Clinical and Dermoscopic Patterns of Basal Cell Carcinoma and Its Mimickers in Skin of Color: A Practical Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bradford, P.T. Skin Cancer in Skin of Color. Dermatol. Nurs. 2009, 21, 170–177, 206; quiz 178. [Google Scholar] [PubMed]
- Karampinis, E.; Lallas, A.; Lazaridou, E.; Errichetti, E.; Apalla, Z. Race-Specific and Skin of Color Dermatoscopic Characteristics of Skin Cancer: A Literature Review. Dermatol. Pract. Concept. 2023, 13, e2023311S. [Google Scholar] [CrossRef]
- Enechukwu, N.A.; Behera, B.; Ding, D.D.; Lallas, A.; Chauhan, P.; Khare, S.; Sławińska, M.; Nisa Akay, B.; Ankad, B.S.; Bhat, Y.J.; et al. Dermoscopy of Cutaneous Neoplasms in Skin of Color—A Systematic Review by the International Dermoscopy Society “Imaging in Skin of Color” Task Force. Dermatol. Pract. Concept. 2023, 13, e2023308S. [Google Scholar] [CrossRef] [PubMed]
- Khare, S.; Behera, B.; Ding, D.D.; Lallas, A.; Chauhan, P.; Enechukwu, N.A.; Sławińska, M.; Akay, B.N.; Ankad, B.S.; Bhat, Y.J.; et al. Dermoscopy of Hair and Scalp Disorders (Trichoscopy) in Skin of Color—A Systematic Review by the International Dermoscopy Society “Imaging in Skin of Color” Task Force. Dermatol. Pract. Concept. 2023, 13, e2023210S. [Google Scholar] [CrossRef]
- Chauhan, P.; Behera, B.; Ding, D.D.; Lallas, A.; Khare, S.; Enechukwu, N.A.; Sławińska, M.; Nisa Akay, B.; Ankad, B.S.; Bhat, Y.J.; et al. Dermoscopy of Infectious Dermatoses (Infectiouscopy) in Skin of Color—A Systematic Review by the International Dermoscopy Society “Imaging in Skin of Color” Task Force. Dermatol. Pract. Concept. 2023, 13, e2023309S. [Google Scholar] [CrossRef]
- Sławińska, M.; Żółkiewicz, J.; Behera, B.; Ding, D.D.; Lallas, A.; Chauhan, P.; Khare, S.; Enechukwu, N.A.; Akay, B.N.; Ankad, B.S.; et al. Dermoscopy of Inflammatory Dermatoses (Inflammoscopy) in Skin of Color—A Systematic Review by the International Dermoscopy Society “Imaging in Skin of Color” Task Force. Dermatol. Pract. Concept. 2023, 13, e2023297S. [Google Scholar] [CrossRef]
- Karampinis, E.; Toli, O.; Georgopoulou, K.-E.; Kampra, E.; Spyridonidou, C.; Roussaki Schulze, A.-V.; Zafiriou, E. Can Artificial Intelligence “Hold” a Dermoscope?—The Evaluation of an Artificial Intelligence Chatbot to Translate the Dermoscopic Language. Diagnostics 2024, 14, 1165. [Google Scholar] [CrossRef]
- Torres, V.; Herane, M.I.; Costa, A.; Martin, J.P.; Troielli, P. Refining the Ideas of “Ethnic” Skin. An. Bras. Dermatol. 2017, 92, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Dhir, A.; Orengo, I.; Bruce, S.; Kolbusz, R.V.; Alford, E.; Goldberg, L. Basal Cell Carcinoma on the Scalp of an Indian Patient. Dermatol. Surg. 1995, 21, 247–250. [Google Scholar] [CrossRef]
- Pandey, S.; Sharma, V.; Titiyal, G.; Satyawali, V. Sequential Occurrence of Basal Cell Carcinoma in Symmetrically Identical Positions of Both Lower Eyelids: A Rare Finding of a Common Skin Cancer. Oman J. Ophthalmol. 2010, 3, 145–147. [Google Scholar] [CrossRef]
- Sen, S.; Bandyopadhyay, D. Periungual Basal Cell Carcinoma: A Case Report with Review of Literature. Indian J. Dermatol. 2011, 56, 220–222. [Google Scholar] [CrossRef] [PubMed]
- Jian-De, H.; Qi-Man, L.; Yu-Yun, Z.; Li-Hua, C.; Chun-Guang, M.; Cheng, T. Successful Treatment of Giant Basal Cell Carcinoma with Topical Imiquimod 5% Cream with Long Term Follow-Up. Indian J. Dermatol. 2014, 59, 575–578. [Google Scholar] [CrossRef] [PubMed]
- Verma, K.; Khaitan, B.; Malhotra, A.; Bansal, A.; Mridha, A. Superficial Basal Cell Carcinoma on Face Treated with 5% Imiquimod Cream. Indian. J. Dermatol. Venereol. Leprol. 2006, 72, 373–375. [Google Scholar] [CrossRef] [PubMed]
- Jetley, S.; Jairajpuri, Z.; Rana, S.; Talikoti, M. Adenoid Basal Cell Carcinoma and Its Mimics. Indian J. Dermatol. 2013, 58, 244. [Google Scholar] [CrossRef]
- Tambe, S.; Ghate, S.; Jerajani, H. Adenoid Type of Basal Cell Carcinoma: Rare Histopathological Variant at an Unusual Location. Indian. J. Dermatol. 2013, 58, 159. [Google Scholar] [CrossRef]
- Nadiminti, U.; Rakkhit, T.; Washington, C. Morpheaform Basal Cell Carcinoma in African Americans. Dermatol. Surg. 2004, 30, 1550–1552. [Google Scholar] [CrossRef]
- Mehta, V.; Balachandran, C. Pigmented Basal Cell Carcinoma Successfully Treated with 5% Imiquimod Cream. Indian J. Dermatol. 2008, 53, 140–141. [Google Scholar] [CrossRef]
- Rao, A. Coexistence of Solid (Nodular) and Differentiated (Adenoid) Basal Cell Carcinoma at the Same Anatomical Site. Indian J. Dermatol. 2015, 60, 524. [Google Scholar] [CrossRef]
- Sarkar, S.; Kunal, P.; Kishore, B.; Ghosh, K. Neglected Basal Cell Carcinoma on Scalp. Indian J. Dermatol. 2016, 61, 85–87. [Google Scholar] [CrossRef]
- Singha, J.; Patel, N. Superficial Basal Cell Carcinoma on the Face Is a Diagnostic Challenge. Indian J. Dermatol. 2016, 61, 236. [Google Scholar] [CrossRef]
- Dongre, A.; Khopkar, U.; Kalyanpad, Y.; Gole, P. Fibroepithelioma of Pinkus in Continuity with Nodular Basal Cell Carcinoma: A Rare Presentation. Indian Dermatol. Online J. 2016, 7, 285–287. [Google Scholar] [CrossRef] [PubMed]
- Usatine, R.P.; Heath, C.R. Basal Cell Carcinoma. J. Fam. Pract. 2021, 71, E11–E12. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, C.; Singh, K.; Pujani, M.; Verma, P.; Chauhan, V. Solitary Nodular Lesion on Forehead in a 56-Year-Old Woman. Indian J. Dermatol. Venereol. Leprol. 2019, 85, 555–558. [Google Scholar] [CrossRef] [PubMed]
- Oram, Y.; Orengo, I.; Alford, E.; Green, L.K.; Rosen, T.; Netscher, D.T. Basal Cell Carcinoma of the Scalp Resulting in Spine Metastasis in a Black Patient. J. Am. Acad. Dermatol. 1994, 31, 916–920. [Google Scholar] [CrossRef] [PubMed]
- Woods, S.G. Basal Cell Carcinoma in the Black Population. Int. J. Dermatol. 1995, 34, 517–518. [Google Scholar] [CrossRef]
- Matsuoka, L.Y.; Schauer, P.K.; Sordillo, P.P. Basal Cell Carcinoma in Black Patients. J. Am. Acad. Dermatol. 1981, 4, 670–672. [Google Scholar] [CrossRef]
- Greenbaum, S.S.; Krull, E.A.; Simmons, E.B. Basal Cell Carcinoma at the Base of the Penis in a Black Patient. J. Am. Acad. Dermatol. 1989, 20, 317–319. [Google Scholar] [CrossRef]
- Chorun, L.; Norris, J.E.C.; Gupta, M. Basal Cell Carcinoma in Blacks. Ann. Plast. Surg. 1994, 33, 90–95. [Google Scholar] [CrossRef]
- Frank, W.; Morris, D. Large Basal Cell Carcinoma in a Black Patient. Plast. Reconstr. Surg. 1995, 96, 493–494. [Google Scholar] [CrossRef]
- Humphreys, T.R.; Goldberg, L.H. A Persistent Dermal Nodule in an African-American Patient. Dermatol. Surg. 1995, 21, 991–992. [Google Scholar] [CrossRef]
- Jahan-Tigh, R.R.; Alston, J.L.; Umphlett, M. Basal Cell Carcinoma with Metastasis to the Lung in an African American Man. J. Am. Acad. Dermatol. 2010, 63, e87–e89. [Google Scholar] [CrossRef]
- Boal, N.S.; Milman, T.; Shields, C.L. A Black-Pigmented Eyelid Nodule in an African American Woman. JAMA Ophthalmol. 2020, 138, 99–100. [Google Scholar] [CrossRef]
- Bigler, C.; Feldman, J.; Hall, E.; Padilla, R.S. Pigmented Basal Cell Carcinoma in Hispanics. J. Am. Acad. Dermatol. 1996, 34, 751–752. [Google Scholar] [CrossRef] [PubMed]
- Deepadarshan, K.; Mallikarjun, M.; Abdu, N.N. Pigmented Basal Cell Carcinoma: A Clinical Variant, Report of Two Cases. J. Clin. Diagn. Res. 2013, 7, 3010–3011. [Google Scholar] [CrossRef]
- Abudu, B.; Cohen, P.R. Pigmented Basal Cell Carcinoma Masquerading as a Melanoma. Cureus 2019, 11, e4369. [Google Scholar] [CrossRef] [PubMed]
- Kara, M.; Colgecen, E.; Yildirim, E. Vulvar Basal Cell Carcinoma. Indian J. Pathol. Microbiol. 2012, 55, 583–584. [Google Scholar] [CrossRef]
- Javidi, Z.; Nahidi, Y.; Meibodi, N.; Maleki, M. Clinicopathological Evaluation of Radiation Induced Basal Cell Carcinoma. Indian J. Dermatol. 2008, 53, 137–139. [Google Scholar] [CrossRef]
- Baruah, B.; Sengupta, S.; Kesari, S.P.; Ilapakurty, B. Pattern of Nonmelanoma Skin Cancers in Sikkim, India: A 3-Year Clinicopathological Review. Indian J. Otolaryngol. Head Neck Surg. 2013, 65, 160–162. [Google Scholar] [CrossRef][Green Version]
- Gupta, R.; Bhaduri, A.; Desai, S.; Das, S.; Menon, V. Malignant Tumors of the Eyelid in India: A Multicenter, Multizone Study on Clinicopathologic Features and Outcomes. Indian J. Ophthalmol. 2020, 68, 2466–2470. [Google Scholar] [CrossRef]
- Kaliki, S.; Bothra, N.; Bejjanki, K.M.; Nayak, A.; Ramappa, G.; Mohamed, A.; Dave, T.V.; Ali, M.J.; Naik, M.N. Malignant Eyelid Tumors in India: A Study of 536 Asian Indian Patients. Ocul. Oncol. Pathol. 2019, 5, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Vinay, K.; Ankad, B.S.; Narayan, R.V.; Chatterjee, D.; Bhat, Y.J.; Neema, S.; Shah, S.; Chauhan, P.; Khare, S.; Rajput, C.; et al. A Multicentric Study on Dermoscopic Patterns and Clinical–Dermoscopic–Histological Correlates of Basal Cell Carcinoma in Indian Skin. Clin. Exp. Dermatol. 2022, 47, 1982–1990. [Google Scholar] [CrossRef] [PubMed]
- Supekar, B.B.; Tomar, S.S.; Wankhade, V.H.; Bhushan, R.; Singh, R.P.; Bhat, D.M. Clinical Spectrum of Cutaneous Malignancies in Central India: A Retrospective Study. Indian J. Dermatol. 2021, 66, 284–290. [Google Scholar] [CrossRef]
- Gupta, R.; Gordon, S.L.; Council, M.L.; Hurst, E.A. Clinical Characteristics of Basal Cell Carcinoma in African Americans: A 10-Year Retrospective Review at a Single Academic Institution. Dermatol. Surg. 2019, 45, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.Y.; Kim, J.M.; Kim, G.W.; Mun, J.H.; Song, M.; Ko, H.C.; Kim, B.S.; Kim, H.S.; Kim, M.B. The Clinical and Histopathological Characteristics of Early-onset Basal Cell Carcinoma in Asians. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Roh, S.-G.; Park, J.; Song, K.-H.; Nam, K.-H.; Yun, S.-K.; Kim, H.-U. Clinical and Histopathological Characteristics of Extra-Facial Basal Cell Carcinoma: Analysis of 35 Patients at the Chonbuk National University Hospital in Korea. Australas. J. Dermatol. 2014, 55, e65–e68. [Google Scholar] [CrossRef]
- Moore, M.G.; Bennett, R.G. Basal Cell Carcinoma in Asians: A Retrospective Analysis of Ten Patients. J. Skin Cancer 2012, 2012, 741397. [Google Scholar] [CrossRef]
- Bin Yap, F.B. Clinical Characteristics of Basal Cell Carcinoma in a Tertiary Hospital in Sarawak, Malaysia. Int. J. Dermatol. 2010, 49, 176–179. [Google Scholar] [CrossRef]
- Kikuchi, A.; Shimizu, H.; Nishikawa, T. Clinical Histopathological Characteristics of Basal Cell Carcinoma in Japanese Patients. Arch. Dermatol. 1996, 132, 320–324. [Google Scholar] [CrossRef]
- Lui, P.C.W.; Fan, Y.S.; Lau, P.P.L.; Chau, T.K.F.; Tang, V.W.L.; Tse, G.M.K.; Yu, A.M.C.; Vong, J.S.L.; Tan, P.H.; Trendell-Smith, N.J. Vulvar Basal Cell Carcinoma in China: A 13-Year Review. Am. J. Obstet. Gynecol. 2009, 200, 514.e1–514.e5. [Google Scholar] [CrossRef]
- Kumar, S.; Mahajan, B.B.; Kaur, S.; Yadav, A.; Singh, N.; Singh, A. A Study of Basal Cell Carcinoma in South Asians for Risk Factor and Clinicopathological Characterization: A Hospital Based Study. J. Skin Cancer 2014, 2014, 173582. [Google Scholar] [CrossRef]
- Tan, E.S.; Ee, M.; Shen, L.; Chua, H.; Chan, Y.; Tan, S. Basal Cell Carcinoma in Singapore: A Prospective Study on Epidemiology and Clinicopathological Characteristics with a Secondary Comparative Analysis between Singaporean Chinese and Caucasian Patients. Australas. J. Dermatol. 2015, 56, 175–179. [Google Scholar] [CrossRef]
- Manci, R.; Dauscher, M.; Marchetti, M.A.; Usatine, R.; Rotemberg, V.; Dusza, S.; Marghoob, A. Features of Skin Cancer in Black Individuals: A Single-Institution Retrospective Cohort Study. Dermatol. Pract. Concept. 2022, 12, e2022075. [Google Scholar] [CrossRef]
- Behera, B.; Kumari, R.; Thappa, D.M.; Gochhait, D.; Srinivas, B.H.; Ayyanar, P. Dermoscopic Features of Basal Cell Carcinoma in Skin of Color: A Retrospective Cross-Sectional Study from Puducherry, South India. Indian J. Dermatol. Venereol. Leprol. 2023, 89, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Rose, C.; Kaddu, S.; El-Sherif, T.F.; Kerl, H. A Distinctive Type of Widespread Congenital Melanocytic Nevus with Large Nodules. J. Am. Acad. Dermatol. 2003, 49, 732–735. [Google Scholar] [CrossRef] [PubMed]
- Bansal, R.K.; Bhaduri, A.S.; Pancholi, Y.J.; Balar, D.B. Cellular Blue Nevus with Nevus Cells in Regional Lymph Nodes: A Lesion That Mimics Melanoma. Indian J. Cancer 1989, 26, 145–150. [Google Scholar]
- Matrakool, P.; Chaisrisawadisuk, S.; Vongviriyangkoon, T. Prognostic Factors and Outcomes of Cutaneous Malignant Melanoma. Ann. Plast. Surg. 2023, 90, 621–625. [Google Scholar] [CrossRef]
- Tan, E.; Chua, S.H.; Lim, J.T.; Goh, C.L. Malignant Melanoma Seen in a Tertiary Dermatological Centre, Singapore. Ann. Acad. Med. Singap. 2001, 30, 414–418. [Google Scholar] [PubMed]
- Suseelan, A.V.; Gupta, I.M. Malignant Melanoma in Nigeria-Pathological Studies. Afr. J. Med. Med. Sci. 1977, 6, 209–214. [Google Scholar]
- Pai, R.; Kini, H.; Kamath, S.; Kumar, S. Giant Hanging Melanoma of the Eyelid Skin. Indian J. Ophthalmol. 2008, 56, 239–240. [Google Scholar] [CrossRef]
- Krishnamurthy, A.; Ramshankar, V.; Majhi, U. The Aesthetic and Oncological Challenges in the Management of an Atypical Nodular Hidradenoma of the Pinna. Indian J. Surg. Oncol. 2014, 5, 148–151. [Google Scholar] [CrossRef][Green Version]
- Kanitakis, J.; Brutzkus, A.; Butnaru, A.C.; Claudy, A. Melanotrichoblastoma. Am. J. Dermatopathol. 2002, 24, 498–501. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, S.; Singh, S.; Khera, S.; Bardia, A. Pigmented Nipple-like Nodule on the Neck. Indian J. Dermatol. Venereol. Leprol. 2019, 85, 235. [Google Scholar] [CrossRef] [PubMed]
- Kamat, G.; Yelikar, B.; Shettar, S.; Karigoudar, M. Pigmented Trichoblastoma with Sebaceous Hyperplasia. Indian J. Dermatol. Venereol. Leprol. 2009, 75, 506–508. [Google Scholar] [CrossRef]
- Goncharuk, V.; Mulvaney, M.; Carlson, J.A. Bednár Tumor Associated with Dermal Melanocytosis: Melanocytic Colonization or Neuroectodermal Multidirectional Differentiation? J. Cutan. Pathol. 2003, 30, 147–151. [Google Scholar] [CrossRef]
- Camara, M.; Pinheiro, P.M.; Jales, R.; da Trindade Neto, P.B.; Costa, J.; Rocha de Sousa, V.L. Multiple Dermatofibromas: Dermoscopic Patterns. Indian J. Dermatol. 2013, 58, 243. [Google Scholar] [CrossRef]
- Vasani, R.J.; Khanna, D.; Singal, A. Cutaneous Vascular Lesions and Their Management in Indian Setting. Dermatol. Ther. 2012, 25, 358–375. [Google Scholar] [CrossRef]
- Shenoy, M.; Girisha, B.; Krishna, S. Chromoblastomycosis: A Case Series and Literature Review. Indian Dermatol. Online J. 2023, 14, 665–669. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Lee, S.M.; Sung, H.S.; Won, C.H.; Chang, S.; Lee, M.W.; Choi, J.-H.; Moon, K.-C. Clinical Analysis of Deep Cutaneous Mycoses: A 12-year Experience at a Single Institution. Mycoses 2012, 55, 501–506. [Google Scholar] [CrossRef]
- Khullar, G.; Saikia, U.; De, D.; Handa, S.; Radotra, B. Predisposing Factors and Histopathological Variants of Cutaneous Squamous Cell Carcinoma: Experience from a North Indian Teaching Hospital. Indian J. Dermatol. Venereol. Leprol. 2016, 82, 273–278. [Google Scholar] [CrossRef]
- Asati, D.; Brahmachari, S.; Kudligi, C.; Gupta, C. Hidradenocarcinoma: A Rare Sweat Gland Neoplasm Presenting as Small Turban Tumor of the Scalp. Indian J. Dermatol. 2015, 60, 421. [Google Scholar] [CrossRef]
- Miller, A.; Siller, A., Jr.; Rodriguez, R.; Gill, P.; Curry, J.L.; Tyring, S.K. A Pearly Nodule on an Indurated Plaque. Dermatol. Online J. 2022, 27, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Mani, M.Z.; Feierabend, C.T. Verrucous Haemangioma. Indian J. Dermatol. Venereol. Leprol. 1982, 48, 116–117. [Google Scholar]
- Khullar, G.; Narang, T.; De, D.; Chougule, A.; Handa, S. Isolated Benign Primary Cutaneous Plasmacytosis in an Adult Indian Male. Dermatol. Online J. 2016, 22. [Google Scholar] [CrossRef]
- Jemisingh, P.; MaalikBabu, A.M.; Arumugam, V.; Palanivel, N. Interesting Case of Cutaneous Metastases to Thoracic Skin from Anaplastic Carcinoma of Thyroid: An Unreported Entity in India. Indian J. Dermatol. 2022, 67, 93. [Google Scholar] [CrossRef] [PubMed]
- Rahima, S.; Najeeba, R. Psoriatic Arthritis with Acral Lentiginous Melanoma: Role for Methotrexate? Indian J. Dermatol. 2013, 58, 492. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, P.P.; Koumarianou, A.A.; Chu, A.C. Pigmented Bowen’s Disease. Br. J. Dermatol. 1998, 138, 515–518. [Google Scholar] [CrossRef]
- Musaddique Ansari, S.; Gupta, A.; Nayak, C. Bowen’s Disease on Two Different Unrelated Anatomical Sites (Genitals and Nail) in Succession in an Immunocompromised Patient. Indian J. Sex. Transm. Dis. AIDS 2022, 43, 189–191. [Google Scholar] [CrossRef]
- Jayaraman, M.; Janaki, V.R.; Yesudian, P. Cutaneous B-Cell Lymphoma. Indian J. Dermatol. Venereol. Leprol. 1995, 61, 317–319. [Google Scholar] [PubMed]
- Nagalla, R.R.; Lee, B.A.; Smith, J.; Kraus, C.N. Eroded Pigmented Anogenital Plaque in an Elderly Woman. JAAD Case Rep. 2023, 34, 52–54. [Google Scholar] [CrossRef]
- Yuki, A.; Takatsuka, S.; Abe, R.; Takenouchi, T. Diagnostic Accuracy of Dermoscopy for 934 Basal Cell Carcinomas: A Single-center Retrospective Study. J. Dermatol. 2023, 50, 64–71. [Google Scholar] [CrossRef]
- Sutedja, E.K.; Ahmed, R.; Sutedja, E.; Rowawi, R.; Suwarsa, O.; Gunawan, H. A Successful Defect Closure after Total Excision of Seborrheic Keratoses with Atypical Clinical Presentation Using Island Pedicle Flap in an Elderly Patient. Int. Med. Case Rep. J. 2021, 14, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Nutan; Dogra, S.; Kanwar, A.J. Bowen Disease over Photoprotected Site in an Indian Male. Dermatol. Online J. 2009, 15, 16. [Google Scholar] [CrossRef]
- McKinley, E.; Valles, R.; Bang, R.; Bocklage, T. Signet-ring Squamous Cell Carcinoma: A Case Report. J. Cutan. Pathol. 1998, 25, 176–181. [Google Scholar] [CrossRef]
- Chatterjee, M.; Chand, K.; Banerjee, S. Extramammary Paget′s Disease. Indian J. Dermatol. Venereol. Leprol. 2005, 71, 417–420. [Google Scholar] [CrossRef]
- Kharkar, V.; Gutte, R.; Khopkar, U.; Mahajan, S.; Chikhalkar, S. Kaposi’s Sarcoma: A Presenting Manifestation of HIV Infection in an Indian. Indian J. Dermatol. Venereol. Leprol. 2009, 75, 391–393. [Google Scholar] [CrossRef]
- Gupta, S.N.; Flaherty, J.P.; Shaw, J.C. Erythema Nodosum Associated with Reactivation Tuberculous Lymphadenitis (Scrofula). Int. J. Dermatol. 2002, 41, 173–175. [Google Scholar] [CrossRef]
- Sharma, V.K.; Kumar, B.; Kaur, I.; Kaur, S. Side Lab Diagnosis of Chromoblastomycosis. Indian J. Dermatol. Venereol. Leprol. 1985, 51, 157–159. [Google Scholar] [PubMed]
- Arora, N.; Goel, A.; Kumar, P.; Bhargava, A. Secondary Cutaneous Mucormycosis—Retrospective Analysis from Tertiary Care Hospitall. Indian J. Otolaryngol. Head Neck Surg. 2023, 75, 1712–1718. [Google Scholar] [CrossRef] [PubMed]
- Lehrhoff, S.; Tzu, J.; Patel, R.; Sanchez, M.; Franks, A.G. Lupus Erythematosus Tumidus with Discoid Lupus Erythematosus-Induced Alopecia of the Scalp. Dermatol. Online J. 2011, 17, 24. [Google Scholar] [CrossRef]
- Heath, C.R. Atopic Dermatitis. J. Fam. Pract. 2021, 70, 252. [Google Scholar] [CrossRef]
- Mittal, R.R.; Bansal, N. Erythrokeratodermia Progressivum Symmetricum. Indian J. Dermatol. Venereol. Leprol. 1998, 64, 126–127. [Google Scholar] [PubMed]
- Mahajan, R.; Kumaran, M.S.; Narang, T.; Handa, S.; Dogra, S. Genital Psoriasis among Indians: A Prospective Cross-Sectional Study. Australas. J. Dermatol. 2015, 56, e18–e20. [Google Scholar] [CrossRef]
- Sinhasan, S.; Jadhav, C.; Bhat, R.; Amaranathan, A. Pilomatrixoma—Presented as Hypopigmented Tender Nodule: Diagnosed by FNAC: A Case Report with Review of Literature. Indian J. Dermatol. 2013, 58, 405. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.; Tay, Y. Tufted Angioma: A Report of Five Cases. Pediatr. Dermatol. 2002, 19, 388–393. [Google Scholar] [CrossRef]
- Nie, J.; Li, Y.; Shen, X.; Liu, Y.; Shi, H.; Lu, Y. Nodular Malignant Melanoma in Vulvar Skin without Pigmentation: A Case Report. BMC Women’s Health 2021, 21, 289. [Google Scholar] [CrossRef]
- Brar, S.; Bano, R.; Puri, N.; Singh, A. A Study on Clinical Spectrum of Lichen Sclerosus in a Tertiary Care Centre in North India. Indian J. Sex. Transm. Dis. AIDS 2022, 43, 43–46. [Google Scholar] [CrossRef]
- Ankad, B.; Sakhare, P.; Prabhu, M. Dermoscopy of Non-Melanocytic and Pink Tumors in Brown Skin: A Descriptive Study. Indian J. Dermatopathol. Diagn. Dermatol. 2017, 4, 41. [Google Scholar] [CrossRef]
- Behera, B.; Kumari, R.; Thappa, D.M.; Gochhait, D.; Srinivas, B.H.; Ayyanar, P. Dermoscopy of Bowen’s Disease: A Case Series of Five Patients. Indian J. Dermatol. Venereol. Leprol. 2021, 87, 576–580. [Google Scholar] [CrossRef] [PubMed]
- Cabo, H.; Salerni, G.; Cohen Sabban, E.; Bollea Garlatti, A.; Orendain, N.; Rodriguez Saa, S.; Marchiori Bakos, R.; Pozzobon, F.C.; Gonzalez, V.M.; Peralta, R.; et al. Dermoscopic Features of Pigmented Bowen Disease: A Multicenter Study on Behalf of the Ibero-Latin American College of Dermatology (CILAD). Dermatol. Pract. Concept. 2024, 14, e2024086. [Google Scholar] [CrossRef]
- Mustari, A.; Chauhan, P.; Chatterjee, D.; Vinay, K. Dermoscopy of Dermatofibrosarcoma Protuberans in Skin of Colour: A Study of Four Cases. Indian J. Dermatol. Venereol. Leprol. 2023, 5, 1–3. [Google Scholar] [CrossRef]
- Kelati, A.; Aqil, N.; Baybay, H.; Gallouj, S.; Mernissi, F.Z. Beyond Classic Dermoscopic Patterns of Dermatofibromas: A Prospective Research Study. J. Med. Case Rep. 2017, 11, 266. [Google Scholar] [CrossRef] [PubMed]
- Giddens, T.; Seiverling, E.; Marghoob, A.; Usatine, R. Absence of Central White Patch in Dermatofibromas Presenting in Darker Skin. JAAD Case Rep. 2022, 21, 63–65. [Google Scholar] [CrossRef] [PubMed]
- Lallas, A.; Reggiani, C.; Argenziano, G.; Kyrgidis, A.; Bakos, R.; Masiero, N.C.M.S.; Scheibe, A.B.; Cabo, H.; Ozdemir, F.; Sortino-Rachou, A.M.; et al. Dermoscopic Nevus Patterns in Skin of Colour: A Prospective, Cross-Sectional, Morphological Study in Individuals with Skin Type V and VI. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 1469–1474. [Google Scholar] [CrossRef]
- Tuma, B.; Yamada, S.; Atallah, Á.N.; Araujo, F.M.; Hirata, S.H. Dermoscopy of Black Skin: A Cross-Sectional Study of Clinical and Dermoscopic Features of Melanocytic Lesions in Individuals with Type V/VI Skin Compared to Those with Type I/II Skin. J. Am. Acad. Dermatol. 2015, 73, 114–119. [Google Scholar] [CrossRef]
- Madankumar, R.; Gumaste, P.V.; Martires, K.; Schaffer, P.R.; Choudhary, S.; Falto-Aizpurua, L.; Arora, H.; Kallis, P.J.; Patel, S.; Damanpour, S.; et al. Acral Melanocytic Lesions in the United States: Prevalence, Awareness, and Dermoscopic Patterns in Skin-of-Color and Non-Hispanic White Patients. J. Am. Acad. Dermatol. 2016, 74, 724–730.e1. [Google Scholar] [CrossRef]
- Sakamoto, S.; Oiso, N.; Narita, T.; Kawada, A. Blue Nevus with a Dermoscopic Appearance of Peripheral Streaks with Branches. Case Rep. Dermatol. 2014, 6, 66–68. [Google Scholar] [CrossRef] [PubMed]
- Behera, B.; Chandrashekar, L.; Thappa, D.M.; Gochhait, D.; Srinivas, B.H.; Ayyanar, P. Dermoscopic Features of Benign Cutaneous Adnexal Tumours in Dark Skin: A Retrospective Study from South India. Australas. J. Dermatol. 2021, 62, E249–E255. [Google Scholar] [CrossRef]
- Errichetti, E.; Ankad, B.S.; Sonthalia, S.; Jha, A.K.; Keshavamurthy, V.; Kyrgidis, A.; Neema, S.; Chatterjee, M.; Kaliyadan, F.; Dogra, S.; et al. Dermoscopy in General Dermatology (Non-Neoplastic Dermatoses) of Skin of Colour: A Comparative Retrospective Study by the International Dermoscopy Society. Eur. J. Dermatol. 2020, 30, 688–698. [Google Scholar] [CrossRef] [PubMed]
- Nwako-Mohamadi, M.K.; Masenga, J.E.; Mavura, D.; Jahanpour, O.F.; Mbwilo, E.; Blum, A. Dermoscopic Features of Psoriasis, Lichen Planus, and Pityriasis Rosea in Patients with Skin Type IV and Darker Attending the Regional Dermatology Training Centre in Northern Tanzania. Dermatol. Pract. Concept. 2019, 9, 44–51. [Google Scholar] [CrossRef]
- Jindal, R.; Chauhan, P.; Sethi, S. Dermoscopy of the Diverse Spectrum of Cutaneous Tuberculosis in the Skin of Color. Dermatol. Pract. Concept. 2022, 12, e2022203. [Google Scholar] [CrossRef]
- Karampinis, E.; Nechalioti, P.-M.; Georgopoulou, K.E.; Goniotakis, G.; Roussaki Schulze, A.V.; Zafiriou, E.; Kouretas, D. Systemic Oxidative Stress Parameters in Skin Cancer Patients and Patients with Benign Lesions. Stresses 2023, 3, 785–812. [Google Scholar] [CrossRef]
- Karampinis, E.; Aloizou, A.-M.; Zafiriou, E.; Bargiota, A.; Skaperda, Z.; Kouretas, D.; Roussaki-Schulze, A.-V. Non-Melanoma Skin Cancer and Vitamin D: The “Lost Sunlight” Paradox and the Oxidative Stress Explanation. Antioxidants 2023, 12, 1107. [Google Scholar] [CrossRef] [PubMed]
- Lallas, A.; Tzellos, T.; Kyrgidis, A.; Apalla, Z.; Zalaudek, I.; Karatolias, A.; Ferrara, G.; Piana, S.; Longo, C.; Moscarella, E.; et al. Accuracy of Dermoscopic Criteria for Discriminating Superficial from Other Subtypes of Basal Cell Carcinoma. J. Am. Acad. Dermatol. 2014, 70, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Scope, A.; Benvenuto-Andrade, C.; Agero, A.L.C.; Marghoob, A.A. Nonmelanocytic Lesions Defying the Two-Step Dermoscopy Algorithm. Dermatol. Surg. 2006, 32, 1398–1406. [Google Scholar] [CrossRef] [PubMed]
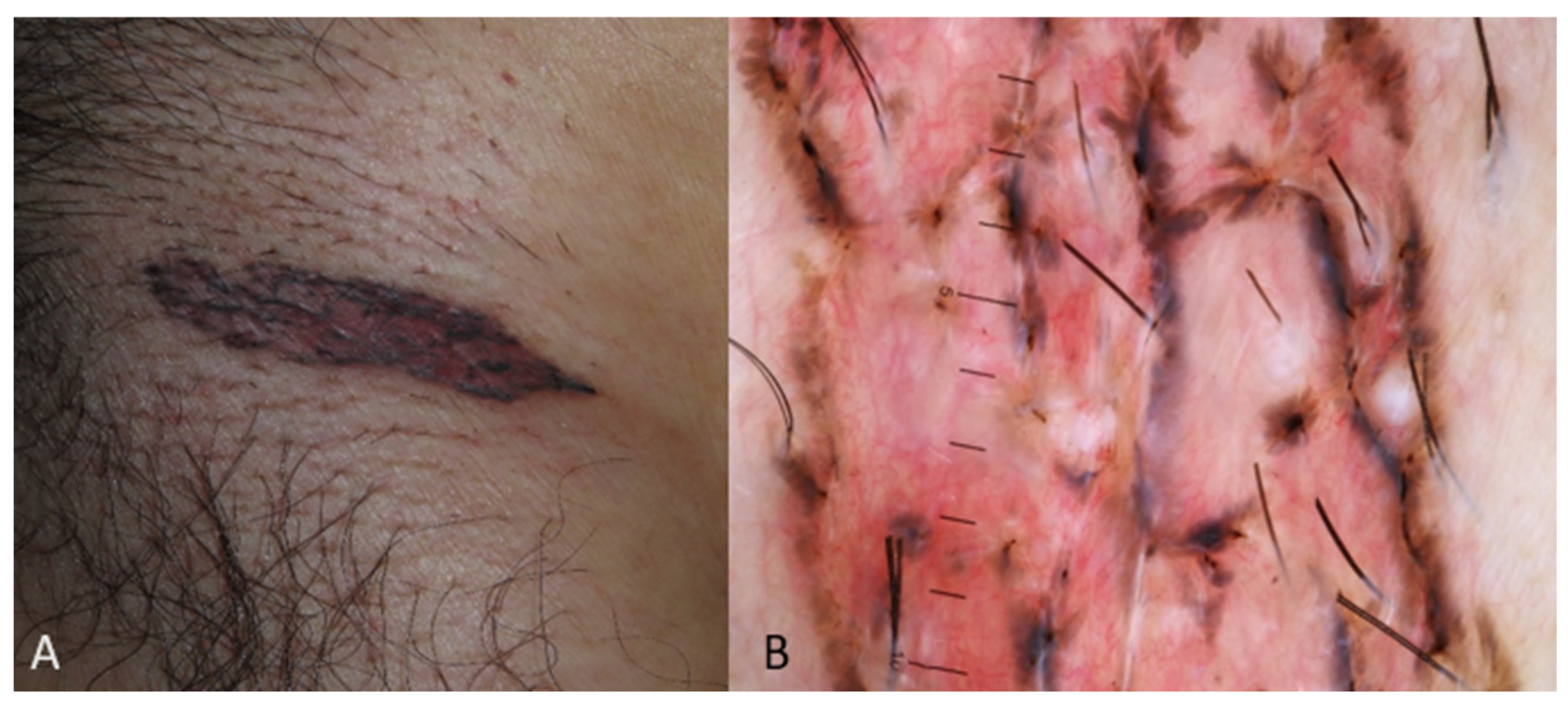
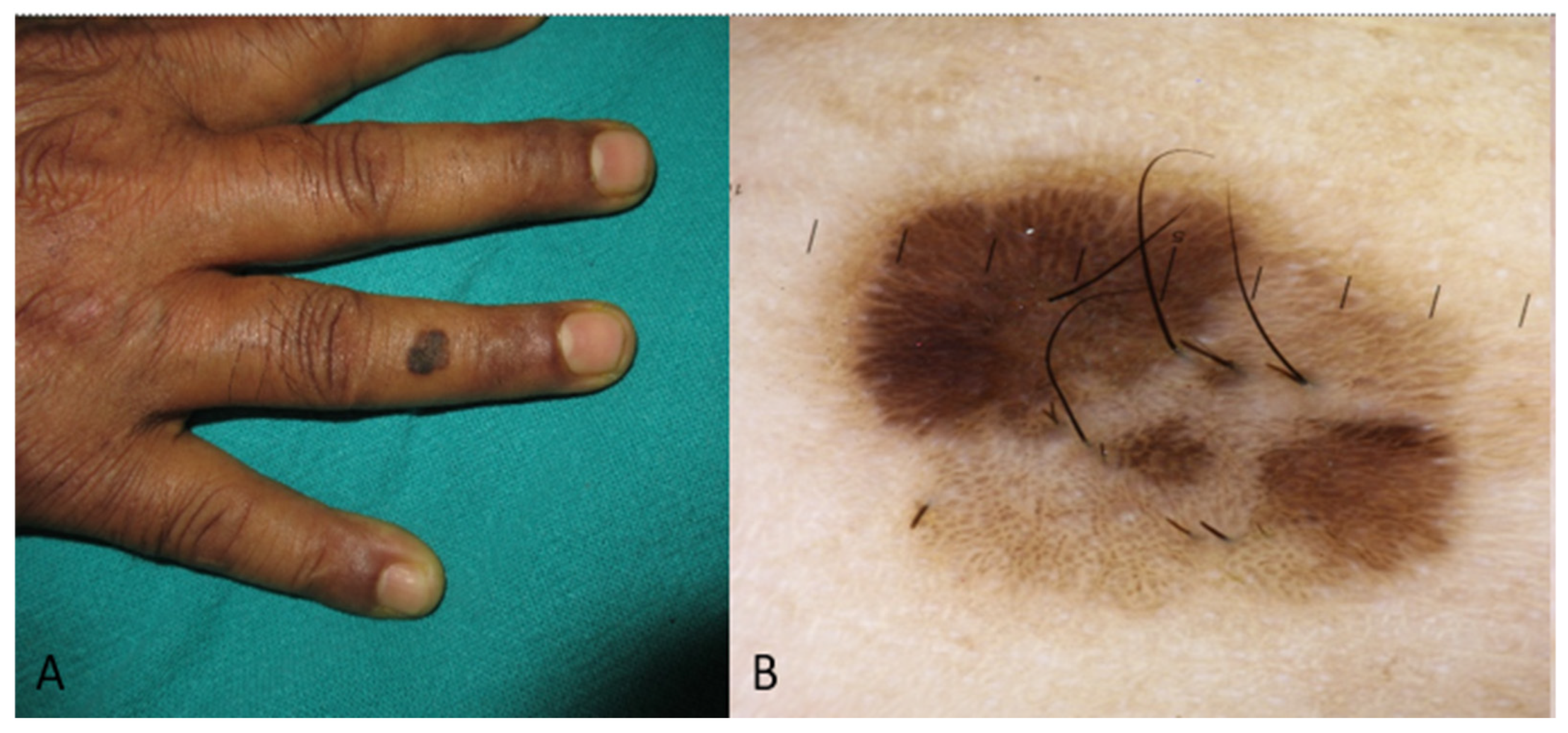
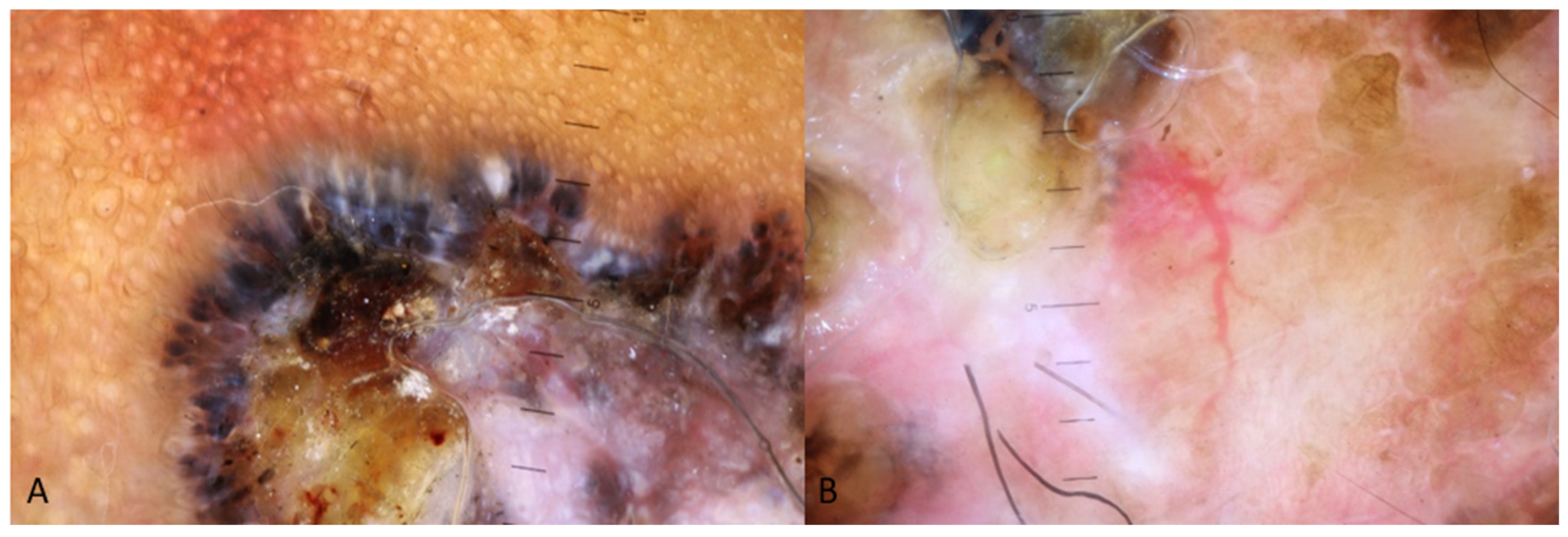
| Type of Study | Nationality | Location of the Lesion | Subtype | Differential Diagnosis (BCC Mimickers) Based on the Authors or Text of the Respective Study | |
|---|---|---|---|---|---|
| Case report | [9] | Indian | Scalp | Nodular non-pigmented | Trauma, nevus sebaceous, radiation dermatitis |
| Case series | [37] | Iran | Scalp (more frequent), forehead, nose and periorbital area | Mostly nodulocystic or micronodular pigmented | SCC |
| Case report | [36] | NM | Vulvar | Adenoid | Extramammary Paget disease, lichen sclerosus, atrophicus and lichen simplex chronicus |
| Case series | [15] | NM | 1o: Lower back 2o: Lumbosacral region | Adenoid | Pre-existing skin condition, indigenous drug intake (containing arsenic), exposure to irradiation or trauma |
| Case report | [14] | NM | Nose | Adenoid | Adenoid cystic carcinoma, metastasis |
| Case series | [38] | Indian | Upper lip (more frequent location) and cheek | Mostly nodular | SCC |
| Case report | [18] | NM | Nose | Nodular and adenoid | SCC, adenoid cystic carcinoma, scrofuloderma and deep mycosis |
| Case report | [19] | Indian | Scalp | Infiltrative | SCC |
| Case report | [20] | NM | Face | Superficial | Eczema, psoriasis, lichen planus, or Bowen’s disease. |
| Case series | [40] | Indian | Eyelid (mostly upper) | NM | SCC, sebaceous gland carcinoma, malignant melanoma, and miscellaneous tumors |
| Case series | [39] | Indian | Eyelid (mostly upper) | NM | sebaceous gland carcinoma, SCC and miscellaneous tumors |
| Case series | [42] | Indian | Forehead (most frequent) followed by cheeks | Mostly nodular followed by ulcerative and pigmented | SCC, melanoma, cutaneous lymphomas, and sarcomas. |
| Case reports | [34] | Indian | Temporal region of scalp and inner canthus of left eye | Pigmented | Melanoma, squamous cell carcinoma, discoid lupus erythematosus and nevus comedonicus |
| Case report | [25] | NS (Black) | Groin region | Micronodular and infiltrative | SCC, metastasis, sarcoma |
| Case reports | [28] | NS (Black) and Asian | Head and neck region (most frequent) | NM | SCC, dermatofibroma protuberans, nevus, melanoma |
| Case report | [29] | NS (Black) | Upper lip | Nodular | SCC, burn, chronic infection |
| Case report | [16] | African American | Nose | Morpheaform | Melanoma, seborrheic keratoses or nevus sebaceous |
| Case report | [31] | African American | Forehead | Infiltrative | SCC, Marjolin ulcer, metastasis |
| Case series | [43] | African Americans | Mostly in head and neck regions | Mostly pigmented followed by infiltrative | Seborrheic keratosis, benign nevus |
| Case report | [32] | African American | Eyelid | Nodular | Benign tumors, such as nevi, blue nevi, seborrheic keratosis, apocrine hidrocystomas, vascular malformations and inflammatory processes (such as a chalazion) Malignant tumors such as melanoma, metastasis, pigmented SCC |
| Case reports | [35] | Hispanics | 1o: Nasal tip 2o: Nasal bridge 3o: Breast | Nodular | Melanoma |
| Case series | [49] | Chinese | Vulvar | Nodular (mostly) | Melanocytic nevus, seborrheic keratosis, malignant melanoma, SCC, adenoid cystic carcinoma |
| BCC Clinical Image in SoC | Main Findings |
|---|---|
| Superficial | Solitary well-defined hyperpigmented plaque or patch or erythematous, indurated, irregular plaque |
| Nodular | Pigmented nodule (usually with pearly appearance) with or without ulcerated area or red giant nodule with or without ulcerated area |
| Adenoid | Mainly ulcerated lesion |
| Morpheaform | Scar-like lesion |
| Infiltrative | Mainly ulcerated lesion |
| BCC dermoscopy image in SoC | Main findings |
| Superficial | Pigmented structures such as maple leaf-like area and spoke wheel-like areas, red-white homogenous area, multiple small erosions, short fine telangiectasia, spoke wheel-like areas [2,3,52,53]. |
| Nodular | Ulceration, blue-white veil, brown to blue-gray ovoid nests, arborising vessels [2,3,49,50,51] |
| Adenoid | - |
| Morpheaform | - |
| Infiltrative | - |
| Macroscopy Image of BCC in Skin of Color Patients | Differential Diagnosis |
|---|---|
| Pigmented nodular lesion | Cutaneous neoplasms: nevi [54], blue nevi [55], melanoma [56,57,58,59], benign appendageal tumors (such as melanotrophoblastoma and hidroadenoma, etc.) [60,61,62,63], dermatofibroma and/or pigmented dermatofibrosarcoma protuberans [64,65], vascular lesions [66] Infectious diseases: deep cutaneous mycosis (such as chromoblastomycosis in endemic regions) [67,68] |
| Ulcerated lesion | Cutaneous neoplasms: squamous cell carcinoma (such as Marjolin’s ulcer) [69], malignant appendageal tumors (such as hidraneosarcoma and sebaceous carcinoma) [70], dermatofibrosarcoma protuberans [71], vascular lesions [72], metastasis [73,74] |
| Solid pigmented plaque patch with or without ulceration | Cutaneous neoplasms: lentigious melanoma [75], pigmented Bowen [76,77], B-lymphoma [78], extramammary Paget [79], seborrheic keratosis and nevus sebaceous [80,81]. |
| Erythematous plaque or patch | Skin cancer entities: squamous cell carcinoma and Bowen disease [82,83], lymphoma and extramammary Paget [84], Kaposi sarcoma [85] Cutaneous infections such as tuberculosis [86], chromoblastomycosis [87], cutaneous mucormycosis [88] Chronic inflammations such as lupus erythematosus [89], dermatitis [90], psoriasis and erythrokeratodermia [91,92] |
| Red nodule with or without ulceration | Cutaneous neoplasms: benign appendageal tumors (pilomatricoma) [93], vascular lesions and angiomas [94], melanoma [95] |
| Scar-like appearance | Scar, dermatofibroma [65], lichen sclerosus [96] |
| Cutaneous Neoplasms | Predominant Dermoscopy Finding |
|---|---|
| SCC | White areas, scales, erosions and ulcerations, polymorphic vascular pattern such as dotted or linear or irregular or serpentine vessels, white and shiny clods [52,97] |
| Bowen’s disease | Blue-gray dots/globules, arranged either peripherally in clusters or linearly, scales, light to dark brown keratotic structureless areas [98,99] |
| Dermatofibroma protuberans | Pigment network, pink background, white structureless area [100] |
| Dermatofibroma | Peripheral pigmented network, white patches (“central scar-like” or “eccentric multiple”), central homogeneous pigmentation [101,102] |
| (Acral) Melanoma | Structureless regions displaying various tones of brown, blue, black and pink colors, erosions or ulceration, parallel and fibrilla ridge pattern of surrounding skin [52] |
| Cutaneous lymphoma | - |
| Extramammary Paget | - |
| Sebaceous keratosis | “Moth eaten” borders, comedo-like openings, milia-like cysts, “fat fingers”, cerebriform pattern, “finger print” pattern, surface white scaling [97] |
| Nevus comedonicus | Not enough data to conclude to predominant dermoscopy findings. Only case reports found |
| Nevi (reticular) | Brown or black color, reticular lines, structureless areas [103,104,105] |
| Blue nevus | Homogenous structureless blue [106] |
| Hemangioma—vascular lesions | Red-purple clods-lagoons, white lines [97] |
| Skin adnexal lesions | Examples: Trichoepithelioma: white homogenous area with milia-like cyst, linear, arborizing and crown vessels Nodular hidradenoma: a white to gray structureless area, erosion and a polymorphous vascular pattern [107] |
| Nevus sebaceous | Papillary to knob-like arrangement on a background ranging from yellow to gray, yellow-white homogeneous region, ovoid nests [107] |
| Metastasis | - |
| Inflammation diseases | |
| Dermatitis (in the context of radiation) | Irregularly distributed, predominantly yellow but also including brown and white scales, purple dots and fabric fibers, brown-black dots against a background, erosions [108] |
| Psoriasis | Dotted vessels, diffuse or patchy distributed white scales, pigmented structures, such as brown, gray, and blue structureless areas, dots, or globules [109] |
| Lichen planus | Blue globules and Wickham striae [109] |
| Facial lichen planus pigmentosus | Brown dots/globules, pseudonetwork, loss of vellus hair [108] |
| Chronic lichen sclerosis | Not enough data to conclude to predominant dermoscopy findings. Only case reports found |
| Infectious diseases | |
| Deep fungal infections Examples: chromoblastomycosis | Not enough data to conclude to predominant dermoscopy findings. Only case reports found |
| Lupus vulgaris | Yellow-orange structureless areas, linear/dotted vessels, white scales, white structureless areas [110] |
| Dermoscopic Features of the Lesion in SoC | Differential Diagnosis |
|---|---|
| Ulceration | BCC, SCC, melanoma, deep fungal infection (chromoblastomycosis) |
| Blue-white veil or blue color | BCC, melanoma, blue nevi |
| Ovoid nests | BCC, nevus sebaceous |
| Arborising vessels | BCC, adnexal tumors (melanotrichoepithelioma) |
| White homogenous/structureless areas | BCC, SCC, dermatofibroma protuberans, nevus sebaceous, skin adnexal tumors (nodular hidradenoma), dermatofibroma, lupus vulgaris |
| Scales | Psoriasis, dermatitis, SCC, Bowen’s disease, lupus vulgaris, deep fungal infection (chromoblastomycosis, etc.) |
| Dotted vessels | Lupus vulgaris, SCC, psoriasis |
| Pigment network | Nevi, melanoma, dermatofibroma protuberans |
| Erosions | SCC, dermatitis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karampinis, E.; Georgopoulou, K.-E.; Kampra, E.; Zafiriou, E.; Lallas, A.; Lazaridou, E.; Apalla, Z.; Behera, B.; Errichetti, E. Clinical and Dermoscopic Patterns of Basal Cell Carcinoma and Its Mimickers in Skin of Color: A Practical Summary. Medicina 2024, 60, 1386. https://doi.org/10.3390/medicina60091386
Karampinis E, Georgopoulou K-E, Kampra E, Zafiriou E, Lallas A, Lazaridou E, Apalla Z, Behera B, Errichetti E. Clinical and Dermoscopic Patterns of Basal Cell Carcinoma and Its Mimickers in Skin of Color: A Practical Summary. Medicina. 2024; 60(9):1386. https://doi.org/10.3390/medicina60091386
Chicago/Turabian StyleKarampinis, Emmanouil, Konstantina-Eirini Georgopoulou, Elli Kampra, Efterpi Zafiriou, Aimilios Lallas, Elizabeth Lazaridou, Zoe Apalla, Biswanath Behera, and Enzo Errichetti. 2024. "Clinical and Dermoscopic Patterns of Basal Cell Carcinoma and Its Mimickers in Skin of Color: A Practical Summary" Medicina 60, no. 9: 1386. https://doi.org/10.3390/medicina60091386
APA StyleKarampinis, E., Georgopoulou, K.-E., Kampra, E., Zafiriou, E., Lallas, A., Lazaridou, E., Apalla, Z., Behera, B., & Errichetti, E. (2024). Clinical and Dermoscopic Patterns of Basal Cell Carcinoma and Its Mimickers in Skin of Color: A Practical Summary. Medicina, 60(9), 1386. https://doi.org/10.3390/medicina60091386









