Abstract
Introduction: Cutaneous squamous cell carcinoma (cSCC) is one of the most frequently occurring types of cancer in humans. Scientometric research is an innovative method for analyzing the research trends in various domains, with great implications in the field of medicine. Materials and Methods: We searched the Web of Science database with the following established query terms: “Squamous cell carcinoma”, “skin”, and “immunohistochemistry”. After applying the inclusion and exclusion criteria, a total of 76 articles were selected. The present study aims to analyze, based on the frequency of use of keywords with scientometric algorithms and map-based distributions, the trends of the research concerning cSCCs in 2017–2022. Results: A graphical representation based on 11 scientometric maps presented the division of the keywords into seven clusters, from which seven categories of research interest were defined. The clusters represent a multidisciplinary approach to the diagnosis and treatment of cSCCs, cancer diagnostics, patient outcomes, histopathological importance, management of cSCCs, role of progression, and adequate treatment of and importance of immunohistochemistry for cSCCs. The distribution of the citations shows the importance of the available research on cSCCs by analyzing the first five most-cited articles included in our study in direct concordance with the seven defined clusters. Conclusion: The scientometric research method reveals the interest of research in the multidisciplinary approach used to obtain the best outcomes for the patient, including a targeted investigation, as well as diagnostic and treatment options. The trends in the research reveal that histopathological diagnostics and immunohistochemistry, combined with molecular techniques, are the most important tools used to establish a personalized diagnosis, thus increasing the quality of life and life expectancy for patients with cSCCs.
1. Introduction
Cutaneous squamous skin carcinomas (cSCCs) are part of the non-melanocytic skin cancers (NMSCs) composed of the basal cell carcinoma (BCC), squamous cell carcinoma (cSCC), and metatypical carcinoma. The cSCC is a skin cancer based on the malignant proliferation of cells that show an increased squamous differentiation [1].
Even though, in every pathology department, the histopathological diagnosis of cSCCs is a routine procedure, it is important to mention that the successful diagnosis of these malignant tumors is also based on the immunohistochemistry panel for establishing the squamous origin of these cells.
The research concerning the diagnosis of NMSCs is continuously evolving, and, as a result of this new form of diagnostics and therapy, novel approaches are being discovered. The histopathological diagnosis of cSCCs remains the most important tool in establishing the best treatment and best outcomes for the patient [1,2].
To define the latest research trends for the histopathological and immunohistochemical diagnosis of cSCCs, we perform a scientometric study based on the scientific literature, in order to emphasize the importance as well as the necessity of the research on this kind of pathology.
2. Materials and Methods
We conducted a scientometric research study based on the available scientific literature by searching the Web Of Science (WoS) database. The WoS database was searched by defining our main research items. We established that the main research items were “squamous cell carcinoma”, “skin”, and “immunohistochemistry”. The research items were selected based on the scientometric algorithm of the research in order to organize our research target. In the WoS, the advanced search option was selected, and the query terms were set to “All fields” with the Boolean “and” value. The query for the study was: “((ALL = (Squamous cell carcinoma)) AND ALL = (skin)) AND ALL = (immunohistochemistry)”. Following the interrogation, a total of 983 articles were presented. The inclusion criteria of the articles in the present study were based on certain publication years (2017–2022), access policy (open access), research domain (oncology, pathology, dermatology, and biochemistry molecular biology), and article type (original research). The exclusion criteria were based on the values of the inclusion criteria outside the established range. The PRISMA diagram (Figure 1) shows the workflow for refining our results. After applying all the inclusion and exclusion criteria, a total of 76 articles were selected for further processing throughout our study. For assessing the impact of and to establish the research directions for squamous cell carcinomas, we used the scientometric method [3,4]. A set of instructions was defined based on the keywords from the 76 articles we assessed [5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80]. In order to obtain a valid research database of articles, the citations of the articles included in our study were analyzed with a threshold minimum of 2 citations/articles. The first five articles with the most citations were analyzed. For the graphic representation, data interpretation, and cluster formation, we used the free software VOSviewer (Version 1.6.17 – 2021 – Nees Jan van Eck and Ludo Waltman – Centre for Science and Technology Studies of Leiden University). VOSviewer is free software that can create maps of scientific data by interrogating scientific databases and creating interconnections between different parameters. The data file regarding the selected research articles from the WoS was exported as a plain text file and uploaded onto VOSviewer [81]. The scientometric analysis for our study was based on 2 attributes: the occurrence attribute and the total link strength attribute. The occurrence refers to the count of usage of the keywords in the selected articles. The total link strength indicates the total strength of the keyword links of a given research article with other research articles. Using those two attributes, we have identified the interconnections and generated the scientometric maps.
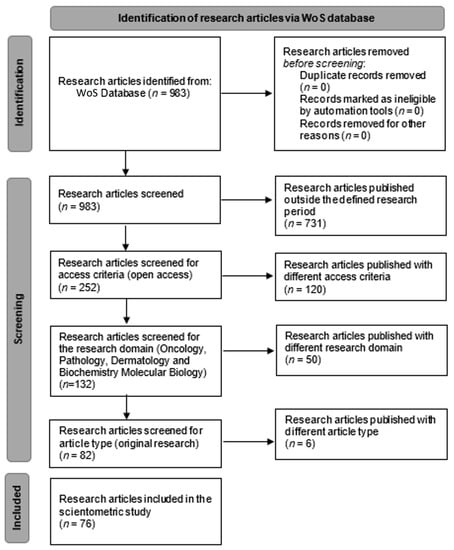
Figure 1.
PRISMA chart of workflow—identification of research articles via WoS database.
3. Results
The data from the selected research articles were uploaded onto VOSviewer and a series of scientometric maps were created based on the keywords obtained from the research articles; the number of occurrences of the keywords was used to represent the relationship between the keywords. A total of 400 keywords were identified from the total of 76 articles. From these keywords, a threshold minimum of two occurrences for each keyword was set. The threshold was met by 77 keywords. The 77 keywords were divided into seven clusters, based on the scientometric algorithms in terms of the occurrences of the keywords and the strength of the connection between them in the selected research articles. All the keywords included in the present study are recognized by the Medical Subject Headings 2022 (MeSH).
4. Cluster Classifications
Cluster 1 is defined by 16 keywords obtained from the selected research articles and is shown in Figure 2 as map-based connections and in Figure 3 as descriptive classifications. Cluster 2 is defined by 15 keywords obtained from the selected research articles and is shown in Figure 2 as map-based connections and in Figure 3 as descriptive classifications. Cluster 3 is defined by 14 keywords obtained from the selected research articles and is shown in Figure 2 as map-based connections and in Figure 3 as descriptive classifications. Cluster 4 is defined by 9 keywords obtained from the selected research articles and is shown in Figure 2 as map-based connections and in Figure 3 as descriptive classifications. Cluster 5 is defined by 10 keywords obtained from the selected research articles and is shown in Figure 2 as map-based connections and in Figure 3 as descriptive classifications. Cluster 6 is defined by 7 keywords obtained from the selected research articles and is shown in Figure 2 as map-based connections and in Figure 3 as descriptive classifications. Cluster 7 is defined by 1 keyword obtained from the selected research articles and is shown in Figure 2 as a map-based connection and in Figure 3 as a descriptive classification.
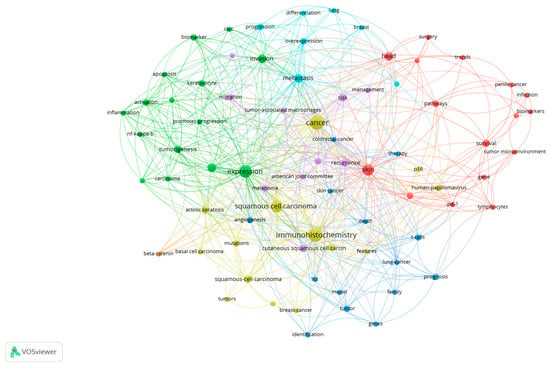
Figure 2.
The scientometric map based on the keywords analyzed from the selected research articles *. (* Colors observed in Figure 2 refer to the defined clusters. Legend of used colors: Cluster 1—red, Cluster 2—green, Cluster 3—blue, Cluster 4—Yellow, Cluster 5—purple, Cluster 6—turquoise, Cluster 7—orange).
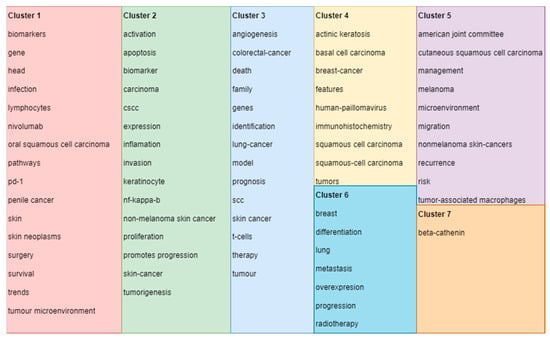
Figure 3.
Clusters’ descriptive classifications of keywords based on the scientometric analysis *. (* Colors observed in Figure 3 refer to the defined clusters. Legend of used colors: Cluster 1—red, Cluster 2—green, Cluster 3—blue, Cluster 4—Yellow, Cluster 5—purple, Cluster 6—turquoise, Cluster 7—orange).
5. Density of the Research Tendency Based on Used Keywords in the Selected Articles
Figure 4 shows the density of keywords used in the selected articles based on their occurrence in the selected articles. The intensity of the yellow–green colors shows the frequency of use of the keywords.
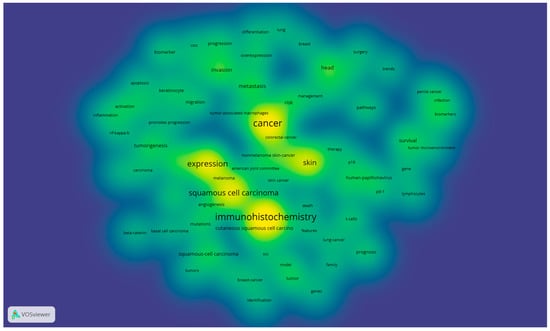
Figure 4.
Density of the main research tendency based on used keywords in the selected articles *. (* Color legend: The intensity of the yellow–green colors shows the frequency of use of the keywords).
6. Keyword Dynamics
The 77 examined keywords were analyzed based on the occurrence and total link strength. Table 1 shows a descriptive statistic of the two analyzed parameters.

Table 1.
Descriptive statistics of the occurrences and total link strength of keywords.
Based on the occurrences of the keywords, a set of the first 10 most commonly used keywords was selected and presented in Figure 5.
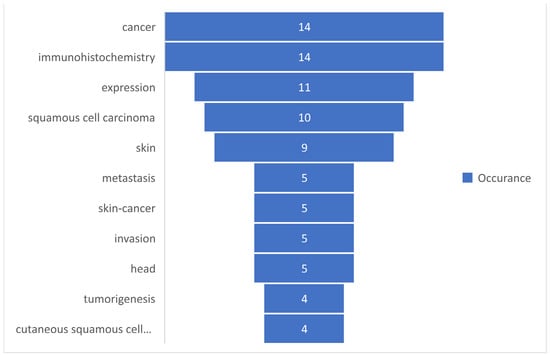
Figure 5.
The most commonly used keywords based on occurrences.
Based on the total link strength, a set of the first 10 most commonly used keywords was selected and presented in Figure 6.
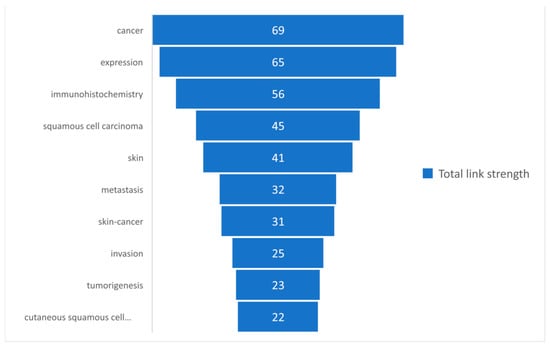
Figure 6.
The most commonly used keywords based on the total link strength.
7. Density of Citations in the WoS of the Selected Articles
Figure 7 presents the citation density of the selected research articles from the WoS. The intensity of the yellow–green colors represents the higher number of citations for the most-cited research articles out of those that were selected.
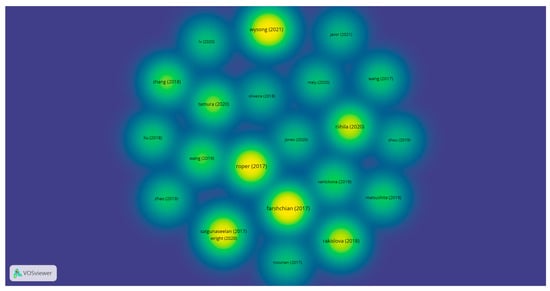
Figure 7.
The citation density of the selected research articles from WoS *. (* Color legend: The intensity of the yellow–green colors represents the higher number of citations for the most-cited research articles out of those that were selected).
8. Discussion
Squamous cell carcinomas (cSCCs) of the skin are one of the most frequently non-melanocytic skin tumors found in humans, after basal cell carcinoma [82]. Due to this fact, a series of parameters starting from clinical appearance, diagnosis, and treatment are continuously being studied to achieve the best outcomes for the patient.
The diagnosis is established mostly on the histopathological excision of the tumor, which is investigated by hematoxylin–eosin (H and E) staining and by immunohistochemistry assets. Immunohistochemistry is constantly developing, so scientific research into the panel, which is best used for the histopathological diagnosis of cSCCs, is always important.
To establish novel histopathological diagnostics, our study initially observed the main points of interest in cSCC research and combined this with immunohistochemistry. For this approach, we used the scientometric method of research. The ideology of this research method is to identify the development and transmission of scientific literature in order to analyze the most up-to-date trends in the field of interest, by using the parameters offered by scientific databases such as Web of Science.
Our study was based on defining the scientometric map based on the investigation of the keywords extracted from the WoS database by the criteria shown in the Section 2. After selecting all the parameters, the scientometric map presented in Figure 2 was generated based on the keywords that met the selected threshold. A total of seven clusters were defined by intercalating the occurrence and total link strength attributes of the analyzed keywords. The clusters defined the seven categories of research interest.
Cluster 1, composed of 16 keywords, shows the strength of the multidisciplinary approach used for cSCCs based on the following keywords: biomarkers, infection, gene, and skin. It also shows direct connections with survival, treatment options (surgery), and trends in finding new perspectives on cSCC management. Cluster 2, composed of 15 keywords, targets cancer diagnostics by establishing a direct connection between the type of cancer, in this case non-melanoma skin cancers; the category in which cSCCs are present; and presenting cell characteristics, such as apoptosis, invasion, keratinocytes, and tumor genesis. Cluster 3 presents a personal and psychological approach, in terms of the patient’s outcome, by selecting and establishing connections between different types of cancers and keywords, such as death and family. By including the keyword “therapy” in this cluster, a greater research interest in the patient outcomes and social integration can be observed. Cluster 4, from the histopathological point of view, defines the intercalation in the research with other non-melanocytic skin cancers, such as basal cell carcinomas (BCCs) of the skin, introducing the necessity of targeted immunohistochemistry for those types of cancers. Regarding cluster 5, it can be observed that the management of cSCCs remains of extremely high interest in research based on the management and recurrence of keywords and according to the American Joint Committee on Cancer. Nevertheless, cluster 6 establishes the role of progression and adequate therapy for this type of cancer in order to avoid metastasis as much as possible, which can affect the breast or even the lungs. Even though cluster 7 is formed by only one keyword, beta-catenin, it has an increased total link strength, which means that it has many connections with other keywords, and by this, it highlights an important research gateway for immunohistochemistry in the diagnosis of cSCCs. As shown in Figure 2 and Figure 3, the clusters observed on the scientometric map have a good distribution, marked by intensive connections between the keyword analyzed.
The density of the main research tendency based on the used keywords in the selected articles is presented in Figure 4, where we can observe that the interest of the scientific research concentrates on skin, cancer, expression, and immunohistochemistry, which justifies the necessity of a correct and good histopathological diagnosis based on adequate IHC panels. Nevertheless, the importance of the multidisciplinary approach to produce a good diagnosis is highlighted by the other keywords that are emerging from the most evident ones.
The keywords-review approach was based on the number of occurrences of each keyword, which was analyzed in the present study, and by the total link strength of those words. A descriptive statistic was presented to observe the tendency of using those keywords. It can be observed in Table 1 that a total of 77 keywords were analyzed, for which the maximum occurrence was 14 and the minimum was 2, with a mean rate of occurrence of 3.16. Regarding the total length strength, the minimum length strength was 3, whereas the maximum was 79. Figure 5 and Figure 6 present the 10 most frequently used keywords that had the highest occurrence and greatest total link strength. It can be observed in Figure 5 that cancer and immunohistochemistry are the most commonly used keywords, followed by expression, squamous cell carcinoma, and skin. Although our research query was defined by skin, immunohistochemistry, and squamous cell carcinoma, the keywords “cancer” and “expression” show that, in terms of the research, a great interest is shown in this type of cancer. The knowledge concerning the total link strength is changing in comparison to the occurrences, but the first four links are maintained, as shown in Figure 6. This is explained by the considerable interest of the research in this domain, based on immunohistochemistry and the expression of different parameters of squamous cell carcinomas.
In terms of citations, the density of the citations is represented in Figure 7. By analyzing the conclusions obtained for the selected research articles, our research clusters are supported by the relevant research articles. Our cluster identification and interpretation are validated by the support of the first most-cited research article. According to Satgunaseelan et al., the immunohistochemical assessment of head and neck cutaneous squamous cell carcinomas with p16 is not associated with a better prognosis. The management of the diagnosis and treatment of SCCs must be in accordance with p16 IHC expression [31]. Riihila et al. demonstrated in their study the importance of complement components C1r and C1s in cutaneous squamous cell carcinomas in terms of progression. This highlights the importance of targeted novel treatments for cSCCs [43]. Roper et al. showed that PD-L1 expression is usually observed in cSCC primary tumors and in TILs (tumor-infiltrating lymphocytes). Additionally, an expression > 5% in primary tumor cells and primary and metastatic TILs is associated with better survival rates in head and neck cSCCs. [16] Wysong et al. demonstrated that their 40-GEP genetic prognostic test, correlated with clinical and pathological findings, may improve the quality of life for the patient, presenting better patient outcomes and adequate treatment and diagnostics [35]. The results presented by Farshchian et al. in their study show the importance of AIM2 in considering the progression of cSCCs. This can lead to potential targeted therapeutic agents in primary invasive and metastatic cSCCs [32].
9. Conclusions
The research interest in studying squamous cell carcinomas (cSCCs) of the skin remains high, as it seeks to target new approaches in the diagnosis and treatment of this pathology. The scientometric research method revealed the necessity of a multidisciplinary approach for attaining the best outcomes for the patient. The identified scientific research directions of and interests in histopathology and immunohistochemistry in terms of diagnostics remain the most important tools used to develop a personalized diagnosis, thus increasing the quality of life and life expectancy for the patients. The limitations of the present study are represented by the fact that only the Web of Science (WoS) database was analyzed in our study.
Author Contributions
Conceptualization, I.G.C. and A.-C.T.; Formal analysis, V.V.; Investigation, A.R., A.-H.S. and A.R.S.; Methodology, R.N., V.V., M.C.P. and R.M.; Resources, I.G.C., A.-H.S. and T.C.C.; Supervision, M.E.C., A.R., A.R.S. and O.S.C.; Validation, M.E.C. and O.S.C.; Visualization, I.G.C. and R.N.; Writing—original draft, I.G.C., A.-C.T., C.E.B. and T.C.C.; Writing—review & editing, I.G.C., C.E.B., M.C.P. and R.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data produced here are available and can be produced upon request.
Acknowledgments
This article is part of a PhD thesis from the Doctoral School of Medicine and Pharmacy within the University of Medicine, Pharmacy, Sciences, and Technology “George Emil Palade” of Targu Mures, titled “Study of the epidemiological, histological and immunohistochemical aspects of non-melanocytic skin cancers”, which will be presented by Iuliu Gabriel Cocuz in 2022.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Elder, D.E.; Massi, D.; Scolyer, R.A.; Willemze, R.; Cancer, L.; Al, E. WHO Classification of Skin Tumours; International Agency for Research on Cancer: Lyon, France, 2018; ISBN 9789283224402. [Google Scholar]
- Caruntu, A.; Moraru, L.; Lupu, M.; Ciubotaru, D.A.; Dumitrescu, M.; Eftimie, L.; Hertzog, R.; Zurac, S.; Caruntu, C.; Voinea, O.C. Assessment of Histological Features in Squamous Cell Carcinoma Involving Head and Neck Skin and Mucosa. J. Clin. Med. 2021, 10, 2343. [Google Scholar] [CrossRef] [PubMed]
- Bucătaru, C.; Săvescu, D.; Repanovici, A.; Blaga, L.; Coman, E.; Cocuz, M.-E. The Implications and Effects of Medical Waste on Development of Sustainable Society—A Brief Review of the Literature. Sustainability 2021, 13, 3300. [Google Scholar] [CrossRef]
- Pantea, I.; Repanovici, A.; Cocuz, M.E. Analysis of Research Directions on the Rehabilitation of Patients with Stroke and Diabetes Using Scientometric Methods. Healthcare 2022, 10, 773. [Google Scholar] [CrossRef] [PubMed]
- Ramezani, M.; Masnadjam, M.; Azizi, A.; Zavattaro, E.; Khazaei, S.; Sadeghi, M. Evaluation of C-Kit (CD117) Expression in Patients with Squamous Cell Carcinoma (SCC) and Basal Cell Carcinoma (BCC) of the Skin. Aims Mol. Sci. 2021, 8, 51–59. [Google Scholar] [CrossRef]
- Vuletic, M.; Jancic, S.; Milenkovic, S.; Paunovic, M.; Milicic, B.; Jancic, N.; Perunicic, B.; Slovic, Z. Clinical -Pathological Significance of Leptin Receptor (LEPR) Expression in Squamous Cell Carcinoma of the Skin. Pathol. Res. Pract. 2020, 216, 153111. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.C.; Nóbrega, V.C.; Marques, M.E.A. Lymphoepithelioma-like Carcinoma of the Skin. An. Bras. Dermatol. 2018, 93, 256–258. [Google Scholar] [CrossRef]
- Ling, B.; Yao, M.; Li, G.; Liu, J.; Liu, B.; Wang, W.; Jiang, B. Clinical Significance of Ring Finger Protein 2 High Expression in Skin Squamous Cell Carcinoma. Oncol. Lett. 2020, 20, 1111–1118. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.L.; Suarez-Bonnet, A.; Mitchell, J.A.; Ramirez, G.A.; Stidworthy, M.F.; Priestnall, S.L. Avian Papilloma and Squamous Cell Carcinoma: A Histopathological, Immunohistochemical and Virological Study. J. Comp. Pathol. 2020, 175, 13–23. [Google Scholar] [CrossRef]
- Qi, J.; Hu, Z.; Xiao, H.; Liu, R.; Guo, W.; Yang, Z.; Ma, K.; Su, S.; Tang, P.; Zhou, X.; et al. SOX10-A Novel Marker for the Differential Diagnosis of Breast Metaplastic Squamous Cell Carcinoma. Cancer Manag. Res. 2020, 12, 4039–4044. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, P.; Wang, X.; Shi, L.; Fan, Z.; Zhang, G.; Yang, D.; Bahavar, C.F.; Zhou, F.; Chen, W.R.; et al. Antitumor Effects of DC Vaccine with ALA-PDT-Induced Immunogenic Apoptotic Cells for Skin Squamous Cell Carcinoma Mice. Technol. Cancer Res. Treat. 2018, 17. [Google Scholar] [CrossRef] [PubMed]
- Tugrul, B.; Soylev, S.; Temiz, P.; Gencoglan, G. Investigation of Effect of Vitamin D Receptor, Calcium-Sensing Receptor and Beta-Catenin on Cutaneous Squamous Cell Carcinoma. Turk. J. Biochem. Turk Biyokim. Derg. 2020, 45, 91–98. [Google Scholar] [CrossRef]
- Matsushita, M.; Iwasaki, T.; Wardhani, L.O.; Kuwamoto, S.; Nonaka, D.; Nagata, K.; Kato, M.; Kitamura, Y.; Hayashi, K. Decreased H3K27me3 Expression Is Associated with Merkel Cell Polyomavirus-Negative Merkel Cell Carcinoma, Especially Combined with Cutaneous Squamous Cell Carcinoma. Anticancer Res. 2019, 39, 5573–5579. [Google Scholar] [CrossRef] [PubMed]
- Kakabadze, M.; Paresishvili, T.; Mardaleishvili, K.; Vadachkoria, Z.; Kipshidze, N.; Jangavadze, M.; Karalashvili, L.; Ghambashidze, K.; Chakhunashvili, D.; Kakabadze, Z. Local Drug Delivery System for the Treatment of Tongue Squamous Cell Carcinoma in Rats. Oncol. Lett. 2022, 23, 13. [Google Scholar] [CrossRef] [PubMed]
- Roper, E.; Lum, T.; Palme, C.E.; Ashford, B.; Ch’ng, S.; Ranson, M.; Boyer, M.; Clark, J.; Gupta, R. PD-L1 Expression Predicts Longer Disease Free Survival in High Risk Head and Neck Cutaneous Squamous Cell Carcinoma. Pathology 2017, 49, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Tervo, S.; Seppala, M.; Rautiainen, M.; Huhtala, H.; Salo, T.; Al-Samadi, A.; Kuopio, T.; Ahtiainen, M.; Tommola, S.; Paavonen, T.; et al. The Expression and Prognostic Relevance of Programmed Cell Death Protein 1 in Tongue Squamous Cell Carcinoma. Apmis 2020, 128, 626–636. [Google Scholar] [CrossRef]
- Farshchian, M.; Nissinen, L.; Siljamaki, E.; Riihila, P.; Piipponen, M.; Kivisaari, A.; Kallajoki, M.; Grenman, R.; Peltonen, J.; Peltonen, S.; et al. Tumor Cell-Specific AIM2 Regulates Growth and Invasion of Cutaneous Squamous Cell Carcinoma. Oncotarget 2017, 8, 45825–45836. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Bae, J.Y.; Zheng, Z.; Park, H.S.; Chung, K.Y.; Roh, M.R.; Jin, Z. Overexpression and Implications of Melanoma-Associated Antigen A12 in Pathogenesis of Human Cutaneous Squamous Cell Carcinoma. Anticancer Res. 2019, 39, 1849–1857. [Google Scholar] [CrossRef]
- Peng, P.; Xiao, Y.; Zhao, Z.; Sun, C.; Wu, D.; Chen, Y.; Zhang, L. Treatment beyond Progression with Chemo-Immunotherapy in an Advanced Esophageal Squamous Cell Carcinoma Patient: A Case Report. Transl. Cancer Res. 2021, 10, 4973–4978. [Google Scholar] [CrossRef]
- Wang, X.; Tao, C.; Yuan, C.; Ren, J.; Yang, M.; Ying, H. AQP3 Small Interfering RNA and PLD2 Small Interfering RNA Inhibit the Proliferation and Promote the Apoptosis of Squamous Cell Carcinoma. Mol. Med. Rep. 2017, 16, 1964–1972. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rakislova, N.; Alemany, L.; Clavero, O.; del Pino, M.; Saco, A.; Quiros, B.; Lloveras, B.; Alejo, M.; Halec, G.; Quint, W.; et al. Differentiated Vulvar Intraepithelial Neoplasia-like and Lichen Sclerosus-like Lesions in HPV-Associated Squamous Cell Carcinomas of the Vulva. Am. J. Surg. Pathol. 2018, 42, 828–835. [Google Scholar] [CrossRef] [PubMed]
- Vanickova, L.; Guran, R.; Kollar, S.; Emri, G.; Krizkova, S.; Do, T.; Heger, Z.; Zitka, O.; Adam, V. Mass Spectrometric Imaging of Cysteine Rich Proteins in Human Skin. Int. J. Biol. Macromol. 2019, 125, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Maly, C.J.; Cumsky, H.J.L.; Costello, C.M.; Schmidt, J.E.; Butterfield, R.J.; Zhang, N.; DiCaudo, D.J.; Nelson, S.A.; Smith, M.L.; Ochoa, S.A.; et al. Prognostic Value of Inositol Polyphosphate-5-Phosphatase Expression in Recurrent and Metastatic Cutaneous Squamous Cell Carcinoma. J. Am. Acad. Dermatol. 2020, 82, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, J.; Sun, J.; Liu, Y.; Liu, D.; Du, L.; Wang, B.; Liu, W. Expression of RAD51 and Its Clinical Impact in Oral Squamous Cell Carcinoma. Anal. Cell. Pathol. 2020, 2020, 1827676. [Google Scholar] [CrossRef] [PubMed]
- Koletsa, T.; Petrakis, G.; Karayannopoulou, G.; Euvrard, S.; Kanitakis, J. MTOR Signalling Pathway-Protein Expression in Post-Transplant Cutaneous Squamous-Cell Carcinomas before and after Conversion to MTOR-Inhibitors. Anticancer Res. 2018, 38, 3319–3322. [Google Scholar] [CrossRef] [PubMed]
- Fortugno, P.; Condorelli, A.G.; Dellambra, E.; Guerra, L.; Cianfarani, F.; Tinaburri, L.; Proto, V.; Luca, D.; Passarelli, F.; Ricci, F.; et al. Multiple Skin Squamous Cell Carcinomas in Junctional Epidermolysis Bullosa due to Altered Laminin-332 Function. Int. J. Mol. Sci. 2020, 21, 1426. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.; Coltart, G.; Shapanis, A.; Healy, C.; Alabdulkareem, A.; Selvendran, S.; Theaker, J.; Sommerlad, M.; Rose-Zerilli, M.; Al-Shamkhani, A.; et al. CD8+CD103+Tissue-Resident Memory T Cells Convey Reduced Protective Immunity in Cutaneous Squamous Cell Carcinoma. J. Immunother. Cancer 2021, 9, e001807. [Google Scholar] [CrossRef] [PubMed]
- Wright, M.F.; Weiss, V.L.; Jr, L.; Schmitz, J.E.; Ely, K.A. Determination of High-Risk HPV Status of Head and Neck Squamous Cell Carcinoma Using the Roche Cobas HPV Test on Cytologic Specimens and Acellular Supernatant Fluid. Cancer Cytopathol. 2020, 128, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Haase, C.; Lethaus, B.; Knuechel-Clarke, R.; Hoelzle, F.; Cassataro, A.; Braunschweig, T. Development of a Rapid Analysis Method for Bone Resection Margins for Oral Squamous Cell Carcinoma by Immunoblotting. Head Neck Pathol. 2018, 12, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Satgunaseelan, L.; Chia, N.; Suh, H.; Virk, S.; Ashford, B.; Lum, T.; Ranson, M.; Clark, J.; Gupta, R. P16 Expression in Cutaneous Squamous Cell Carcinoma of the Head and Neck Is Not Associated with Integration of High Risk HPV DNA or Prognosis. Pathology 2017, 49, 494–498. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Li, G.; Wang, D.; Sun, X.; Li, X. Cytokeratin Expression in Epidermal Stem Cells in Skin Adnexal Tumors. Oncol. Lett. 2019, 17, 927–932. [Google Scholar] [CrossRef] [PubMed]
- Nissinen, L.; Siljamaki, E.; Riihila, P.; Piipponen, M.; Farshchian, M.; Kivisaari, A.; Kallajoki, M.; Raiko, L.; Peltonen, J.; Peltonen, S.; et al. Expression of Claudin-11 by Tumor Cells in Cutaneous Squamous Cell Carcinoma Is Dependent on the Activity of P38 Delta. Exp. Dermatol. 2017, 26, 771–777. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Wu, K.; Qin, X.; Yuan, J.; Yan, M.; Zhang, J.; Wang, L.; Ji, T.; Cao, W.; Chen, W. A Novel Tumor Suppressor SPINK5 Serves as an Independent Prognostic Predictor for Patients with Head and Neck Squamous Cell Carcinoma. Cancer Manag. Res. 2020, 12, 4855–4869. [Google Scholar] [CrossRef] [PubMed]
- Wysong, A.; Newman, J.G.; Covington, K.R.; Kurley, S.J.; Ibrahim, S.F.; Farberg, A.S.; Bar, A.; Cleaver, N.J.; Somani, A.-K.; Panther, D.; et al. Validation of a 40-Gene Expression Profile Test to Predict Metastatic Risk in Localized High-Risk Cutaneous Squamous Cell Carcinoma. J. Am. Acad. Dermatol. 2021, 84, 361–369. [Google Scholar] [CrossRef]
- Ackermann, K.; Wallner, S.; Brochhausen, C.; Schreml, S. Expression Profiles of ASIC1/2 and TRPV1/4 in Common Skin Tumors. Int. J. Mol. Sci. 2021, 22, 6024. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Guo, Y.; Huang, Y.; Xue, H.; Bai, S.; Zhu, J.; Xia, X.; Shen, B.; Fang, W. Effects of Insulin-like Growth Factor Binding Protein 3 on Apoptosis of Cutaneous Squamous Cell Carcinoma Cells. Oncotargets Ther. 2018, 11, 6569–6576. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Cao, Y.; Wang, G.; Luo, Z. Expression of FOXC2, PinX1, Ki-67 and Cyclin D1 in Cutaneous Cell Carcinoma. Oncol. Lett. 2017, 14, 635–638. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, K.; Chen, Y.; Zhou, J.; Liang, Y.; Yang, X.; Li, X.; Cao, Y.; Wang, D.; Luo, L.; et al. Mutational Landscape of Penile Squamous Cell Carcinoma in a Chinese Population. Int. J. Cancer 2019, 145, 1280–1289. [Google Scholar] [CrossRef]
- Hu, M.; Tang, Y.; Long, G.; Zhang, D.; Kresak, J.L.; Lai, J. Primary Extracranial Meningioma of Mastoid in a Patient with History of Skin Squamous Cell Carcinoma, Lung Adenocarcinoma and Prostatic Carcinoma. Anticancer Res. 2019, 39, 3197–3201. [Google Scholar] [CrossRef] [PubMed]
- Huisman, B.W.; Cankat, M.; Bosse, T.; Vahrmeijer, A.L.; Rissmann, R.; Burggraaf, J.; Sier, C.F.M.; Poelgeest, M.I.E.V. Poelgeest Integrin Alpha v Beta 6 as a Target for Tumor-Specific Imaging of Vulvar Squamous Cell Carcinoma and Adjacent Premalignant Lesions. Cancers 2021, 13, 6006. [Google Scholar] [CrossRef]
- Alameda, J.P.; Garcia-Garcia, V.A.; Lopez, S.; Hernando, A.; Page, A.; Navarro, M.; Moreno-Maldonado, R.; Paramio, J.M.; Ramirez, A.; Garcia-Fernandez, R.A.; et al. CYLD Inhibits the Development of Skin Squamous Cell Tumors in Immunocompetent Mice. Int. J. Mol. Sci. 2021, 22, 6736. [Google Scholar] [CrossRef]
- Riihila, P.; Viiklepp, K.; Nissinen, L.; Farshchian, M.; Kallajoki, M.; Kivisaari, A.; Meri, S.; Peltonen, J.; Peltonen, S.; Kähäri, V.-M. Tumour-Cell-Derived Complement Components C1r and C1s Promote Growth of Cutaneous Squamous Cell Carcinoma. Br. J. Dermatol. 2020, 182, 658–670. [Google Scholar] [CrossRef] [PubMed]
- Miola, A.C.; Castilho, M.A.; Schmitt, J.V.; Esther, M. Helio Amante Contribution to Characterization of Skin Field Cancerization Activity: Morphometric, Chromatin Texture, Proliferation, and Apoptosis Aspects. An. Bras. Dermatol. 2019, 94, 698–703. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhou, Y.; Li, C.; Xu, S.; Li, M.; Liu, W.; Ma, Y.; Wang, H. The Expression and Prognostic Value of FGF2, FGFR3, and FGFBP1 in Esophageal Squamous Cell Carcinoma. Anal. Cell. Pathol. 2020, 2020, 2872479. [Google Scholar] [CrossRef] [PubMed]
- Gaitskell, K.; Nassar, S.; Ibrahim, H. Merkel Cell Carcinoma with Divergent Differentiation. Clin. Exp. Dermatol. 2020, 45, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Javor, S.; Gasparini, G.; Biatta, C.M.; Cozzani, E.; Cabiddu, F.; Ravetti, J.L.; Vellone, V.G.; Parodi, A. P53 Staining Index and Zonal Staining Patterns in Actinic Keratoses. Arch. Dermatol. Res. 2021, 313, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Takashima, Y.; Murakami, T.; Inoue, T.; Hagiyama, M.; Yoneshige, A.; Nishimura, S.; Akagi, M.; Ito, A. Manifestation of Osteoblastic Phenotypes in the Sarcomatous Component of Epithelial Carcinoma and Sarcomatoid Carcinoma. Tumor Biol. 2017, 39. [Google Scholar] [CrossRef]
- Tamura, R.; Yoshihara, K.; Nakaoka, H.; Yachida, N.; Yamaguchi, M.; Suda, K.; Ishiguro, T.; Nishino, K.; Ichikawa, H.; Homma, K.; et al. XCL1 Expression Correlates with CD8-Positive T Cells Infiltration and PD-L1 Expression in Squamous Cell Carcinoma Arising from Mature Cystic Teratoma of the Ovary. Oncogene 2020, 39, 3541–3554. [Google Scholar] [CrossRef]
- Caley, M.P.; Martins, V.L.; Moore, K.; Lashari, M.; Nissinen, L.; Kahari, V.-M.; Alexander, S.; Jones, E.; Harwood, C.A.; Jones, J.; et al. Loss of the Laminin Subunit Alpha-3 Induces Cell Invasion and Macrophage Infiltration in Cutaneous Squamous Cell Carcinoma. Br. J. Dermatol. 2021, 184, 923–934. [Google Scholar] [CrossRef]
- Yu, L.; Liu, J.; Zhang, T.-D.; Zheng, X.-F.; Luo, D.-L.; Zhu, W.-L.; Qiu, X.-W.; Guo, L.-L. Decreased TMEM40 Expression Is Associated with Malignant Behavior of Cutaneous Squamous Cell Carcinoma and Inhibits Tumor Progression. Oncol. Lett. 2021, 22, 606. [Google Scholar] [CrossRef]
- Guerrero-Aspizua, S.; Gonzalez-Masa, A.; Conti, C.J.; Garcia, M.; Chacon-Solano, E.; Larcher, F. Rio Humanization of Tumor Stroma by Tissue Engineering as a Tool to Improve Squamous Cell Carcinoma Xenograft. Int. J. Mol. Sci. 2020, 21, 1951. [Google Scholar] [CrossRef]
- Harms, P.W.; Verhaegen, M.E.; Hu, K.; Hrycaj, S.M.; Chan, M.P.; Liu, C.-J.; Grachtchouk, M.; Patel, R.M.; Udager, A.M.; Dlugosz, A.A. Genomic Evidence Suggests That Cutaneous Neuroendocrine Carcinomas Can Arise from Squamous Dysplastic Precursors. Mod. Pathol. 2022, 35, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Ruiz, E.; Toll, A.; Garcia-Diez, I.; Andrades, E.; Ferrandiz-Pulido, C.; Masferrer, E.; Yebenes, M.; Jaka, A.; Gimeno, J.; Gimeno, R.; et al. The Polycomb Proteins RING1B and EZH2 Repress the Tumoral Pro-Inflammatory Function in Metastasizing Primary Cutaneous Squamous Cell Carcinoma. Carcinogenesis 2018, 39, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Kim, J.-H.; Han, J.H.; Cho, N.H.; Kim, S.J.; Kim, S.I.; Choo, S.H.; Kim, J.S.; Park, B.; Kwon, J.E. TERT Promoter Mutations in Penile Squamous Cell Carcinoma: High Frequency in Non-HPV-Related Type and Association with Favorable Clinicopathologic Features. J. Cancer Res. Clin. Oncol. 2021, 147, 1125–1135. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Ballah, T.; Nottage, M.; Hay, K.; Chua, B.; Kenny, L.; Thomas, P.; Teng, M.; Keller, J.; Le, T.; et al. A Prospective Study Investigating the Efficacy and Toxicity of Definitive ChemoRadiation and ImmunOtherapy (CRIO) in Locally And/or Regionally Advanced Unresectable Cutaneous Squamous Cell Carcinoma. Radiat. Oncol. 2021, 16, 69. [Google Scholar] [CrossRef] [PubMed]
- Guimera, N.; Alemany, L.; Halec, G.; Pawlita, M.; Wain, G.V.; Santos, J.; Azike, J.E.; Jenkins, D.; de Sanjose, S.; Quint, W.; et al. Human Papillomavirus 16 Is an Aetiological Factor of Scrotal Cancer. Br. J. Cancer 2017, 116, 1218–1222. [Google Scholar] [CrossRef]
- Chang, C.-T.; Soo, W.-N.; Chen, Y.-H.; Shyur, L.-F. Essential Oil of Mentha Aquatica Var. Kenting Water Mint Suppresses Two-Stage Skin Carcinogenesis Accelerated by BRAF Inhibitor Vemurafenib. Molecules 2019, 24, 2344. [Google Scholar] [CrossRef]
- Kok, M.K.; Chambers, J.K.; Ong, S.M.; Nakayama, H.; Uchida, K. Hierarchical Cluster Analysis of Cytokeratins and Stem Cell Expression Profiles of Canine Cutaneous Epithelial Tumors. Vet. Pathol. 2018, 55, 821–837. [Google Scholar] [CrossRef]
- Ghiciuc, C.M.; Strat, A.L.; Ochiuz, L.; Lupusoru, C.E.; Ignat, M.; Vasile, A.; Grigorovici, A.; Stoleriu, I.; Solcan, C.I. Inhibition of Bcl-2 and Cox-2 Protein Expression after Local Application of a New Carmustine-Loaded Clinoptilolite-Based Delivery System in a Chemically Induced Skin Cancer Model in Mice. Molecules 2017, 22, 2014. [Google Scholar] [CrossRef]
- Kim, S.A.; Ryu, Y.W.; Kwon, J.I.; Choe, M.S.; Jung, J.W.; Cho, J.W. Differential Expression of Cyclin D1, Ki-67, PRb, and P53 in Psoriatic Skin Lesions and Normal Skin. Mol. Med. Rep. 2018, 17, 735–742. [Google Scholar] [CrossRef]
- Rita, T.; Tizuko, C.; Cardili, L.; Ribeiro, D.A.; Silva, M.S.; Korinfsky, J.P.; Plapler, H. The Role of Dimethoate and UV-B on Skin of Wistar Rats. Anticancer Res. 2019, 39, 5179–5184. [Google Scholar] [CrossRef]
- Peng, W.; Bruijn, H.S.D.; Hagen, T.L.M.T.; Dam, G.M.V.; Roodenburg, J.L.N.; Berg, K.; Witjes, M.J.H.; Robinson, D.J. Targeted Photodynamic Therapy of Human Head and Neck Squamous Cell Carcinoma with Anti-Epidermal Growth Factor Receptor Antibody Cetuximab and Photosensitizer IR700DX in the Mouse Skin-Fold Window Chamber Model. Photochem. Photobiol. 2020, 96, 708–717. [Google Scholar] [CrossRef] [PubMed]
- Brenn, T. Soft Tissue Special Issue: Cutaneous Pleomorphic Spindle Cell Tumors. Head Neck Pathol. 2020, 14, 109–120. [Google Scholar] [CrossRef]
- Menz, A.; Bauer, R.; Kluth, M.; Marie, C.; Gorbokon, N.; Viehweger, F.; Lennartz, M.; Volkl, C.; Fraune, C.; Uhlig, R.; et al. Diagnostic and Prognostic Impact of Cytokeratin 19 Expression Analysis in Human Tumors: A Tissue Microarray Study of 13,172 Tumors. Hum. Pathol. 2021, 115, 19–36. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Lee, K.H.; Lee, G.K.; Lee, S.-H.; Lim, K.Y.; Joo, J.; Go, Y.J.; Lee, J.S.; Han, J.-Y. Randomized Phase II Study of Afatinib plus Simvastatin versus Afatinib Alone in Previously Treated Patients with Advanced Nonadenocarcinomatous Non-Small Cell Lung Cancer. Cancer Res. Treat. 2017, 49, 1001–1011. [Google Scholar] [CrossRef] [PubMed]
- Niu, D.; Bai, Y.; Yao, Q.; Hou, W.; Zhou, L.; Huang, X.; Zhao, C. Expression and Significance of AQP3 in Cutaneous Lesions. Anal. Cell. Pathol. 2021, 2021, 7866471. [Google Scholar] [CrossRef] [PubMed]
- Zolfaghari, M.A.; Karimi, A.; Kalantari, E.; Korourian, A.; Ghanadan, A.; Kamyab, K.; Esmaili, N.; Razavi, A.N.E.; Madjd, Z. A Comparative Study of Long Interspersed Element-1 Protein Immunoreactivity in Cutaneous Malignancies. BMC Cancer 2020, 20, 567. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-F.; Shen, D.-F.; Zhao, S.; Ren, T.-R.; Gao, Y.; Shi, S.; Wu, J.-C.; Sun, H.-Z.; Zheng, H.-C. Expression Pattern and Level of ING5 Protein in Normal and Cancer Tissues. Oncol. Lett. 2019, 17, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Tabata, S.; Murata, M.; Takasawa, A.; Fukuda, A.; Ogasawara, J.; Koseki, T.; Nakano, K.; Segawa, K.; Morita, R.; Hasegawa, T.; et al. Cytological Findings of Langerhans Cell Sarcoma in a Case of Quintuple Cancer. Diagn. Cytopathol. 2017, 45, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Gou, W.-F.; Yang, X.-F.; Shen, D.-F.; Zhao, S.; Sun, H.-Z.; Luo, J.-S.; Zheng, H.-C. Immunohistochemical Profile of ING3 Protein in Normal and Cancerous Tissues. Oncol. Lett. 2017, 13, 1631–1636. [Google Scholar] [CrossRef]
- Singer, K.; Dettmer, K.; Unger, P.; Schoenhammer, G.; Renner, K.; Peter, K.; Siska, P.J.; Bemeburg, M.; Herr, W.; Oefner, P.J.; et al. Topical Diclofenac Reprograms Metabolism and Immune Cell Infiltration in Actinic Keratosis. Front. Oncol. 2019, 9, 605. [Google Scholar] [CrossRef]
- Li, X.-J.; Liu, P.; Tian, W.-W.; Li, Z.-F.; Liu, B.-G.; Sun, J.-F. Mechanisms of CXCR7 Induction in Malignant Melanoma Development. Oncol. Lett. 2017, 14, 4106–4114. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhao, S.; Zheng, H.-C. MRNA and Protein of P33ING1 in Normal and Cancer Tissues. Transl. Cancer Res. 2020, 9, 3623–3633. [Google Scholar] [CrossRef]
- Baumgartner, E.; Ullman, D.; Jones, J.A.; Fasciano, D.; Atherton, D.S.; Pavlidakey, P.; Peker, D. Postradiation Histiocytic Sarcoma in the Setting of Muir-Torre Syndrome. Case Rep. Pathol. 2018, 2018, 5947870. [Google Scholar] [CrossRef] [PubMed]
- Fujii, E.; Funahashi, S.; Taniguchi, K.; Kawai, S.; Nakano, K.; Kato, A.; Suzuki, M. Tissue-Specific Effects of an Anti-Desmoglein-3 ADCC Antibody due to Expression of the Target Antigen and Physiological Characteristics of the Mouse Vagina. J. Toxicol. Pathol. 2020, 33, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Ghazi, N.; Saghravanian, N.; Ghazi, A.; Shakeri, M.T.; Khajehbahrami, H. CD44 Expression in Dysplastic and Non-Dysplastic Oral Lichen Planus. Int. J. Cancer Manag. 2020, 13, e98061. [Google Scholar] [CrossRef]
- Tang, T.; Zhang, D.-L. Detection of P53 and Bcl-2 Expression in Cutaneous Hemangioma through the Quantum Dot Technique. Oncol. Lett. 2017, 13, 2937–2944. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kollmann, D.; Ignatova, D.; Jedamzik, J.; Chang, Y.-T.; Jomrich, G.; Baierl, A.; Kazakov, D.; Michal, M.; French, L.E.; Hoetzenecker, W.; et al. PD-L1 Expression Is an Independent Predictor of Favorable Outcome in Patients with Localized Esophageal Adenocarcinoma. Oncoimmunology 2018, 7, e1435226. [Google Scholar] [CrossRef] [PubMed]
- Langton, A.K.; Ayer, J.; Griffiths, T.W.; Rashdan, E.; Naidoo, K.; Caley, M.P.; Birch-Machin, M.A.; O’Toole, E.A.; Watson, R.E.B.; Griffiths, C.E.M. Distinctive Clinical and Histological Characteristics of Atrophic and Hypertrophic Facial Photoageing. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 762–768. [Google Scholar] [CrossRef]
- Apprich, V.; Licka, T.; Freiler, S.; Gabriel, C. Equine Hoof Canker: Bovine Papillomavirus Infection Is Not Associated with Impaired Keratinocyte Differentiation. Vet. Pathol. 2020, 57, 525–534. [Google Scholar] [CrossRef] [PubMed]
- VOSviewer—Visualizing Scientific Landscapes. Available online: https://www.vosviewer.com/ (accessed on 13 August 2022).
- Fania, L.; Didona, D.; Di Pietro, F.R.; Verkhovskaia, S.; Morese, R.; Paolino, G.; Donati, M.; Ricci, F.; Coco, V.; Ricci, F.; et al. Cutaneous Squamous Cell Carcinoma: From Pathophysiology to Novel Therapeutic Approaches. Biomedicines 2021, 9, 171. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).