helicobacter pylori Antigens and Its Relevance in Gastric Disorders. Gastrointest. Disord. 2025, 7, 6. [Google Scholar] [CrossRef]
- Cui, Y.; Yang, M.; Zhu, J.; Zhang, H.; Duan, Z.; Wang, S.; Liao, Z.; Liu, W. Developments in Diagnostic Applications of Saliva in Human Organ Diseases. Med. Nov. Technol. Devices 2022, 13, 100115. [Google Scholar] [CrossRef]
- Okuyama, K.; Yanamoto, S. Saliva in Balancing Oral and Systemic Health, Oral Cancer, and Beyond: A Narrative Review. Cancers 2024, 16, 4276. [Google Scholar] [CrossRef] [PubMed]
- Vila, T.; Rizk, A.M.; Sultan, A.S.; Jabra-Rizk, M.A. The Power of Saliva: Antimicrobial and Beyond. PLoS Pathog. 2019, 15, e1008058. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, M.; Soria, S.A.; Pires, J.R.; Sant’Ana, A.C.P.; Freire, M. Natural and Induced Immune Responses in Oral Cavity and Saliva. BMC Immunol. 2025, 26, 34. [Google Scholar] [CrossRef]
- Alarcón-Sánchez, M.A.; Rodríguez-Montaño, R.; Mosaddad, S.A.; Heboyan, A. Levels of IL-1β, MMP-8, and MMP-9 in the Saliva of Subjects With Periodontitis: A Systematic Review and Meta-Analysis. Clin. Lab. Anal. 2025, 39, e70040. [Google Scholar] [CrossRef]
- Toby Thomas, J.; Joseph, B.; Waltimo, T.; Anil, S. Matrix Metalloproteinases (MMPs) in Periodontium: Is It a Boon or a Bane? In Dentistry; Sufaru, I.-G., Mihaela Solomon, S., Eds.; IntechOpen: London, UK, 2024; Volume 20, ISBN 9780850142730, 9780850142747. [Google Scholar]
- Sorsa, T.; Tjäderhane, L.; Salo, T. Matrix Metalloproteinases (MMPs) in Oral Diseases. Oral Dis. 2004, 10, 311–318. [Google Scholar] [CrossRef]
- Ruan, Z.; Xie, J.; Yu, J.; Yin, L.; Nesheli, D.N.; Ye, W. The Association between Poor Dental Health and Gastric Cancer Risk: A Nationwide Cohort and Sibling-Controlled Study. BMC Med. 2025, 23, 434. [Google Scholar] [CrossRef]
- Jordão, H.W.; McKenna, G.; McMenamin, Ú.C.; Kunzmann, A.T.; Murray, L.J.; Coleman, H.G. The Association between Self-reported Poor Oral Health and Gastrointestinal Cancer Risk in the UK Biobank: A Large Prospective Cohort Study. UEG J. 2019, 7, 1241–1249. [Google Scholar] [CrossRef]
- Luo, T.; Li, J.; Pu, K.; Yang, G. Association between Periodontitis and Gastrointestinal Cancer Risk and Prognosis: Evidence from a Nested Case–Control Study in Southwest China. Eur. J. Med. Res. 2025, 30, 225. [Google Scholar] [CrossRef]
- Krajden, S.; Fuksa, M.; Anderson, J.; Kempston, J.; Boccia, A.; Petrea, C.; Babida, C.; Karmali, M.; Penner, J.L. Examination of Human Stomach Biopsies, Saliva, and Dental Plaque for Campylobacter pylori. J. Clin. Microbiol. 1989, 27, 1397–1398. [Google Scholar] [CrossRef] [PubMed]
- Coker, O.O.; Dai, Z.; Nie, Y.; Zhao, G.; Cao, L.; Nakatsu, G.; Wu, W.K.; Wong, S.H.; Chen, Z.; Sung, J.J.Y.; et al. Mucosal Microbiome Dysbiosis in Gastric Carcinogenesis. Gut 2018, 67, 1024–1032. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.M.; Pereira-Marques, J.; Pinto-Ribeiro, I.; Costa, J.L.; Carneiro, F.; Machado, J.C.; Figueiredo, C. Gastric Microbial Community Profiling Reveals a Dysbiotic Cancer-Associated Microbiota. Gut 2018, 67, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Sorino, J.; Della Mura, M.; Ingravallo, G.; Cazzato, G.; Pizzimenti, C.; Zuccalà, V.; Pepe, L.; Germanà, E.; Martini, M.; Ieni, A.; et al. Fusobacterium nucleatum and Gastric Cancer: An Emerging Connection. Int. J. Mol. Sci. 2025, 26, 7915. [Google Scholar] [CrossRef]
- Castellarin, M.; Warren, R.L.; Freeman, J.D.; Dreolini, L.; Krzywinski, M.; Strauss, J.; Barnes, R.; Watson, P.; Allen-Vercoe, E.; Moore, R.A.; et al. Fusobacterium nucleatum Infection Is Prevalent in Human Colorectal Carcinoma. Genome Res. 2012, 22, 299–306. [Google Scholar] [CrossRef]
- Zhao, T.; Wang, X.; Fu, L.; Yang, K. Fusobacterium nucleatum: A New Player in Regulation of Cancer Development and Therapeutic Response. Cancer Drug Resist. 2022, 5, 424–438. [Google Scholar] [CrossRef]
- Slade, G.D.; Offenbacher, S.; Beck, J.D.; Heiss, G.; Pankow, J.S. Acute-Phase Inflammatory Response to Periodontal Disease in the US Population. J. Dent. Res. 2000, 79, 49–57. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, Inflammation, and Cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef]
- Salazar, C.R.; Francois, F.; Li, Y.; Corby, P.; Hays, R.; Leung, C.; Bedi, S.; Segers, S.; Queiroz, E.; Sun, J.; et al. Association between Oral Health and Gastric Precancerous Lesions. Carcinogenesis 2012, 33, 399–403. [Google Scholar] [CrossRef]
- Zhou, Y.; Luo, G.-H. Porphyromonas gingivalis and Digestive System Cancers. World J. Clin. Cases 2019, 7, 819–829. [Google Scholar] [CrossRef]
- Tongtawee, T.; Wattanawongdon, W.; Simawaranon, T. Effects of Periodontal Therapy on Eradication and Recurrence of Helicobacter pylori Infection after Successful Treatment. J. Int. Med. Res. 2019, 47, 875–883. [Google Scholar] [CrossRef]
- Meyer, M.S.; Joshipura, K.; Giovannucci, E.; Michaud, D.S. A Review of the Relationship between Tooth Loss, Periodontal Disease, and Cancer. Cancer Causes Control 2008, 19, 895–907. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Segers, S.; Hayes, R.B. Periodontal Disease, Porphyromonas Gingivalis Serum Antibody Levels and Orodigestive Cancer Mortality. Carcinogenesis 2012, 33, 1055–1058. [Google Scholar] [CrossRef]
- Lofgren, J.L.; Whary, M.T.; Ge, Z.; Muthupalani, S.; Taylor, N.S.; Mobley, M.; Potter, A.; Varro, A.; Eibach, D.; Suerbaum, S.; et al. Lack of Commensal Flora in Helicobacter pylori–Infected INS-GAS Mice Reduces Gastritis and Delays Intraepithelial Neoplasia. Gastroenterology 2011, 140, 210–220.e4. [Google Scholar] [CrossRef] [PubMed]
- Kostic, A.D.; Chun, E.; Robertson, L.; Glickman, J.N.; Gallini, C.A.; Michaud, M.; Clancy, T.E.; Chung, D.C.; Lochhead, P.; Hold, G.L.; et al. Fusobacterium nucleatum Potentiates Intestinal Tumorigenesis and Modulates the Tumor-Immune Microenvironment. Cell Host Microbe 2013, 14, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Buti, L.; Spooner, E.; Van Der Veen, A.G.; Rappuoli, R.; Covacci, A.; Ploegh, H.L. Helicobacter pylori Cytotoxin-Associated Gene A (CagA) Subverts the Apoptosis-Stimulating Protein of P53 (ASPP2) Tumor Suppressor Pathway of the Host. Proc. Natl. Acad. Sci. USA 2011, 108, 9238–9243. [Google Scholar] [CrossRef]
- Chmielewski, M.; Pilloni, A.; Cuozzo, A.; D’Albis, G.; D’Elia, G.; Papi, P.; Marini, L. The 2018 Classification of Periodontitis: Challenges from Clinical Perspective. Dent. J. 2025, 13, 361. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).