Abstract
Context: Several recent randomized controlled trials (RCTs) have reported on the survival benefits of poly (ADP-ribose) polymerase inhibitors (PARPi) compared to standard-of-care (SOC) treatment (enzalutamide, abiraterone, or docetaxel) in patients with metastatic castration-resistant prostate cancer (mCRPC). However, there is a limited integrated analysis of high-quality evidence comparing the efficacy and safety of PARPi and SOC treatments in this context. Objective: This study aims to comprehensively analyze the survival benefits and adverse events associated with PARPi and SOC treatments through a head-to-head meta-analysis in mCRPC. Evidence acquisition: A systematic review search was conducted in PubMed, Embase, Clinical trials, and the Central Cochrane Registry in July 2023. RCTs were assessed following the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines. The systematic review was prospectively registered on PROSPERO (CRD42023441034). Evidence synthesis: A total of 8 studies, encompassing 2341 cases in the PARPi treatment arm and 1810 cases in the controlled arm, were included in the qualitative synthesis. The hazard ratio (HR) for radiographic progression-free survival (rPFS) and overall survival (OS) were 0.74 (95% CI, 0.61–0.90) and 0.89 (95% CI, 0.80–0.99), respectively, in the intention-to-treatment patients. For subgroup analysis, HRs for rPFS and OS in the BRCA-mutated subgroup were 0.39 (95% CI, 0.28–0.55) and 0.62 (95% CI, 0.38–0.99), while in the HRR-mutated subgroup, HR for rPFS was 0.57 (95% CI, 0.48–0.69) and for OS was 0.77 (95% CI, 0.64–0.93). The odds ratio (OR) for all grades of adverse events (AEs) and AEs with severity of at least grade 3 were 3.86 (95% CI, 2.53–5.90) and 2.30 (95% CI, 1.63–3.26), respectively. Conclusions: PARP inhibitors demonstrate greater effectiveness than SOC treatments in HRR/BRCA-positive patients with mCRPC. Further research is required to explore ways to reduce adverse event rates and investigate the efficacy of HRR/BRCA-negative patients.
1. Introduction
A significant proportion of patients inevitably progress to a state of metastatic castration resistance, which represents the terminal clinical phase in the intricate trajectory of prostate cancer evolution. In such cases, the standard-of-care (SOC) treatment options commonly employed include chemotherapy, with docetaxel or cabazitaxel, and second-generation antihormonal therapy, encompassing abiraterone or enzalutamide [,]. However, the meager survival benefits provided by these current therapeutic strategies have spurred an intensified exploration for alternative or combinatorial drugs, ranging from radiotherapy to immunotherapy and microbiota-targeted therapy [,,]. Nevertheless, it is disheartening to note that these endeavors have not succeeded in significantly improving overall survival outcomes for patients with mCRPC [,,].
Recently, the success of PARP inhibitors in other solid tumors has sparked interest in their potential application in mCRPC treatment, leading to multiple RCTs comparing PARP inhibitors with SOC treatment [,,,]. As mutations of homologous recombination repair (HRR) genes (e.g., BRCA1/2) are found in approximately one-quarter of cases of mCRPC, multiple randomized clinical trials have been conducted to explore the efficacy and safety of PARP inhibitors compared to SOC treatments [,,,,,,,,,,,,]. Notably, due to the relatively better survival benefits observed in patients with BRCA1/2/ATM alterations in the treatment arm of the PROfound trial, olaparib has been approved and recommended for those with HRR gene alterations who have progressed to mCRPC after receiving previous next-generation hormonal drug treatment []. In addition, other PARP inhibitors, such as talazoparib, niraparib, veliparib, and rucaparib, have also been tested in clinical trials for mCRPC and have reported encouraging data on the therapeutic performance of PARP inhibitors in terms of survival, further demonstrating the tremendous potential and necessity to use them in treating this lethal malignancy [,,,].
Despite having similar strict inclusion criteria, these high-quality randomized controlled trials (RCTs) have displayed inconsistent results regarding survival benefits and adverse events, partly due to disparities in participant numbers, HRR gene mutation status, treatment durations, and other factors. For instance, KEYLYNK-010 reported limited survival benefits and an unfavorable side effect profile of the treatment arm with a PARP inhibitor, while TRITON3 showed advantageous radiographic progression-free survival (rPFS) over the SOC arm and comparable rates of grade 3 or higher adverse events [,]. Moreover, although similar improvements in rPFS were observed for patients with BRCA1/2 alterations in all RCTs, it remains controversial whether PARP inhibitors can be applied to mCRPC in patients without BRCA or even HRR gene mutations, despite recent evidence of novel mechanisms beyond inhibition of DNA repair in tumor cells [,].
Therefore, we performed this systematic review and meta-analysis to integrate all updated high-quality RCTs, aimed at analyzing primary endpoints and adverse events and exploring the efficacy of PARP inhibitors among specific patient subgroups. By synthesizing and integrating the available evidence, we hope that this study will provide guidance for defining the appropriate scope of PARP inhibitor application and support clinicians in making informed decisions regarding treatment options for mCRPC.
2. Materials and Methods
2.1. Search Strategy and Data Extraction
The review protocol was registered on PROSPERO (CRD42023441034) and conducted following the Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines []. PubMed, Embase, Clinical trials, and the Central Cochrane Registry were searched for RCTs using PARP inhibitors to treat mCRPC and published before 15 July 2023. The search keywords were as follows: (“PARP” OR “PARP inhibitors” OR “Olaparib” OR “talazoparib” OR “rucaparib” OR “niraparib” OR “veliparib”) AND (“mCRPC” OR “metastatic castration-resistant prostate cancer”). Next, two independent reviewers performed a literature screening. Of note, 4 publications of RCTs belong to the same study PROfound (NCT02987543), and two publications of RCTs belong to the same study with clinical trial number NCT01972217. We carefully compared all the publications affiliated with the same RCT and used the most updated and complete data.
2.2. Inclusion and Exclusion Criteria
The following studies were included: (1) randomized controlled trials; (2) patients with histologically or cytologically diagnosed mCRPC; (3) studies exploring the comparison between PARPi and SOC; (4) SOC treatment group studied was novel hormonal agents (NHA); (5) studies reported data on rPFS or OS.
The following studies were excluded: (1) single-arm trials; (2) reviews, letters, case reports, and protocols; (3) pharmacokinetics studies; (4) studies that do not provide data on relevant evaluation indicators; (5) non-English language.
2.3. Measure of Effect
OS and rPFS assessed by a blinded independent central review were the primary endpoints of interest. Other endpoints included in this meta-analysis were time to first subsequent therapy (TFST), time to PSA progression (TTPP), PSA response rate (a confirmed PSA decrease of at least 50%, PSA RR), and objective response rate (ORR). For safety analysis, rates of all grades, ≥3 grades, and serious treatment-emergent adverse events (AEs) were analyzed (graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.03). The key results of the meta-analyses are summarized in Supplementary Table S1.
2.4. Risk of Bias Assessment
The risk of bias for individual nonrandomized studies was analyzed in accordance with Cochrane recommendations using RevMan 5.3 by two independent reviewers. Studies with a significant risk of bias were excluded from the quantitative synthesis.
2.5. Statistical Analysis
We performed a meta-analysis of the mCRPC studies. The hazard ratio (HR) was calculated to evaluate the OS, rPFS, and TFST. The odds ratio (OR) was estimated to evaluate the PSA response and ORR, as well as AEs. All estimates were expressed with their 95% confidence intervals (CIs). On this basis, we divided the patients into four subgroups according to their HRR and BRCA mutation status to discuss whether the different mutation statuses would have different outcomes in terms of efficacy benefit. All meta-analyses were performed using a random-effects model and produced into forest plots using Cochrane Collaboration ReviewManager software (RevMan 5.3). We used R 4.2.3 to conduct a sensitivity analysis by eliminating one by one method to evaluate the consistency of the results. p < 0.05 (two-tailed) was considered statistically significant, and I2 > 50% was defined as high heterogeneity.
3. Results
3.1. Study Selection
The initial search identified 1476 publications, and a total of 1225 publications remained after the elimination of the duplicates. Then, 1147 articles were excluded after screening the titles and abstracts, and full-text reviews were performed on 78 articles. According to the selection criteria, we filtered eight studies comprising 4151 patients for inclusion in this meta-analysis. The entire process of initial screening and the reasons for excluding studies are illustrated in Figure 1. The characteristics of the included studies and patients are shown in Table 1 and Table 2. All these studies were published between 2018 and 2023, and were multicenter, prospective, large-scale clinical RCTs. Each risk of bias for the included studies was analyzed using RevMan 5.3 according to Cochrane recommendations, and the methodological quality of most studies was deemed good (Figures S1 and S2).
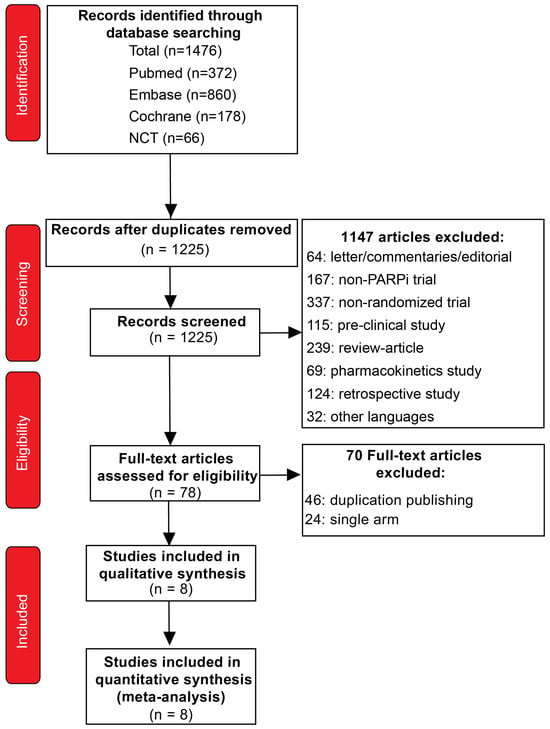
Figure 1.
The PRISMA flow chart, detailing the article selection process.

Table 1.
Characteristics of the studies included.

Table 2.
Characteristics of the patients included. * Patients with bone disease only.
3.2. Efficacy
3.2.1. rPFS
This meta-analysis was conducted on trials reporting rPFS as the primary endpoint and not differentiating between mutation status. The results showed that the PARPi treatment demonstrated superior efficacy (HR, 0.74; 95% CI, 0.61–0.90) compared to the control treatment (Figure 2A). Sensitivity analyses were performed, and the results showed good concordance between the trials (Figure S3A).
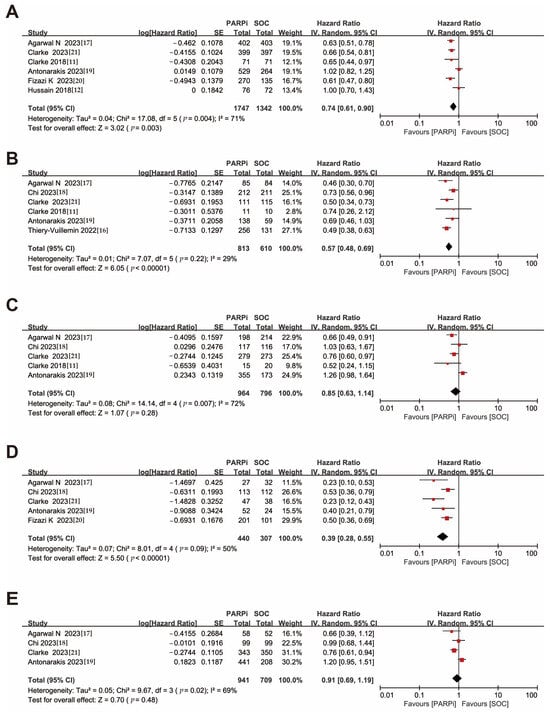
Figure 2.
Forest plots showing the association of rPFS in mCRPC patients with or without DNA damage repair gene mutation. (A). Overall patients. (B). HRR gene-mutated patients. (C). Non-HRR gene-mutated patients. (D). BRCA gene-mutated patients. (E). Non-BRCA gene-mutated patients. rPFS: radiographic progression-free survival; mCRPC: metastatic castration-resistant prostate cancer; HRR: homologous recombination repair [,,,,,,,].
A similar favorable result for PARPi was obtained among patients with HRR gene mutations (HR, 0.57; 95% CI, 0.48–0.69), while no significant advantage of PARPi over standard therapy was demonstrated (HR, 0.85; 95% CI, 0.63–1.14) in populations without detectable HRR gene mutations (Figure 2B,C).
Notably, sensitivity analysis showed that even after excluding KEYLYNK-010, PARPi still exhibited improved efficacy in patients without HRR gene alterations (HR, 0.74; 95% CI, 0.62–0.88), underscoring the need for further exploration of PARPi efficacy in a wider range of patients (Figure S3B).
For patients with BRCA gene mutations, the PARPi treatment group showed a significant advantage (HR, 0.39; 95% CI, 0.28–0.55). In contrast, PARPi did not demonstrate therapeutic benefits for patients without BRCA gene mutations (HR, 0.91; 95% CI, 0.69–1.19) (Figure 2D,E), and the sensitivity analysis likewise showed that the KEYLYNK-010 had a large impact on the results (Figure S3C,D).
3.2.2. OS
OS was considered a secondary outcome in the studies included in this meta-analysis. For the overall patient population, even though individual trials had negative results, a conservative model was used to derive a slight benefit for PARPi treatment compared to NHA (HR, 0.89; 95% CI, 0.80–0.99) (Figure 3A). Considering the very low heterogeneity of the original study, which became statistically significant after meta-analysis with a larger sample size (Figure S3E).
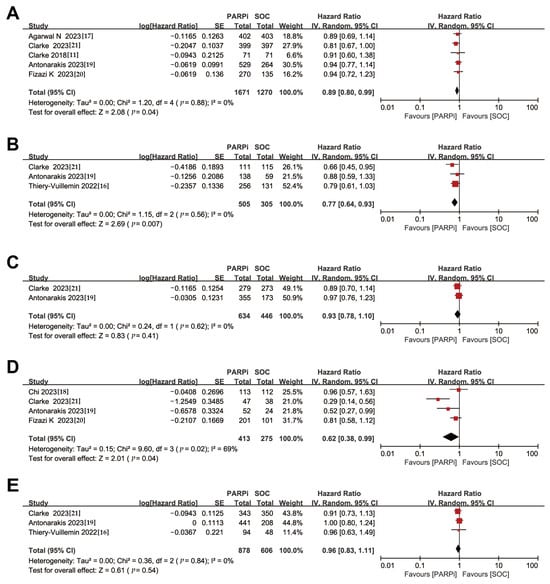
Figure 3.
Forest plots showing the association of OS in mCRPC patients with or without DNA damage repair gene mutation. (A). Overall patients. (B). HRR gene-mutated patients. (C). Non-HRR gene-mutated patients. (D). BRCA gene-mutated patients. (E). Non-BRCA gene-mutated patients. OS: overall survival; mCRPC: metastatic castration-resistant prostate cancer; HRR: homologous recombination repair [,,,,,,].
Similar to the findings for rPFS, in the subgroup of patients with HRR gene mutations, PARPi treatment demonstrated improved overall survival (HR, 0.77; 95% CI, 0.64–0.93), while no significant improvements were observed in non-HRR gene-mutated patients (HR, 0.93; 95% CI, 0.78–1.10) (Figure 3B,C).
In the BRCA gene-mutated subgroup, PARPi treatment also showed a therapeutic advantage (HR, 0.62; 95% CI, 0.38–0.99), but the evidence for this advantage in patients without BRCA gene mutations is currently inconclusive (HR, 0.96; 95% CI, 0.83–1.11) (Figure 3D,E). Nevertheless, sensitivity analyses showed heterogeneity in this finding (Figure S3F), possibly due to studies with insufficient OS maturation. This emphasizes the need for more clinical trials to follow up on whether PARPi is effective in improving overall survival in patients without BRCA gene mutations.
3.2.3. Disease Progression and Relief
Based on the comprehensive information disclosed in the included studies, we evaluated four outcomes related to disease progression and relief in the intention-to-treat populations: TFST, TTPP, PSA RR, and ORR. In terms of disease progression, both TFST (HR, 0.72; 95% CI, 0.57–0.89) and TTPP (HR, 0.73; 95% CI, 0.54–0.98) were significantly reduced in the PARPi treatment arm. As for disease relief, PSA RR (OR, 1.52; 95% CI, 1.10–2.10) and ORR (OR, 1.97; 95% CI, 1.27–3.04) also favored the PARPi treatment (Figure S4A–D). Despite substantial heterogeneity among the studies, the consistent conclusions consistently favored the selection of PARPi treatment (Figure S3G–J).
3.3. Safety
All eight included studies reported overall adverse events and grade ≥ 3 adverse events, with five studies reporting serious adverse events. The PARPi treatment group had a higher risk of all grades of adverse events (OR, 3.86; 95% CI, 2.53–5.90) compared to the SOC treatment group, as well as a higher risk of grade ≥3 adverse events (OR, 2.30; 95% CI, 1.63–3.26) and serious adverse events (OR, 1.49; 95% CI, 1.25–1.76) (Figure 4A–C). We ranked the types of side effects based on their frequencies and ultimately identified four hematologic-related side effects, hypertension, and fatigue, as indicators for further analysis. Among these, anemia and fatigue had the highest incidence rates, with odds ratios of all grades of adverse events of (OR, 6.01; 95% CI, 3.78–9.56) and (OR, 1.45; 95% CI, 1.22–1.74), and odds ratios for grade ≥3 adverse events of (OR, 9.73; 95% CI, 5.44–17.41) and (OR, 1.12; 95% CI, 0.76–1.65), respectively (Figure S5A–D). Of interest, hypertension did not significantly differ in either overall (OR, 0.76; 95% CI, 0.47–1.23) or grade ≥ 3 adverse events (OR, 0.75; 95% CI, 0.51–1.11) (Figure S5E,F).
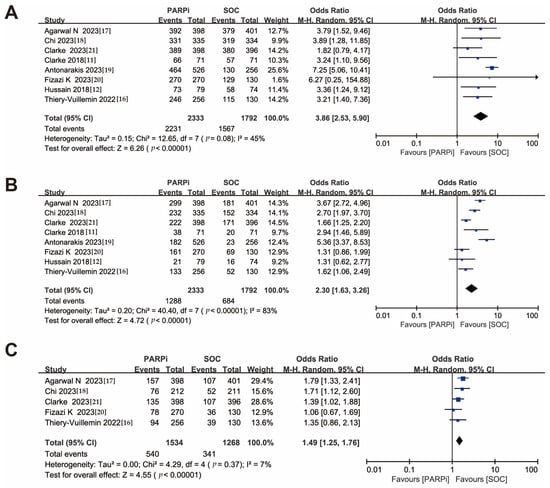
Figure 4.
Forest plots showing the association of treatment safety in mCRPC patients. (A). All grades of adverse event. (B). Grade ≥ 3 of adverse event. (C). Serious adverse event. mCRPC: metastatic castration-resistant prostate cancer [,,,,,,,].
PARPi treatment also showed a higher risk for the remaining three hematologic-related side effects: Thrombocytopenia (OR, 5.45; 95% CI, 2.61–11.40), neutropenia (OR, 3.96; 95% CI, 1.77–8.86), and leukopenia (OR, 5.28; 95% CI, 3.30–8.43) (Figure S5G,I,K). In grade ≥ 3 adverse events, thrombocytopenia (OR, 5.45; 95% CI, 2.90–10.21) and neutropenia (OR, 4.50; 95% CI, 1.18–17.16) showed similar trends (Figure S5H,J), but the association with leukopenia (OR, 5.48; 95% CI, 0.34–88.82) was not statistically significant, especially after removing the studies leading to heterogeneity (Figure S5L).
4. Discussion
In contrast to recent published systematic reviews [,], which primarily involve a constrained number of trials, often lacking randomization, potentially leading to incomplete or biased conclusions, this exhaustive meta-analysis, encompassing a corpus of eight meticulously executed prospective randomized controlled trials (RCTs), unequivocally substantiates that the application of poly (ADP-ribose) polymerase (PARP) inhibitors in the therapeutic management of metastatic castration-resistant prostate cancer (mCRPC) confers remarkable enhancements in both overall survival (OS) and radiographic progression-free survival (rPFS) across all patient cohorts. This notable improvement is observed not only among the general population but is especially pronounced in individuals exhibiting BRCA/homologous recombination repair (HRR) mutations.
BRCA1/2, the foremost DNA repair gene loci to be identified, as well as the most widely acknowledged marker for mutation testing concerning the application of PARP inhibitors, have played a pivotal role in this field [,]. Efforts have also been made to investigate other genes involved in homologous recombination repair (HRR) prior to the initiation of PARP inhibitor treatment, such as ATM, CDK12, CHEK2, and many others [,,]. However, a consensus linking specific mutations to the application of PARP inhibitors, apart from BRCA1/2, has yet to be reached. Our findings indicate that patients who tested positive for mutations in the BRCA or HRR genes can derive therapeutic benefits from PARP inhibitor treatment, although some heterogeneity was observed in OS within the subgroup of BRCA-mutated patients. Importantly, the variability in the examination of HRR genes across different trials impairs the efficacy of conclusions establishing the subgroup of patients with HRR mutations as “potentially profited patients”.
Even in prostate cancer cases lacking HRR alterations, the combination therapy of PARP inhibitors with androgen receptor inhibitors (ARi) holds great promise due to the synergistic treatment effects observed []. According to our results, although statistically significant improvements in survival outcomes were not seen in the subgroup of patients without BRCA/HRR mutations, sensitivity analysis pointed towards potential survival benefits for this subgroup upon exclusion of the KEYLYNK-010 study. This finding may be attributed to the use of pembrolizumab in the KEYLYNK-010 study. Similar sensitivity analyses were also conducted for time to prostate-specific antigen radiographic progression (TTPP) and prostate-specific antigen response rate (PSARR), both of which showcased the narrowing impact of PD-1 blockade therapy on the conclusions regarding survival benefits.
It has been postulated that patients without HRR mutations may still derive potential benefits from PARP inhibition []. On the one hand, PARP inhibitors can attenuate the transcriptional activity of the androgen receptor (AR), thereby enhancing the inhibitory effects of ARi on AR pathways [,]. On the other hand, AR itself serves as a transcriptional factor that promotes DNA damage response and HRR by facilitating the accumulation of γH2AX and RAD51 foci [,,]. Consequently, the obstruction of AR signaling in patients undergoing androgen deprivation therapy (ADT) compromises HRR and leads to compensatory PARP activity. Thus, the inhibition of AR becomes synthetically lethal when combined with PARP inhibition [,]. Additionally, other yet undiscovered mutation loci may induce sensitivity to PARP inhibition in prostate cancer [], which may explain why some mCRPC patients lacking deleterious HRR mutations still respond to PARP inhibitors []. Therefore, further delineation and more detailed studies of the HRR-negative population are warranted, as are additional fundamental research endeavors to uncover new mechanisms and improve the identification of the patient population suitable for PARP inhibitors.
Our comprehensive meta-analysis unequivocally demonstrates the substantial advantages conferred by PARP inhibitors over standard-of-care treatment in the improvement of rPFS and OS among mCRPC patients with any HRR mutation, thereby underscoring the immense therapeutic potential of PARP inhibitors in a stratified manner based on HRR gene-mutation signatures. Nonetheless, while our overall findings in the subgroup of patients without BRCA/HRR mutations do not advocate for the routine application of PARP inhibitors, sensitivity analysis reminds us that further exploration necessitates more profound and comprehensive pre-clinical and clinical evidence. Furthermore, the concept of “patient-centered clinical trials” has gained significant traction in recent times []. Despite the general responsiveness of BRCA/HRR-positive patients to PARP inhibitors, a subset of these patients fails to derive a survival benefit. The reasons for this subset of patients warrant in-depth subgroup analyses. Collectively, these findings underscore the necessity and significance of molecular testing in guiding patient management and emphasize the importance of establishing treatment frameworks that incorporate precisely targeted therapies for mCRPC patients.
However, it is important to acknowledge a concurrent elevation in the incidence of treatment-emergent adverse events. Regarding safety, the rates of overall adverse events (AEs), grade ≥ 3 AEs, and serious AEs were all higher in the PARP inhibitor treatment arm as compared to the standard-of-care treatment arm. The most frequently observed adverse effects encompassed fatigue, anemia, hypertension, thrombocytopenia, nausea, neutropenia, and others, mirroring the occurrences reported in previous studies involving other solid tumors [,]. Generally, these side effects can be effectively managed through supportive measures such as transfusion of blood components and growth factor therapy, as well as dose reduction and interruption when necessary. Notably, the incidences of treatment-emergent hypertension were similar irrespective of the utilization of PARP inhibitors, thereby offering an alternative treatment option for individuals who cannot tolerate the elevated blood pressure associated with ARi.
5. Conclusions
This meta-analysis presents compelling and robust data indicating the favorable clinical efficacy and tolerability of PARP inhibitor (PARPi) treatment, both as a monotherapy and in combination therapy, for the management of refractory metastatic castration-resistant prostate cancer (mCRPC) characterized by BRCA/HRR mutations. To further enhance therapy selection and optimize treatment outcomes, it is imperative to dedicate resources to comprehensively investigate and comprehend predictive markers and signatures associated with treatment response and resistance to PARP inhibitors. This endeavor will enable the development of personalized treatment approaches tailored to individual patients, maximizing therapeutic benefit.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/medicina59122198/s1, Figure S1: Risk of bias summary of the included studies; Figure S2: Risk of bias graph of the included studies; Figure S3: Forest plots showing the sensitivity analysis of treatment efficacy in mCRPC patients by sequential omitting each included study, (A) rPFS based on intention-to-treat patients, (B) rPFS based on Non-HRR mutated patients, (C) rPFS based on BRCA mutated patients, (D) rPFS based on Non-BRCA mutated patients, (E) OS based on intention-to-treat patients, (F) OS based on BRCA mutated patients, (G) TFST based on intention-to-treat patients, (H) TTPP based on intention-to-treat patients, (I) PSA RR based on intention-to-treat patients, and (J) ORR based on intention-to-treat patients; Figure S4: Forest plots showing the association of treatment efficacy in mCRPC patients, (A) TFST, (B) TTPP, (C) PSA RR, and (D) ORR; Figure S5: Forest plots showing the association of adverse events with a high incidence rate in mCRPC patients, (A) All grades of anemia, (B) Grade ≥ 3 of anemia, (C) All grades of fatigue, (D) Grade ≥ 3 of fatigue, (E) All grades of hypertension, (F) Grade ≥ 3 of hypertension, (G) All grades of thrombocytopenia, (H) Grade ≥ 3 of thrombocytopenia, (I) All grades of neutropenia, (J) Grade ≥ 3 of neutropenia, (K) All grades of leukopenia, and (L) Grade ≥ 3 of leukopenia; Table S1: Meta-analysis of the primary efficacy and safety of PAPRi for the treatment of mCRPC.
Author Contributions
Conceptualization: Z.W. (Zhihua Wang); data curation: L.L., Y.J., Y.T. and X.G.; formal analysis: Z.C., Y.T., X.H. and C.Z.; methodology: L.L. and Y.J.; software: Z.W. (Zefeng Wang); statistical analysis: Z.C. and Z.W. (Zefeng Wang); validation: Y.W., W.Y. and F.C.; visualization: Z.C. and Z.W. (Zefeng Wang); writing—original draft: Z.C. and L.L.; writing—review and editing: Z.W. (Zefeng Wang), F.C. and Z.W. (Zhihua Wang); supervision: Z.W. (Zhihua Wang). All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
Zhihua Wang certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (e.g., employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
References
- Chang, A.J.; Autio, K.A.; Roach, M., 3rd; Scher, H.I. High-risk prostate cancer-classification and therapy. Nat. Rev. Clin. Oncol. 2014, 11, 308–323. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Dehm, S.M.; Sharifi, N. Targeting the Androgen Signaling Axis in Prostate Cancer. J. Clin. Oncol. 2023, 41, 4267. [Google Scholar] [CrossRef] [PubMed]
- Pernigoni, N.; Guo, C.; Gallagher, L.; Yuan, W.; Colucci, M.; Troiani, M.; Liu, L.; Maraccani, L.; Guccini, I.; Migliorini, D.; et al. The potential role of the microbiota in prostate cancer pathogenesis and treatment. Nat. Rev. Urol. 2023, 20, 706–718. [Google Scholar] [CrossRef] [PubMed]
- Gorchakov, A.A.; Kulemzin, S.V.; Kochneva, G.V.; Taranin, A.V. Challenges and Prospects of Chimeric Antigen Receptor T-cell Therapy for Metastatic Prostate Cancer. Eur. Urol. 2020, 77, 299–308. [Google Scholar] [CrossRef]
- Hamdy, F.C.; Donovan, J.L.; Lane, J.A.; Metcalfe, C.; Davis, M.; Turner, E.L.; Martin, R.M.; Young, G.L.; Walsh, E.I.; Bryant, R.J.; et al. Fifteen-Year Outcomes after Monitoring, Surgery, or Radiotherapy for Prostate Cancer. N. Engl. J. Med. 2023, 388, 1547–1558. [Google Scholar] [CrossRef] [PubMed]
- Golan, T.; Hammel, P.; Reni, M.; Van Cutsem, E.; Macarulla, T.; Hall, M.J.; Park, J.-O.; Hochhauser, D.; Arnold, D.; Oh, D.-Y.; et al. Maintenance Olaparib for Germline BRCA-Mutated Metastatic Pancreatic Cancer. N. Engl. J. Med. 2019, 381, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Loibl, S.; O’Shaughnessy, J.; Untch, M.; Sikov, W.M.; Rugo, H.S.; McKee, M.D.; Huober, J.; Golshan, M.; von Minckwitz, G.; Maag, D.; et al. Addition of the PARP inhibitor veliparib plus carboplatin or carboplatin alone to standard neoadjuvant chemotherapy in triple-negative breast cancer (BrighTNess): A randomised, phase 3 trial. Lancet Oncol. 2018, 19, 497–509. [Google Scholar] [CrossRef] [PubMed]
- González-Martín, A.; Pothuri, B.; Vergote, I.; DePont Christensen, R.; Graybill, W.; Mirza, M.R.; McCormick, C.; Lorusso, D.; Hoskins, P.; Freyer, G.; et al. Niraparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N. Engl. J. Med. 2019, 381, 2391–2402. [Google Scholar] [CrossRef]
- Niazi, M.; Jahangir, A.; Sahra, S.; Sattar, S.; Asti, D.; Bershadskiy, A. Efficacy of PARP Inhibitors as Maintenance Therapy for Metastatic Castration-Resistant Prostate Cancer: A Meta-Analysis of Randomized Controlled Trials. Oncology 2021, 35, 708–715. [Google Scholar]
- Saad, F.; Thiery-Vuillemin, A.; Wiechno, P.; Alekseev, B.; Sala, N.; Jones, R.; Kocak, I.; Chiuri, V.E.; Jassem, J.; Flechon, A.; et al. Patient-reported outcomes with olaparib plus abiraterone versus placebo plus abiraterone for metastatic castration-resistant prostate cancer: A randomised, double-blind, phase 2 trial. Lancet Oncol. 2022, 23, 1297–1307. [Google Scholar] [CrossRef]
- Clarke, N.; Wiechno, P.; Alekseev, B.; Sala, N.; Jones, R.; Kocak, I.; Chiuri, V.E.; Jassem, J.; Fléchon, A.; Redfern, C.; et al. Olaparib combined with abiraterone in patients with metastatic castration-resistant prostate cancer: A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2018, 19, 975–986. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Daignault-Newton, S.; Twardowski, P.W.; Albany, C.; Stein, M.N.; Kunju, L.P.; Siddiqui, J.; Wu, Y.-M.; Robinson, D.; Lonigro, R.J.; et al. Targeting Androgen Receptor and DNA Repair in Metastatic Castration-Resistant Prostate Cancer: Results from NCI 9012. J. Clin. Oncol. 2018, 36, 991–999. [Google Scholar] [CrossRef] [PubMed]
- De Bono, J.; Mateo, J.; Fizazi, K.; Saad, F.; Shore, N.; Sandhu, S.; Chi, K.N.; Sartor, O.; Agarwal, N.; Olmos, D.; et al. Olaparib for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2020, 382, 2091–2102. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Mateo, J.; Fizazi, K.; Saad, F.; Shore, N.; Sandhu, S.; Chi, K.N.; Sartor, O.; Agarwal, N.; Olmos, D.; et al. Survival with Olaparib in Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2020, 383, 2345–2357. [Google Scholar] [CrossRef] [PubMed]
- Roubaud, G.; Ozguroglu, M.; Penel, N.; Matsubara, N.; Mehra, N.; Kolinsky, M.P.; Procopio, G.; Feyerabend, S.; Joung, J.Y.; Gravis, G.; et al. Olaparib tolerability and common adverse-event management in patients with metastatic castration-resistant prostate cancer: Further analyses from the PROfound study. Eur. J. Cancer. 2022, 170, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Thiery-Vuillemin, A.; de Bono, J.; Hussain, M.; Roubaud, G.; Procopio, G.; Shore, N.; Fizazi, K.; Dos Anjos, G.; Gravis, G.; Joung, J.Y.; et al. Pain and health-related quality of life with olaparib versus physician’s choice of next-generation hormonal drug in patients with metastatic castration-resistant prostate cancer with homologous recombination repair gene alterations (PROfound): An open-label, randomised, phase 3 trial. Lancet Oncol. 2022, 23, 393–405. [Google Scholar] [PubMed]
- Agarwal, N.; Azad, A.; Carles, J.; Fay, A.P.; Matsubara, N.; Heinrich, D.; Szczylik, C.; De Giorgi, U.; Joung, J.Y.; Fong, P.C.C.; et al. Talazoparib plus enzalutamide in men with first-line metastatic castration-resistant prostate cancer (TALAPRO-2): A randomised, placebo-controlled, phase 3 trial. Lancet 2023, 402, 291–303. [Google Scholar] [CrossRef]
- Chi, K.N.; Rathkopf, D.; Smith, M.R.; Efstathiou, E.; Attard, G.; Olmos, D.; Lee, J.Y.; Small, E.J.; Gomes, A.J.P.d.S.; Roubaud, G.; et al. Niraparib and Abiraterone Acetate for Metastatic Castration-Resistant Prostate Cancer. J. Clin. Oncol. 2023, 41, 3339–3351. [Google Scholar] [CrossRef]
- Antonarakis, E.S.; Park, S.H.; Goh, J.C.; Shin, S.J.; Lee, J.L.; Mehra, N.; McDermott, R.; Sala-Gonzalez, N.; Fong, P.C.; Greil, R.; et al. Pembrolizumab Plus Olaparib for Patients with Previously Treated and Biomarker-Unselected Metastatic Castration-Resistant Prostate Cancer: The Randomized, Open-Label, Phase III KEYLYNK-010 Trial. J. Clin. Oncol. 2023, 41, 3839–3850. [Google Scholar] [CrossRef]
- Fizazi, K.; Piulats, J.M.; Reaume, M.N.; Ostler, P.; McDermott, R.; Gingerich, J.R.; Pintus, E.; Sridhar, S.S.; Bambury, R.M.; Emmenegger, U.; et al. Rucaparib or Physician’s Choice in Metastatic Prostate Cancer. N. Engl. J. Med. 2023, 388, 719–732. [Google Scholar] [CrossRef]
- Clarke, N.W.; Armstrong, A.J.; Thiery-Vuillemin, A.; Oya, M.; Shore, N.D.; Procopio, G.; Guedes, J.D.C.; Arslan, C.; Mehra, N.; Parnis, F.; et al. Final overall survival (OS) in PROpel: Abiraterone (abi) and olaparib (ola) versus abiraterone and placebo (pbo) as first-line (1L) therapy for metastatic castration-resistant prostate cancer (mCRPC). J. Clin. Oncol. 2023, 41, LBA16. [Google Scholar] [CrossRef]
- Clarke, N.W.; Armstrong, A.J.; Thiery-Vuillemin, A.; Oya, M.; Shore, N.; Loredo, E.; Procopio, G.; de Menezes, J.; Girotto, G.; Arslan, C.; et al. Abiraterone and Olaparib for Metastatic Castration-Resistant Prostate Cancer. NEJM Evid. 2022, 1, EVIDoa2200043. [Google Scholar] [CrossRef]
- Zhang, Z.; Diao, L.; Zhang, C.; Wang, F.; Guan, X.; Yao, X. Use of PARP inhibitors in prostate cancer: From specific to broader application. Front. Endocrinol. 2023, 14, 1164067. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Messina, C.; Giunta, E.F.; Signori, A.; Rebuzzi, S.E.; Banna, G.L.; Maniam, A.; Buti, S.; Cattrini, C.; Fornarini, G.; Bauckneht, M.; et al. Combining PARP Inhibitors and Androgen Receptor Signalling Inhibitors in Metastatic Prostate Cancer: A Quantitative Synthesis and Meta-analysis. Eur. Urol. Oncol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Giesen, A.; Baekelandt, L.; Devlies, W.; Devos, G.; Dumez, H.; Everaerts, W.; Claessens, F.; Joniau, S. Double trouble for prostate cancer: Synergistic action of AR blockade and PARPi in non-HRR mutated patients. Front. Oncol. 2023, 13, 1265812. [Google Scholar] [CrossRef] [PubMed]
- Lord, C.J.; Ashworth, A. Mechanisms of resistance to therapies targeting BRCA-mutant cancers. Nat. Med. 2013, 19, 1381–1388. [Google Scholar] [CrossRef] [PubMed]
- Andronikou, C.; Rottenberg, S. Studying PAR-Dependent Chromatin Remodeling to Tackle PARPi Resistance. Trends Mol. Med. 2021, 27, 630–642. [Google Scholar] [CrossRef]
- Concannon, K.; Morris, B.B.; Gay, C.M.; Byers, L.A. Combining targeted DNA repair inhibition and immune-oncology approaches for enhanced tumor control. Mol. Cell 2023, 83, 660–680. [Google Scholar] [CrossRef]
- Chou, J.; Quigley, D.A.; Robinson, T.M.; Feng, F.Y.; Ashworth, A. Transcription-Associated Cyclin-Dependent Kinases as Targets and Biomarkers for Cancer Therapy. Cancer Discov. 2020, 10, 351–370. [Google Scholar] [CrossRef]
- Cheng, H.H.; Sokolova, A.O.; Schaeffer, E.M.; Small, E.J.; Higano, C.S. Germline and Somatic Mutations in Prostate Cancer for the Clinician. J. Natl. Compr. Canc. Netw. 2019, 17, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Bienkowski, M.; Tomasik, B.; Braun, M.; Jassem, J. PARP inhibitors for metastatic castration-resistant prostate cancer: Biological rationale and current evidence. Cancer Treat Rev. 2022, 104, 102359. [Google Scholar] [CrossRef] [PubMed]
- Sciarra, A.; Frisenda, M.; Bevilacqua, G.; Gentilucci, A.; Cattarino, S.; Mariotti, G.; Del Giudice, F.; Di Pierro, G.B.; Viscuso, P.; Casale, P.; et al. How the Analysis of the Pathogenetic Variants of DDR Genes Will Change the Management of Prostate Cancer Patients. Int. J. Mol. Sci. 2022, 24, 674. [Google Scholar] [CrossRef] [PubMed]
- Schiewer, M.J.; Goodwin, J.F.; Han, S.; Brenner, J.C.; Augello, M.A.; Dean, J.L.; Liu, F.; Planck, J.L.; Ravindranathan, P.; Chinnaiyan, A.M.; et al. Dual roles of PARP-1 promote cancer growth and progression. Cancer Discov. 2012, 2, 1134–1149. [Google Scholar] [CrossRef]
- Cai, M.; Song, X.L.; Li, X.A.; Chen, M.; Guo, J.; Yang, D.H.; Chen, Z.; Zhao, S.-C. Current therapy and drug resistance in metastatic castration-resistant prostate cancer. Drug Resist. Updat. 2023, 68, 100962. [Google Scholar] [CrossRef] [PubMed]
- Gui, B.; Gui, F.; Takai, T.; Feng, C.; Bai, X.; Fazli, L.; Dong, X.; Liu, S.; Zhang, X.; Zhang, W.; et al. Selective targeting of PARP-2 inhibits androgen receptor signaling and prostate cancer growth through disruption of FOXA1 function. Proc. Natl. Acad. Sci. USA 2019, 116, 14573–14582. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chang, W.; Yang, G.; Ren, C.; Park, S.; Karantanos, T.; Karanika, S.; Wang, J.; Yin, J.; Shah, P.K.; et al. Targeting poly(ADP-ribose) polymerase and the c-Myb-regulated DNA damage response pathway in castration-resistant prostate cancer. Sci. Signal 2014, 7, ra47. [Google Scholar] [CrossRef] [PubMed]
- Hankey, W.; Chen, Z.; Wang, Q. Shaping Chromatin States in Prostate Cancer by Pioneer Transcription Factors. Cancer Res. 2020, 80, 2427–2436. [Google Scholar] [CrossRef]
- Li, L.; Karanika, S.; Yang, G.; Wang, J.; Park, S.; Broom, B.M.; Manyam, G.C.; Wu, W.; Luo, Y.; Basourakos, S.; et al. Androgen receptor inhibitor-induced “BRCAness” and PARP inhibition are synthetically lethal for castration-resistant prostate cancer. Sci. Signal 2017, 10, 7479. [Google Scholar] [CrossRef]
- Asim, M.; Tarish, F.; Zecchini, H.I.; Sanjiv, K.; Gelali, E.; Massie, C.E.; Baridi, A.; Warren, A.Y.; Zhao, W.; Ogris, C.; et al. Synthetic lethality between androgen receptor signalling and the PARP pathway in prostate cancer. Nat. Commun. 2017, 8, 374. [Google Scholar] [CrossRef]
- Tsujino, T.; Takai, T.; Hinohara, K.; Gui, F.; Tsutsumi, T.; Bai, X.; Miao, C.; Feng, C.; Gui, B.; Sztupinszki, Z.; et al. CRISPR screens reveal genetic determinants of PARP inhibitor sensitivity and resistance in prostate cancer. Nat. Commun. 2023, 14, 252. [Google Scholar] [CrossRef]
- Liu, S.-Y.M.; Tu, H.-Y.; Wei, X.-W.; Yan, H.-H.; Dong, X.-R.; Cui, J.-W.; Zhou, Z.; Xu, C.-R.; Zheng, M.-Y.; Li, Y.-S.; et al. First-line pyrotinib in advanced HER2-mutant non-small-cell lung cancer: A patient-centric phase 2 trial. Nat. Med. 2023, 29, 2079–2086. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).