Abstract
Background and Objectives: There has been an increasing interest in the use of non-pharmacological approaches for the multidimensional treatment of chronic pain. The aim of this systematic review was to assess the effectiveness of mindfulness-based therapies and Guided Imagery (GI) interventions in managing chronic non-cancer pain and related outcomes. Materials and Methods: Searching three electronic databases (Web of Science, PubMed, and Scopus) and following the PRISMA guidelines, a systematic review was performed on Randomized Controlled Trials (RCTs) and pilot RCTs investigating mindfulness or GI interventions in adult patients with chronic non-cancer pain. The Cochrane Risk of Bias Tool was utilized to assess the quality of the evidence, with outcomes encompassing pain intensity, opioid consumption, and non-sensorial dimensions of pain. Results: Twenty-six trials met the inclusion criteria, with most of them exhibiting a moderate to high risk of bias. A wide diversity of chronic pain types were under analysis. Amongst the mindfulness interventions, and besides the classical programs, Mindfulness-Oriented Recovery Enhancement (MORE) emerges as an approach that improves interoception. Six trials demonstrated that mindfulness techniques resulted in a significant reduction in pain intensity, and three trials also reported significant outcomes with GI. Evidence supports a significant improvement in non-sensory dimensions of pain in ten trials using mindfulness and in two trials involving GI. Significant effects on opioid consumption were reported in four mindfulness-based trials, whereas one study involving GI found a small effect with that variable. Conclusions: This study supports the evidence of benefits of both mindfulness techniques and GI interventions in the management of chronic non-cancer pain. Regarding the various mindfulness interventions, a specific emphasis on the positive results of MORE should be highlighted. Future studies should focus on specific pain types, explore different durations of the mindfulness and GI interventions, and evaluate emotion-related outcomes.
1. Introduction
According to the International Association for the Study of Pain (IASP), pain consists of an unpleasant emotional and sensory experience linked to actual or potential tissue damage, driving individuals to seek medical attention [1,2]. Chronic Pain (CP) is considered a pathological condition characterized by its persistence beyond the healing period, typically around 3 months [2,3,4]. The source of the physical pain may or may not be identified [5,6]. CP’s prevalence is very diverse, ranging from 11% to 63% [7,8,9,10].
The biopsychosocial model describes CP as a dynamic interplay of physiological, emotional, and social factors [11], including vulnerability, conditioned responses, and emotional–cognitive states, impairing the quality of life and with an economic impact [12]. In this context, sensory variables are important to assess, namely pain intensity, but also emotional variables, particularly anxiety and depression. More complex aspects are also to be considered, namely life meaning, stress, sleep quality, well-being, opioid consumption, unpleasantness, acceptance, catastrophizing, and interference [13,14,15,16]. This last parameter can be defined as a “construct of the self-reported consequences of pain on activities” and “satisfaction in social relationships with family and friends and enjoyment of participation in work and social activities” [17]. Herein, besides the classical pharmacological approaches, a multidimensional approach of CP should be considered in personalized and integrative pain management [18,19]. Due to analgesics’ side effects [20], the inability to promote pain relief for some CP patients [21], or the invasiveness of low-resolution surgical procedures [7], pain clinics could benefit from the inclusion of Cognitive Behavioral Therapies (CBTs), such as mindfulness approaches, in an effort to reduce suffering.
Mindfulness has been defined as the awareness that arises through paying attention in a particular way: on purpose, in the present moment, and non-judgmentally [22]. This approach empowers CP patients to change their perception of pain, fostering coping skills, reducing suffering, and consequently improving their overall quality of life [23,24,25]. Mindfulness interventions can be used alongside other treatments or even as a stand-alone approach [25] through several approaches, including group-based programs, retreats, comprehensive treatment programs, such as cognitive behavioral stress management and acceptance and commitment therapy, and via internet and smartphone apps [23]. The most currently used programs classically last for 8 weeks and include Mindfulness-Based Stress Reduction (MBSR), Mindfulness-Based Cognitive Therapy (MBCT), and Mindfulness Self Compassion (MSC). Recently, Mindfulness-Based Relapse Prevention (MBRP) and Mindfulness-Oriented Recovery Enhancement (MORE) are also used in specific situations.
MBSR aids patients coping with various challenges through body awareness and acceptance in weekly sessions, a retreat day, and home-based sessions [26,27,28,29,30]. MBCT combines cognitive therapy and psychoeducation to foster the acceptance of unwanted feelings and thoughts, emphasizing metacognitive awareness [27,31,32], which may prevent depression relapses [33,34]. MSC provides tools for better and proactive self-care, allowing for relief from suffering, including CP [35,36,37,38]. MBRP is an intervention to reduce the probability and intensity of relapse by identifying its risk factors and increasing awareness, exposure, and behavioral flexibility in daily cognitive and emotional experiences [39,40,41]. MORE uses social–behavioral learning theory to enhance participant motivation and engagement, and, by combining CBT, psychological concepts, and mindfulness in a group program, it uniquely addresses and improves psychiatric symptoms, physical pain, and addictive behavior [42,43,44,45,46]. Spiritualized Mindfulness (SPM) is a combined mindfulness and spiritualization technique which aims to cultivate a spiritual feeling through framing and explaining the technique, followed by guided meditation focused on the spirituality–breath connection [47]. In spite of the benefits, mindfulness can lead to increased false-memory recall, temporary increases in pain, and agitation or anxiety derived from the increased awareness of bodily sensations in certain patients [48] and, herein, specific features need to be considered, such as individual characteristics, preferences, and medical history.
Another integrative mind–body intervention directed to CP that emerged recently is Guided Imagery (GI) [49,50]. This intervention is different from mindfulness [51,52] as it incorporates techniques such as the generation or recalling of mental images and/or verbal suggestions using, for example, storytelling, drawing, or interpretation of dreams. Thus, the success of this technique depends namely on the therapist leading the patient to achieve the desired response, particularly in pain reduction [53], and on the patient’s focus and relaxation, achieved by availing oneself of techniques, such as diaphragmatic breathing or progressive muscle relaxation [54,55]. GI reduces stress, pain, anxiety, analgesic intake, blood pressure, heart frequency, and fear, while improving sleep, immunity, psychological well-being, and energy [54,56,57,58,59,60,61]. The efficacy of GI remains unclear in regard to pain treatment other than cancer and musculoskeletal pain [50]. Therefore, since this is considered an effective, feasible, safe, and accessible cognitive behavioral tool [53], its benefits in non-cancer CP should be deeply evaluated.
The aim of this systematic review was to assess the effectiveness of mindfulness therapies and GI interventions in managing non-cancer CP and related outcomes. In this review, non-cancer CP was primarily attributed to osteoarticular causes [21,62,63], with the lower back being the most common location of CP [62,64]. By synthesizing the best available evidence, this review aims to provide valuable guidance to healthcare providers and individuals seeking evidence-based interventions for chronic non-cancer pain management.
2. Materials and Methods
A systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The PICO question for this review was “In chronic non-cancer pain subjects (P), does the use of mindfulness therapies and/or GI interventions (I) compared to standard care or other interventions (C) result in improved pain management and related outcomes (O)?”. If yes, which one is better?
A search in three electronic bibliographic databases, which included Web of Science, PubMed, and Scopus, was carried out in October 2022. The search strategy was built up combining search words (Mindfulness, Imagery, Psychotherapy, Chronic, Pain, Chronic Pain) and MeSH terms (Mindfulness; Imagery, Psychotherapy; Chronic Pain). The search strategy used for PubMed was the following: (“Mindfulness” [Mesh] OR “Imagery, Psychotherapy” [Mesh]) AND “Chronic Pain” [Mesh]; for Scopus we used: (Mindfulness OR Imagery) AND Psychotherapy AND chronic AND pain; and for Web of Science the keywords were: “Mindfulness” AND “Imagery” AND “Chronic Pain”. The last strategy was adopted to optimize data extraction from databases.
The inclusion criteria applied comprised: adult human participants diagnosed with chronic non-cancer pain; participants who have completed a mindfulness or GI intervention; measures of pain intensity and/or pain-related outcomes; published in English; a sample size of at least 10 participants per group; Randomized Controlled Trials (RCTs; including pilots if the final study was not published). The exclusion criteria included studies in which participants had cancer-related pain or who had not completed a mindfulness or GI intervention, as well as the duplicated studies, not including measures of pain intensity and/or pain-related outcomes, observational studies, case reports, and case series.
Titles and abstracts were screened by one of the authors to assess their relevance and alignment with the objective of this systematic review. After this initial selection, a full-text review was conducted and information from each selected study was extracted, including the characteristics of the participants and the conclusions drawn. The studies were then systematically evaluated, ensuring that only studies with the appropriate methodology and outcomes were included in the systematic review and that the results were valid and reliable.
Regarding the data synthesis process, the first step involved conducting a thorough extraction of the relevant data from each study, including the participants’ characteristics, number of participants, the intervention details, and the outcome measures. These data were then organized in a chart and subdivided into classical mindfulness interventions, novel mindfulness interventions (studies that used MORE), and in GI studies. Subsequently, a qualitative analysis of the findings was conducted to identify patterns and trends in the data including risk of bias. This consisted in grouping the studies based on their similarities and differences and conducting a critical appraisal of the strengths and limitations of each study by using the Cochrane Risk of Bias Tool [65].
The search, selection, evaluation, and extraction process were then reviewed by the other two authors, and the analysis and categorization of results was performed by all the authors involved in this systematic review. If any discrepancies were present, the solution was found through consensus.
Regarding the degree of agreement between the authors of this review, in the initial analysis made by two of them (BM and DP), the k was 100%. When comparing the studies accepted for inclusion by these authors and the ones accepted by the third author (IT), the k was 97% due to disagreement with one of the trials that was excluded due to the use of both interventions simultaneously (mindfulness and GI).
3. Results
The PRISMA flow-chart is depicted in Figure 1. The initial electronic search assembled 444 references, of which 45 were removed since they were duplicated records. From the 399 articles remaining, 373 were excluded due to the non-fulfillment of the inclusion criteria and/or due to some of their features being part of the exclusion criteria. Of them, 326 were excluded due to their target population being patients with cancer-related pain or who had not completed a mindfulness or GI intervention or due to lack of inclusion of mindfulness or GI methods; 2 were excluded because the full text was written in German; 45 were excluded since the study design was not consistent with an RCT or a pilot of an RCT.
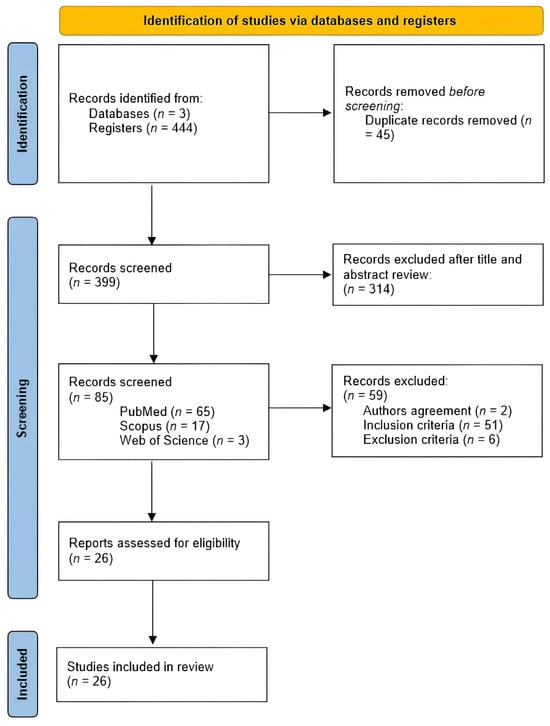
Figure 1.
PRISMA flow-chart of literature search and study screening and inclusion.
After the review process was completed, 26 studies were included in this review, 7 pilot RCTs (27%) and 19 RCTs (73%).
Table 1 displays the selected studies. All the trials included various pain-related outcomes: intensity, interference, unpleasantness, acceptance, and catastrophizing. Regarding pain intensity measurement, some studies assessed intensity using a Numeric Rating Scale (NRS), on a scale from 0 (“no pain”) to 10 (“worst pain imaginable”) [47,66,67,68,69,70,71,72,73,74,75,76,77,78,79], by asking the patients the one number that best describes their pain, modifying if they were assessing the average, worst, or current pain. Meanwhile, others utilized a Visual Analogue Scale (VAS) [38,80,81,82], also on a scale from 0 (“no pain”) to 10 (“worst pain”). Additionally, some studies used an NRS but used different scales. Cooperman et al. [83] made this assessment with subscales of the RAND 36-Item Short Form Health Survey, with the scores for each factor ranging from 0 to 100, where higher scores indicated less pain. Lewandowski et al. [84] used the Wong–Baker FACES scale to evaluate pain intensity at baseline but employed another method to assess this parameter throughout the study, which utilized descriptor word groups. Baird et al. [85] used the pain scale from the Arthritis Impact Measures (AIMS2) to assess this. None of the studies defined the interpretation of the intensity as low, moderate, or high.

Table 1.
Concise overview of findings from randomized controlled clinical trials employing mind–body therapies on chronic non-cancer pain.
Furthermore, most of them also focused on some emotional variables, namely depression, anxiety, meaning in life, stress, and sleep disturbances, as well as quality of life.
In terms of interventions, four trials used MORE [70,71,72,83], considered as the novel mindfulness intervention, eighteen trials used more classical mindfulness interventions (two Mindfulness Meditation (MM) [76,78], six MBSR [66,67,74,77,80,86], one MBCT [68], one MSC [38], one Mindfulness in Action (MIA) [69], one mindfulness-based pain management program [88], one SPM [47], one online mindfulness intervention [73], two non-specified mindfulness-based therapies [75,87], and two other mindfulness approaches [79,82]), and four trials used GI [81,84,85,89].
The participating adult populations were from a great variety of origin countries (50% from the USA [47,66,67,70,71,72,78,79,82,83,84,85,86], 15.4% from the United Kingdom [73,75,77,88], 7.7% from Spain [38,81], 3,8% from Sweden [74], 7.7% from Australia [68,87], 3.8% from the United Arab Emirates [76], 3.8% from Denmark [80], 3.8% from Ireland [69], and 3.8% from Germany [89]), with 38.3% of them being European countries, and included a pronounced variety of CP types. A total of 2964 patients (2762 in mindfulness trials and 202 in GI interventions) were included in the analyzed studies. The studies with the smallest sample have 28 patients [85,88], and the study with the largest sample has 342 patients [80]. The duration of the CP was more than 8 years in 11 trials [66,68,70,71,73,74,75,76,79,80,84,86] and less than 8 years, but more than 3 months, in 9 trials [38,67,69,77,78,81,82,83,89], while 6 trials did not report the duration of pain [47,72,85,87,88].
Regarding study results, in all four of the MORE trials [70,71,72,83], the intervention was significantly better at improving pain intensity and opioid consumption and/or craving than control. One of them reported an improvement in pain interference and in stress levels [72], one described a significant reduction in anxiety and depression levels [83], and one reported a significant improvement in both pain interference and depression [70] and one in positive affect, meaning in life, and savoring [71].
The trials that focused on MM showed that this intervention led to a significant reduction in pain intensity [76,78]. In the Williams, Day et al. trial [78], MM did not significantly reduce anxiety and depression, but it was able to reduce pain interference more than the other interventions.
Day, Ward et al. [68] focused on MBCT and showed a significant decrease in pain intensity, although it was not different from the other groups. They also demonstrated a significant reduction in pain interference and depression levels compared with the other interventions, as well as a significant difference in opioid use between the pre-treatment phase and the 3-month follow-up period.
Out of six trials that studied the effects of MBSR, two of them showed that MBSR led to a significant decrease in pain intensity [66,74], while four verified a pain reduction that did not differ from the control group [67,77,80,86]. Burns, Jensen et al. [66] showed that MBSR led to a more pronounced reduction in pain interference, depressive symptoms, and sleep disturbance than the control. Henriksson et al. [74] showed a small effect size of MBSR on pain interference, although better than the control, and a significant decrease in affective distress and pain acceptance than control. In the Morone et al. trial [86], MBSR showed a more pronounced decrease in catastrophizing than control but not in depression. In Cherkin’s study [67], MBSR significantly improved pain bothersomeness and anxiety and depression levels, although CBT demonstrated better results in these last two variables. The results of la Cour and Petersen [80] showed a significant improvement in anxiety and pain acceptance than the control, which was not verified in catastrophizing and depression. In the Ussher et al. trial [74], MBSR led to a significant reduction in distress and in pain interference in the clinic setting when compared with control.
With respect to the remaining trials of mindfulness interventions, two did not assess the effects of the intervention on pain intensity [47,88], one showed a significant decrease in this variable with the respective intervention [79], and the other six did not show differences between groups for the same variable [38,69,73,75,82,87]. In the Zgierska et al. trial [79], the intervention led to a significant increase in pain acceptance, but no better than control, which also happened regarding mindful attention, perceived stress, and opioid dosage used. In the Torrijos-Zarcero et al. study [38], MSC led to a significant decrease in anxiety levels, pain interference, and depressive symptoms, and a higher increase in pain acceptance, in comparison to CBT. MSC significantly reduced pain catastrophizing. Polaski et al. [82] showed that the Meditation and Exercise Trial (MedExT) did not lead to any significant improvement in anxiety levels. In the Hearn and Finlay trial [73], the mindfulness online intervention led to a significant improvement in anxiety levels and pain catastrophizing, with this effect on pain unpleasantness only in T2 level lesions. Dowd et al. [69] showed that MIA significantly improved catastrophizing, pain acceptance, and pain interference but not anxiety and depression levels. The Howarth et al. trial [75] did not demonstrate significant differences in anxiety and depression levels, Cathcart et al. [87] did not assess emotional variables after treatment. Brown and Jones [88] showed a statistically significant improvement in mental health and in affective clinical pain score with a mindfulness-based pain management program.
Regarding the GI studies, two of them reported a significant reduction in pain intensity [81,85]. In another study, since the variables evaluated were different, pain intensity was not assessed directly, but in the intervention group pain became changeable and there was no recurrence of constant pain [84]. Furthermore, the Alexander Technique (AT) demonstrated a better efficacy in improving pain intensity and satisfaction levels than GI [89]. In the Onieva-Zafra et al. study [81] there was a better improvement in depression levels with GI than with control. Two trials [84,85] did not evaluate emotional variables.
Concerning the methodological quality, eleven studies were classified as having a “high risk” of bias, eleven as “moderate risk”, and four as “low risk”. A great percentage of this risk arose from the impossibility of blinding the participants and the investigators due to the nature of the interventions applied (Figure 2).
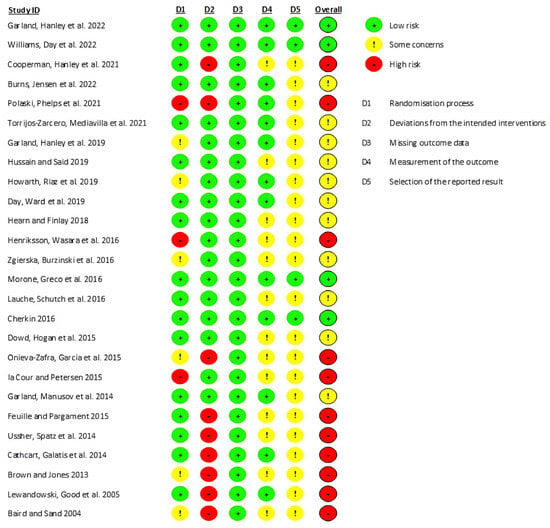
Figure 2.
Evidence quality assessment of the studies included in this review using the Cochrane Risk of Bias Tool. The risk of bias is represented in five categories and with its overall score for each study. A code of colors is used: green-low risk of bias; yellow-some concerns about bias; red-high risk of bias [38,47,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89].
4. Discussion
4.1. General Findings
To the best of our knowledge, this is the first systematic review addressing mindfulness and GI interventions in chronic non-malignant pain. Overall, mindfulness-based interventions demonstrated a more useful role in improving emotional outcomes than pain intensity, while GI interventions were found to be useful in reducing pain intensity. Since only two GI-focused studies [81,89] addressed their impact on emotion outcomes, we cannot definitely state whether these interventions may have a beneficial effect on those variables. However, those two trials showed potential in aiding patients at an emotional level.
The studies analyzed in this systematic review were highly variable across multiple factors, namely in the pain type and duration of the intervention. Regarding the duration of the intervention, it should be noted that there is currently consideration for shortening of the 8-week period of classical MBSR or MBCT programs. This is because the same intervention type may potentially improve pain-related outcomes within a shorter timeframe. This was mainly evident when comparing the effects on emotional variables between the MBSR trials with an 8-week protocol [66,67,74,80,86] and the brief MBSR intervention [77]. The findings of a recent trial [90] indicated that varying durations of MM sessions, whether 10 min or 30 min over a period of two weeks, did not significantly influence the impact on mental well-being. Although this investigation only focused on one mindfulness intervention, and the target population was healthy, it may reinforce the evidence seen in this review. This is interesting as the duration of the interventions was previously viewed as a limitation of the classical mindfulness programs.
All trials focusing on a novel mindfulness intervention demonstrated improvements compared to the control in at least one of the components studied [70,71,72,83]. Regarding the trials that focused on classical mindfulness interventions, five showed no significant difference in the effects of intervention compared to the control group [69,75,78,82,87]. This might happen because three of those trials are pilots and may have had a lower number of participants than the one needed to achieve significant results [75,82,87]. In the MM trial [78], this could be attributed to the fact that the control employed is a hypnosis technique, which has shown similar or better results compared to MM in other trials [91,92]. Regarding MIA [69], the lack of significance in results could arise from the similar benefits it shares with psychoeducation programs, since another trial that studied a similar online treatment demonstrated significant differences in pain-related outcomes when compared with a waiting list control, although it also exhibits a higher rate of adherence [93]. Since MIA was used in a diverse group of patients with varying types of pain, there could have been variable efficacy across these different pain types. MIA could have been effective in one type of pain, but ineffective in another, which could eventually lead to a non-significant result compared to the control.
On the other hand, thirteen studies showed that mindfulness was better than the control in at least one of the variables studied [38,47,66,67,68,73,74,76,77,79,80,86,88].
Regarding the GI studies, three of them reported positive outcomes in several of the variables under investigation [81,84,85]. In the study that compared the AT with GI, the intervention did not achieve results as good as those observed with AT [89].
4.2. Effects on Pain Intensity
In all MORE trials there was a significant reduction in pain intensity when compared with supportive group psychotherapy or treatment as usual [70,71,72,83]. The studies on classical mindfulness reported a significant decrease in pain intensity for MM alone [76] or combined with cognitive behavior [79]. However, in two other MM trials, this effect on pain did not happen [68,78]. This may be related to the control being either hypnosis [78] or MBCT [68], that may be equivalent to MM. Due to similarities, the observed outcomes do not appear to be attributable to treatment duration, pain type, or the specific population under study.
Patients undergoing MBSR reported decreased pain intensity compared to the control in only one trial [74], whereas in other five trials this was not observed [66,67,77,80,86]. These differences may derive from the different procedures used in the trial that demonstrated significant results that used audio files and 10 min mindfulness exercises. Although this may appear promising, since there is only one trial using this procedure, there is sparse evidence to conclude about the true value for CP intensity. In general, MBSR does not seem to lead to a significant improvement of pain intensity. Therefore, care must be taken when using it with the main purpose of improving this aspect.
MSC [38] demonstrated a significant ability to improve pain levels. However, not all mindfulness techniques yield the same results. There were no significant differences observed in trials that studied MedExT for treating chronic low back pain [82], as well as for online mindfulness intervention [73], MIA [69], an unspecified mindfulness-based intervention [75], and mindfulness-based interventions with unspecified details [87].
It is important to notice that, although mindfulness techniques were developed with the intent of reducing pain intensity, it has been demonstrated that they are more effective in improving non-sensorial dimensions of pain. Additionally, while mindfulness can have an important role in CP management, improving quality of life by enhancing pain acceptance, its effectiveness may vary from person to person, and its efficacy is dependent on the specific treatment approach. In this context, MORE seems to be the most suitable to reduce pain perception, while other techniques should be chosen carefully when pain reduction is the primary goal. MSC interventions had promising results, but further studies are needed to better evaluate its effects on pain.
A significant reduction in pain was reported with GI alone, either with 4 days of treatment [84] or over a 4-week period [81], indicating a short-term benefit that can be extended according to patients’ needs. The combination of GI and Progressive Muscle Relaxation (PMR) was also found to be beneficial when compared with a control group [85]. However, when compared with the AT, GI was found to be inferior regarding pain intensity [89]. This also suggests that the employment of GI-based methods can lead to a significant decrease in CP perception and the treatment choice should be based on patients’ needs, as happens with other mind–body interventions.
4.3. Effects on Non-Sensorial Dimensions of Pain
Although quality of life was not assessed in MORE studies, three of them demonstrated a significant reduction in depression and/or anxiety/stress levels [70,72,83], two trials showed a reduction in pain interference [70,72] and one study reported a significant improvement in positive affect [71]. This demonstrated that MORE is an important therapy to approach the complex and multidimensional nature of pain and related emotional variables, and, therefore, should be particularly considered for CP patients experiencing effects on their mental well-being. These results are in accordance with a previous meta-analysis on CP, along with addictive behavior and psychiatric distress [46].
MM did not demonstrate superiority to hypnosis [78] nor to MBCT [68] in improving pain interference, anxiety, and depression. However, when administered alongside CBT [79], mindfulness led to a significant improvement in pain acceptance, aligning with the objectives of this therapeutic approach. Three of four MBSR trials demonstrated significant improvements in quality of life directly [67,74,80]. Furthermore, MBSR led to a significant improvement in pain-related outcomes, such as pain interference, pain acceptance, and pain catastrophizing and a significant reduction in anxiety/distress levels [67,74,77,80]. MSC managed to significantly decrease anxiety and depression levels, as well as reduce pain interference and catastrophizing and increase pain acceptance [38].
No significant improvements in quality of life [83], anxiety/stress, and depression were observed in the trials with a non-specified mindfulness intervention when compared with the control [75,87]. It is important to note that patients should receive the most appropriate and personalized treatment to achieve better results, especially when managing such a complex problem as CP. Sometimes, relying solely on one approach may not be sufficient, and patients may lose confidence in mind–body therapies. This situation can be exacerbated if the patient has already experienced failed treatments, including pharmacological ones. Consequently, not only may emotional variables related to pain fail to improve but anxiety, depression, catastrophizing, and sleep disturbances could worsen, further intensifying the experience of pain. In this context, it becomes exceedingly important to select the appropriate treatment from the beginning.
Mindfulness interventions were typically conducted in person. However, advancements in new technologies have now enabled alternative approaches, allowing for greater flexibility and accessibility. Virtual platforms and mobile applications, for instance, have revolutionized the way mindfulness practices can be delivered, reaching a wider audience and accommodating various schedules. This shift towards technology-mediated interventions has expanded the possibilities for individuals to engage in mindfulness training from the comfort of their own environments, promoting convenience and potentially enhancing the integration of mindfulness into their daily lives. Furthermore, online mindfulness also allows a significant reduction in depression levels and severity, as well as in pain unpleasantness and catastrophizing, although it did not demonstrate a significant effect on quality of life [73]. Again, caution must be taken when opting for an online approach in the complexity of CP. Ideally, patients should be initially seen in person for a comprehensive first evaluation of their current situation. Subsequently, follow-up therapy can be conducted conveniently online for those for whom it is the only feasible way to continue treatment.
Selecting the most suitable mind–body therapy for each patient is crucial, as not all approaches will effectively address the concerns voiced by individuals. For instance, a therapy that focuses on relaxation might be more beneficial for someone experiencing stress-related issues, while another individual with CP might find greater relief through techniques emphasizing pain management and physical rehabilitation. Tailoring the choice of therapy to the specific needs and preferences of each patient enhances the likelihood of positive outcomes and contributes to a more personalized and effective healthcare approach. In this context, MIA was not able to significantly impact pain acceptance, interference, and catastrophizing nor anxiety and depression levels compared with psychoeducation [69]. The reason may also lie in different uses of evidence-based protocols; instead, audio-visual treatment was used.
Although MedExT proved to be useful in reducing low back pain intensity and unpleasantness, this modality did not improve anxiety levels [82]. This could be attributed to the intervention’s potential to offer greater benefits in reducing anxiety levels when patients initially have higher baseline anxiety compared to those who participated in the trial [82]. Furthermore, the lack of a significant result may be due to the limited number of participants, which may have not provided enough statistical power to detect differences that might actually exist.
STM was able to significantly reduce pain-related stress [47]. Although the number of trials directly assessing quality of life were not substantial, those that did suggest that these interventions can inherently benefit this component. Moreover, it seems that they can have a significant positive impact on pain-related outcomes as well as mental health.
Only two GI interventions analyzed emotional variables [81,89]. One showed improvement in satisfaction levels [89] and the other demonstrated a significant amelioration in depression when compared with the control [81]. Since only two of these trials targeted emotional variables, it is challenging to draw definitive conclusions regarding the impact of these interventions on emotions. GI is a relatively less comprehensive therapy, and the multidimensional and complex disease of CP may also require a more holistic mind–body approach. A MORE intervention, for example, which encompasses a wider range of techniques, from mindfulness practices to reappraisal and savoring skills, could be more suitable in addressing the various dimensions of CP. On the other hand, GI’s focus is based on imagery techniques which, although diversified, cannot reach as far as mindfulness when approaching the various pain dimensions.
4.4. Opioid Consumption
Understanding opioid consumption is of paramount importance due to its consequences on public health, society, and economy. Opioid abuse not only poses significant risks to individual health but also contributes to societal issues such as addiction, overdose deaths, and economic burdens. Regarding MORE interventions, they were able to significantly reduce craving and/or consumption of opioids in all four trials included in our results [66,67,68,69]. The trials of classical mindfulness interventions did not demonstrate any significant difference in this outcome when compared with the control [68,78,79]. Given this, there is a potential of MORE in improving variables linked to opioid consumption. However, more studies focused on classical mindfulness treatments are required to better evaluate their effects on this important outcome.
4.5. Cancer Pain
Ultimately, extensive research has been conducted regarding mind–body approaches in the context of cancer-related pain. A comprehensive systematic review and meta-analysis [94], encompassing mindfulness interventions alongside GI interventions, unveiled noteworthy findings. Notably, a discernible and favorable influence on this particular outcome was observed. More specifically, mindfulness interventions led to significant results in favor of the intervention, while GI interventions showed only a small or even nil effect in favor. These results in cancer pain are similar to those found in our study on chronic non-cancer pain, but the quality of the evidence garnered remains limited and a substantial degree of heterogeneity within the studies was evident. In another systematic review and meta-analysis, there were also moderate to large effect size improvements in pain and opioid-related outcomes with mindfulness but not with GI [95]. Bearing in mind these results and the ones analyzed in this review, most evidence points to a lack of effect of GI in opioid-related outcomes. However, this reinforces the important role of mindfulness in improving these outcomes and therefore it gives a better and greater opportunity in life to these patients.
4.6. Limitations
This review has some limitations. Although the type of study included has some of the highest levels of evidence, the sole use of RCTs could lead to the loss of some crucial information on the topic studied. The bias evaluation showed that most studies included have a high or moderate risk of bias, mostly due to lack of allocation concealment, which, given the interventions studied, is often impractical to implement, and blind assessment of outcomes. The latter is a characteristic that could be improved in future trials. Additionally, given the low number of RCTs with intervention groups exposed to GI, it was not possible to exclude trials that did not consider emotional variables, an essential component to study regarding integrative therapies. Furthermore, the heterogeneity of the target population and of the duration of techniques (especially between mindfulness interventions and GI ones) is moderate/high. Finally, a meta-analysis was not possible due to limited eligible studies, high methodological heterogeneity, and a substantial risk of bias in the available research. These factors hindered the reliable aggregation of data and the generation of meaningful conclusions through meta-analytic techniques.
4.7. Strengths
This review demonstrates several strengths in its methodology. It exhibits robustness through well-defined inclusion and exclusion criteria, systematic adherence to PRISMA guidelines in the literature search, and rigorous validation of study selection and data extraction by multiple reviewers. Additionally, the review employs the Cochrane Risk of Bias Tool for quality assessment, furnishes comprehensive study and patient characteristics, and ensures a conflict-of-interest-free evaluation by all reviewers.
4.8. Future Research
It will be important for further studies to increase their focus not only on the influence of these interventions on pain-related outcomes but also on other dimensions of pain, such as the emotional variables related to it, especially with GI. Given the well-known close relationship between emotions and pain [96,97,98,99], exploring these aspects could provide valuable insights into the effectiveness of interventions like GI. Future studies in CP management should explore the integration of GI with other therapeutic approaches to ensure a comprehensive approach to managing the multidimensional nature of pain. Targeting these components in the future may help obtain to a better understanding of the true potential of mindfulness and GI in the control of CP.
As stated in the review from Eric Garland et al. [95], there should be an effort to at least blind assessors and/or investigators in order to decrease the risk of bias in these types of studies. Since the blinding of participants is very difficult to achieve with mind–body interventions, alternative methods of minimizing bias, such as blinded assessment of outcomes, should be considered in future studies.
It will also be important to study each technique with different durations to assess if it is possible to achieve similar or comparable results in a shorter period, without including subsequent work-at-home sessions. This could make it easier and comfortable for patients to adhere to these techniques. Focusing on more homogenous groups of patients regarding the type of pain and its duration and comparing a classic mindfulness intervention with a GI intervention could provide relevant information about which technique is more effective for chronic non-malignant pain.
5. Conclusions
In conclusion, this systematic review underscores that both mindfulness therapies and GI interventions demonstrate promise in managing chronic non-cancer pain and related outcomes. GI interventions exhibited significant reductions mainly in pain intensity. Notably, mindfulness additionally demonstrated significant improvements in pain intensity and emotional variables such as depression and anxiety/stress levels. This, and a beneficial effect in opioid consumption, are especially evident when mindfulness is incorporated with the MORE technique, emerging as a superior choice, offering valuable insights for healthcare providers and individuals seeking evidence-based interventions for chronic non-cancer pain management. Overall, integrating these interventions into chronic non-cancer pain management strategies could offer valuable benefits, but individualized treatment approaches tailored to patient needs and preferences remain important.
Author Contributions
Conceptualization, B.M.P., I.T. and D.H.P.; methodology, B.M.P., I.T. and D.H.P.; writing—original draft preparation, B.M.P., I.T. and D.H.P.; writing—review and editing, B.M.P., I.T. and D.H.P.; supervision, I.T. and D.H.P.; project administration, I.T. and D.H.P. All authors have read and agreed to the published version of the manuscript.
Funding
This study was performed within the aims of Catedra de Medicina da Dor of the Faculty of Medicine of Porto, Portugal.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. The original data explored in the review are stated in the article and additional inquiries can be directed to the respective contributing authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mäntyselkä, P.; Kumpusalo, E.; Ahonen, R.; Kumpusalo, A.; Kauhanen, J.; Viinamäki, H.; Halonen, P.; Takala, J. Pain as a reason to visit the doctor: A study in Finnish primary health care. Pain 2001, 89, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Raja, S.N.; Carr, D.B.; Cohen, M.; Finnerup, N.B.; Flor, H.; Gibson, S.; Keefe, F.J.; Mogil, J.S.; Ringkamp, M.; Sluka, K.A.; et al. The revised International Association for the Study of Pain definition of pain: Concepts, challenges, and compromises. Pain 2020, 161, 1976–1982. [Google Scholar] [CrossRef]
- Nicholas, M.; Vlaeyen, J.W.S.; Rief, W.; Barke, A.; Aziz, Q.; Benoliel, R.; Cohen, M.; Evers, S.; Giamberardino, M.A.; Goebel, A.; et al. The IASP classification of chronic pain for ICD-11: Chronic primary pain. Pain 2019, 160, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Treede, R.D.; Rief, W.; Barke, A.; Aziz, Q.; Bennett, M.I.; Benoliel, R.; Cohen, M.; Evers, S.; Finnerup, N.B.; First, M.B.; et al. Chronic pain as a symptom or a disease: The IASP Classification of Chronic Pain for the International Classification of Diseases (ICD-11). Pain 2019, 160, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Engel, G.L. The need for a new medical model: A challenge for biomedicine. Science 1977, 196, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Hylands-White, N.; Duarte, R.V.; Raphael, J.H. An overview of treatment approaches for chronic pain management. Rheumatol. Int. 2017, 37, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.P.; Vase, L.; Hooten, W.M. Chronic pain: An update on burden, best practices, and new advances. Lancet 2021, 397, 2082–2097. [Google Scholar] [CrossRef] [PubMed]
- Dahlhamer, J.; Lucas, J.; Zelaya, C.; Nahin, R.; Mackey, S.; DeBar, L.; Kerns, R.; Von Korff, M.; Porter, L.; Helmick, C. Prevalence of Chronic Pain and High-Impact Chronic Pain among Adults—United States, 2016. MMWR. Morb. Mortal. Wkly. Rep. 2018, 67, 1001–1006. [Google Scholar] [CrossRef] [PubMed]
- Johannes, C.B.; Le, T.K.; Zhou, X.; Johnston, J.A.; Dworkin, R.H. The prevalence of chronic pain in United States adults: Results of an Internet-based survey. J. Pain 2010, 11, 1230–1239. [Google Scholar] [CrossRef]
- Nahin, R.L. Estimates of pain prevalence and severity in adults: United States, 2012. J Pain 2015, 16, 769–780. [Google Scholar] [CrossRef] [PubMed]
- Steglitz, J.; Buscemi, J.; Ferguson, M.J. The future of pain research, education, and treatment: A summary of the IOM report “Relieving pain in America: A blueprint for transforming prevention, care, education, and research”. Transl. Behav. Med. 2012, 2, 6–8. [Google Scholar] [CrossRef] [PubMed]
- Rice, A.S.C.; Smith, B.H.; Blyth, F.M. Pain and the global burden of disease. Pain 2016, 157, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Gatchel, R.J.; McGeary, D.D.; McGeary, C.A.; Lippe, B. Interdisciplinary chronic pain management: Past, present, and future. Am. Psychol. 2014, 69, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.P.; Turk, D.C. Contributions of psychology to the understanding and treatment of people with chronic pain: Why it matters to ALL psychologists. Am. Psychol. 2014, 69, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, A.; Mackey, S. Outcomes in pain medicine: A brief review. Pain Ther. 2012, 1, 5. [Google Scholar] [CrossRef] [PubMed]
- Meints, S.M.; Edwards, R.R. Evaluating psychosocial contributions to chronic pain outcomes. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 87, 168–182. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, R.; Bourbonnais, D.; Higgins, J.; Mireault, M.; Harris, P.G.; Danino, M.A. Pain interference may be an important link between pain severity, impairment, and self-reported disability in participants with wrist/hand pain. J. Hand Ther. 2020, 33, 562–570.e561. [Google Scholar] [CrossRef] [PubMed]
- Clauw, D.J.; Essex, M.N.; Pitman, V.; Jones, K.D. Reframing chronic pain as a disease, not a symptom: Rationale and implications for pain management. Postgrad. Med. 2019, 131, 185–198. [Google Scholar] [CrossRef]
- Loeser. The Role of Chronic Pain Clinics in Managing Back Pain; Raven Press: New York, NY, USA, 1991; pp. 221–229. [Google Scholar]
- Voon, P.; Karamouzian, M.; Kerr, T. Chronic pain and opioid misuse: A review of reviews. Subst. Abus. Treat. Prev. Policy 2017, 12, 36. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Atcheson, R. Opioid and chronic non-cancer pain. J. Anaesthesiol. Clin. Pharmacol. 2013, 29, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Kabat-Zinn, J. Wherever You Go There You Are: Mindfulness Meditation in Everyday Life; Hyperion: New York, NY, USA, 1994. [Google Scholar]
- Creswell, J.D. Mindfulness Interventions. Annu. Rev. Psychol. 2017, 68, 491–516. [Google Scholar] [CrossRef] [PubMed]
- Kabat-Zinn, J. An outpatient program in behavioral medicine for chronic pain patients based on the practice of mindfulness meditation: Theoretical considerations and preliminary results. Gen. Hosp. Psychiatry 1982, 4, 33–47. [Google Scholar] [CrossRef]
- Majeed, M.H.; Ali, A.A.; Sudak, D.M. Mindfulness-based interventions for chronic pain: Evidence and applications. Asian J. Psychiatr. 2018, 32, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Chaskalson, M.; Hadley, S.G. Mindfulness in Organizations. Foundations, Research and Applications; Reb, J., Atkins, P.W.B., Eds.; Cambridge University Press: Cambridge, UK, 2015; Volume 3. [Google Scholar]
- Janssen, M.; Heerkens, Y.; Kuijer, W.; van der Heijden, B.; Engels, J. Effects of Mindfulness-Based Stress Reduction on employees’ mental health: A systematic review. PLoS ONE 2018, 13, e0191332. [Google Scholar] [CrossRef] [PubMed]
- Kabat-Zinn, J. Full Catastrophe Living: Using the Wisdom of Your Body and Mind to Face Stress, Pain and Illness; Delacorte: New York, NY, USA, 1990. [Google Scholar]
- Khoury, B.; Sharma, M.; Rush, S.E.; Fournier, C. Mindfulness-based stress reduction for healthy individuals: A meta-analysis. J. Psychosom. Res. 2015, 78, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Rosenzweig, S.; Greeson, J.M.; Reibel, D.K.; Green, J.S.; Jasser, S.A.; Beasley, D. Mindfulness-based stress reduction for chronic pain conditions: Variation in treatment outcomes and role of home meditation practice. J. Psychosom. Res. 2010, 68, 29–36. [Google Scholar] [CrossRef]
- Segal, Z.; Williams, J.; Teasdale, J. Mindfulness-Based Cognitive Therapy for Depression: A New Approach to Preventing Relapse; The Guildford Press: New York, NY, USA, 2002. [Google Scholar]
- Sipe, W.E.; Eisendrath, S.J. Mindfulness-based cognitive therapy: Theory and practice. Can. J. Psychiatry 2012, 57, 63–69. [Google Scholar] [CrossRef]
- Kuyken, W.; Warren, F.C.; Taylor, R.S.; Whalley, B.; Crane, C.; Bondolfi, G.; Hayes, R.; Huijbers, M.; Ma, H.; Schweizer, S.; et al. Efficacy of Mindfulness-Based Cognitive Therapy in Prevention of Depressive Relapse: An Individual Patient Data Meta-analysis From Randomized Trials. JAMA Psychiatry 2016, 73, 565–574. [Google Scholar] [CrossRef]
- Piet, J.; Hougaard, E. The effect of mindfulness-based cognitive therapy for prevention of relapse in recurrent major depressive disorder: A systematic review and meta-analysis. Clin. Psychol. Rev. 2011, 31, 1032–1040. [Google Scholar] [CrossRef] [PubMed]
- Delaney, M.C. Caring for the caregivers: Evaluation of the effect of an eight-week pilot mindful self-compassion (MSC) training program on nurses’ compassion fatigue and resilience. PLoS ONE 2018, 13, e0207261. [Google Scholar] [CrossRef] [PubMed]
- Neff, K.D. Compassion and Wisdom in Psychotherapy; Guilford Press: New York, NY, USA, 2012. [Google Scholar]
- Neff, K.D. Self-Compassion: Theory, Method, Research, and Intervention. Annu. Rev. Psychol. 2023, 74, 193–218. [Google Scholar] [CrossRef] [PubMed]
- Torrijos-Zarcero, M.; Mediavilla, R.; Rodriguez-Vega, B.; Del Rio-Dieguez, M.; Lopez-Alvarez, I.; Rocamora-Gonzalez, C.; Palao-Tarrero, A. Mindful Self-Compassion program for chronic pain patients: A randomized controlled trial. Eur. J. Pain 2021, 25, 930–944. [Google Scholar] [CrossRef] [PubMed]
- Bowen, S.; Witkiewitz, K.; Clifasefi, S.L.; Grow, J.; Chawla, N.; Hsu, S.H.; Carroll, H.A.; Harrop, E.; Collins, S.E.; Lustyk, M.K.; et al. Relative efficacy of mindfulness-based relapse prevention, standard relapse prevention, and treatment as usual for substance use disorders: A randomized clinical trial. JAMA Psychiatry 2014, 71, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Daley, D.; Marlatt, G. Overcoming Your Alcohol or Drug Problem: Effective Recovery Strategies: Therapist Guide, 2nd ed.; Oxford University Press: New York, NY, USA, 2006. [Google Scholar]
- Marlatt, G.; Gordon, J. Relapse Prevention: Maintenance Strategies in the Treatment of Addictive Behaviors; Guilford Press: New York, NY, USA, 1985. [Google Scholar]
- Garland, E.L. Mindfulness-Oriented Recovery Enhancement for Addiction, Stress, and Pain; NASW Press: Washington, DC, USA, 2013. [Google Scholar]
- Garland, E.L. Mindful Positive Emotion Regulation as a Treatment for Addiction: From Hedonic Pleasure to Self-Transcendent Meaning. Curr. Opin. Behav. Sci. 2021, 39, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Garland, E.L.; Fredrickson, B.L. Positive psychological states in the arc from mindfulness to self-transcendence: Extensions of the Mindfulness-to-Meaning Theory and applications to addiction and chronic pain treatment. Curr. Opin. Psychol. 2019, 28, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Hanley, A.W.; Nakamura, Y.; Garland, E.L. The Nondual Awareness Dimensional Assessment (NADA): New tools to assess nondual traits and states of consciousness occurring within and beyond the context of meditation. Psychol. Assess. 2018, 30, 1625–1639. [Google Scholar] [CrossRef] [PubMed]
- Parisi, A.; Roberts, R.L.; Hanley, A.W.; Garland, E.L. Mindfulness-Oriented Recovery Enhancement for Addictive Behavior, Psychiatric Distress, and Chronic Pain: A Multilevel Meta-Analysis of Randomized Controlled Trials. Mindfulness 2022, 13, 2396–2412. [Google Scholar] [CrossRef] [PubMed]
- Feuille, M.; Pargament, K. Pain, mindfulness, and spirituality: A randomized controlled trial comparing effects of mindfulness and relaxation on pain-related outcomes in migraineurs. J. Health Psychol. 2015, 20, 1090–1106. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.M.; Mickes, L.; Stolarz-Fantino, S.; Evrard, M.; Fantino, E. Increased False-Memory Susceptibility After Mindfulness Meditation. Psychol. Sci. 2015, 26, 1567–1573. [Google Scholar] [CrossRef] [PubMed]
- Nelson, E.A.; Dowsey, M.M.; Knowles, S.R.; Castle, D.J.; Salzberg, M.R.; Monshat, K.; Dunin, A.J.; Choong, P.F. Systematic review of the efficacy of pre-surgical mind-body based therapies on post-operative outcome measures. Complement. Ther. Med. 2013, 21, 697–711. [Google Scholar] [CrossRef] [PubMed]
- Posadzki, P.; Lewandowski, W.; Terry, R.; Ernst, E.; Stearns, A. Guided imagery for non-musculoskeletal pain: A systematic review of randomized clinical trials. J. Pain Symptom Manag. 2012, 44, 95–104. [Google Scholar] [CrossRef]
- Luberto, C.M.; Hall, D.L.; Park, E.R.; Haramati, A.; Cotton, S. A Perspective on the Similarities and Differences Between Mindfulness and Relaxation. Glob. Adv. Health Med. 2020, 9, 2164956120905597. [Google Scholar] [CrossRef] [PubMed]
- Morton, M.L. A Systematic Review of Mindfulness Interventions on Psychophysiological Responses to Acute Stress. Mindfulness 2020, 11, 2039–2054. [Google Scholar] [CrossRef]
- Kaplun, A.; Alperovitch-Najenson, D.; Kalichman, L. Effect of Guided Imagery on Pain and Health-Related Quality of Life in Musculoskeletal Medicine: A Comprehensive Narrative Review. Curr. Pain Headache Rep. 2021, 25, 76. [Google Scholar] [CrossRef] [PubMed]
- Felix, M.; Ferreira, M.B.G.; da Cruz, L.F.; Barbosa, M.H. Relaxation Therapy with Guided Imagery for Postoperative Pain Management: An Integrative Review. Pain Manag. Nurs. 2019, 20, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Kosslyn, S.M.; Ganis, G.; Thompson, W.L. Neural foundations of imagery. Nat. Rev. Neurosci. 2001, 2, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Baird, C.L.; Murawski, M.M.; Wu, J. Efficacy of guided imagery with relaxation for osteoarthritis symptoms and medication intake. Pain Manag. Nurs. 2010, 11, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Francis, A.J. Relaxation and imagery for chronic, nonmalignant pain: Effects on pain symptoms, quality of life, and mental health. Pain Manag. Nurs. 2010, 11, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, W.; Jacobson, A. Bridging the gap between mind and body: A biobehavioral model of the effects of guided imagery on pain, pain disability, and depression. Pain Manag. Nurs. 2013, 14, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, W.; Jacobson, A.; Palmieri, P.A.; Alexander, T.; Zeller, R. Biological mechanisms related to the effectiveness of guided imagery for chronic pain. Biol. Res. Nurs. 2011, 13, 364–375. [Google Scholar] [CrossRef]
- Menzies, V.; Lyon, D.E.; Elswick, R.K., Jr.; McCain, N.L.; Gray, D.P. Effects of guided imagery on biobehavioral factors in women with fibromyalgia. J. Behav. Med. 2014, 37, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.M.; Davies, A.; Griffiths, G. Facilitating comfort for hospitalized patients using non-pharmacological measures: Preliminary development of clinical practice guidelines. Int. J. Nurs. Pract. 2009, 15, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, L.F.; Costa-Pereira, A.; Mendonca, L.; Dias, C.C.; Castro-Lopes, J.M. Epidemiology of chronic pain: A population-based nationwide study on its prevalence, characteristics and associated disability in Portugal. J. Pain 2012, 13, 773–783. [Google Scholar] [CrossRef]
- Gouveia, B.; Fonseca, S.; Pozza, D.H.; Xara, D.; Sa Rodrigues, A. Relationship between Postoperative Pain and Sociocultural Level in Major Orthopedic Surgery. Adv. Orthop. 2022, 2022, 7867719. [Google Scholar] [CrossRef] [PubMed]
- Deyo, R.A.; Mirza, S.K.; Martin, B.I. Back pain prevalence and visit rates: Estimates from U.S. national surveys, 2002. Spine 2006, 31, 2724–2727. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savovic, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Burns, J.W.; Jensen, M.P.; Thorn, B.; Lillis, T.A.; Carmody, J.; Newman, A.K.; Keefe, F. Cognitive therapy, mindfulness-based stress reduction, and behavior therapy for the treatment of chronic pain: Randomized controlled trial. Pain 2022, 163, 376–389. [Google Scholar] [CrossRef] [PubMed]
- Cherkin, D.C.; Sherman, K.J.; Balderson, B.H.; Cook, A.J.; Anderson, M.L.; Hawkes, R.J.; Hansen, K.E.; Turner, J.A. Effect of Mindfulness-Based Stress Reduction vs Cognitive Behavioral Therapy or Usual Care on Back Pain and Functional Limitations in Adults with Chronic Low Back Pain: A Randomized Clinical Trial. JAMA 2016, 315, 1240–1249. [Google Scholar] [CrossRef] [PubMed]
- Day, M.A.; Ward, L.C.; Ehde, D.M.; Thorn, B.E.; Burns, J.; Barnier, A.; Mattingley, J.B.; Jensen, M.P. A Pilot Randomized Controlled Trial Comparing Mindfulness Meditation, Cognitive Therapy, and Mindfulness-Based Cognitive Therapy for Chronic Low Back Pain. Pain Med. 2019, 20, 2134–2148. [Google Scholar] [CrossRef] [PubMed]
- Dowd, H.; Hogan, M.J.; McGuire, B.E.; Davis, M.C.; Sarma, K.M.; Fish, R.A.; Zautra, A.J. Comparison of an Online Mindfulness-based Cognitive Therapy Intervention with Online Pain Management Psychoeducation: A Randomized Controlled Study. Clin. J. Pain 2015, 31, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Garland, E.L.; Hanley, A.W.; Nakamura, Y.; Barrett, J.W.; Baker, A.K.; Reese, S.E.; Riquino, M.R.; Froeliger, B.; Donaldson, G.W. Mindfulness-Oriented Recovery Enhancement vs Supportive Group Therapy for Co-occurring Opioid Misuse and Chronic Pain in Primary Care: A Randomized Clinical Trial. JAMA Intern. Med. 2022, 182, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Garland, E.L.; Hanley, A.W.; Riquino, M.R.; Reese, S.E.; Baker, A.K.; Salas, K.; Yack, B.P.; Bedford, C.E.; Bryan, M.A.; Atchley, R.; et al. Mindfulness-oriented recovery enhancement reduces opioid misuse risk via analgesic and positive psychological mechanisms: A randomized controlled trial. J. Consult. Clin. Psychol. 2019, 87, 927–940. [Google Scholar] [CrossRef] [PubMed]
- Garland, E.L.; Manusov, E.G.; Froeliger, B.; Kelly, A.; Williams, J.M.; Howard, M.O. Mindfulness-oriented recovery enhancement for chronic pain and prescription opioid misuse: Results from an early-stage randomized controlled trial. J. Consult. Clin. Psychol. 2014, 82, 448–459. [Google Scholar] [CrossRef] [PubMed]
- Hearn, J.H.; Finlay, K.A. Internet-delivered mindfulness for people with depression and chronic pain following spinal cord injury: A randomized, controlled feasibility trial. Spinal Cord 2018, 56, 750–761. [Google Scholar] [CrossRef] [PubMed]
- Henriksson, J.; Wasara, E.; Ronnlund, M. Effects of Eight-Week-Web-Based Mindfulness Training on Pain Intensity, Pain Acceptance, and Life Satisfaction in Individuals with Chronic Pain. Psychol. Rep. 2016, 119, 586–607. [Google Scholar] [CrossRef] [PubMed]
- Howarth, A.; Riaz, M.; Perkins-Porras, L.; Smith, J.G.; Subramaniam, J.; Copland, C.; Hurley, M.; Beith, I.; Ussher, M. Pilot randomised controlled trial of a brief mindfulness-based intervention for those with persistent pain. J. Behav. Med. 2019, 42, 999–1014. [Google Scholar] [CrossRef] [PubMed]
- Hussain, N.; Said, A.S.A. Mindfulness-Based Meditation versus Progressive Relaxation Meditation: Impact on Chronic Pain in Older Female Patients with Diabetic Neuropathy. J. Evid. Based Integr. Med. 2019, 24, 2515690X19876599. [Google Scholar] [CrossRef] [PubMed]
- Ussher, M.; Spatz, A.; Copland, C.; Nicolaou, A.; Cargill, A.; Amini-Tabrizi, N.; McCracken, L.M. Immediate effects of a brief mindfulness-based body scan on patients with chronic pain. J. Behav. Med. 2014, 37, 127–134. [Google Scholar] [CrossRef]
- Williams, R.M.; Day, M.A.; Ehde, D.M.; Turner, A.P.; Ciol, M.A.; Gertz, K.J.; Patterson, D.; Hakimian, S.; Suri, P.; Jensen, M.P. Effects of hypnosis vs mindfulness meditation vs education on chronic pain intensity and secondary outcomes in veterans: A randomized clinical trial. Pain 2022, 163, 1905–1918. [Google Scholar] [CrossRef] [PubMed]
- Zgierska, A.E.; Burzinski, C.A.; Cox, J.; Kloke, J.; Stegner, A.; Cook, D.B.; Singles, J.; Mirgain, S.; Coe, C.L.; Backonja, M. Mindfulness Meditation and Cognitive Behavioral Therapy Intervention Reduces Pain Severity and Sensitivity in Opioid-Treated Chronic Low Back Pain: Pilot Findings from a Randomized Controlled Trial. Pain Med. 2016, 17, 1865–1881. [Google Scholar] [CrossRef] [PubMed]
- la Cour, P.; Petersen, M. Effects of mindfulness meditation on chronic pain: A randomized controlled trial. Pain Med. 2015, 16, 641–652. [Google Scholar] [CrossRef] [PubMed]
- Onieva-Zafra, M.D.; Garcia, L.H.; Del Valle, M.G. Effectiveness of guided imagery relaxation on levels of pain and depression in patients diagnosed with fibromyalgia. Holist. Nurs. Pract. 2015, 29, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Polaski, A.M.; Phelps, A.L.; Smith, T.J.; Helm, E.R.; Morone, N.E.; Szucs, K.A.; Kostek, M.C.; Kolber, B.J. Integrated Meditation and Exercise Therapy: A Randomized Controlled Pilot of a Combined Nonpharmacological Intervention Focused on Reducing Disability and Pain in Patients with Chronic Low Back Pain. Pain Med. 2021, 22, 444–458. [Google Scholar] [CrossRef] [PubMed]
- Cooperman, N.A.; Hanley, A.W.; Kline, A.; Garland, E.L. A pilot randomized clinical trial of mindfulness-oriented recovery enhancement as an adjunct to methadone treatment for people with opioid use disorder and chronic pain: Impact on illicit drug use, health, and well-being. J. Subst. Abus. Treat. 2021, 127, 108468. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, W.; Good, M.; Draucker, C.B. Changes in the meaning of pain with the use of guided imagery. Pain Manag. Nurs. 2005, 6, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Baird, C.L.; Sands, L. A pilot study of the effectiveness of guided imagery with progressive muscle relaxation to reduce chronic pain and mobility difficulties of osteoarthritis. Pain Manag. Nurs. 2004, 5, 97–104. [Google Scholar] [CrossRef]
- Morone, N.E.; Greco, C.M.; Moore, C.G.; Rollman, B.L.; Lane, B.; Morrow, L.A.; Glynn, N.W.; Weiner, D.K. A Mind-Body Program for Older Adults with Chronic Low Back Pain: A Randomized Clinical Trial. JAMA Intern. Med. 2016, 176, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Cathcart, S.; Galatis, N.; Immink, M.; Proeve, M.; Petkov, J. Brief mindfulness-based therapy for chronic tension-type headache: A randomized controlled pilot study. Behav. Cogn. Psychother. 2014, 42, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.A.; Jones, A.K. Psychobiological correlates of improved mental health in patients with musculoskeletal pain after a mindfulness-based pain management program. Clin. J. Pain 2013, 29, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Lauche, R.; Schuth, M.; Schwickert, M.; Ludtke, R.; Musial, F.; Michalsen, A.; Dobos, G.; Choi, K.E. Efficacy of the Alexander Technique in treating chronic non-specific neck pain: A randomized controlled trial. Clin Rehabil. 2016, 30, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Fincham, G.W.; Mavor, K.; Dritschel, B. Effects of Mindfulness Meditation Duration and Type on Well-being: An Online Dose-Ranging Randomized Controlled Trial. Mindfulness 2023, 14, 1171–1182. [Google Scholar] [CrossRef] [PubMed]
- Grover, M.P. An experimental investigation of the effects and mechanisms of mindfulness meditation versus self-hypnosis versus an attention control on cold pressor outcomes. Mindfulness 2021, 12, 923–935. [Google Scholar] [CrossRef]
- Swain, N.R. A comparison of therapist-present or therapist-free delivery of very brief mindfulness and hypnosis for acute experimental pain. J. Psychol. 2014, 43, 22–28. [Google Scholar]
- Dear, B.F.; Titov, N.; Perry, K.N.; Johnston, L.; Wootton, B.M.; Terides, M.D.; Rapee, R.M.; Hudson, J.L. The Pain Course: A randomised controlled trial of a clinician-guided Internet-delivered cognitive behaviour therapy program for managing chronic pain and emotional well-being. Pain 2013, 154, 942–950. [Google Scholar] [CrossRef] [PubMed]
- Danon, N.; Al-Gobari, M.; Burnand, B.; Rodondi, P.Y. Are mind-body therapies effective for relieving cancer-related pain in adults? A systematic review and meta-analysis. Psychooncology 2022, 31, 345–371. [Google Scholar] [CrossRef] [PubMed]
- Garland, E.L.; Brintz, C.E.; Hanley, A.W.; Roseen, E.J.; Atchley, R.M.; Gaylord, S.A.; Faurot, K.R.; Yaffe, J.; Fiander, M.; Keefe, F.J. Mind-Body Therapies for Opioid-Treated Pain: A Systematic Review and Meta-analysis. JAMA Intern. Med. 2020, 180, 91–105. [Google Scholar] [CrossRef] [PubMed]
- Arola, H.M.; Nicholls, E.; Mallen, C.; Thomas, E. Self-reported pain interference and symptoms of anxiety and depression in community-dwelling older adults: Can a temporal relationship be determined? Eur. J. Pain 2010, 14, 966–971. [Google Scholar] [CrossRef] [PubMed]
- Bair, M.J.; Robinson, R.L.; Katon, W.; Kroenke, K. Depression and pain comorbidity: A literature review. Arch. Intern. Med. 2003, 163, 2433–2445. [Google Scholar] [CrossRef] [PubMed]
- Kroenke, K.; Wu, J.; Bair, M.J.; Krebs, E.E.; Damush, T.M.; Tu, W. Reciprocal relationship between pain and depression: A 12-month longitudinal analysis in primary care. J. Pain 2011, 12, 964–973. [Google Scholar] [CrossRef]
- Lewandowski Holley, A.; Law, E.F.; Zhou, C.; Murphy, L.; Clarke, G.; Palermo, T.M. Reciprocal longitudinal associations between pain and depressive symptoms in adolescents. Eur. J. Pain 2013, 17, 1058–1067. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).