Abstract
Background and Objectives: The aim was to compare the intra and postoperative outcomes between the Enhanced Recovery After Surgery (ERAS) protocol versus the standard of care protocol (SCP) in patients who underwent radical cystectomy performed by a single surgeon. Materials and Methods: A retrospective comparative study was conducted including patients who underwent radical cystectomy from 2017 to 2020. Length of stay (LOS), incidence of ileus, early postoperative complications, and number of re-hospitalizations within 30 days were considered as primary comparative outcomes of the study. Results: Data were collected for 91 patients who underwent cystectomy, and 70 and 21 patients followed the SCP and ERAS protocol, respectively. The mean age of the patients was 70.6 (SD 9.5) years. Although there was a statistically significant difference in time to flatus (TTF) [3 (2.7–3) vs. 1 (1–2 IQR) days, p < 0.001, in the SC hospital and in the ERAS center respectively], no difference was reported in time to first defecation (TTD) [5 (4–6) vs. 4 (3–5.8), p = 0.086 respectively]. The median LOS in the SCP group was 12 (IQR 11–13) days vs. 9 (IQR 8–13 p = 0.024). In the postoperative period, patients reported 22 complications (37% in SCP and 42.8% in ERAS group, p = 0.48). Conclusions: The study reveals how even partial adherence to the ERAS protocols leads to similar outcomes when compared to SCP. As a single surgeon series, our study confirmed the role of surgeons in reducing complications and improving surgical outcomes.
1. Introduction
Radical cystectomy (RC) with pelvic lymph node dissection is the gold standard treatment for muscle-invasive bladder cancer [1,2,3]. This procedure, despite the surgical experience gained over the years, is not free from complications both during the operative practice and peri- and postoperative time. The interest of research in the urological field is focused on finding a common guideline for the management of patients who undergo RC to improve the postoperative recovery. Enhanced recovery after surgery (ERAS) [4,5], theorized by Kehlet, consists of evidence-based interventions applied during pre-, intra- and postoperative time to reduce the physical stress due to surgery, to accelerate patient rehabilitation, and reduce hospitalization time. Although the ERAS protocol was born for colorectal surgery, several studies have shown a possible role of this protocol, even in the case of RC, the postoperative recovery [6]. This study aims to compare patients who underwent cystectomy who followed the ERAS protocol with patients who followed the usual care protocol analyzing the return to normal bowel functions, length of stay, and early- and late-postoperative complication incidence.
2. Materials and Methods
A retrospective comparative study was conducted including consecutive patients who underwent radical cystectomy in two urological centers. We collected the data regarding the hospitalization of patients undergoing RC, with an open approach in a standard care center from 2017 to 2019 (non-ERAS protocol), and the data of patients from a second center undergoing the same surgery, but with a certified ERAS protocol. The ERAS certification was achieved in 2020 (ERAS items are present in Table 1, according to the ERAS guidelines). Inclusion criteria were consecutive patients who underwent RC for muscle invasive bladder cancer performed by the same surgeon. For each patient, all of the preoperative characteristics such as age, sex, comorbidities, blood tests, ECOG (Eastern Cooperative Oncology Group), ASA (American Society of Anesthesiologists), and CCI (Charlson Comorbity Index) were considered. We compared the postoperative parameters of the two centers (ERAS and non-ERAS) analyzing the differences in length of stay (LOS), time to flatus (TTF), time to defecation (TTD), presence of ileus, positioning of the nasogastric tube (NGT) and its maintaining time, onset of complications, early or late, using the Clavien–Dindo (CD) score, and the presence of re-hospitalizations. The study was conducted on the available retrospective data and according to our ethical committee, approval is not required.

Table 1.
A summary of each ERAS item and their respective level of evidence and grade of recommendation derived from both the cystectomy and the colorectal literature.
Statistical Analysis
The means and standard errors of the mean were reported for continuous variables assumed to be normally distributed. The remaining continuous variables were summarized by their median values and interquartile ranges. Data are compared with the Student’s t-test, Mann–Whitney, and Chi-square for the normal, non-normal, and nominal variables, respectively. A value of p < 0.05 was considered significant. The sample size was not calculated because of the retrospective nature of the study.
3. Results
Data were collected from 91 consecutive patients who underwent cystectomy, 70 of them followed the standard care protocol (SCP) and 21 the ERAS protocol. The mean age of the patients was 70.6 ± 9.5 years. Pre-operative blood analyses of the two subgroups did not demonstrate statistically significant differences except for the albumin serum levels, which was higher in the SPC center (Table 2. There was a statistically significant difference between the incidences of comorbidities, with a greater rate of in-patients treated with the SCP (78.5% vs. 28.5%, p < 0.001). The performance status was evaluated with the ECOG score (Table 3. The ASA score was higher in the SCP patients (ASA ≥ 3: 97.1% vs. 47.6%). Operating time (OR) and estimated blood loss (EBL) were also reported in Table 2. Although there was a statistically significant difference in TTF (3 (2.7–3) vs. 1 (1–2) p < 0.001, in standard care hospital vs. ERAS hospital), no difference were recorded in the two groups for the time to first defecation (TTD) (5 (4–6) vs. 4 (3–5.8), p = 0.086, respectively) (Figure 1). The percentage of patients without postoperative NGT is significantly different between the two groups (10.2% vs. 90.4% in non-ERAS centers and in the ERAS patients, respectively). This can be explained by the fact that in the SPC center, the NGT was routinely inserted and maintained after the surgical procedure. There was a difference in the ileus rate, with a higher incidence in the ERAS center (23.8 % vs. 4.1 %, p = 0.037) (Table 4). The difference can be explained by the fact that NGT was routinely inserted and maintained in the SPC center, reducing the rate of postoperative nausea. The median length of stay (LOS) of patients in the non-ERAS center was 12 days (IQR 11–13) and in the ERAS center, it was 9 days (IQR 8–13.4) (p = 0.024). In the postoperative period, 22 (37%) complications have been recorded in the non-ERAS center (68% grade 1 and 2 according to the Clavien–Dindo classification), while nine (43%) complications were recorded in the ERAS center (100% grade 1, 2) without significant differences between the two groups (p = 0.48). Table 4 reports the rate of postoperative complications according to CD classification. According to these findings, the incidence rate of late postoperative complications was significantly higher in the ERAS patients compared to the usual care patients (35% vs. 12.2%, respectively; p = 0.03). The mean follow-up time after surgery was 15.8 (IQR 7.3–21.8) months and 7 (IQR 4.3–9.8) in the SCP and ERAS groups, respectively (p = 0.01).

Table 2.
The patient characteristics.

Table 3.
The pre-operative blood analyses.
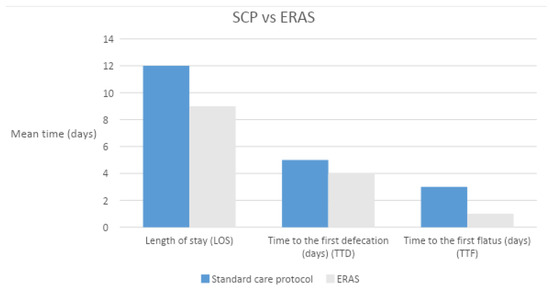
Figure 1.
The standard care protocol vs. the ERAS protocol: LOS, TTD, TTF.

Table 4.
The postoperative characteristics.
4. Discussion
In all surgical areas, there is increasing attention on the hospitalization time, influenced by the onset of postoperative complications. The ERAS protocol was born to improve the clinical outcomes of surgery and reduce the economic pressure of patients in many developing countries [7,8]. Since the introduction of ERAS into colorectal surgery, several preoperative, intraoperative, and postoperative steps have been implemented to improve patient rehabilitation. The benefits of the ERAS protocols are similar in many surgeries such as bariatric, esophageal, and other gastrointestinal surgeries [9,10,11]. In urological practice, the use of the ERAS protocol is limited for one of the most challenging interventions, both for surgeons and patients, RC. [4,12]. The ERAS protocol consists of 21 items including pre-, intra-, and postoperative approach [6]. The literature refers to many patients undergoing radical cystectomy that benefit from enhancements in perioperative management. Unfortunately, sometimes, the protocol is hard to apply for its rigidity. The aim of our study was to evaluate the difference in outcome in the usual care patients comparing the ERAS patients and the effectiveness of the ERAS protocol by comparing specific parameters. Our data indicate that there was no substantial improvement in the postoperative course between patients who followed the ERAS protocol and those undergoing usual care. The population examined in the non-ERAS center was characterized by higher comorbidities and higher ASA score. Although the TTF was shorter in patients undergoing the ERAS protocol [3 days (2.7–3) vs. 1 day (1–2) p < 0.001, respectively], the same cannot be said for the TTD time, which was comparable. The ERAS protocols were also introduced to reduce postoperative ileus [13]. There are many definitions of the ileus in the literature. We defined post operative ileus after radical cystectomy, the reduction in bowel movement, intolerance of oral diet, and vomiting, requiring cessation of oral intake with the necessity of nasogastric tube insertion [14]. There is no univocal criterion for the insertion of NGT, and it is always upon clinical judgment. Standardization would be helpful to compare the results of future studies on postoperative ileus [15]. Our data demonstrated a lower incidence of the ileus in the usual care population compared to the ERAS protocol population. Despite this result, the TTF was significantly shorter in the ERAS group. Another purpose of the ERAS protocol is to minimize the hospitalization time: such a target was confirmed in our study. Furthermore, the hospitalization time is influenced by postoperative complications evaluated with the CD classification, both in the short- and long-term. Furthermore, for this parameter, there is no evidence of substantial and statistically significant differences. Although the ERAS protocol is today of great interest in urological surgery, however, there are conflicting data in the literature on its ability to influence perioperative outcomes [15,16]. Many studies have shown that ERAS courses can reduce the length of stay [15,17,18,19,20,21], while some refute this thesis [21,22,23,24]; the same was true in the evaluation of the reduction in time to the recovery of bowel activity and in the readmission rate [19,20,21,22,23,25,26]. The study does not want to discredit the ERAS protocol, but to rather understand whether there are unassessed parameters that could influence the postoperative course. These parameters can be subjective and hardly standardizable as, for instance, the surgeon experience and the number of interventions by the center. The surgical technique, the manual skills, and the number of interventions can influence the operative course as they affect various parameters such as the operative time, the time of ileal–ileal anastomosis and the suture technique, blood losses, and the incidence of intraoperative complications. The high number of interventions of a center is associated with a greater confidence with the type of patient, with their needs, knowledge of time for the postoperative steps such as mobilization, which is essential for a rapid recovery of the patient, and prompt identification of deviations from the patient in a normal postoperative course. This hypothesis was already explored by previous studies and in 2016, Moschini et al. reported the published results into a systematic review [27,28]. According to their results, the surgical volume is affecting outcomes after radical cystectomy in terms of perioperative complications. The main limitation of this study was the initial retrospective design. A larger number of patients needs to be included in future trials to reach significance and stronger results. However, we reported this number of patients because this is exactly the required amount necessary to obtain the ERAS certification.
5. Conclusions
The application of the ERAS protocol in patients undergoing RC should improve the timing of rehabilitation and reduce LOS and the hospitalization costs. Our study surprisingly showed that our surgical technique resized the results expected from the ERAS Society protocol to accelerate the postoperative recovery time and reduce the early- and late-postoperative complications. Therefore, a precise, accurate, and fast surgical procedure with reduced blood loss that tends to minimize postoperative complications is able to determine the patient’s recovery on its own, regardless of strict protocols. In other words, the ERAS protocols are certainly useful to speed up recovery, but does not seem to be able to disregard a good surgical technique.
Author Contributions
Conceptualization, A.S.; Methodology, G.T.; Formal analysis, N.P.; Investigation, A.A.; Resources, E.D.; Data curation, N.P.; Writing—original draft preparation, G.T., D.B., P.M. and S.G.; Writing—review and editing, V.F., A.S., R.G. and M.V.; Supervision, A.S., C.P., R.B. and V.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Ethics Committee of A.O.U. P. Paolo Giaccone of Palermo, Italy. Protocol code 03/2022, date of approval 15 March 2022.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this manuscript are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Donald, E.L. Guidelines for Perioperative Care in Esophagectomy: Enhanced Recovery After Surgery (ERAS®) Society Recommendations. World J. Surg. 2019, 43, 299–330. [Google Scholar]
- Kulkarni, G.S.; Hakenberg, O.W.; Gschwend, J.E.; Thalmann, G.; Kassouf, W.; Kamat, A.; Zlotta, A. An Updated Critical Analysis of the Treatment Strategy for Newly Diagnosed High-grade T1 (Previously T1G3) Bladder Cancer. Eur. Urol. 2010, 57, 60–70. [Google Scholar] [CrossRef]
- Khadhouri, S.; Gallagher, K.M.; MacKenzie, K.R.; Shah, T.T.; Gao, C.; Moore, S.; Zimmermann, E.F.; Edison, E.; Jefferies, M.; Nambiar, A.; et al. The IDENTIFY study: The investigation and detection of urological neoplasia in patients referred with suspected urinary tract cancer—A multicentre observational study. Br. J. Urol. 2021, 128, 440–450. [Google Scholar] [CrossRef]
- Cerantola, Y.; Valerio, M.; Persson, B. Guidelines for perioperative care after radical cystectomy for bladder cancer: Enhanced Recovery After Surgery (ERAS(®)) society recommendations. Clin. Nutr. 2013, 32, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Claps, F.; Mir, M.C.; Zargar, H. Molecular markers of systemic therapy response in urothelial carcinoma. Asian J. Urol. 2021, 8, 376–390. [Google Scholar] [CrossRef] [PubMed]
- Chenam, A.; Chan, K.G. Enhanced Recovery after Surgery for Radical Cystectomy. Cancer Treat. Res. 2018, 175, 215–239. [Google Scholar]
- Chunxiao, W.; Fengchun, W. Application of enhanced recovery after surgery in patients undergoing radical cystectomy. J. Int. Med. Res. 2018, 46, 5011–5018. [Google Scholar]
- Kehlet, H. Multimodal approach to control postoperative pathophysiology and rehabilitation. Br. J. Anaesth. 1997, 78, 606–617. [Google Scholar] [CrossRef]
- Pisarska, M.; Małczak, P.; Major, P.; Wysocki, M.; Budzyński, A.; Pędziwiatr, M. Enhanced recovery after surgery protocol in oesophageal cancer surgery: Systematic review and meta-analysis. PLoS ONE 2017, 12, e0174382. [Google Scholar] [CrossRef]
- Małczak, P.; Pisarska-Adamczyk, M.; Piotr, M.; Wysocki, M.; Budzyński, A.; Pędziwiatr, M. Enhanced Recovery after Bariatric Surgery: Systematic Review and Meta-Analysis. Obes. Surg. 2016, 27, 226–235. [Google Scholar] [CrossRef]
- Pędziwiatr, M.; Mavrikis, J.; Witowski, J. Current status of enhanced recovery after surgery (ERAS) protocol in gastrointestinal surgery. Med. Oncol. 2018, 35, 95. [Google Scholar] [CrossRef] [PubMed]
- Connor, M.F.; Ali, C.C. Defining postoperative ileus and associated risk factors in patients undergoing radical cystectomy with an Enhanced Recovery AfterSurgery (ERAS) program. Can. Urol. Assoc. 2021, 15, 33–39. [Google Scholar]
- Godden, A.R.; Marshall, M.J. Ultrasonography guided rectus sheath catheters vs. epidural analgesia for open colorectal cancer surgery in a single centre. Ann. R. Coll. Surg. Engl. 2013, 95, 591–594. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, J.A.; McIntosh, A.G.; Strehlow, R.; Lawrence, V.A.; Parekh, D.J.; Svatek, R.S. Definition, incidence, risk factors, and prevention of paralytic ileus following radical cystectomy: A systematic review. Eur. Urol. 2013, 64, 588–597. [Google Scholar] [CrossRef]
- Pruthi, R.S.; Chun, J.; Richman, M. Reducing time to oral diet and hospital discharge in patients undergoing radical cystectomy using a perioperative care plan. Urology 2003, 62, 661–665. [Google Scholar] [CrossRef]
- Xu, W.; Daneshmand, S.; Bazargani, S.T.; Cai, J.; Miranda, G.; Schuckman, A.K.; Djaladat, H. Postoperative Pain Management after Radical Cystectomy: Comparing Traditional versus Enhanced Recovery Protocol Pathway. J. Urol. 2015, 194, 1209–1213. [Google Scholar] [CrossRef]
- Arumainayagam, N.; McGrath, J.; Jefferson, K.P.; Gillatt, D.A. Introduction of an enhanced recovery protocol for radical cystectomy. Br. J. Urol. 2008, 101, 698–701. [Google Scholar] [CrossRef]
- Daneshmand, S.; Ahmadi, H.; Schuckman, A.K.; Mitra, A.P.; Cai, J.; Miranda, G.; Djaladat, H. Enhanced Recovery Protocol after Radical Cystectomy for Bladder Cancer. J. Urol. 2014, 192, 50–56. [Google Scholar] [CrossRef]
- Smith, J.; Meng, Z.W.; Lockyer, R.; Dudderidge, T.; McGrath, J.; Hayes, M.; Birch, B. Evolution of the Southampton Enhanced Recovery Programme for radical cystectomy and the aggregation of marginal gains. Br. J. Urol. 2014, 114, 375–383. [Google Scholar] [CrossRef]
- Collins, J.W.; Adding, C.; Hosseini, A.; Nyberg, T.; Pini, G.; Dey, L.; Wiklund, P.N. Introducing an enhanced recovery programme to an established totally intracorporeal robot-assisted radical cystectomy service. Scand. J. Urol. 2015, 50, 39–46. [Google Scholar] [CrossRef]
- Guan, X.; Liu, L.; Lei, X. A comparative study of fast-track versus [corrected] conventional surgery in patients undergoing laparoscopic radical cystectomy and ileal conduit diversion: Chinese experience. Sci. Rep. 2014, 4, 6820. [Google Scholar] [CrossRef] [PubMed]
- Mukhtar, S.; Ayres, B.E.; Issa, R.; Swinn, M.J.; Perry, M.J.A. Challenging boundaries: An enhanced recovery programme for radical cystectomy. Ann. R. Coll. Surg. Engl. 2013, 95, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Cerruto, M.A.; De Marco, V.; D’Elia, C. Fast track surgery to reduce short-term complications following radical cystectomy and intestinal urinary diversion with Vescica Ileale Padovana neobladder: Proposal for a tailored enhanced recovery protocol and preliminary report from a pilot study. Urol. Int. 2014, 92, 41–49. [Google Scholar] [CrossRef]
- Persson, B.; Carringer, M.; Andrén, O.; Andersson, S.-O.; Carlsson, J.; Ljungqvist, O. Initial experiences with the enhanced recovery after surgery (ERAS®) protocol in open radical cystectomy. Scand. J. Urol. 2014, 49, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Claps, F.; van de Kamp, M.W.; Mayr, R.; Bostrom, P.J.; Boormans, J.L.; Eckstein, M.; Mertens, L.S.; Boevé, E.R.; Neuzillet, Y.; Burger, M.; et al. Risk factors associated with positive surgical margins’ location at radical cystectomy and their impact on bladder cancer survival. World J. Urol. 2021, 39, 4363–4371. [Google Scholar] [CrossRef]
- Grass, F.; Slieker, J.; Jurt, J.; Kummer, A.; Solà, J.; Hahnloser, D.; Demartines, N.; Hübner, M. Postoperative ileus in an enhanced recovery pathway—A retrospective cohort study. Int. J. Color. Dis. 2017, 32, 675–681. [Google Scholar] [CrossRef]
- Moschini, M.; Simone, G.; Stenzl, A.; Gill, I.S.; Catto, J. Critical Review of Outcomes from Radical Cystectomy: Can Complications from Radical Cystectomy Be Reduced by Surgical Volume and Robotic Surgery? Eur. Urol. Focus 2016, 2, 19–29. [Google Scholar] [CrossRef]
- Claps, F.; Rai, S.; Mir, M.C.; van Rhijn, B.W.; Mazzon, G.; Davis, L.E.; Valadon, C.L.; Silvestri, T.; Rizzo, M.; Ankem, M.; et al. Prognostic value of preoperative albumin-to-fibrinogen ratio (AFR) in patients with bladder cancer treated with radical cystectomy. Urol. Oncol. Semin. Orig. Investig. 2021, 39, 835.e9–835.e17. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).