Glutathione Peroxidase 4 in Blunt Snout Bream (Megalobrama amblycephala) Regulates Ferroptosis and Inflammation in Response to Aeromonas hydrophila Infection
Abstract
1. Introduction
2. Material and Methods
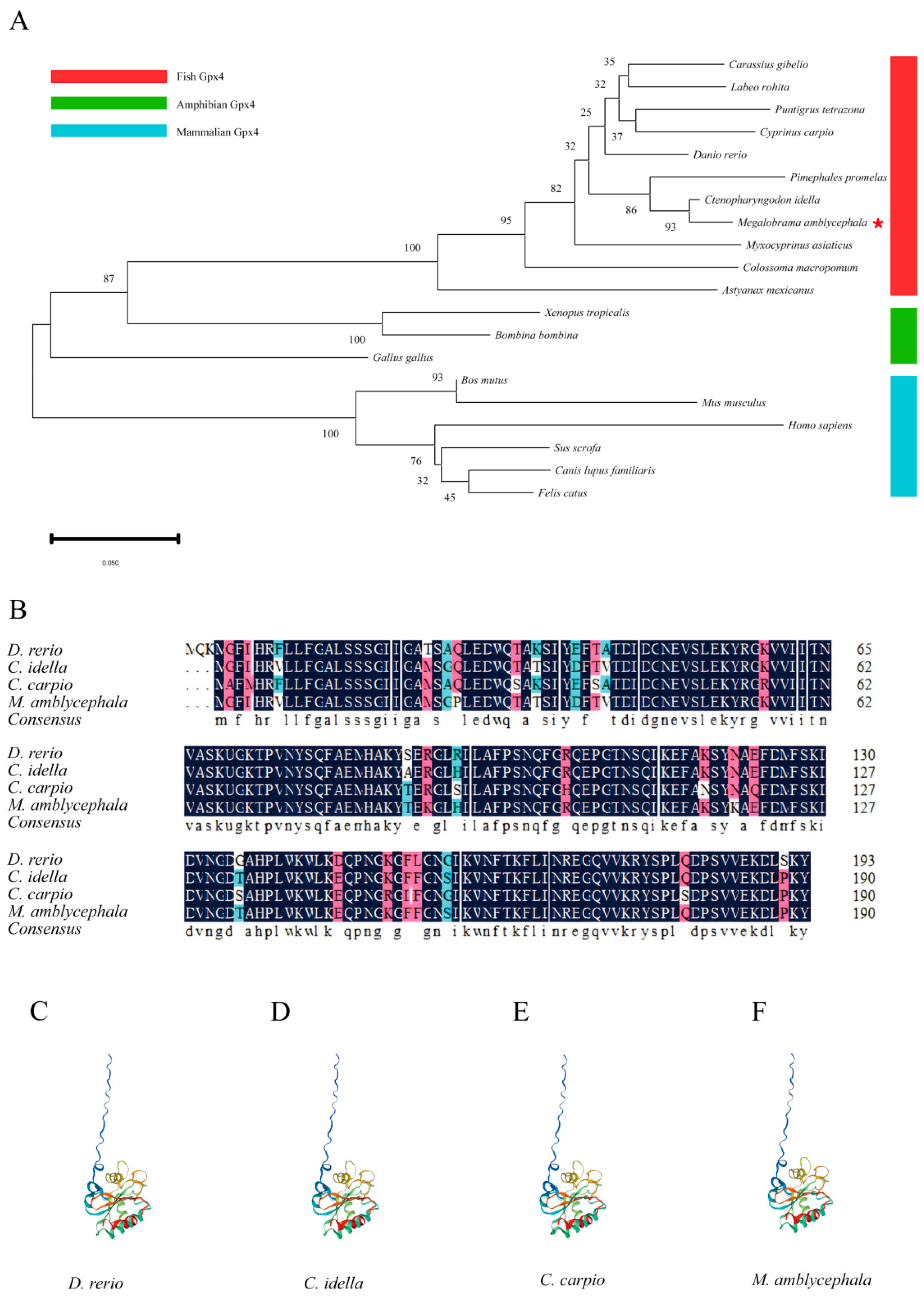
2.1. Phylogenetic Analysis, Homologous Alignment, and Protein Structure
2.2. Bacterial Challenge and Sample Collection
2.3. Plasmid Construction
2.4. Cell Culture and Transfection
2.5. Gene Overexpresstion
2.6. Bacterial Infection and Ferroptosis-Inducer RSL3 Treatment
2.7. RNA Extraction and Real-Time Quantitative PCR (RT-qPCR)
2.8. CCK-8 Cell Proliferation Assay
2.9. Measurement of MDA Content, GSH/GSSG Ratio, and ROS Generation
2.10. Statistical Analysis
3. Results
3.1. Characterization of Gpx4
3.2. Expression of gpx4 During Embryonic Development, in Healthy Fish and After A. hydrophila Infection
3.3. gpx4 Overexpression Inhibited Expression of Inflammatory Factors
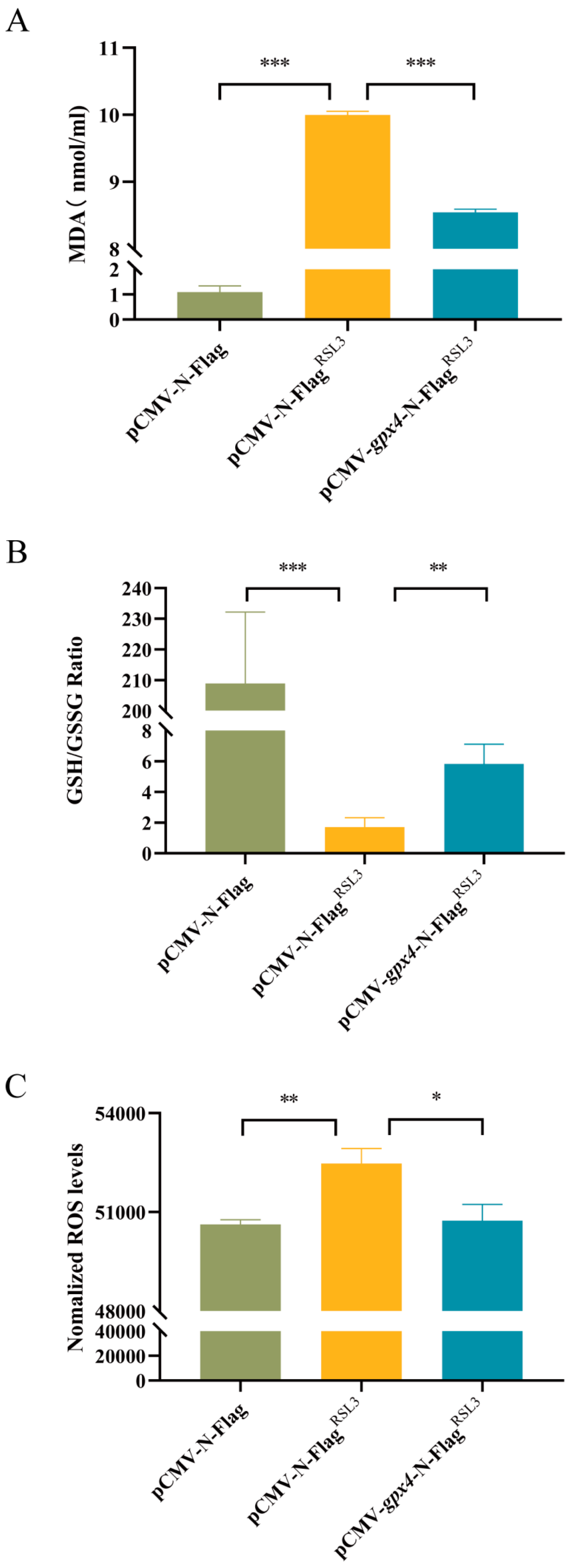
3.4. Overexpression of gpx4 Inhibits RSL3-Induced Ferroptosis in L8824 Cells
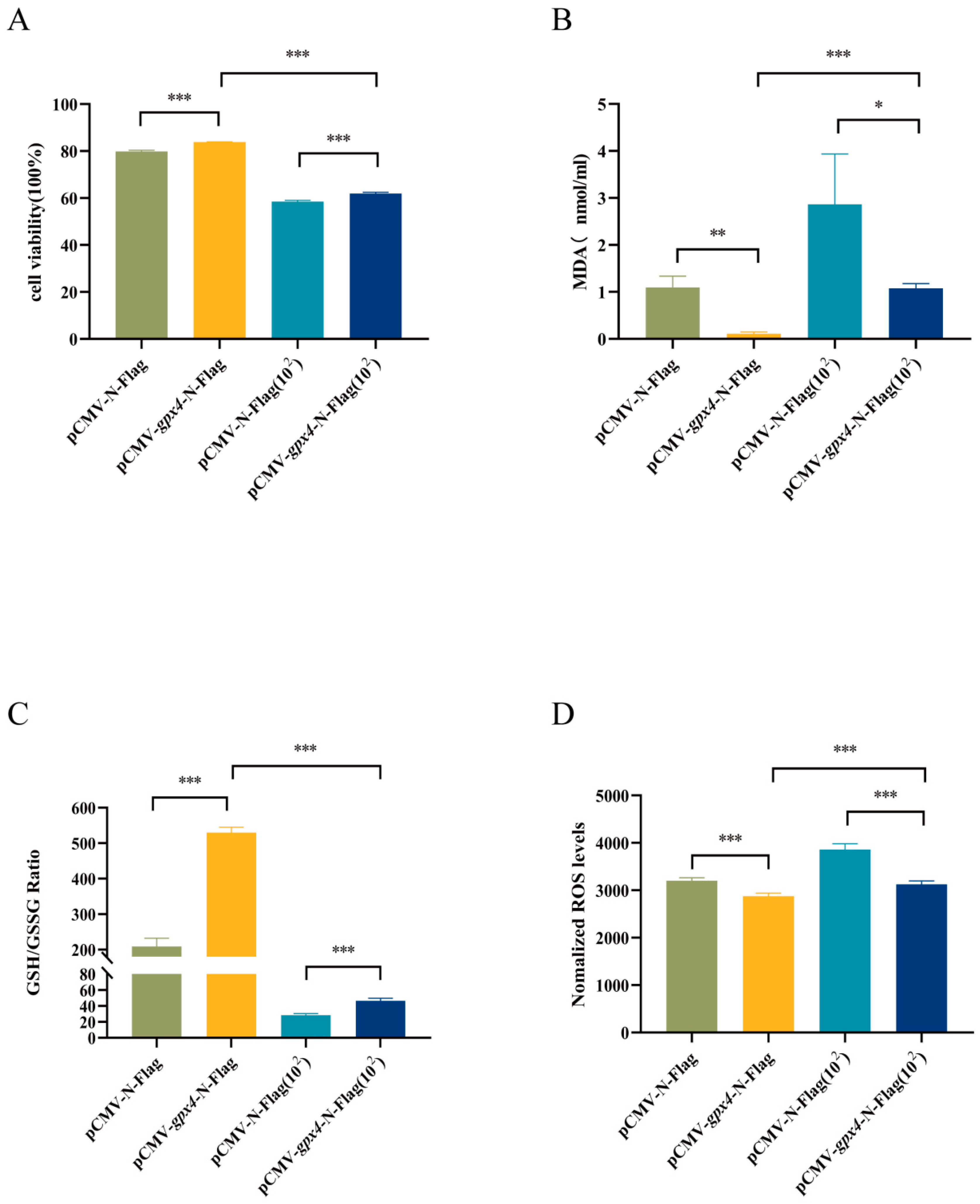
3.5. gpx4 Overexpression Enhanced Cell Viability and Inhibited Ferroptosis Induced by A. hydrophila Infection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kozińska, A. Dominant pathogenic species of mesophilic aeromonads isolated from diseased and healthy fish cultured in Poland. J. Fish. Dis. 2007, 30, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Awan, F.; Dong, Y.; Wang, N.; Liu, J.; Ma, K.; Liu, Y. The fight for invincibility: Environmental stress response mechanisms and Aeromonas hydrophila. Microb. Pathog. 2018, 116, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Kou, T.; Peng, L.; Munang, H.M.; Peng, B. Fructose promotes crucian carp survival against Aeromonas hydrophila infection. Front. Immunol. 2022, 13, 865560. [Google Scholar] [CrossRef]
- Sahoo, P.K.; Mahapatra, K.D.; Saha, J.N.; Barat, A.; Sahoo, M.; Mohanty, B.R.; Gjerde, B.; Odegård, J.; Rye, M.; Salte, R. Family association between immune parameters and resistance to Aeromonas hydrophila infection in the Indian major carp Labeo rohita. Fish Shellfish Immunol. 2008, 25, 163–169. [Google Scholar] [CrossRef]
- Fu, X.; Ding, Z.; Fan, J.; Wang, H.; Zhou, F.; Cui, L.; Boxiang, C.; Wang, W.; Liu, H. Characterization, promoter analysis and expression of the interleukin-6 gene in blunt snout bream Megalobrama amblycephala. Fish. Physiol. Biochem. 2016, 42, 1527–1540. [Google Scholar] [CrossRef]
- Ding, M.; Fan, J.; Wang, W.; Wang, H.; Liu, H. Molecular characterization, expression and antimicrobial activity of complement factor D in Megalobrama amblycephala. Fish Shellfish Immunol. 2019, 89, 43–51. [Google Scholar] [CrossRef]
- Rodríguez, I.; Novoa, B.; Figueras, A. Immune response of zebrafish (Danio rerio) against a newly isolated bacterial pathogen Aeromonas hydrophila. Fish Shellfish Immunol. 2008, 25, 239–249. [Google Scholar] [CrossRef]
- Hatha, M.; Vivekanandhan, A.A.; Joice, G.J. Christol, Antibiotic resistance pattern of motile aeromonads from farm raised fresh water fish. Int. J. Food Microbiol. 2005, 98, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Doukas, V.; Athanassopoulou, F.; Karagouni, E.; Dotsika, E. Short communication Aeromonas hydrophila infection in cultured sea bass. Dicentrarchus labrax L. and Puntazzo puntazzo Cuvier from the Aegean Sea. J. Fish. Dis. 1998, 21, 317–320. [Google Scholar] [CrossRef]
- Robello, E.; Galatro, A.; Puntarulo, S. Labile iron pool and ferritin content in developing rat brain gamma-irradiated in utero. Neurotoxicology 2009, 30, 430–435. [Google Scholar] [CrossRef]
- Tang, D.; Chen, X.; Kang, R.; Kroemer, G. Ferroptosis: Molecular mechanisms and health implications. Cell Res. 2021, 31, 107–125. [Google Scholar] [CrossRef]
- Wu, G.; Fang, Y.Z.; Yang, S.; Lupton, J.R.; Turner, N.D. Glutathione metabolism and its implications for health. J. Nutr. 2004, 134, 489–492. [Google Scholar] [CrossRef] [PubMed]
- Pompella, A.; Visvikis, A.; Paolicchi, A.; De Tata, V.; Casini, A.F. The changing faces of glutathione, a cellular protagonist. Biochem. Pharmacol. 2003, 66, 1499–1503. [Google Scholar] [CrossRef] [PubMed]
- Bielack, S.S.; Kempf-Bielack, B.; Delling, G.; Exner, G.U.; Flege, S.; Helmke, K.; Kotz, R.; Salzer-Kuntschik, M.; Werner, M.; Winkelmann, W.; et al. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: An analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J. Clin. Oncol. 2002, 20, 776–790. [Google Scholar] [CrossRef]
- Chen, X.; Kang, R.; Kroemer, G.; Tang, D. Ferroptosis in infection, inflammation, and immunity. J. Exp. Med. 2021, 218, e20210518. [Google Scholar] [CrossRef] [PubMed]
- Ursini, F.; Maiorino, M.; Valente, M.; Ferri, L.; Gregolin, C. Purification from pig liver of a protein which protects liposomes and biomembranes from peroxidative degradation and exhibits glutathione peroxidase activity on phosphatidylcholine hydroperoxides. Biochim. Biophys. Acta 1982, 710, 197–211. [Google Scholar] [CrossRef]
- Tang, D.; Kang, R.; Berghe, T.V.; Vandenabeele, P.; Kroemer, G. The molecular machinery of regulated cell death. Cell Res. 2019, 29, 347–364. [Google Scholar] [CrossRef]
- Wang, Y.; Quan, F.; Cao, Q.; Lin, Y.; Yue, C.; Bi, R.; Cui, X.; Yang, H.; Yang, Y.; Birnbaumer, L.; et al. Quercetin alleviates acute kidney injury by inhibiting ferroptosis. J. Adv. Res. 2021, 28, 231–243. [Google Scholar] [CrossRef]
- Li, C.; Deng, X.; Xie, X.; Liu, Y.; Angeli, J.P.F.; Lai, L. Activation of glutathione peroxidase 4 as a novel anti-inflammatory strategy. Front. Pharmacol. 2018, 9, 1120. [Google Scholar] [CrossRef]
- Kang, R.; Zeng, L.; Zhu, S.; Xie, Y.; Liu, J.; Wen, Q.; Cao, L.; Xie, M.; Ran, Q.; Kroemer, G.; et al. Lipid peroxidation drives gasdermin D-mediated pyroptosis in lethal polymicrobial sepsis. Cell Host Microbe 2018, 24, 97–108.e4. [Google Scholar] [CrossRef]
- Yang, K.; Ma, W.; Liang, H.; Ouyang, Q.; Tang, C.; Lai, L. Dynamic simulations on the arachidonic acid metabolic network. PLoS Comput. Biol. 2007, 3, e55. [Google Scholar] [CrossRef]
- Yang, K.; Bai, H.; Ouyang, Q.; Lai, L.; Tang, C. Finding multiple target optimal intervention in disease-related molecular network. Mol. Syst. Biol. 2008, 4, 228. [Google Scholar] [CrossRef]
- Pei, J.; Yin, N.; Ma, X.; Lai, L. Systems biology brings new dimensions for structure-based drug design. J. Am. Chem. Soc. 2014, 136, 11556–11565. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Liu, Y.; Lai, L. Diverse ways of perturbing the human arachidonic acid metabolic network to control inflammation. Acc. Chem. Res. 2015, 48, 2242–2250. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Kang, R.; Klionsky, D.J.; Tang, D. GPX4 in cell death, autophagy, and disease. Autophagy 2023, 19, 2621–2638. [Google Scholar] [CrossRef]
- Fang, J.; Yuan, Q.; Du, Z.; Zhang, Q.; Yang, L.; Wang, M.; Yang, W.; Yuan, C.; Yu, J.; Wu, G.; et al. Overexpression of GPX4 attenuates cognitive dysfunction through inhibiting hippocampus ferroptosis and neuroinflammation after traumatic brain injury. Free Radic. Biol. Med. 2023, 204, 68–81. [Google Scholar] [CrossRef] [PubMed]
- Amaral, E.P.; Foreman, T.W.; Namasivayam, S.; Hilligan, K.L.; Kauffman, K.D.; Bomfim, C.C.B.; Costa, D.L.; Barreto-Duarte, B.; Gurgel-Rocha, C.; Santana, M.F.; et al. GPX4 regulates cellular necrosis and host resistance in Mycobacterium tuberculosis infection. J. Exp. Med. 2022, 219, e20220504. [Google Scholar] [CrossRef]
- Sun, H.; Wang, D.; Ren, J.; Liu, J.; Wang, Z.; Wang, X.; Zhang, A.; Yang, K.; Yang, M.; Zhou, H. Vitamin D ameliorates Aeromonas hydrophila-induced iron-dependent oxidative damage of grass carp splenic macrophages by manipulating Nrf2-mediated antioxidant pathway. Fish Shellfish Immunol. 2023, 142, 109145. [Google Scholar] [CrossRef]
- Xu, T.; Cui, J.; Xu, R.; Cao, J.; Guo, M.Y. Microplastics induced inflammation and apoptosis via ferroptosis and the NF-κB pathway in carp. Aquat. Toxicol. 2023, 262, 106659. [Google Scholar] [CrossRef]
- Long, X.; He, K.; Zhang, M.; Li, M.; Wang, Z.; Wang, C.; Dong, X.; Shao, J.; Gan, L.; Hu, X.; et al. Temporal correlations of ferroptosis, inflammation and oxidative stress under acute ammonia exposure in brain tissue of yellow catfish (Pelteobagrus fulvidraco). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2023, 271, 109693. [Google Scholar] [CrossRef]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef]
- Wang, G.; Sun, Q.; Wang, H.; Liu, H. Identification and characterization of circRNAs in the liver of blunt snout bream (Megalobrama amblycephala) infected with Aeromonas hydrophila. Dev. Comp. Immunol. 2021, 124, 104185. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Hu, H.; Wei, W.; Wang, W.; Liu, H. Identification and characterization of microRNAs in the liver of blunt snout bream (Megalobrama amblycephala) infected by Aeromonas hydrophila. Int. J. Mol. Sci. 2016, 17, 1972. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Zhou, D.; Ding, N.Z.; Teng, C.B.; Yan, X.C.; Liang, Y. Common carp mef2 genes: Evolution and expression. Genes 2019, 10, 588. [Google Scholar] [CrossRef]
- Deng, H.; Zeng, L.; Chang, K.; Lv, Y.; Du, H.; Lu, S.; Liu, Y.; Zhou, P.; Mao, H.; Hu, C. Grass carp (Ctenopharyngodon idellus) Cdc25a down-regulates IFN 1 expression by reducing TBK1 phosphorylation. Dev. Comp. Immunol. 2021, 118, 104014. [Google Scholar] [CrossRef] [PubMed]
- Brigelius-Flohé, R.; Maiorino, M. Glutathione peroxidases. Biochim. Biophys. Acta 2013, 1830, 3289–3303. [Google Scholar] [CrossRef]
- Xie, X.; Chen, M.; Zhu, A. Molecular characterization and functional analysis of two phospholipid hydroperoxide isoforms from Larimichthys crocea under Vibrio parahaemolyticus challenge. Fish Shellfish Immunol. 2018, 78, 259–269. [Google Scholar] [CrossRef]
- Thompson, J.L.; See, V.H.; Thomas, P.M.; Schuller, K.A. Cloning and characterization of two glutathione peroxidase cDNAs from southern bluefin tuna (Thunnus maccoyii). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2010, 156, 287–297. [Google Scholar] [CrossRef]
- Tan, H.; Yue, T.; Xu, Y.; Zhao, J.; Xing, B. Microplastics reduce lipid digestion in simulated human gastrointestinal system. Environ. Sci. Technol. 2020, 54, 12285–12294. [Google Scholar] [CrossRef]
- Yin, L.; Liu, H.; Cui, H.; Chen, B.; Li, L.; Wu, F. Impacts of polystyrene microplastics on the behavior and metabolism in a marine demersal teleost, black rockfish (Sebastes schlegelii). J. Hazard. Mater. 2019, 380, 120861. [Google Scholar] [CrossRef]
- Chen, Q.; Lv, W.; Jiao, Y.; Liu, Z.; Li, Y.; Cai, M.; Wu, D.; Zhou, W.; Zhao, Y. Effects of exposure to waterborne polystyrene microspheres on lipid metabolism in the hepatopancreas of juvenile redclaw crayfish, Cherax quadricarinatus. Aquat. Toxicol. 2020, 224, 105497. [Google Scholar] [CrossRef]
- Heymann, F.; Tacke, F. Immunology in the liver--from homeostasis to disease. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 88–110. [Google Scholar] [CrossRef]
- Trivedi, P.J.; Adams, D.H. Gut-liver immunity. Hepatol. J. 2016, 64, 1187–1189. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Song, Y.L.; Wang, B.; Zhang, X.Y.; Zhang, X.J.; Wang, Y.L.; Cheng, Y.Y.; Chen, D.D.; Xia, X.Q.; Lu, Y.S.; et al. Fish gut-liver immunity during homeostasis or inflammation revealed by integrative transcriptome and proteome studies. Sci. Rep. 2016, 6, 36048. [Google Scholar] [CrossRef] [PubMed]
- Mayr, L.; Grabherr, F.; Schwärzler, J.; Reitmeier, I.; Sommer, F.; Gehmacher, T.; Niederreiter, L.; He, G.W.; Ruder, B.; Kunz, K.T.R.; et al. Dietary lipids fuel GPX4-restricted enteritis resembling Crohn’s disease. Nat. Commun. 2020, 11, 1775. [Google Scholar] [CrossRef]
- Shen, K.; Wang, X.; Wang, Y.; Jia, Y.; Zhang, Y.; Wang, K.; Luo, L.; Cai, W.; Li, J.; Li, S.; et al. miR-125b-5p in adipose derived stem cells exosome alleviates pulmonary microvascular endothelial cells ferroptosis via Keap1/Nrf2/GPX4 in sepsis lung injury. Redox Biol. 2023, 62, 102655. [Google Scholar] [CrossRef] [PubMed]
- Shou, Y.; Yang, L.; Yang, Y.; Xu, J. Inhibition of keratinocyte ferroptosis suppresses psoriatic inflammation. Cell Death Dis. 2021, 12, 1009. [Google Scholar] [CrossRef]
- Amaral, E.P.; Costa, D.L.; Namasivayam, S.; Riteau, N.; Kamenyeva, O.; Mittereder, L.; Mayer-Barber, K.D.; Andrade, B.B.; Sher, A. A major role for ferroptosis in Mycobacterium tuberculosis-induced cell death and tissue necrosis. J. Exp. Med. 2019, 216, 556–570. [Google Scholar] [CrossRef]
- Dong, H.; Qiang, Z.; Chai, D.; Peng, J.; Xia, Y.; Hu, R.; Jiang, H. Nrf2 inhibits ferroptosis and protects against acute lung injury due to intestinal ischemia reperfusion via regulating SLC7A11 and HO-1. Aging 2020, 12, 12943–12959. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhai, Y.; Chen, J.; Xu, X.; Wang, H. Kaempferol ameliorates oxygen-glucose deprivation/reoxygenation-induced neuronal ferroptosis by activating Nrf2/SLC7A11/GPX4 axis. Biomolecules 2021, 11, 923. [Google Scholar] [CrossRef]
- Bai, Q.; Liu, J.; Wang, G. Ferroptosis, a regulated neuronal cell death type after intracerebral hemorrhage. Front. Cell Neurosci. 2020, 14, 591874. [Google Scholar] [CrossRef]
- Shintoku, R.; Takigawa, Y.; Yamada, K.; Kubota, C.; Yoshimoto, Y.; Takeuchi, T.; Koshiishi, I.; Torii, S. Lipoxygenase-mediated generation of lipid peroxides enhances ferroptosis induced by erastin and RSL3. Cancer Sci. 2017, 108, 2187–2194. [Google Scholar] [CrossRef] [PubMed]
- Endale, H.T.; Tesfaye, W.; Mengstie, T.A. ROS induced lipid peroxidation and their role in ferroptosis. Front. Cell Dev. Biol. 2023, 11, 1226044. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sun, Q.; Wang, G.; Wang, H.; Liu, H. The effects of blunt snout bream (Megalobrama amblycephala) IL-6 trans-signaling on immunity and iron metabolism via JAK/STAT3 pathway. Dev. Comp. Immunol. 2022, 131, 104372. [Google Scholar] [CrossRef]
- Wang, J.; Sun, Q.; Zhang, J.; Wang, H.; Liu, H. Classical signaling and trans-signaling pathways stimulated by Megalobrama amblycephala IL-6 and IL-6R. Int. J. Mol. Sci. 2022, 23, 2019. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Wang, G.; Wu, D.; Song, A.; Wang, H.; Liu, H. Blunt snout bream (Megalobrama amblycephala) circARHGEF15 enhances antibacterial immunity against Aeromonas hydrophila infection via ceRNA regulatory axis. Aquaculture 2024, 590, 741080. [Google Scholar] [CrossRef]
- Yang, W.S.; Stockwell, B.R. Synthetic lethal screening identifies compounds activating iron-dependent, nonapoptotic cell death in oncogenic-RAS-harboring cancer cells. Chem. Biol. 2008, 15, 234–245. [Google Scholar] [CrossRef]
- Chen, X.; Kang, R.; Kroemer, G.; Tang, D. Broadening horizons: The role of ferroptosis in cancer. Nat. Rev. Clin. Oncol. 2021, 18, 280–296. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, Z.; Zhou, X.; Zhao, Z.; Zhao, R.; Xu, X.; Kong, X.; Ren, J.; Yao, X.; Wen, Q.; et al. Microglia and macrophage exhibit attenuated inflammatory response and ferroptosis resistance after RSL3 stimulation via increasing Nrf2 expression. J. Neuroinflamm. 2021, 18, 249. [Google Scholar] [CrossRef]
- Jiang, C.; Shi, Q.; Yang, J.; Ren, H.; Zhang, L.; Chen, S.; Si, J.; Liu, Y.; Sha, D.; Xu, B.; et al. Ceria nanozyme coordination with curcumin for treatment of sepsis-induced cardiac injury by inhibiting ferroptosis and inflammation. J. Adv. Res. 2024, 63, 159–170. [Google Scholar] [CrossRef]
- Xing, G.; Meng, L.; Cao, S.; Liu, S.; Wu, J.; Li, Q.; Huang, W.; Zhang, L. PPARα alleviates iron overload-induced ferroptosis in mouse liver. EMBO Rep. 2022, 23, e52280. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, S.; Li, H.; Lou, L.; Huang, Q.; Zhang, Z.; Mo, J.; Li, M.; Lu, J.; Zhu, K.; Chu, Y.; et al. Inhibition of STAT3-ferroptosis negative regulatory axis suppresses tumor growth and alleviates chemoresistance in gastric cancer. Redox Biol. 2022, 52, 102317. [Google Scholar] [CrossRef]
- Stockwell, B.R. Ferroptosis turns 10: Emerging mechanisms, physiological functions, and therapeutic applications. Cell 2022, 185, 2401–2421. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.J.; Yin, M.; Wang, Z.J.; Wang, X.P. Transplanted neural stem cells: Playing a neuroprotective role by ceruloplasmin in the substantia nigra of PD model rats? Oxid. Med. Cell Longev. 2015, 2015, 618631. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.S.; Deng, Y.; Guo, S.L.; Li, J.Q.; Zhou, Y.C.; Liao, J.; Wu, D.D.; Lan, W.F. Endothelial cell ferroptosis mediates monocrotaline-induced pulmonary hypertension in rats by modulating NLRP3 inflammasome activation. Sci. Rep. 2022, 12, 3056. [Google Scholar] [CrossRef]
- Chen, H.; Qian, Y.; Jiang, C.; Tang, L.; Yu, J.; Zhang, L.; Dai, Y.; Jiang, G. Butyrate ameliorated ferroptosis in ulcerative colitis through modulating Nrf2/GPX4 signal pathway and improving intestinal barrier. Biochim. Biophys. Acta Mol. Basis Dis. 2024, 1870, 166984. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, M.; Wang, H.; Liu, H. Glutathione Peroxidase 4 in Blunt Snout Bream (Megalobrama amblycephala) Regulates Ferroptosis and Inflammation in Response to Aeromonas hydrophila Infection. Curr. Issues Mol. Biol. 2025, 47, 326. https://doi.org/10.3390/cimb47050326
He M, Wang H, Liu H. Glutathione Peroxidase 4 in Blunt Snout Bream (Megalobrama amblycephala) Regulates Ferroptosis and Inflammation in Response to Aeromonas hydrophila Infection. Current Issues in Molecular Biology. 2025; 47(5):326. https://doi.org/10.3390/cimb47050326
Chicago/Turabian StyleHe, Miao, Huanling Wang, and Hong Liu. 2025. "Glutathione Peroxidase 4 in Blunt Snout Bream (Megalobrama amblycephala) Regulates Ferroptosis and Inflammation in Response to Aeromonas hydrophila Infection" Current Issues in Molecular Biology 47, no. 5: 326. https://doi.org/10.3390/cimb47050326
APA StyleHe, M., Wang, H., & Liu, H. (2025). Glutathione Peroxidase 4 in Blunt Snout Bream (Megalobrama amblycephala) Regulates Ferroptosis and Inflammation in Response to Aeromonas hydrophila Infection. Current Issues in Molecular Biology, 47(5), 326. https://doi.org/10.3390/cimb47050326





