Superficial Femoral Artery Recanalization Using Fiber Optic RealShape Technology
Abstract
1. Introduction
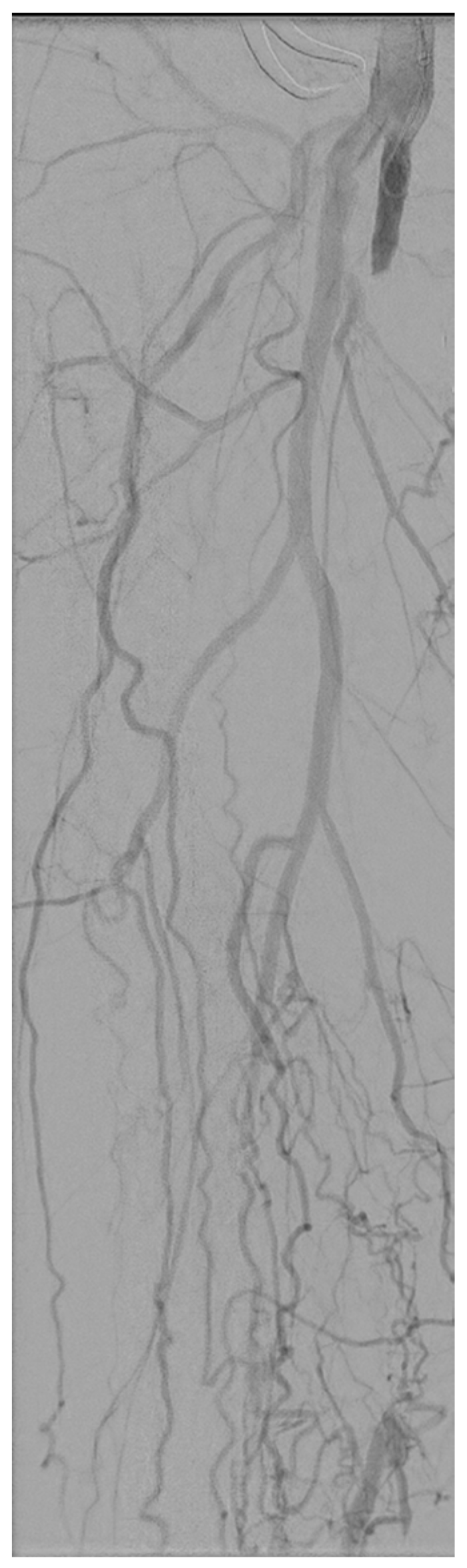
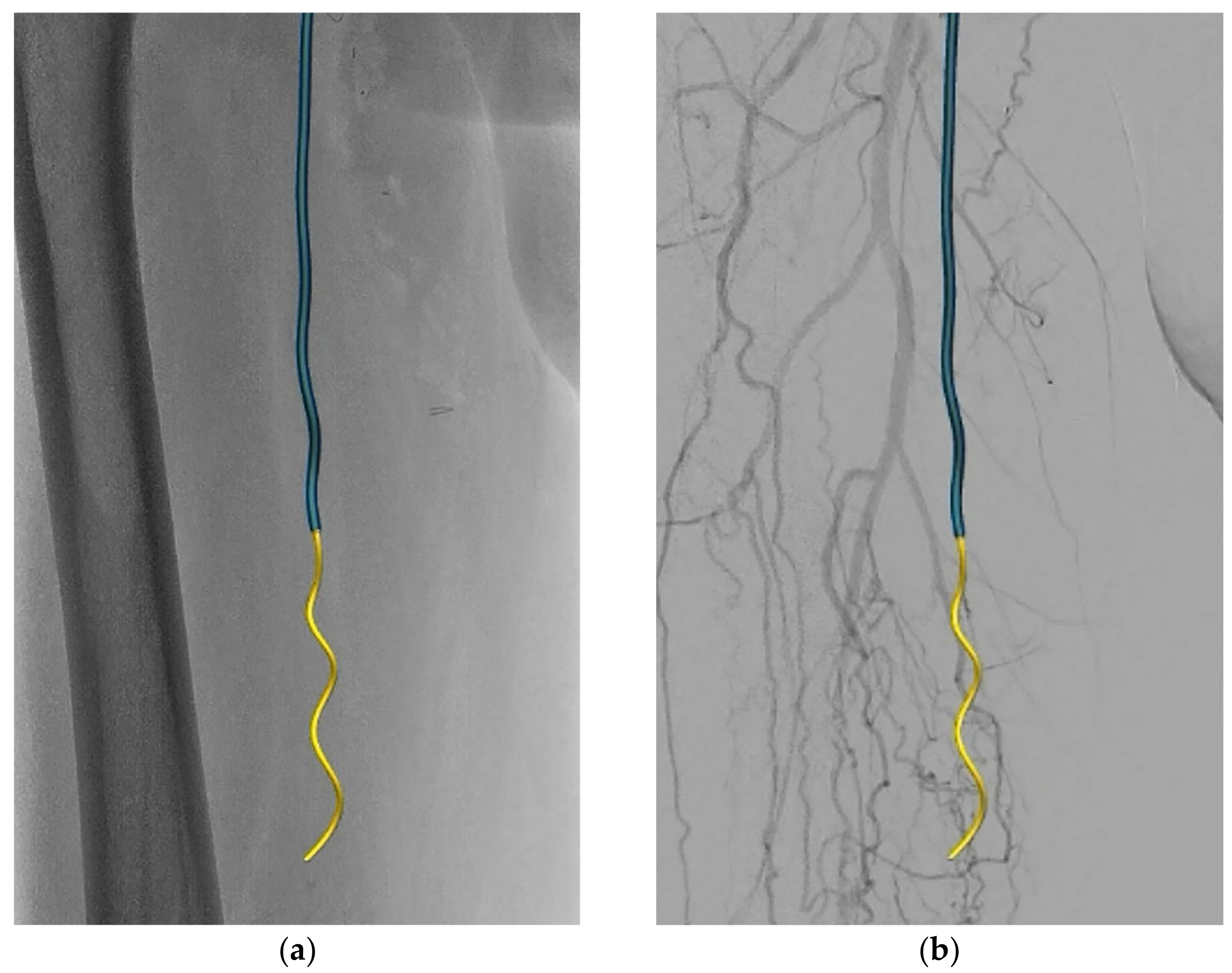
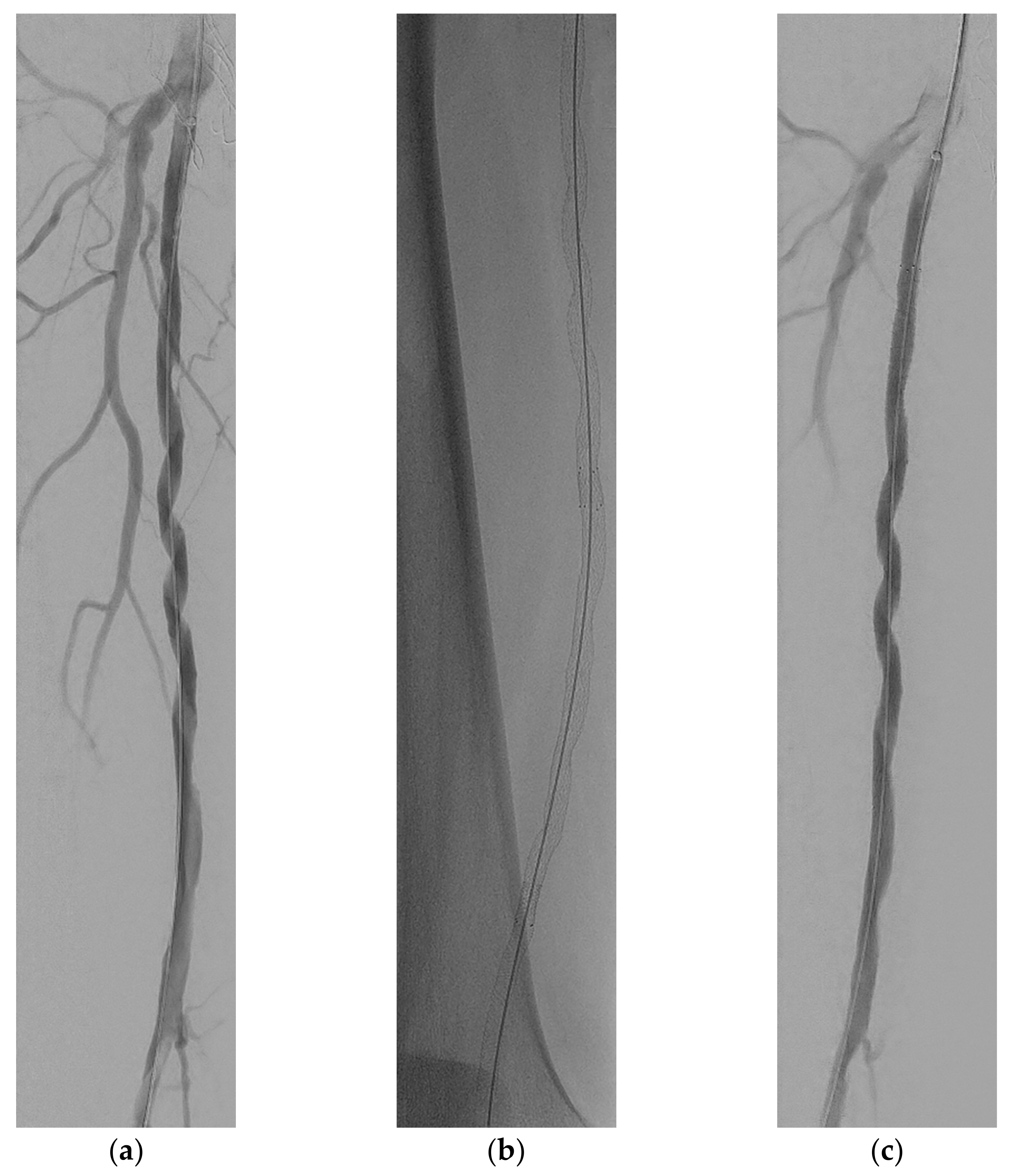
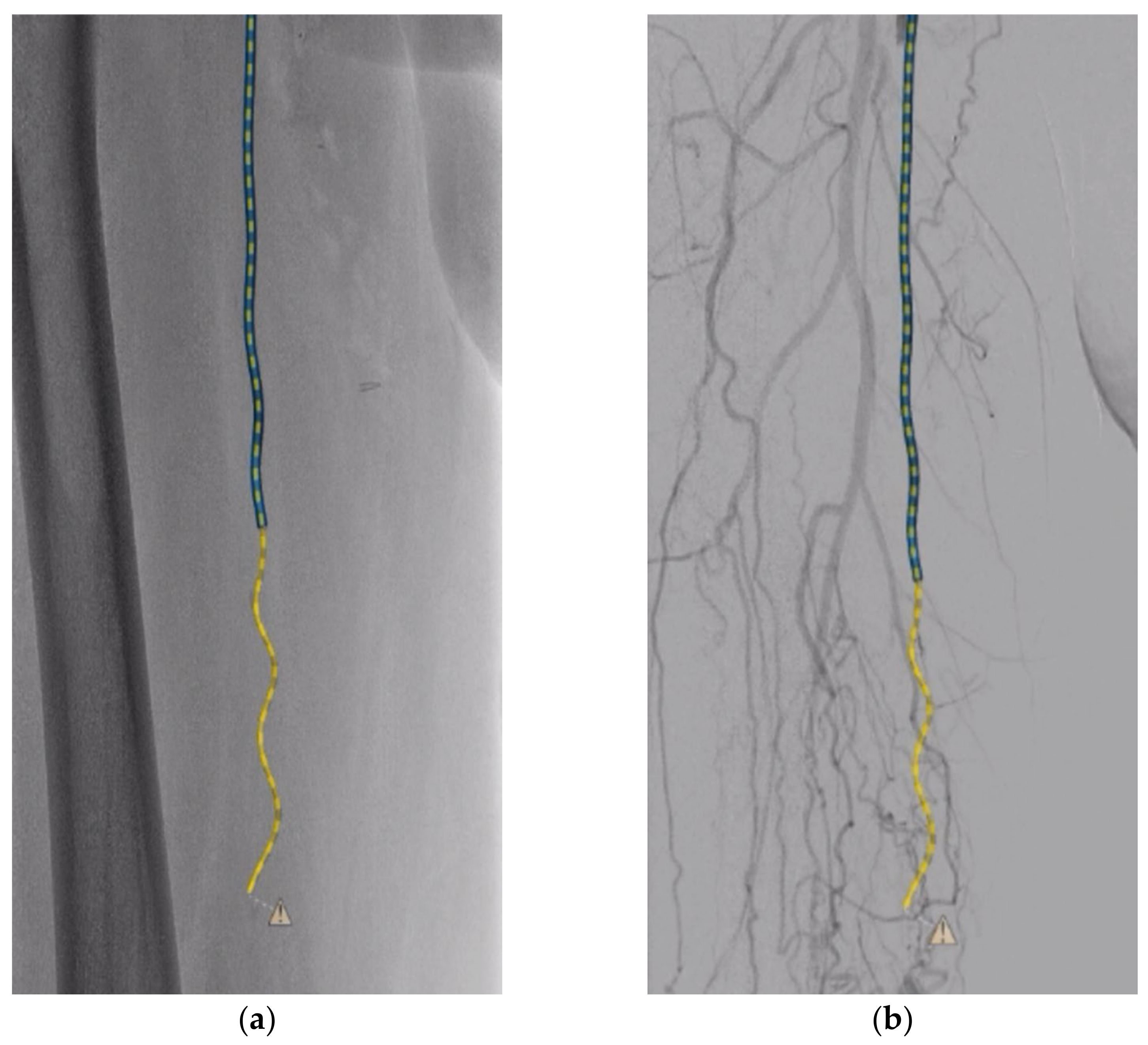
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ketteler, E.R.; Brown, K.R. Radiation Exposure in Endovascular Procedures. J. Vasc. Surg. 2011, 53, 35S–38S. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.R.; Loganovsky, K. Low Dose or Low Dose Rate Ionizing Radiation-Induced Health Effect in the Human. J. Environ. Radioact. 2018, 192, 32–47. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.; Kang, S.; Ha, M.; Kim, J.; Jun, J.K.; Kong, K.A.; Lee, W.J. Health Effects from Occupational Radiation Exposure among Fluoroscopy-Guided Interventional Medical Workers: A Systematic Review. J. Vasc. Interv. Radiol. 2018, 29, 353–366. [Google Scholar] [CrossRef] [PubMed]
- Jansen, M.; Khandige, A.; Kobeiter, H.; Vonken, E.-J.; Hazenberg, C.; van Herwaarden, J. Three Dimensional Visualisation of Endovascular Guidewires and Catheters Based on Laser Light Instead of Fluoroscopy with Fiber Optic RealShape Technology: Preclinical Results. Eur. J. Vasc. Endovasc. Surg. 2020, 60, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Van Herwaarden, J.A.; Jansen, M.M.; Vonken, E.P.A.; Bloemert-Tuin, T.; Bullens, R.W.M.; de Borst, G.J.; Hazenberg, C.E.V.B. First in Human Clinical Feasibility Study of Endovascular Navigation with Fiber Optic RealShape (FORS) Technology. Eur. J. Vasc. Endovasc. Surg. 2021, 61, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Boc, V.; Boc, A.; Zdešar, U.; Blinc, A. Patients’ Radiation Doses during Percutaneous Endovascular Procedures in Arteries of the Lower Limbs. Vasa 2019, 48, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Goldsweig, A.M.; Kennedy, K.F.; Abbott, J.D.; Jones, W.S.; Velagapudi, P.; Rutar, F.J.; Curtis, J.C.; Tsai, T.T.; Aronow, H.D. Patient Radiation Dosage During Lower Extremity Endovascular Intervention. JACC Cardiovasc. Interv. 2019, 12, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Guillou, M.; Maurel, B.; Necib, H.; Vent, P.-A.; Costargent, A.; Chaillou, P.; Gouëffic, Y.; Kaladji, A. Comparison of Radiation Exposure during Endovascular Treatment of Peripheral Arterial Disease with Flat-Panel Detectors on Mobile C-Arm versus Fixed Systems. Ann. Vasc. Surg. 2018, 47, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Sigterman, T.A.; Bolt, L.J.J.; Snoeijs, M.G.; Krasznai, A.G.; Heijboer, R.; Schurink, G.-W.H.; Bouwman, L.H. Radiation Exposure during Percutaneous Transluminal Angioplasty for Symptomatic Peripheral Arterial Disease. Ann. Vasc. Surg. 2016, 33, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Segal, E.; Weinberg, I.; Leichter, I.; Klimov, A.; Giri, J.; Bloom, A.I. Patient Radiation Exposure during Percutaneous Endovascular Revascularization of the Lower Extremity. J. Vasc. Surg. 2013, 58, 1556–1562. [Google Scholar] [CrossRef] [PubMed]
- Majewska, N.; Blaszak, M.A.; Juszkat, R.; Frankiewicz, M.; Makalowski, M.; Majewski, W. Patients’ Radiation Doses during the Implantation of Stents in Carotid, Renal, Iliac, Femoral and Popliteal Arteries. Eur. J. Vasc. Endovasc. Surg. 2011, 41, 372–377. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bolia, A.; Miles, K.A.; Brennan, J.; Bell, P.R.F. Percutaneous Transluminal Angioplasty of Occlusions of the Femoral and Popliteal Arteries by Subintimal Dissection. Cardiovasc. Interv. Radiol. 1990, 13, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Reekers, J.A.; Bolia, A. Percutaneous Intentional Extraluminal (Subintimal) Recanalization: How to Do It Yourself. Eur. J. Radiol. 1998, 28, 192–198. [Google Scholar] [CrossRef]
- Stonebridge, P.A.; Suttie, S.A.; Ross, R.; Dick, J. Spiral Laminar Flow: A Survey of a Three-Dimensional Arterial Flow Pattern in a Group of Volunteers. Eur. J. Vasc. Endovasc. Surg. 2016, 52, 674–680. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; He, Y.; Corti, A.; Gallo, D.; Casarin, S.; Rozowsky, J.M.; Migliavacca, F.; Berceli, S.; Chiastra, C. Baseline Local Hemodynamics as Predictor of Lumen Remodeling at 1-Year Follow-up in Stented Superficial Femoral Arteries. Sci. Rep. 2021, 11, 1613. [Google Scholar] [CrossRef] [PubMed]
- Gökgöl, C.; Diehm, N.; Räber, L.; Büchler, P. Prediction of Restenosis Based on Hemodynamical Markers in Revascularized Femoro-Popliteal Arteries during Leg Flexion. Biomech. Model. Mechanobiol. 2019, 18, 1883–1893. [Google Scholar] [CrossRef] [PubMed]
- Kokkalis, E.; Aristokleous, N.; Houston, J.G. Haemodynamics and Flow Modification Stents for Peripheral Arterial Disease: A Review. Ann. Biomed. Eng. 2016, 44, 466–476. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, T.M.; Zeller, T.; Nakamura, M.; Gaines, P.A. Treatment of Femoropopliteal Lesions with the BioMimics 3D Vascular Stent System: Two-Year Results from the MIMICS-2 Trial. J. Endovasc. Ther. 2021, 28, 236–245. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klaassen, J.; van Herwaarden, J.A.; Teraa, M.; Hazenberg, C.E.V.B. Superficial Femoral Artery Recanalization Using Fiber Optic RealShape Technology. Medicina 2022, 58, 961. https://doi.org/10.3390/medicina58070961
Klaassen J, van Herwaarden JA, Teraa M, Hazenberg CEVB. Superficial Femoral Artery Recanalization Using Fiber Optic RealShape Technology. Medicina. 2022; 58(7):961. https://doi.org/10.3390/medicina58070961
Chicago/Turabian StyleKlaassen, Jurre, Joost A. van Herwaarden, Martin Teraa, and Constantijn E. V. B. Hazenberg. 2022. "Superficial Femoral Artery Recanalization Using Fiber Optic RealShape Technology" Medicina 58, no. 7: 961. https://doi.org/10.3390/medicina58070961
APA StyleKlaassen, J., van Herwaarden, J. A., Teraa, M., & Hazenberg, C. E. V. B. (2022). Superficial Femoral Artery Recanalization Using Fiber Optic RealShape Technology. Medicina, 58(7), 961. https://doi.org/10.3390/medicina58070961