Abstract
Prion diseases are progressive and irreversible neurodegenerative disorders with a low incidence (1.5–2 cases per million per year). Genetic (10–15%), acquired (anecdotal) and sporadic (85%) forms of the disease have been described. The clinical spectrum of prion diseases is very varied, although the most common symptoms are rapidly progressive dementia, cerebellar ataxia and myoclonus. Mean life expectancy from the onset of symptoms is 6 months. There are currently diagnostic criteria based on clinical phenotype, as well as neuroimaging biomarkers (magnetic resonance imaging), neurophysiological tests (electroencephalogram and polysomnogram), and cerebrospinal fluid biomarkers (14-3-3 protein and real-time quaking-induced conversion (RT-QuIC)). The sensitivity and specificity of some of these tests (electroencephalogram and 14-3-3 protein) is under debate and the applicability of other tests, such as RT-QuIC, is not universal. However, the usefulness of these biomarkers beyond the most frequent prion disease, sporadic Creutzfeldt–Jakob disease, remains unclear. Therefore, research is being carried out on new, more efficient cerebrospinal fluid biomarkers (total tau, ratio total tau/phosphorylated tau and neurofilament light chain) and potential blood biomarkers (neurofilament light chain, among others) to try to universalize access to early diagnosis in the case of prion diseases.
1. Introduction
Prion diseases, also known as transmissible spongiform encephalopathies, are rapidly progressive and irremediably fatal neurodegenerative disorders. The average life expectancy is six months, but great variability in the duration of the clinical course of the disease has been described, lasting from weeks to years [1,2,3]. The cause is the aggregation of a misfolded prion protein scrapie (PrPSc). PrPSc is the abnormal conformational isoform of the normal cellular prion protein (PrPc) located on the cell surface of central nervous system neurons, the exact function of which remains unknown [4]. PrPSc is able to propagate and to aggregate in the brain tissue. PrPsc is neurotoxic and its accumulation leads to synaptic degeneration and disorganization, which induces neuronal loss and spongiform changes. Indeed, a reduction of over 30% in the relative synaptic index has been reported in prion disease-affected brains [1,3,5].
Prion diseases are classified into sporadic (85%), genetic (10–15%) (due to mutations in the prion protein gene (PRNP)) and acquired (exceptional) forms (Figure 1). Prion diseases show a huge variety of cognitive, motor and neuropsychiatric symptoms. Almost 90% of the sporadic cases are due to sporadic Creutzfeldt–Jakob disease (sCJD) with an incidence close to 1.5–2 cases per million persons per year [3]. In addition to familial Creutzfeldt–Jakob disease, genetic causes include familial fatal insomnia (FFI) (of which there is also a very rare sporadic form), Gerstmann–Straüssler–Scheincker (GSS) and Huntington-disease-like 1 (HDL1) [6,7]. In 1996, variant Creutzfeldt–Jakob (vCJD), a zoonotic prion disease acquired by consumption of cattle contaminated by bovine spongiform encephalopathy, was described for the first time in the United Kingdom [8,9,10]. Fortunately, sanitary measures and food chain regulation have contributed to the near disappearance of vCJD [8,9]. Regardless of the type of prionopathy, early diagnosis is a challenge due to the great phenotypic variability, with the most frequent symptoms being rapidly progressive dementia, cerebellar ataxia and myoclonus [3].
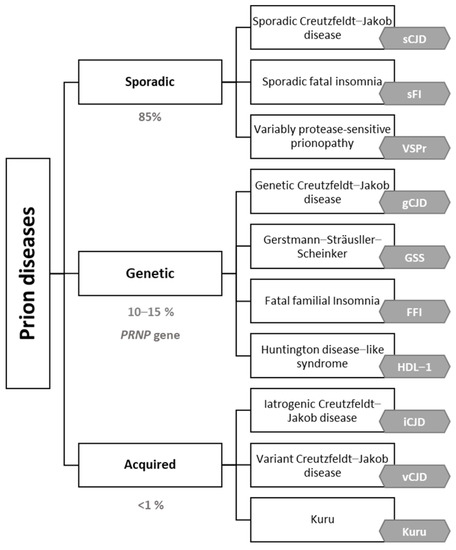
Figure 1.
Prion diseases etiologic classification adapted by the authors [10,11,12,13].
Currently, the definitive diagnosis of prion diseases requires postmortem anatomopathological examination or brain biopsy, which is not feasible in clinical practice [1,3] (Table 1). Postmortem neuropathological study is also necessary to classify cases into the different subtypes of sCJD. Different clinicopathological subtypes of sCJD, defined by methionine/valine polymorphism at codon 129 of the PRNP gene and the type (based on the size of protease-resistant fragments) of PrPSc accumulated in the brain, have been described [3,14]. Despite the development of new diagnostic tools, postmortem anatomopathological study is the gold standard technique to confirm, or completely rule out, the diagnosis of a prion disease and, therefore, classifications that relate clinical and anatomical changes are still relevant. The molecular subtype of sCJD is an important prognostic marker for patient survival [3].

Table 1.
Diagnostic criteria for probable and definite categories of prion diseases.
In recent decades, efforts have been made to advance the use of biomarkers to allow an early diagnosis of the different prionopathies. Neurophysiological and neuroimaging biomarkers and different cerebrospinal fluid (CSF) analytes (14-3-3 protein and total tau) have been progressively incorporated into the diagnostic criteria. Special mention should be made of the real-time quaking-induced conversion (RT-QuIC) technique for the detection of prion protein in CSF, a technique with the most promising results for the accurate premortem diagnosis of sCJD and other prionopathies, which is already incorporated into the latest diagnostic criteria [3] (Table 1). Despite its promising results, the RT-QuIC technique is not universally available; therefore, research into other more accessible biomarkers with potentially high diagnostic performance has continued over the last few years.
This narrative review aims to examine the currently accepted neurophysiological, neuroimaging and cerebrospinal fluid (CSF) biomarkers for diagnosis and to investigate alternative CSF and peripheral blood biomarkers that have been recently proposed (Figure 2). We highlight those biomarkers that are more easily accessible, including blood biomarkers, which would truly represent a diagnostic revolution in prionopathies. The existing differences in biomarker performance between different prionopathies will also be emphasized, although it is true that most of the work reviewed has focused on sCJD. The examination, in combination, of current diagnostic criteria and of multimodal diagnostic biomarkers (e.g., neurophysiological, neuroimaging, genetic, CSF and plasma analytes), available both for use in clinical practice and in the development phase, not only limited to CJD but also to the rest of the prionopathies, is considered of special interest in this review.
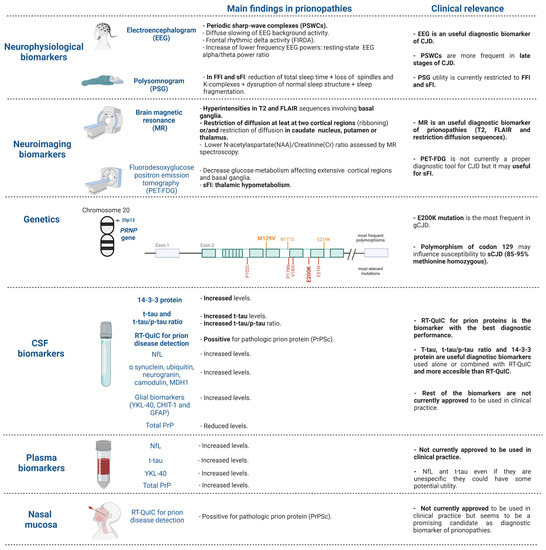
Figure 2.
Diagnostic biomarkers for prion diseases in use in clinical practice and summary of the most promising biomarkers under investigation. Figure created with biorender.com (accessed on 18 February 2022). CJD: Creutzfeldt–Jakob diseases, sCJD: sporadic Creutzfeldt–Jakob disease; gCJD: genetic Creutzfeldt–Jakob disease, FFI: fatal familial insomnia, sFI: sporadic fatal insomnia.
2. Neurophysiological Biomarkers
2.1. Electroencephalogram (EEG)
Periodic sharp-wave complexes (PSWCs), generalized and/or lateralized complexes, characterized by strictly periodic cerebral potentials at a frequency of 1 Hz, are the typical EEG finding commonly associated with sCJD. PSWCs tend to disappear during sleep and may be attenuated by some psychotropic drugs [15]. PSWCs are observed in 67–95% of patients with sCJD, with a sensitivity of 65% and specificity of 90% [16,17]. In turn, the main background frequency and the α/θ power ratio in quantitative EEG seems to be related to clinical progression and has been suggested as a useful tool for follow-up monitoring in prion diseases [18]. EEG could also be useful for the detection of non-convulsive status epilepticus, which, although infrequent, has an increased risk in some prionopathies, such as sCJD [3].
Advantages: EEG is an economically accessible and safe technique. It is available in most healthcare centers and can be performed repeatedly during clinical follow-up if required.
Limitations: The diagnosis performance of EEG improves with the clinical course of the disease. Therefore, it is a biomarker that is mainly related to the late symptomatic phases of sCJD [13,15]. In early stages of the disease, unspecific alterations, such as diffuse slowing and frontal rhythmic delta activity (FIRDA), are more frequent [15]. Among the other limitations, it should be noted that similar alterations have been described in other neurodegenerative dementias, such as Alzheimer’s disease (AD) and Lewy body dementia (LBD), although less frequently. It is also noteworthy that the probability of detection of PSWC is much lower in other prionopathies, such as FFI or GSS, and even in the MV2, VV2 and MM2 forms of sCJD [3,17,19].
2.2. Polysomnogram (PSG)
In cases of familial or sporadic fatal insomnia the demonstration of an early and progressive reduction in total sleep time, the loss of sleep spindles and K-complexes, the disruption of normal sleep structure, sleep fragmentation, and periods of subwakefulness interrupted by brief episodes of REM sleep, with or without atonia, often associated with dream enactment behavior, is a diagnostic criterion [20]. In the case of sCJD, sleep anomalies are not recognized as a diagnostic criterion, although it is common to detect loss of normal sleep EEG architecture, sleep-disordered breathing [21] and periodic leg movement disorders in the PSG [22].
Advantages: A non-invasive technique with high diagnostic performance for both sporadic and familial fatal insomnia.
Limitations: Not universally accessible and not useful for differential diagnosis of the most frequent causes of rapidly progressive neurodegenerative dementias apart from fatal insomnia.
3. Neuroimaging Biomarkers
3.1. Brain Magnetic Resonance (MR)
The presence of hyperintensities in T2 and fluid-attenuated inversion recovery imaging (FLAIR) sequences are frequent in the MR images of sCJD specially involving basal ganglia [16], as well as the restriction of diffusion (DWI) in at least two cortical regions (ribboning) or/and restricted diffusion predominantly in the caudate nucleus, putamen and/or thalamus [3,17,23,24]. However, these typical signs on MR images are not pathognomonic of sCJD and could also be induced, though rarely, by toxic metabolic encephalopathies, progressive multifocal dementia, autoimmune encephalitis, CNS lymphoma, vasculitis and infectious etiologies [24].
The MR pattern could also be useful, mainly when combined with PRNP polymorphisms, to differentiate sCJD molecular subtypes [17]. In the MM1 subtype of sCJD, the caudate nucleus has unilateral or bilateral asymmetrical involvement, and the involvement of the thalamus is more frequent in VV2 and MV2 subtypes [25]. The presence of a pulvinar sign (high signal on FLAIR and DWI) is highly suggestive of vCJD [3,26]. Moreover, altered diffusion in the striatum, thalamus and frontal and occipital cortices has been reported in GSS [6]. The sensitivity of sCJD diagnosis using MR varies according to different studies between 80 and 92%; the same is true for specificity, with a range between 74 and 98% [3,27,28]. Recently, the use of MR spectroscopy to determine the N-acetylaspartate (NAA)/creatine (Cr) ratio has been suggested as a useful parameter for predicting the clinical course in sCJD, as lower NAA/Cr is related to shorter disease duration [29].
Advantages: Structural neuroimaging is a mandatory test for the differential diagnosis of cognitive impairment, and, in the case of rapidly progressive dementias, the MR evaluation is crucial. Changes in restriction occur early in the context of sCJD [3,30]. In contrast with EEG, MR diffusion abnormalities are an early phenomenon, being detectable at least one year before the onset of symptoms in asymptomatic PRNP mutation carriers [31], making it useful for early diagnosis.
Limitations: At the time to perform the MR, a clinical suspicion of possible prion disease must be reported because is advisable to perform an MR evaluation with a specific protocol including diffusion sequences (DWI/ADC).
3.2. Fluorodesoxyglucose Positron Emission Tomography (PET-FDG)
Decreased glucose metabolism in the neocortex affecting extensive cortical regions (frontal, parietal and occipital cortices) and basal ganglia has been reported in sCJD but does not seem to be useful for differential diagnosis with other neurodegenerative dementias [28]. However, the hypometabolism of medial temporal area seems to be significantly less frequent compared to other neurodegenerative dementias [32]. Instead, it may be useful for the diagnosis of infrequent forms of sporadic fatal insomnia, where hypometabolism in the thalamic region is early and characteristic [3,6].
Advantages: It could be useful for helping with the diagnosis of sporadic fatal insomnia.
Limitations: It is an expensive test that is not usually performed in cognitive decline screening. There are no specific hypometabolism patterns that could be useful for diagnosing sCJD or performing a differential diagnosis with other prionopathies.
4. Genetics
PRNP gene sequencing is the primary diagnostic technique in genetic prion disease. PRNP mutations account for 10–15% of all human prion syndromes [3]. The detection of PRNP gene mutations can be performed by sequencing DNA from patient blood specimens or a decedent’s unfixed autopsy tissue. All the genetic forms of prion disease are linked to PRNP mutations and include point mutations, octapeptide repeat insertions and deletions. Many different mutations have been linked to genetic CJD, although the most common worldwide is E200K [2]. The penetrance of PRNP mutations is assumed to be close to 100% although real-life data are lacking. In the specific case of the E200K mutation, the most widespread significant variability has been detected with penetrance ranging from 60 to 90% among different populations [2]. The D178N mutation is present in all families with FFI. In individuals of European ancestry, five variants account for up to 85% of the pathogenic PRNP variants (Table 2). Therefore, the first step in genetic analysis is to determine whether these variants exist, and, if not, the entire gene should be sequenced [2]. However, in specific geographic regions with higher prevalence of gCJD, an adaptation of the preliminary genetic analysis could be advisable.

Table 2.
Summary of the most frequent PRNP variants. Adapted from Ladogana et al., 2018 [2].
In addition, the polymorphism of codon 129 of the PRNP gene has potential relevance as it may influence susceptibility to both variant and sporadic forms of CJD: 85–95% of sCJD cases are methionine homozygous at codon 129, compared to 49% in healthy controls [2,17]. However, the codon 129 polymorphism has primarily been investigated in research studies and is not currently used in the diagnostic work-up of prion disease. Interestingly, stratifying patients by codon 129 polymorphism could have a possible role in future clinical trials [17].
5. Cerebrospinal Fluid (CSF) Biomarkers
5.1. Biochemical Analysis
No differences in the quantity of proteins, glucose concentration and total cell number assessed in CSF are detected in prion diseases compared to controls [33].
Advantages: The existence of significant biochemical alterations in CSF may help to check for other etiologies, e.g., inflammatory.
Limitations: Lumbar puncture is considered an invasive test, even though it is currently performed in clinical practice for the early diagnosis of neurodegenerative disorders, such as AD.
5.2. CSF Surrogate Biomarkers
5.2.1. 14-3-3 Protein
The gamma-isoform of the 14-3-3 protein (14-3-3 gamma) expressed in neurons could be a specific marker for neuronal damage and is useful for sCJD diagnosis [34,35,36]. The pathological mechanisms leading to the accumulation of 14-3-3 protein in CSF are not fully understood; however, neuronal loss followed by cell lysis is assumed to cause increase in 14-3-3 levels [37]. Currently, the detection of increased 14-3-3 protein in CSF is used as a molecular diagnostic criterion for patients that are clinically compatible with sCJD [37]. The diagnosis performance is lower for genetic forms of prion diseases and for sporadic fatal insomnia [38,39]. The diagnostic performance of quantitative enzyme-linked immunosorbent (ELISA) assay for 14-3-3 is higher in comparison with western blotting (WB) [34,40]. Combination with other surrogate biomarkers, such as the determination of total tau (t-tau) and the ratio of t-tau/phosphorylated tau (p-tau), significantly increases the specificity [14,36,41,42,43].
Advantages: It has high sensitivity (86–97%) for sCJD [44,45,46] (Figure 3). Combination with t-tau and the ratio of t-tau/p-tau has very good sensitivity and specificity and is more accessible compared to other techniques, such as RT-QuIC.
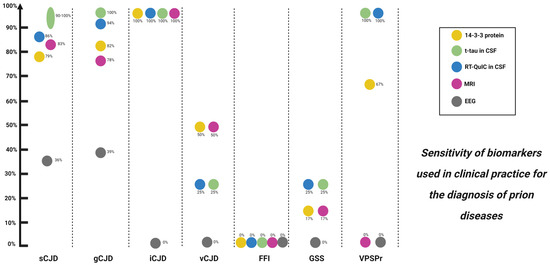
Figure 3.
Biomarkers used in clinical practice and their diagnostic sensitivity for prion diseases. sCJD: sporadic Creutzfeldt–Jakob disease; gCJD: genetic Creutzfeldt–Jakob disease; iCJD: iatrogenic Creutzfeldt–Jakob disease; vCJD: variant Creutzfeldt–Jakob disease; FFI: fatal familial insomnia; GSS: Gerstmann–Sträusller–Scheinker; VPSPr: variably protease-sensitive prionopathy; MRI: magnetic resonance imaging; EEG: electroencephalogram.
Limitations: Protein 14-3-3 is not as specific as was initially thought [16]; specificity could vary between 75.6 and 91% for sCJD [42]. Acute neurological conditions, such as stroke, status epilepticus or inflammatory encephalopathies, can also increase 14-3-3 protein levels [47]. In turn, the approach shows poor performance in the diagnosis of infrequent prion diseases.
5.2.2. Total Tau (t-tau) and Total Tau/Phosphorylated Tau (t-tau/p-tau) Ratio
Elevation of CSF t-tau levels is correlated with axonal neurodegeneration rate in many different neurological conditions, while p-tau is increased in AD but not in other neurodegenerative disorders. The determination of levels of t-tau and the ratio of t-tau/p-tau could be useful for the diagnosis of CJD, preferably combined with other diagnostic tools [16,48]. Sensitivity and specificity vary depending on the cut-off point established for both t-tau and t-tau/p-tau ratio values but very high sensitivity (85%) and specificity (98.6%) can be achieved, both of which are higher compared to the 14-3-3 protein performance [16,49,50,51,52,53]. In turn, the most highly elevated levels of t-tau are observed in the MM1, MV1 and VV2 types with classical symptomatology [54]. Therefore, it has been suggested that t-tau can be used in the diagnostic assessment of prion protein type when the codon 129 genotype is known and could provide valuable information for physicians about the prognosis [55]. Increase in t-tau and t-tau/p-tau is related to a shortened life expectancy, which could be explained because t-tau reflects neuronal damage [1,48,54,56] and t-tau levels continue to increase during the progression of the disease [57]. Levels of t-tau correlate with disease burden as assessed by cortical involvement evaluated by DWI sequence of MR [58].
Advantages: High specificity for t-tau and t-tau/p-tau ratio for sCJD. Diagnostic performance improves for both genetic and sporadic CJD if combined with 14-3-3 protein [43,59,60]
Limitations: t-tau could be increased in other neurodegenerative conditions but not as much as in prionopathies.
5.2.3. Neurofilament Light Chain Protein (NfL)
NfL is a neuronal cytoskeleton component and is released when there is neuronal damage in a wide range of conditions, making this a very good biomarker of neurodegeneration [61,62]. NfL is increased in all sCJD subtypes, including those which typically show low values of t-tau and negative protein 14-3-3 (e.g., sCJD MV2K, MM2C and gCJD E200K) [5,63]. It has outstanding sensitivity to detect sCJD, higher than 95%, but a very low specificity of 43.1% [14,64].
Limitations: Low specificity makes it less useful compared to the determination of t-tau or the combination of t-tau or ratio t-tau/p-tau + 14-3-3 protein in the differential diagnosis of rapidly progressive dementias [14].
Advantages: Due to high sensitivity, this could be useful combined with other surrogate biomarkers for the early diagnosis of prionopathies.
5.2.4. Other Biomarkers
Alpha synuclein, a neuronal protein especially abundant at presynaptic regions, stands out among other promising surrogate biomarkers. Its levels are increased in both genetic and sporadic forms of CJD, with good diagnostic performance (sensitivity 98% and specificity 97%); in addition, there is an inverse correlation between alpha synuclein levels and disease duration in CJD [61,65,66,67,68]. In turn, neurogranin, related to synaptic plasticity, has been shown to be increased in sCJD compared to controls and other neurodegenerative dementias with a diagnostic yield similar to 14-3-3 protein in the early stages of the disease, without significant variations with disease progression [5,10]. It has been speculated that neurogranin levels could be useful to differentiate between different subtypes of CJD (different concentrations having been reported according to the clinicopathological subtypes) [5]. Interestingly, it has been reported that ubiquitin, which marks neuritic damage, dysfunctional protostasis and neuroinflammation, has higher concentrations in CJD than in controls and other neurodegenerative dementias, and especially in less frequent forms of sCJD, such as MM(V)1 [69,70]. One study has also shown higher levels of calmodulin, a ubiquitous calcium-binding protein, in sCJD compared to other neurodegenerative dementias, particularly in those with higher levels of t-tau [71].
Markers related to oxidative stress have also been postulated, as is the case for mitochondrial malate dehydrogenase 1 (MDH1). Increase in MDH1 would have a sensitivity of 97.5% and specificity of 95.6% for the diagnosis of sCJD with positive correlation for t-tau and 14-3-3 protein concentrations according to preliminary data from several studies with small or very heterogeneous samples [72,73]; however, this must be confirmed in future studies prior to its future possible use in clinical practice.
A single study indicates that glial biomarkers, such as YKL-40, CHIT-1 and GFAP, are significantly increased in the VV2 form of CJD compared to other neurodegenerative dementias and are positively correlated with symptomatic progression of the disease [10,74]. An increase in these biomarkers has even been observed in presymptomatic cases of GSS [74].
Biomarkers related to iron metabolism, such as transferrin, have also been studied. A single study has postulated that elevated transferrin could be used in combination with t-tau to increase its diagnostic performance [75].
Advantages: The discovery of new biomarkers related to different pathophysiological processes can help us to better understand the disease itself and thus identify possible therapeutic targets.
Limitations: At present, none of these biomarkers are approved for use, either in isolation, or in combination with core biomarkers, in neurogenerative diseases. Further studies are needed to confirm the benefit of their application in clinical practice.
5.3. CSF Prion Proteins
5.3.1. Total PrP
Total PrP levels in the CSF of patients with prion disease tend to be reduced compared to controls. It has been speculated that this may result from the sequestration of soluble monomeric protein into aggregates in the brain (analogous to the proposed mechanism for reduction of CSF Aβ1-42 in AD) [65,76,77]. Specificity for diagnosing prionopathies of reduced total PrP in CSF seems to be moderate and unlikely to be used for differential diagnosis with other causes of rapidly progressive dementia, at least if not combined with surrogate CSF biomarkers, such as 14-3-3 protein and t-tau or ratio of t-tau/p-tau [37,61,78]. On the other hand, it has been suggested that it could have potential applicability for monitoring response to future disease-modifying treatments because there are significant differences in genetic forms of CJD between PRNP mutation carriers and non-carriers years before symptom onset, and levels of test-retest of total PrP are stable during follow-up [79,80].
Advantages: This biomarker directly reflects the pathophysiology of prion diseases and therefore has a potential role in the follow-up of possible future treatments.
Limitations: It has suboptimal performance, no better than that described for surrogate biomarkers with applications in clinical practice. Therefore, it is unlikely to be indicated for use as a diagnostic biomarker in the future.
5.3.2. Prion Real-Time Quaking-Induced Conversion (RT-QuIC)
Aside from brain biopsy, RT-QuIC, an ultrasensitive in vitro PrPSc amplification assay (Figure 4), is the only disease-specific antemortem diagnostic biomarker that directly detects the pathological prion protein (PrPSc) and since 2018 has been incorporated in the current diagnostic criteria [17,41,42,62,81,82,83]. A very high sensitivity (80–96%) and virtually full specificity (99–100%) has been reported for RT-QuIC for sCJD [13] (Figure 3). However, the sensitivity is lower in MM1, the most frequent subtype of sCJD [41], and is even lower in genetic forms of CJD, GSS and FFI, as well as in sporadic fatal insomnia. The most plausible explanation for this finding is strain variability [76,81]. Nevertheless, the RT-QuIC performance continues to be higher compared to 14-3-3 protein and to the combination of 14-3-3 protein and t-tau or t-tau/p-tau ratio for genetic forms of CJD, GSS and FFI [77]. However, it has not demonstrated an ability to differentiate between different subtypes of sCJD [84]. In turn, RT-QuIC presents increased costs and less inter-laboratory standardization data is available compared to the use of approved surrogate biomarkers (14-3-3 protein and t-tau) [85].
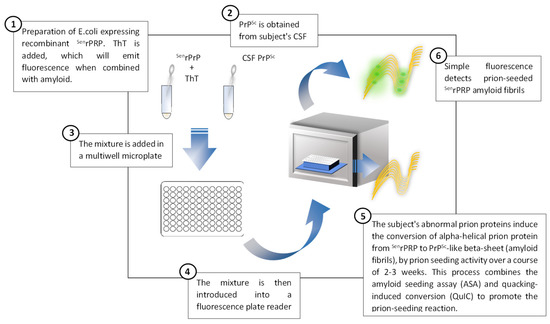
Figure 4.
Description of the prion real-time quaking-induced conversion technique applied to the diagnosis of prion diseases, adapted from Orrù et al., 2017 [86].
Advantages: The ability to detect prion protein with high sensitivity and specificity of almost 100%. Included as a diagnostic criterion.
Limitations: Technically more complex than the determination of other surrogate biomarkers that are measurable in CSF (14-3-3 protein and total tau), and accessible in fewer hospitals. Poorer performance for atypical variants of sCJD and genetic forms of CJD, GSS and familial and/or sporadic fatal insomnia.
6. Plasma Biomarkers
6.1. NfL
Increased levels of plasma NfL have been reported in CJD, significantly higher than in other neurodegenerative dementias (e.g., AD, LBD and frontotemporal dementia -FTD-) and obviously compared to controls. One study has shown an AUC of 0.93 to discriminate CJD from non-CJD dementias [87] and another has reported a sensitivity of 100% and specificity of 85.5% for the diagnosis of different prionopathies (sporadic, genetic and iatrogenic CJD and GSS) [61,88]. However, it has been suggested that performance of NfL is better in CSF compared to plasma [89]. The increase in plasma NfL occurs in the early stages of the disease, before symptom onset, and it seems to be always altered if the prion conversion assay is positive in CSF. The levels of NfL continue to increase with the symptomatic progression of the disease [88,90,91].
Advantages: An accessible biomarker with a huge number of studies suggesting its utility for discriminating neurodegenerative vs. non-neurodegenerative dementias. Altered from the early stages of disease, so therefore useful for early diagnosis.
Limitations: Not a specific biomarker of prion diseases; also increased in other neurodegenerative dementias, although not to the same extent.
6.2. t-tau
Plasma t-tau levels are higher in CJD, in sporadic, iatrogenic and genetic forms, compared not only to controls, but also significantly when compared to other neurodegenerative dementias [25,39,41]. t-tau is particularly increased in sCJD patients who are homozygous for methionine at codon 129 of the PRNP gene [92]. Plasma t-tau seems to have a moderate association with disease duration, offering a moderate survival prediction capacity [93]. One study has reported a sensitivity of 84.6% and specificity of 96.2% [88], but it seems that both sensitivity and specificity are lower in general for plasma t-tau compared to CSF t-tau [89].
Advantages: This could be useful to discriminate from non-neurodegenerative causes of cognitive decline.
Limitations: It is a non-specific biomarker of neurodegeneration that does not clearly improve performance compared to use of plasma NfL.
6.3. YKL-40
Increased levels of plasma YKL-40 have been detected in different sporadic and genetic forms of prionopathies compared to controls. Higher levels are detected in later stages of the disease [94]. Due to the limited capacity to discriminate from other neurodegenerative dementias and moderate ability to distinguish from healthy controls, YKL-40 does not seem to be a good diagnostic biomarker but could have a role in disease monitoring [94].
Advantages: Could be a potential plasma biomarker related to progression of the disease.
Limitations: Very limited capacity to discriminate prion diseases from other neurodegenerative diseases; doubtful utility as an additional biomarker for diagnosis.
6.4. MicroRNA
The blood microRNA profile has been suggested as a complementary test to use, together with plasma neurodegeneration biomarkers. One study, with a small sample size, has shown different microRNA expression in sCJD compared not only to controls but also to AD [95].
Advantages: Could be another non-invasive biomarker of sCJD.
Limitations: Very limited data; does not seem to be applicable in clinical practice, at least in the short term.
6.5. Total Prion Protein (t-PrP)
The existence of vascular pathology with loss of integrity of the blood brain barrier (BBB) has been described in more than 40% of brains with prion disease. With loss of BBB integrity, it is expected that dying endothelial cells in the intracranial capillary vascular system, and specially dying neurons, can release PrP to the blood circulation system. Increased levels of plasma t-PrP have been reported in sporadic, genetic and variant CJD. However, increase in plasma t-PrP is not specific to prion diseases because it has also been reported in other conditions, such as AD, FTD, or LBD [96]. Therefore, it seems to be useful to discriminate between neurodegenerative and non-neurodegenerative dementias, but less clearly between prion diseases and other neurodegenerative disorders, even though higher levels have been reported in classical sCJD compared to other neurodegenerative dementias [96]. Interestingly, there is a dissociation between t-PrP CSF and plasma levels in sCJD, which are increased in plasma but decreased in CSF [62,96]. The presence of higher t-PrP levels in sCJD cases harboring MM and VV at PRNP codon 129 compared to MV carriers has also been reported, suggesting a better performance for homozygous carriers [88]. A mild association has been reported between CSF markers of neuronal injury (14-3-3 protein and NfL) and plasmatic t-PrP, suggesting that plasma t-PrP might be regarded as a biomarker of neurodegeneration, in contrast to CSF t-PrP, which probably reflects pathogenic PrP aggregation occurring in prion disease patients. Non-association has been reported between plasma t-PrP and the stage or disease duration, which argues against a potential use of this marker for prognostic purposes [96].
Advantages: It is possible to assess this biomarker in an accessible fluid, so invasive techniques are not required. The increase in t-PrP in the plasma of CJD (sporadic, acquired and genetic) can be detected from the early stages and does not vary between different stages of the disease, making it potentially useful as an early diagnosis biomarker.
Limitations: It is not specific for CJD diagnosis, and we do not have information about less prevalent prionopathies. This biomarker seems to be more related to neurodegeneration.
7. Nasal Mucosa
RT-QuIC performed in the samples obtained by nasal brushing, a less invasive technique compared to lumbar puncture, has shown a very high diagnosis accuracy according to preliminary data, with a sensitivity of 97% and specificity of 100%; this is a better performance compared to RT-QuIC in CSF [97,98]. It has been suggested that the combination of CSF RT-QuIC and RT-QuIC from a sample of nasal mucosa could increase the sensitivity in the early diagnosis of CJD to almost 100% [42,61].
Advantages: Obtaining a nasal mucosal sample would be safer and better tolerated by patients than lumbar puncture. If the preliminary data are confirmed, it could potentially replace CSF as a sample for RT-QuIC, or, at least, and this is ultimately more likely, allow increased diagnostic confidence in those subjects with high suspicion of prion disease with negative RT-QuIC in CSF, with the ability to detect some false negatives.
Limitations: Only preliminary data are available that do not allow evaluation of its practical clinical applicability. In many subjects, lumbar puncture will continue to be performed for screening of other neurodegenerative dementias; therefore, it seems implausible that nasal mucosa sampling can universally replace lumbar puncture for RT-QuIC study, at least not until other biomarkers with good diagnosis performance for other neurodegenerative dementias are developed for use in nasal mucosa.
8. Future Directions
The combination of neuroimaging (structural magnetic resonance) and CSF RT-QuIC for prion protein, t-tau, t-tau/p-tau ratio and 14-3-3 protein appears to be the most cost-effective combination for the early diagnosis of different prion diseases. Now that t-tau and t-tau/p-tau ratio are frequently assessed in mild cognitive impairment, considering whether abnormally high levels of one or both are highly suggestive of prion disease is essential. Qualitative analysis of EEG seems to be less useful for early diagnosis. However, research in the applicability of quantitative EEG to detect changes from earlier stages of the disease (including presymptomatic) could substantially increase its diagnostic performance, as well as reduce interobserver variability. PSG continues to be essential for the diagnosis of sFI and FFI, and review of EEG during PSG could potentially increase the capacity to detect PSWCs in CJD patients. Future studies to assess the performance of routine EEG vs. PSG-EEG (qualitative and quantitative analyses) would be of interest.
The performance of CSF biomarkers is very good, but their assessment still requires an invasive test (lumbar puncture) that is usually performed later after symptom onset. The identification of diagnostic biomarkers in more accessible fluids, such as peripheral blood, is currently being investigated. The most promising plasma biomarkers are very sensitive and non-specific (NfL and t-tau) and require ultra-sensitive analysis techniques that are unavailable in most centers. Nevertheless, despite technical limitations, peripheral blood is the best potential source for future biomarkers that, ideally, should also directly reflect prion pathology. PrPSc measured in exosomes, small extracellular vesicles capable of crossing the blood-brain barrier, in both peripheral blood and CSF, are being suggested as possible biomarkers for the accurate diagnosis of prion diseases, but validation studies are still lacking [99,100]. Even if the novel strategy of PrPSc determination in exosomes in peripheral blood fails, efforts to identify other biomarkers with the same, or at least very close, performance to RT-QuIC in CSF, the best available diagnostic biomarker, in more accessible fluids, such as peripheral blood and/or mucosa, will remain a priority.
Genetic prion diseases, on the other hand, are not only candidates for the application of potential future disease-modifying treatments (such as antisense oligonucleotides) but also allow the identification of prognostic and/or disease progression biomarkers. Confirming that periodic plasma NfL determination can predict up to two years in advance the onset of symptoms [90], and that higher t-tau levels are associated with a faster disease course, could help to better select potential candidates for future clinical trials. The availability of objective biomarkers of progression is essential to consider how to measure response in future clinical trials, as these are pathologies with widely varying clinical phenotypes and age of symptom onset [101]. Therefore, the identification of accessible prognostic biomarkers to be measured repeatedly in the population at risk is an undeniable need.
9. Conclusions
The early and accurate diagnosis of prion diseases with a clinically progressive course and without modifying treatment is essential. Therefore, the availability of biomarkers that can provide near-total diagnostic certainty is crucial. The fact that some of these biomarkers are found in high concentrations from an early stage (even in asymptomatic carriers in the genetic forms) can help us to better identify subjects that could be candidates for future therapeutic strategies.
Among the biomarkers available in clinical practice, RT-QuIC in cerebrospinal fluid, is the diagnostic biomarker with the best performance for early diagnosis; preliminary results of the same technique in other tissues, such as nasal mucosa, are also very promising. However, along with the determination of RT-QuIC, it is advisable to also request the determination of 14-3-3 protein, t-tau and p-tau, since diagnostic sensitivity will be further increased by their use without any decrease in specificity.
In recent years, there have been fewer advances in neuroimaging and neurophysiology biomarkers, but they are no less relevant. Structural neuroimaging (MR should be used preferably) within the etiological study of any neurodegenerative dementia is mandatory and will therefore continue to have a relevant role. Neurophysiological tests will continue to play an essential role in the diagnosis of sporadic or fatal familial insomnia, and the electroencephalogram, which is non-invasive and inexpensive, can remain as an additional diagnostic tool in centers with fewer resources.
For the time being, it does not seem that plasma biomarkers will be applied for the diagnosis of prion diseases due to the inferior performance of all biomarkers studied in the blood in comparison to cerebrospinal fluid to discriminate prion diseases from other neurodegenerative dementias. However, they could be useful for monitoring the response to a possible disease-modifying treatment, since assay protocols based on peripheral blood biomarkers as a target response will always be easier to perform than those based on CSF biomarkers.
Author Contributions
Conceptualization, M.A.; methodology, M.A. and I.R.; investigation, M.A. and I.R.; resources, M.A.; data curation, M.A. and I.R.; writing—original draft preparation, M.A. and I.R.; writing—review and editing, M.A., M.V.Z. and M.M.; visualization, M.A., M.V.Z. and M.M.; supervision, M.A., M.V.Z. and M.M.; project administration, M.A.; funding acquisition, M.A. All authors have read and agreed to the published version of the manuscript.
Funding
MA acknowledges support from a Río Hortega Fellowship (CM19/00066) by the Carlos III Health Institute.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Olivia Belbin, from the Memory Unit of Hospital Santa Creu i Sant Pau, has provided the license of Biorender.com to include one of the figures in this paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Llorens, F.; Rübsamen, N.; Hermann, P.; Schmitz, M.; Villar-Piqué, A.; Goebel, S.; Karch, A.; Zerr, I. A Prognostic Model for Overall Survival in Sporadic Creutzfeldt-Jakob Disease. Alzheimer’s Dement. 2020, 16, 1438–1447. [Google Scholar] [CrossRef] [PubMed]
- Ladogana, A.; Kovacs, G.G. Genetic Creutzfeldt-Jakob Disease. Handb. Clin. Neurol. 2018, 153, 219–242. [Google Scholar] [CrossRef] [PubMed]
- Hermann, P.; Appleby, B.; Brandel, J.-P.; Caughey, B.; Collins, S.; Geschwind, M.D.; Green, A.; Haïk, S.; Kovacs, G.G.; Ladogana, A.; et al. Biomarkers and Diagnostic Guidelines for Sporadic Creutzfeldt-Jakob Disease. Lancet Neurol. 2021, 20, 235–246. [Google Scholar] [CrossRef]
- Atkinson, C.J.; Zhang, K.; Munn, A.L.; Wiegmans, A.; Wei, M.Q. Prion Protein Scrapie and the Normal Cellular Prion Protein. Prion 2016, 10, 63–82. [Google Scholar] [CrossRef]
- Blennow, K.; Diaz-Lucena, D.; Zetterberg, H.; Villar-Pique, A.; Karch, A.; Vidal, E.; Hermann, P.; Schmitz, M.; Ferrer Abizanda, I.; Zerr, I.; et al. CSF Neurogranin as a Neuronal Damage Marker in CJD: A Comparative Study with AD. J. Neurol. Neurosurg. Psychiatry 2019, 90, 846–853. [Google Scholar] [CrossRef]
- Marino, S.; Morabito, R.; De Salvo, S.; Bonanno, L.; Bramanti, A.; Pollicino, P.; Giorgianni, R.; Bramanti, P. Quantitative, Functional MRI and Neurophysiological Markers in a Case of Gerstmann-Sträussler-Scheinker Syndrome. Funct. Neurol. 2017, 32, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.C.; Xiang, F.; Monaghan, J.; Han, D.; Zhang, Z.; Edström, L.; Anvret, M.; Prusiner, S.B. Huntington Disease Phenocopy Is a Familial Prion Disease. Am. J. Hum. Genet. 2001, 69, 1385–1388. [Google Scholar] [CrossRef] [PubMed]
- Brandel, J.-P.; Knight, R. Variant Creutzfeldt-Jakob Disease. Handb. Clin. Neurol. 2018, 153, 191–205. [Google Scholar] [CrossRef] [PubMed]
- Spencer, M.D.; Knight, R.S.G.; Will, R.G. First Hundred Cases of Variant Creutzfeldt-Jakob Disease: Retrospective Case Note Review of Early Psychiatric and Neurological Features. BMJ 2002, 324, 1479–1482. [Google Scholar] [CrossRef]
- Mok, T.H.; Mead, S. Preclinical Biomarkers of Prion Infection and Neurodegeneration. Curr. Opin. Neurobiol. 2020, 61, 82–88. [Google Scholar] [CrossRef]
- Hill, A.F.; Joiner, S.; Wadsworth, J.D.F.; Sidle, K.C.L.; Bell, J.E.; Budka, H.; Ironside, J.W.; Collinge, J. Molecular Classification of Sporadic Creutzfeldt–Jakob Disease. Brain 2003, 126, 1333–1346. [Google Scholar] [CrossRef] [PubMed]
- Safar, J.G. Molecular Pathogenesis of Sporadic Prion Diseases in Man. Prion 2012, 6, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Ascari, L.M.; Rocha, S.C.; Gonçalves, P.B.; Vieira, T.C.R.G.; Cordeiro, Y. Challenges and Advances in Antemortem Diagnosis of Human Transmissible Spongiform Encephalopathies. Front. Bioeng. Biotechnol. 2020, 8, 585896. [Google Scholar] [CrossRef] [PubMed]
- Abu-Rumeileh, S.; Baiardi, S.; Polischi, B.; Mammana, A.; Franceschini, A.; Green, A.; Capellari, S.; Parchi, P. Diagnostic Value of Surrogate CSF Biomarkers for Creutzfeldt-Jakob Disease in the Era of RT-QuIC. J. Neurol. 2019, 266, 3136–3143. [Google Scholar] [CrossRef] [PubMed]
- Wieser, H.G.; Schindler, K.; Zumsteg, D. EEG in Creutzfeldt-Jakob Disease. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2006, 117, 935–951. [Google Scholar] [CrossRef]
- Skillbäck, T.; Rosén, C.; Asztely, F.; Mattsson, N.; Blennow, K.; Zetterberg, H. Diagnostic Performance of Cerebrospinal Fluid Total Tau and Phosphorylated Tau in Creutzfeldt-Jakob Disease: Results from the Swedish Mortality Registry. JAMA Neurol. 2014, 71, 476–483. [Google Scholar] [CrossRef]
- Figgie, M.P.J.; Appleby, B.S. Clinical Use of Improved Diagnostic Testing for Detection of Prion Disease. Viruses 2021, 13, 789. [Google Scholar] [CrossRef] [PubMed]
- Franko, E.; Wehner, T.; Joly, O.; Lowe, J.; Porter, M.-C.; Kenny, J.; Thompson, A.; Rudge, P.; Collinge, J.; Mead, S. Quantitative EEG Parameters Correlate with the Progression of Human Prion Diseases. J. Neurol. Neurosurg. Psychiatry 2016, 87, 1061–1067. [Google Scholar] [CrossRef]
- Matsubayashi, T.; Akaza, M.; Hayashi, Y.; Hamaguchi, T.; Yamada, M.; Shimohata, T.; Yokota, T.; Sanjo, N. Focal Sharp Waves Are a Specific Early-Stage Marker of the MM2-Cortical Form of Sporadic Creutzfeldt-Jakob Disease. Prion 2020, 14, 207–213. [Google Scholar] [CrossRef]
- Wu, L.-Y.; Zhan, S.-Q.; Huang, Z.-Y.; Zhang, B.; Wang, T.; Liu, C.-F.; Lu, H.; Dong, X.-P.; Wu, Z.-Y.; Zhang, J.-W.; et al. Expert Consensus on Clinical Diagnostic Criteria for Fatal Familial Insomnia. Chin. Med. J. 2018, 131, 1613–1617. [Google Scholar] [CrossRef]
- Kang, P.; de Bruin, G.S.; Wang, L.H.; Ward, B.A.; Ances, B.M.; Lim, M.M.; Bucelli, R.C. Sleep Pathology in Creutzfeldt-Jakob Disease. J. Clin. Sleep Med. JCSM Off. Publ. Am. Acad. Sleep Med. 2016, 12, 1033–1039. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Shao, J.; Lang, Y.; Lv, Y.; Cui, L. Clinical Manifestations and Polysomnography-Based Analysis in Nine Cases of Probable Sporadic Creutzfeldt-Jakob Disease. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2021, 42, 4209–4219. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, R.G.; Wroe, S.J.; Collinge, J.; Yousry, T.A.; Jäger, H.R. Neuroimaging Findings in Human Prion Disease. J. Neurol. Neurosurg. Psychiatry 2007, 78, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Sacco, S.; Paoletti, M.; Staffaroni, A.M.; Kang, H.; Rojas, J.; Marx, G.; Goh, S.-Y.; Luisa Mandelli, M.; Allen, I.E.; Kramer, J.H.; et al. Multimodal MRI Staging for Tracking Progression and Clinical-Imaging Correlation in Sporadic Creutzfeldt-Jakob Disease. NeuroImage. Clin. 2021, 30, 102523. [Google Scholar] [CrossRef] [PubMed]
- Baiardi, S.; Magherini, A.; Capellari, S.; Redaelli, V.; Ladogana, A.; Rossi, M.; Tagliavini, F.; Pocchiari, M.; Giaccone, G.; Parchi, P. Towards an Early Clinical Diagnosis of Sporadic CJD VV2 (Ataxic Type). J. Neurol. Neurosurg. Psychiatry 2017, 88, 764–772. [Google Scholar] [CrossRef]
- Ioannides, P.; Karacostas, D. Neuroimaging in Human Prion Disease: Searching in the Mist. World J. Radiol. 2009, 1, 45–49. [Google Scholar] [CrossRef]
- Divya, K.P.; Menon, R.N.; Thomas, B.; Nair, M. A Hospital-Based Registry of Creutzfeldt-Jakob Disease: Can Neuroimaging Serve as a Surrogate Biomarker? Neurol. India 2016, 64, 411–418. [Google Scholar] [CrossRef]
- Rudge, P.; Hyare, H.; Green, A.; Collinge, J.; Mead, S. Imaging and CSF Analyses Effectively Distinguish CJD from Its Mimics. J. Neurol. Neurosurg. Psychiatry 2018, 89, 461–466. [Google Scholar] [CrossRef]
- Kim, J.H.; Choi, B.S.; Jung, C.; Chang, Y.; Kim, S. Diffusion-Weighted Imaging and Magnetic Resonance Spectroscopy of Sporadic Creutzfeldt-Jakob Disease: Correlation with Clinical Course. Neuroradiology 2011, 53, 939–945. [Google Scholar] [CrossRef]
- Shiga, Y.; Miyazawa, K.; Sato, S.; Fukushima, R.; Shibuya, S.; Sato, Y.; Konno, H.; Doh-ura, K.; Mugikura, S.; Tamura, H.; et al. Diffusion-Weighted MRI Abnormalities as an Early Diagnostic Marker for Creutzfeldt-Jakob Disease. Neurology 2004, 63, 443–449. [Google Scholar] [CrossRef]
- Lee, H.; Rosenmann, H.; Chapman, J.; Kingsley, P.B.; Hoffmann, C.; Cohen, O.S.; Kahana, E.; Korczyn, A.D.; Prohovnik, I. Thalamo-Striatal Diffusion Reductions Precede Disease Onset in Prion Mutation Carriers. Brain 2009, 132, 2680–2687. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.-J.; Cho, S.-S.; Jeong, B.-H.; Kim, Y.-S.; Seo, S.W.; Na, D.L.; Geschwind, M.D.; Jeong, Y. Glucose Metabolism in Sporadic Creutzfeldt-Jakob Disease: A Statistical Parametric Mapping Analysis of (18) F-FDG PET. Eur. J. Neurol. 2012, 19, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Green, A.J.E.; Ramljak, S.; Müller, W.E.G.; Knight, R.S.G.; Schröder, H.C. 14-3-3 in the Cerebrospinal Fluid of Patients with Variant and Sporadic Creutzfeldt-Jakob Disease Measured Using Capture Assay Able to Detect Low Levels of 14-3-3 Protein. Neurosci. Lett. 2002, 324, 57–60. [Google Scholar] [CrossRef]
- Matsui, Y.; Satoh, K.; Miyazaki, T.; Shirabe, S.; Atarashi, R.; Mutsukura, K.; Satoh, A.; Kataoka, Y.; Nishida, N. High Sensitivity of an ELISA Kit for Detection of the Gamma-Isoform of 14-3-3 Proteins: Usefulness in Laboratory Diagnosis of Human Prion Disease. BMC Neurol. 2011, 11, 120. [Google Scholar] [CrossRef] [PubMed]
- Castellani, R.J.; Colucci, M.; Xie, Z.; Zou, W.; Li, C.; Parchi, P.; Capellari, S.; Pastore, M.; Rahbar, M.H.; Chen, S.G.; et al. Sensitivity of 14-3-3 Protein Test Varies in Subtypes of Sporadic Creutzfeldt-Jakob Disease. Neurology 2004, 63, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Leitão, M.J.; Baldeiras, I.; Almeida, M.R.; Ribeiro, M.H.; Santos, A.C.; Ribeiro, M.; Tomás, J.; Rocha, S.; Santana, I.; Oliveira, C.R. CSF Tau Proteins Reduce Misdiagnosis of Sporadic Creutzfeldt-Jakob Disease Suspected Cases with Inconclusive 14-3-3 Result. J. Neurol. 2016, 263, 1847–1861. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.; Cartier, L.; Matamala, J.M.; Hernández, N.; Woehlbier, U.; Hetz, C. Altered Prion Protein Expression Pattern in CSF as a Biomarker for Creutzfeldt-Jakob Disease. PLoS ONE 2012, 7, e36159. [Google Scholar] [CrossRef]
- Ladogana, A.; Sanchez-Juan, P.; Mitrová, E.; Green, A.; Cuadrado-Corrales, N.; Sánchez-Valle, R.; Koscova, S.; Aguzzi, A.; Sklaviadis, T.; Kulczycki, J.; et al. Cerebrospinal Fluid Biomarkers in Human Genetic Transmissible Spongiform Encephalopathies. J. Neurol. 2009, 256, 1620–1628. [Google Scholar] [CrossRef]
- Higuma, M.; Sanjo, N.; Satoh, K.; Shiga, Y.; Sakai, K.; Nozaki, I.; Hamaguchi, T.; Nakamura, Y.; Kitamoto, T.; Shirabe, S.; et al. Relationships between Clinicopathological Features and Cerebrospinal Fluid Biomarkers in Japanese Patients with Genetic Prion Diseases. PLoS ONE 2013, 8, e60003. [Google Scholar] [CrossRef]
- Leitão, M.J.; Baldeiras, I.; Almeida, M.R.; Ribeiro, M.H.; Santos, A.C.; Ribeiro, M.; Tomás, J.; Rocha, S.; Santana, I.; Oliveira, C.R. Sporadic Creutzfeldt-Jakob Disease Diagnostic Accuracy Is Improved by a New CSF ELISA 14-3-3γ Assay. Neuroscience 2016, 322, 398–407. [Google Scholar] [CrossRef]
- Lattanzio, F.; Abu-Rumeileh, S.; Franceschini, A.; Kai, H.; Amore, G.; Poggiolini, I.; Rossi, M.; Baiardi, S.; McGuire, L.; Ladogana, A.; et al. Prion-Specific and Surrogate CSF Biomarkers in Creutzfeldt-Jakob Disease: Diagnostic Accuracy in Relation to Molecular Subtypes and Analysis of Neuropathological Correlates of p-Tau and Aβ42 Levels. Acta Neuropathol. 2017, 133, 559–578. [Google Scholar] [CrossRef] [PubMed]
- Zanusso, G.; Fiorini, M.; Ferrari, S.; Gajofatto, A.; Cagnin, A.; Galassi, A.; Richelli, S.; Monaco, S. Cerebrospinal Fluid Markers in Sporadic Creutzfeldt-Jakob Disease. Int. J. Mol. Sci. 2011, 12, 6281–6292. [Google Scholar] [CrossRef] [PubMed]
- Hyeon, J.W.; Kim, S.Y.; Lee, J.; Park, J.S.; Hwang, K.J.; Lee, S.M.; An, S.A.; Lee, M.K.; Ju, Y.R. Alternative Application of Tau Protein in Creutzfeldt-Jakob Disease Diagnosis: Improvement for Weakly Positive 14-3-3 Protein in the Laboratory. Sci. Rep. 2015, 5, 15283. [Google Scholar] [CrossRef] [PubMed]
- Chohan, G.; Pennington, C.; Mackenzie, J.M.; Andrews, M.; Everington, D.; Will, R.G.; Knight, R.S.G.; Green, A.J.E. The Role of Cerebrospinal Fluid 14-3-3 and Other Proteins in the Diagnosis of Sporadic Creutzfeldt-Jakob Disease in the UK: A 10-Year Review. J. Neurol. Neurosurg. Psychiatry 2010, 81, 1243–1248. [Google Scholar] [CrossRef] [PubMed]
- Lemstra, A.W.; van Meegen, M.T.; Vreyling, J.P.; Meijerink, P.H.; Jansen, G.H.; Bulk, S.; Baas, F.; van Gool, W.A. 14-3-3 Testing in Diagnosing Creutzfeldt-Jakob Disease: A Prospective Study in 112 Patients. Neurology 2000, 55, 514–516. [Google Scholar] [CrossRef]
- Schmitz, M.; Ebert, E.; Stoeck, K.; Karch, A.; Collins, S.; Calero, M.; Sklaviadis, T.; Laplanche, J.-L.; Golanska, E.; Baldeiras, I.; et al. Validation of 14-3-3 Protein as a Marker in Sporadic Creutzfeldt-Jakob Disease Diagnostic. Mol. Neurobiol. 2016, 53, 2189–2199. [Google Scholar] [CrossRef]
- Stoeck, K.; Sanchez-Juan, P.; Gawinecka, J.; Green, A.; Ladogana, A.; Pocchiari, M.; Sanchez-Valle, R.; Mitrova, E.; Sklaviadis, T.; Kulczycki, J.; et al. Cerebrospinal Fluid Biomarker Supported Diagnosis of Creutzfeldt-Jakob Disease and Rapid Dementias: A Longitudinal Multicentre Study over 10 Years. Brain 2012, 135, 3051–3061. [Google Scholar] [CrossRef]
- Llorens, F.; Karch, A.; Golanska, E.; Schmitz, M.; Lange, P.; Sikorska, B.; Liberski, P.P.; Zerr, I. Cerebrospinal Fluid Biomarker-Based Diagnosis of Sporadic Creutzfeldt-Jakob Disease: A Validation Study for Previously Established Cutoffs. Dement. Geriatr. Cogn. Disord. 2017, 43, 71–80. [Google Scholar] [CrossRef]
- Hamlin, C.; Puoti, G.; Berri, S.; Sting, E.; Harris, C.; Cohen, M.; Spear, C.; Bizzi, A.; Debanne, S.M.; Rowland, D.Y. A Comparison of Tau and 14-3-3 Protein in the Diagnosis of Creutzfeldt-Jakob Disease. Neurology 2012, 79, 547–552. [Google Scholar] [CrossRef]
- Coulthart, M.B.; Jansen, G.H.; Olsen, E.; Godal, D.L.; Connolly, T.; Choi, B.C.K.; Wang, Z.; Cashman, N.R. Diagnostic Accuracy of Cerebrospinal Fluid Protein Markers for Sporadic Creutzfeldt-Jakob Disease in Canada: A 6-Year Prospective Study. BMC Neurol. 2011, 11, 133. [Google Scholar] [CrossRef]
- Foucault-Fruchard, L.; Delaye, J.B.; Morange, V.; Beaufils, E.; Duwicquet, C.; Quadrio, I.; Balageas, A.C.; Dufour-Rainfray, D. An Automated Alert System Based on the P-Tau/Tau Ratio to Quickly Inform Health Professionals upon a Suspected Case of Sporadic Creutzfeldt-Jakob Disease. J. Neurol. Sci. 2020, 415, 116971. [Google Scholar] [CrossRef] [PubMed]
- Llorens, F.; Villar-Piqué, A.; Hermann, P.; Schmitz, M.; Goebel, S.; Waniek, K.; Lachmann, I.; Zerr, I. Cerebrospinal Fluid Non-Phosphorylated Tau in the Differential Diagnosis of Creutzfeldt-Jakob Disease: A Comparative Prospective Study with 14-3-3. J. Neurol. 2020, 267, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Meiner, Z.; Kahana, E.; Baitcher, F.; Korczyn, A.D.; Chapman, J.; Cohen, O.S.; Milo, R.; Aharon-Perez, J.; Abramsky, O.; Gabizon, R.; et al. Tau and 14-3-3 of Genetic and Sporadic Creutzfeldt-Jakob Disease Patients in Israel. J. Neurol. 2011, 258, 255–262. [Google Scholar] [CrossRef]
- Gmitterová, K.; Heinemann, U.; Krasnianski, A.; Gawinecka, J.; Zerr, I. Cerebrospinal Fluid Markers in the Differentiation of Molecular Subtypes of Sporadic Creutzfeldt-Jakob Disease. Eur. J. Neurol. 2016, 23, 1126–1133. [Google Scholar] [CrossRef] [PubMed]
- Karch, A.; Hermann, P.; Ponto, C.; Schmitz, M.; Arora, A.; Zafar, S.; Llorens, F.; Müller-Heine, A.; Zerr, I. Cerebrospinal Fluid Tau Levels Are a Marker for Molecular Subtype in Sporadic Creutzfeldt-Jakob Disease. Neurobiol. Aging 2015, 36, 1964–1968. [Google Scholar] [CrossRef] [PubMed]
- Karch, A.; Llorens, F.; Schmitz, M.; Arora, A.S.; Zafar, S.; Lange, P.; Schmidt, C.; Zerr, I. Stratification by Genetic and Demographic Characteristics Improves Diagnostic Accuracy of Cerebrospinal Fluid Biomarkers in Rapidly Progressive Dementia. J. Alzheimer’s Dis. 2016, 54, 1385–1393. [Google Scholar] [CrossRef]
- Satoh, K.; Shirabe, S.; Eguchi, H.; Tsujino, A.; Motomura, M.; Satoh, A.; Tsujihata, M.; Eguchi, K. Chronological Changes in MRI and CSF Biochemical Markers in Creutzfeldt-Jakob Disease Patients. Dement. Geriatr. Cogn. Disord. 2007, 23, 372–381. [Google Scholar] [CrossRef]
- Cohen, O.S.; Chapman, J.; Korczyn, A.D.; Siaw, O.L.; Warman-Alaluf, N.; Nitsan, Z.; Appel, S.; Kahana, E.; Rosenmann, H.; Hoffmann, C. CSF Tau Correlates with the Degree of Cortical Involvement in E200K Familial Creutzfeldt-Jakob Disease. Neurosci. Lett. 2016, 634, 76–78. [Google Scholar] [CrossRef]
- Van Everbroeck, B.; Quoilin, S.; Boons, J.; Martin, J.J.; Cras, P. A Prospective Study of CSF Markers in 250 Patients with Possible Creutzfeldt-Jakob Disease. J. Neurol. Neurosurg. Psychiatry 2003, 74, 1210–1214. [Google Scholar] [CrossRef]
- Wang, G.-R.; Gao, C.; Shi, Q.; Zhou, W.; Chen, J.-M.; Dong, C.-F.; Shi, S.; Wang, X.; Wei, Y.; Jiang, H.-Y.; et al. Elevated Levels of Tau Protein in Cerebrospinal Fluid of Patients with Probable Creutzfeldt-Jakob Disease. Am. J. Med. Sci. 2010, 340, 291–295. [Google Scholar] [CrossRef]
- Thompson, A.G.B.; Mead, S.H. Review: Fluid Biomarkers in the Human Prion Diseases. Mol. Cell. Neurosci. 2019, 97, 81–92. [Google Scholar] [CrossRef]
- Zerr, I.; Schmitz, M.; Karch, A.; Villar-Piqué, A.; Kanata, E.; Golanska, E.; Díaz-Lucena, D.; Karsanidou, A.; Hermann, P.; Knipper, T.; et al. Cerebrospinal Fluid Neurofilament Light Levels in Neurodegenerative Dementia: Evaluation of Diagnostic Accuracy in the Differential Diagnosis of Prion Diseases. Alzheimer’s Dement. 2018, 14, 751–763. [Google Scholar] [CrossRef] [PubMed]
- Kanata, E.; Golanska, E.; Villar-Piqué, A.; Karsanidou, A.; Dafou, D.; Xanthopoulos, K.; Schmitz, M.; Ferrer, I.; Karch, A.; Sikorska, B.; et al. Cerebrospinal Fluid Neurofilament Light in Suspected Sporadic Creutzfeldt-Jakob Disease. J. Clin. Neurosci. Off. J. Neurosurg. Soc. Australas. 2019, 60, 124–127. [Google Scholar] [CrossRef] [PubMed]
- Abu-Rumeileh, S.; Capellari, S.; Stanzani-Maserati, M.; Polischi, B.; Martinelli, P.; Caroppo, P.; Ladogana, A.; Parchi, P. The CSF Neurofilament Light Signature in Rapidly Progressive Neurodegenerative Dementias. Alzheimer’s Res. Ther. 2018, 10, 3. [Google Scholar] [CrossRef] [PubMed]
- Llorens, F.; Kruse, N.; Schmitz, M.; Shafiq, M.; da Cunha, J.E.G.; Gotzman, N.; Zafar, S.; Thune, K.; de Oliveira, J.R.M.; Mollenhauer, B.; et al. Quantification of CSF Biomarkers Using an Electrochemiluminescence-Based Detection System in the Differential Diagnosis of AD and SCJD. J. Neurol. 2015, 262, 2305–2311. [Google Scholar] [CrossRef] [PubMed]
- Llorens, F.; Kruse, N.; Schmitz, M.; Gotzmann, N.; Golanska, E.; Thüne, K.; Zejneli, O.; Kanata, E.; Knipper, T.; Cramm, M.; et al. Evaluation of α-Synuclein as a Novel Cerebrospinal Fluid Biomarker in Different Forms of Prion Diseases. Alzheimer’s Dement. 2017, 13, 710–719. [Google Scholar] [CrossRef] [PubMed]
- Oeckl, P.; Metzger, F.; Nagl, M.; von Arnim, C.A.F.; Halbgebauer, S.; Steinacker, P.; Ludolph, A.C.; Otto, M. Alpha-, Beta-, and Gamma-Synuclein Quantification in Cerebrospinal Fluid by Multiple Reaction Monitoring Reveals Increased Concentrations in Alzheimer’s and Creutzfeldt-Jakob Disease but No Alteration in Synucleinopathies. Mol. Cell. Proteom. 2016, 15, 3126–3138. [Google Scholar] [CrossRef] [PubMed]
- Mastrangelo, A.; Baiardi, S.; Zenesini, C.; Poleggi, A.; Mammana, A.; Polischi, B.; Ladogana, A.; Capellari, S.; Parchi, P. Diagnostic and Prognostic Performance of CSF α-Synuclein in Prion Disease in the Context of Rapidly Progressive Dementia. Alzheimer’s Dement. 2021, 13, e12214. [Google Scholar] [CrossRef]
- Abu-Rumeileh, S.; Oeckl, P.; Baiardi, S.; Halbgebauer, S.; Steinacker, P.; Capellari, S.; Otto, M.; Parchi, P. CSF Ubiquitin Levels Are Higher in Alzheimer’s Disease than in Frontotemporal Dementia and Reflect the Molecular Subtype in Prion Disease. Biomolecules 2020, 10, 497. [Google Scholar] [CrossRef]
- Steinacker, P.; Rist, W.; Swiatek-de-Lange, M.; Lehnert, S.; Jesse, S.; Pabst, A.; Tumani, H.; von Arnim, C.A.F.; Mitrova, E.; Kretzschmar, H.A.; et al. Ubiquitin as Potential Cerebrospinal Fluid Marker of Creutzfeldt-Jakob Disease. Proteomics 2010, 10, 81–89. [Google Scholar] [CrossRef]
- Chen, C.; Hu, C.; Zhou, W.; Chen, J.; Shi, Q.; Xiao, K.; Wang, Y.; Dong, X.-P. Calmodulin Level Is Significantly Increased in the Cerebrospinal Fluid of Patients with Sporadic Creutzfeldt-Jakob Disease. Eur. J. Neurol. 2021, 28, 1134–1141. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, M.; Llorens, F.; Pracht, A.; Thom, T.; Correia, Â.; Zafar, S.; Ferrer, I.; Zerr, I. Regulation of Human Cerebrospinal Fluid Malate Dehydrogenase 1 in Sporadic Creutzfeldt-Jakob Disease Patients. Aging 2016, 8, 2927–2935. [Google Scholar] [CrossRef] [PubMed]
- Zerr, I.; Villar-Piqué, A.; Schmitz, V.E.; Poleggi, A.; Pocchiari, M.; Sánchez-Valle, R.; Calero, M.; Calero, O.; Baldeiras, I.; Santana, I.; et al. Evaluation of Human Cerebrospinal Fluid Malate Dehydrogenase 1 as a Marker in Genetic Prion Disease Patients. Biomolecules 2019, 9, 800. [Google Scholar] [CrossRef] [PubMed]
- Abu-Rumeileh, S.; Steinacker, P.; Polischi, B.; Mammana, A.; Bartoletti-Stella, A.; Oeckl, P.; Baiardi, S.; Zenesini, C.; Huss, A.; Cortelli, P.; et al. CSF Biomarkers of Neuroinflammation in Distinct Forms and Subtypes of Neurodegenerative Dementia. Alzheimer’s Res. Ther. 2019, 12, 2. [Google Scholar] [CrossRef] [PubMed]
- Herbst, A.; McIlwain, S.; Schmidt, J.J.; Aiken, J.M.; Page, C.D.; Li, L. Prion Disease Diagnosis by Proteomic Profiling. J. Proteome Res. 2009, 8, 1030–1036. [Google Scholar] [CrossRef][Green Version]
- Foutz, A.; Appleby, B.S.; Hamlin, C.; Liu, X.; Yang, S.; Cohen, Y.; Chen, W.; Blevins, J.; Fausett, C.; Wang, H.; et al. Diagnostic and Prognostic Value of Human Prion Detection in Cerebrospinal Fluid. Ann. Neurol. 2017, 81, 79–92. [Google Scholar] [CrossRef]
- Sano, K.; Satoh, K.; Atarashi, R.; Takashima, H.; Iwasaki, Y.; Yoshida, M.; Sanjo, N.; Murai, H.; Mizusawa, H.; Schmitz, M.; et al. Early Detection of Abnormal Prion Protein in Genetic Human Prion Diseases Now Possible Using Real-Time QUIC Assay. PLoS ONE 2013, 8, e54915. [Google Scholar] [CrossRef]
- Dorey, A.; Tholance, Y.; Vighetto, A.; Perret-Liaudet, A.; Lachman, I.; Krolak-Salmon, P.; Wagner, U.; Struyfs, H.; De Deyn, P.P.; El-Moualij, B.; et al. Association of Cerebrospinal Fluid Prion Protein Levels and the Distinction between Alzheimer Disease and Creutzfeldt-Jakob Disease. JAMA Neurol. 2015, 72, 267–275. [Google Scholar] [CrossRef]
- Vallabh, S.M.; Minikel, E.V.; Williams, V.J.; Carlyle, B.C.; McManus, A.J.; Wennick, C.D.; Bolling, A.; Trombetta, B.A.; Urick, D.; Nobuhara, C.K.; et al. Cerebrospinal Fluid and Plasma Biomarkers in Individuals at Risk for Genetic Prion Disease. BMC Med. 2020, 18, 140. [Google Scholar] [CrossRef]
- Villar-Piqué, A.; Schmitz, M.; Lachmann, I.; Karch, A.; Calero, O.; Stehmann, C.; Sarros, S.; Ladogana, A.; Poleggi, A.; Santana, I.; et al. Cerebrospinal Fluid Total Prion Protein in the Spectrum of Prion Diseases. Mol. Neurobiol. 2019, 56, 2811–2821. [Google Scholar] [CrossRef]
- Rhoads, D.D.; Wrona, A.; Foutz, A.; Blevins, J.; Glisic, K.; Person, M.; Maddox, R.A.; Belay, E.D.; Schonberger, L.B.; Tatsuoka, C.; et al. Diagnosis of Prion Diseases by RT-QuIC Results in Improved Surveillance. Neurology 2020, 95, e1017–e1026. [Google Scholar] [CrossRef] [PubMed]
- Connor, A.; Wang, H.; Appleby, B.S.; Rhoads, D.D. Clinical Laboratory Tests Used to Aid in Diagnosis of Human Prion Disease. J. Clin. Microbiol. 2019, 57, e00769-19. [Google Scholar] [CrossRef] [PubMed]
- Atarashi, R.; Satoh, K.; Sano, K.; Fuse, T.; Yamaguchi, N.; Ishibashi, D.; Matsubara, T.; Nakagaki, T.; Yamanaka, H.; Shirabe, S.; et al. Ultrasensitive Human Prion Detection in Cerebrospinal Fluid by Real-Time Quaking-Induced Conversion. Nat. Med. 2011, 17, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Piconi, G.; Peden, A.H.; Barria, M.A.; Green, A.J.E. Epitope Mapping of the Protease Resistant Products of RT-QuIC Does Not Allow the Discrimination of SCJD Subtypes. PLoS ONE 2019, 14, e0218509. [Google Scholar] [CrossRef]
- Llorens, F.; Kruse, N.; Karch, A.; Schmitz, M.; Zafar, S.; Gotzmann, N.; Sun, T.; Köchy, S.; Knipper, T.; Cramm, M.; et al. Validation of α-Synuclein as a CSF Biomarker for Sporadic Creutzfeldt-Jakob Disease. Mol. Neurobiol. 2018, 55, 2249–2257. [Google Scholar] [CrossRef]
- Orrù, C.D.; Groveman, B.R.; Hughson, A.G.; Manca, M.; Raymond, L.D.; Raymond, G.J.; Campbell, K.J.; Anson, K.J.; Kraus, A.; Caughey, B. RT-QuIC Assays for Prion Disease Detection and Diagnostics. Methods Mol. Biol. 2017, 1658, 185–203. [Google Scholar] [CrossRef]
- Zerr, I.; Villar-Piqué, A.; Hermann, P.; Schmitz, M.; Varges, D.; Ferrer, I.; Riggert, J.; Zetterberg, H.; Blennow, K.; Llorens, F. Diagnostic and Prognostic Value of Plasma Neurofilament Light and Total-Tau in Sporadic Creutzfeldt-Jakob Disease. Alzheimer’s Res. Ther. 2021, 13, 86. [Google Scholar] [CrossRef]
- Steinacker, P.; Blennow, K.; Halbgebauer, S.; Shi, S.; Ruf, V.; Oeckl, P.; Giese, A.; Kuhle, J.; Slivarichova, D.; Zetterberg, H.; et al. Neurofilaments in Blood and CSF for Diagnosis and Prediction of Onset in Creutzfeldt-Jakob Disease. Sci. Rep. 2016, 6, 38737. [Google Scholar] [CrossRef]
- Abu-Rumeileh, S.; Baiardi, S.; Ladogana, A.; Zenesini, C.; Bartoletti-Stella, A.; Poleggi, A.; Mammana, A.; Polischi, B.; Pocchiari, M.; Capellari, S.; et al. Comparison between Plasma and Cerebrospinal Fluid Biomarkers for the Early Diagnosis and Association with Survival in Prion Disease. J. Neurol. Neurosurg. Psychiatry 2020, 91, 1181–1188. [Google Scholar] [CrossRef]
- Thompson, A.G.B.; Anastasiadis, P.; Druyeh, R.; Whitworth, I.; Nayak, A.; Nihat, A.; Mok, T.H.; Rudge, P.; Wadsworth, J.D.F.; Rohrer, J.; et al. Evaluation of Plasma Tau and Neurofilament Light Chain Biomarkers in a 12-Year Clinical Cohort of Human Prion Diseases. Mol. Psychiatry 2021, 26, 5955–5966. [Google Scholar] [CrossRef]
- Abu-Rumeileh, S.; Parchi, P. Cerebrospinal Fluid and Blood Neurofilament Light Chain Protein in Prion Disease and Other Rapidly Progressive Dementias: Current State of the Art. Front. Neurosci. 2021, 15, 648743. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.G.B.; Luk, C.; Heslegrave, A.J.; Zetterberg, H.; Mead, S.H.; Collinge, J.; Jackson, G.S. Neurofilament Light Chain and Tau Concentrations Are Markedly Increased in the Serum of Patients with Sporadic Creutzfeldt-Jakob Disease, and Tau Correlates with Rate of Disease Progression. J. Neurol. Neurosurg. Psychiatry 2018, 89, 955–961. [Google Scholar] [CrossRef] [PubMed]
- Noguchi-Shinohara, M.; Hamaguchi, T.; Nozaki, I.; Sakai, K.; Yamada, M. Serum Tau Protein as a Marker for the Diagnosis of Creutzfeldt-Jakob Disease. J. Neurol. 2011, 258, 1464–1468. [Google Scholar] [CrossRef] [PubMed]
- Villar-Piqué, A.; Schmitz, M.; Hermann, P.; Goebel, S.; Bunck, T.; Varges, D.; Ferrer, I.; Riggert, J.; Llorens, F.; Zerr, I. Plasma YKL-40 in the Spectrum of Neurodegenerative Dementia. J. Neuroinflamm. 2019, 16, 145. [Google Scholar] [CrossRef]
- Norsworthy, P.J.; Thompson, A.G.B.; Mok, T.H.; Guntoro, F.; Dabin, L.C.; Nihat, A.; Paterson, R.W.; Schott, J.M.; Collinge, J.; Mead, S.; et al. A Blood MiRNA Signature Associates with Sporadic Creutzfeldt-Jakob Disease Diagnosis. Nat. Commun. 2020, 11, 3960. [Google Scholar] [CrossRef]
- Llorens, F.; Villar-Piqué, A.; Schmitz, M.; Diaz-Lucena, D.; Wohlhage, M.; Hermann, P.; Goebel, S.; Schmidt, I.; Glatzel, M.; Hauw, J.-J.; et al. Plasma Total Prion Protein as a Potential Biomarker for Neurodegenerative Dementia: Diagnostic Accuracy in the Spectrum of Prion Diseases. Neuropathol. Appl. Neurobiol. 2020, 46, 240–254. [Google Scholar] [CrossRef]
- Behaeghe, O.; Mangelschots, E.; De Vil, B.; Cras, P. A Systematic Review Comparing the Diagnostic Value of 14-3-3 Protein in the Cerebrospinal Fluid, RT-QuIC and RT-QuIC on Nasal Brushing in Sporadic Creutzfeldt-Jakob Disease. Acta Neurol. Belg. 2018, 118, 395–403. [Google Scholar] [CrossRef]
- Di Fede, G.; Giaccone, G.; Salmona, M.; Tagliavini, F. Translational Research in Alzheimer’s and Prion Diseases. J. Alzheimer’s Dis. 2018, 62, 1247–1259. [Google Scholar] [CrossRef]
- López-Pérez, Ó.; Sanz-Rubio, D.; Hernaiz, A.; Betancor, M.; Otero, A.; Castilla, J.; Andréoletti, O.; Badiola, J.J.; Zaragoza, P.; Bolea, R.; et al. Cerebrospinal Fluid and Plasma Small Extracellular Vesicles and MiRNAs as Biomarkers for Prion Diseases. Int. J. Mol. Sci. 2021, 22, 6822. [Google Scholar] [CrossRef]
- Vassileff, N.; Cheng, L.; Hill, A.F. Extracellular Vesicles—Propagators of Neuropathology and Sources of Potential Biomarkers and Therapeutics for Neurodegenerative Diseases. J. Cell Sci. 2020, 133. [Google Scholar] [CrossRef]
- Minikel, E.V.; Vallabh, S.M.; Orseth, M.C.; Brandel, J.-P.; Haïk, S.; Laplanche, J.-L.; Zerr, I.; Parchi, P.; Capellari, S.; Safar, J.; et al. Age at Onset in Genetic Prion Disease and the Design of Preventive Clinical Trials. Neurology 2019, 93, e125–e134. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).